Abstract
Spinal cord stimulation (SCS) has been described as a valuable neuromodulator procedure in the management of chronic medically untreated neuropathic pain. Although the use of this technique has been published in many papers, a question still remains regarding its applicability in pregnant patients. The goal of this paper is to discuss the risks, complications, and results as well as the prognosis of SCS in pregnant patients. We performed a systematic review from 1967 to 2018 using the databases MEDLINE, LILACS, SciELO, PubMed, and BIREME, utilizing language as selection criteria. Eighteen studies that met our criteria were found and tabulated. SCS is a reversible and adjustable surgical procedure, which results in patients that demonstrated a significant effect in the reduction of pain intensity in pregnant patients. The etiologies most frequent were complex regional pain and failed back pain syndromes, which together represented 94% of analyzed cases. The technical complications most frequent were lead migration (3%, n = 1). Regarding the risks, the authors did not show significative factors among the categorical variables that can suggest a teratogenicity, while the maternal risks have been associated to the consequences of technical complications due to, among other factors, improvement of abdominal pressure during pregnancy and delivery. Finally, although there are not significative cohorts of pregnant patients, the procedure is still an effective surgical approach of neuropathic pain associated to lower rates of complications and significative improvement in the quality of life of patients during pregnancy.
Keywords: Neuropathic pain, pain management, pregnancy, spinal cord stimulation
Introduction
The physiological and body changes of pregnancy associated to nonobstetrical painful conditions, preexisting or developed during pregnancy, imply in the significant reduction in the quality of life in these women.[1,2,3,4,5,6,7,8] Although the pharmacological and surgical managements of pain have been shown a significant development, some considerations in pregnant patients, who complain of pain, such as the potential risk of teratogenicity and fetal toxicity of treatment, complications during pregnancy, and outcome of neonate and pregnant, remain as a lack in knowledge of this common complaint. Although the first description of spinal cord stimulation (SCS) had been performed in 1967[9] and it has been estimated that higher than 12,000 SCS systems are sold every year in worldwide associated to many essays showing significant results in a wide range of pain disorders,[10] the first description of this technique in pregnant patients occurred only in 1999.[11] Therefore, the adequate surgical management of medically refractory neuropathic pain in pregnant patients performed by SCS system still has been described as a challenge.
This study aims to clarify the maternal and neonate risks, complications, and prognosis about the use of SCS in the treatment of chronic neuropathic pain during pregnancy described in the literature at the moment, emphasizing the results of SCS regarding the control of pain and teratogenicity of procedure.
Methods
Systematic bibliographical consultation was performed from 1967 (first description of SCS[9]) to 2018, using as keywords “Spinal Cord Stimulation,” “Pregnant,” “Pregnancy,” and “Pregnancy Outcome” on the databases MEDLINE, LILACS, SciELO, PubMed, and BIREME, utilizing language as selection criteria, choosing preferably recent articles in Portuguese, Spanish, or English and only articles based on clinical studies [Figure 1].
Figure 1.
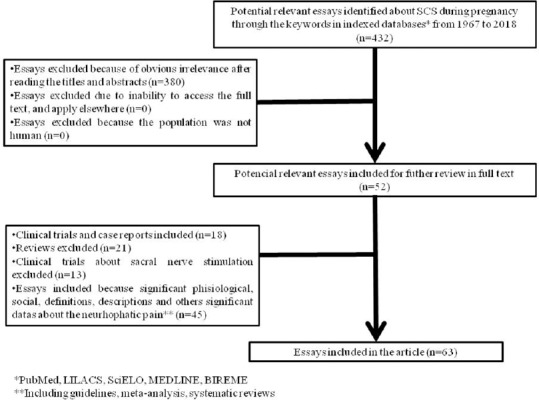
Selection of articles for base this article
Consideration in nonsurgical management of pain during pregnancy
Although the obstetric pain during pregnancy is a common complaint in pregnant patient, the nonobstetric pain has been described associated to higher rates of reduction in the quality of life in these patients. Among the nonobstetric pain, the migraine, musculoskeletal, pelvicoabdominal, rheumatologic, and neuropathic pain syndromes have been described commonly in the literature.[1,7,12,13,14] Currently, the management of neuropathic and other painful syndromes is based on the multidisciplinary team and occupational therapies are associated to pharmacological and surgical treatments.[1,2,7,8,15,16,17,18,19] Although most acute pain cases during pregnancy had treated fastly, the problem arises when pain does not present improvement and becomes chronic or when the patient with a history of chronic pain becomes pregnant implying in the use of therapeutic modalities in combination and consequently the increase in the teratogenicity and fetal toxicity, either because of the availability of safe drugs or because of lack of knowledge of the problem.[1,2,8]
The teratogenic risks in the pharmacological treatment of pain have been estimated around from 2% to 3% of all birth defects, excluding the use of “social” drugs (mainly alcohol).[20] Although the actual recommendation is to restrict, in pregnant patients, the use of drugs unnecessarily, many authors have been described the high rates of pharmacological treatment, illicit and “social” drugs during pregnancy, mainly in the 1st week in which the diagnosis of pregnancy is still unknown.[1,7,20,21,22,23,24]
The teratogenicity and toxicity of drugs resulting in abnormal development of neonate can be different depending on the gestational week in that occurred the exposure.[20,21] While the cardiovascular system has been described associated to teratogenic effects during the 3rd and 4th weeks of gestation, central nervous and skeletal system have been associated to defects in the exposure from the beginning of the 3rd week to the end of pregnancy and into the neonatal period.[20,21,25]
Finally, based on many studies with regard to the teratogenic effects and side effects of pharmacological treatment during pregnancy, it has been shown in the literature a significant enhancement in the surgical management of medically refractory pain in pregnant patients, among which the SCS has been gaining prominence (focus of the present essay).
Results
Eighteen studies composed by 25 pregnant patients and 32 pregnancies were included and were individually and comparatively analyzed in this systematic review [Tables 1 and 2]. Based on these studies, the mean age of the patients at SCS system implantation and at pregnancy diagnosis was 30 ± 4.04 and 33.5 ± 4.0 years, respectively. Although 92% (n = 23/25) of the patients presented the SCS system implanted previously the pregnancy discovered, 8% (n = 1/25) presented the surgical implantation performed during the 1st and 8th week after the conception.[26,27]
Table 1.
Clinical features and prognostic of spinal cord stimulation in pregnant patients affected by neuropathic pain syndromes
| Authors/year | n | Patient age | Gestational age (weeks) | Etiology | Location of SCS system | Results in pain control | Complications | Outcome | Follow-up | Treatment associated | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASSI (years) | AP (years) | Prenatal | Postnatal | Pregnant | Neonate | Delivery | ||||||||
| Segal, 1999[11] | 1 | 30 | 31 | SCS system implanted previously | CRPS | Leads: C2-C3 cervical level, IPG: NR | Pain relief higher than 90% of baseline | None | None | Healthy | Healthy | Full-term baby, type: NR | 13 months | None |
| Hanson and Goodman, 2006[34] | 1* | 34 | 37 | SCS system implanted previously | CRPS | Leads: C3 cervical level, IPG: LUB | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: NR | NR | Epidural analgesia: Bupivacaine 0.125% 10 mL, fentanyl: 5l g/mL, and epinephrine: 1:600.000 |
| Saxena and Eljamel, 2009[35] | 1 | 21 | 30 | SCS system implanted previously | Neuritis of unknown infection | Lead: T6 thoracic level, IPG: AAW | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: NR | NR | In the 28th week, the IPG was repositioned to AAW |
| Bernardini et al., 2010[36] | 2 | 33 | 34 | SCS system implanted previously and turned off during pregnancy | CRPS | Leads: T9-T10 thoracic level, IPG: LUB | Pain relief higher than 75% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Vaginal | 48 months | None |
| 34 | 39 | SCS system implanted previously and turned off during pregnancy | CRPS | Leads: C4 cervical level, IPG: LUB | Pain relief higher than 80% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Caesarean* | 48 months | Acetaminophen | ||
| Sommerfield et al., 2010[37] | 1 | 33 | 35 | SCS system implanted previously | FBSS | 3 leads: T8 thoracic level, IPG: LUB | Pain relief higher than 50% of baseline | IUGR | None | Healthy | Healthy | Preterm baby, type: Cesarean | NR | Once a day: Bisoprolol 10 mg, morphine 40 mg, diazepam 15 mg, and tramadol 400 mg |
| Takeshima et al., 2010[38] | 1** | 28 | 34 | SCS system implanted previously | FBSS | Leads: T12 thoracic level, IPG: AAW | Pain relief higher than 50% of baseline | None | Lead breakage after third vaginal birth | Healthy | Healthy | Full-term baby, type: Vaginal | 50 months | None |
| Yoo et al., 2010[27] | 1 | 32 | 32 | ~8 weeks | CRPS | Leads: T9-T10 thoracic level, IPG: AAW | Pain relief higher than 50% of baseline | Aborted | None | Healthy | Aborted | NR | 1, 5 months | Twice a day: Propranolol 20 mg, mirtazapine 30 mg, and tramadol 50 mg Once a day: buspirone 15 mg, solifenacin succinate 5 mg, and mefenamic acid 250 mg Ethyl loflazepate: 1 mg, sodium tianeptine: 12.5 mg |
| Fedoroff et al., 2012[39] | 1*** | 31 | 34 | SCS system implanted previously and turn off during pregnancy | FBSS | Leads: T10 thoracic level, IPG: AAW | Pain relief higher than 50% of baseline | Hardware malfunction | None | Healthy | Healthy | Full-term baby Preterm baby, type: Both cesarean | ~36 months | Six times a day: Oxycodone 5 mg, acetaminophen 325 mg Three times a day: Gabapentin 300 mg |
| Gredilla et al., 2012[40] | 1 | 31 | 32 | SCS system implanted previously | FBSS | Leads: C2 + T8 thoracic level, IPG: NR | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, Type: cesarean | NR | None |
| Domínguez Suárez et al., 2012[41] | 1 | 34 | 36 | SCS system implanted previously | CRPS | Leads: T7-T8 thoracic level, IPG: NR | None | None | None | Healthy | NR | NR | NR | None |
| Ito et al., 2013[5] | 1 | 35 | 40 | SCS system implanted previously | CRPS | Leads: T10-T11 thoracic level, IPG: LUB | Pain relief higher than 50% of baseline | Aborted**** | None | Healthy | Healthy | Full-term baby, type: cesarean | ~48 months | None |
| Das and McCrory, 2014[42] | 1 | 32 | 33 | SCS system implanted previously and turn off during pregnancy | FBSS | Leads: T9-T10 thoracic level, IPG: RUB | Pain relief higher than 50% of baseline | Hypertension | None | Healthy | Healthy | Gestational week birth: NR, type: Cesarean | 4 months | Oxycontin |
| Edelbroek and Terheggen, 2015[4] | 1 | 35 | 36 | SCS system implanted previously | FBSS | Leads: T8-T9 thoracic level, IPG: NR | Pain relief higher than 50% of baseline | Aborted***** | None | Healthy | Healthy ***** | Full-term baby, type: Caesarean | ~48 months | None |
| Young et al., 2015[43] | 7 | 26 | 29 | SCS system implanted previously and turn off during pregnancy | CRPS | Leads: thoracic level, IPG: AAW | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Pre-term baby, type: Cesarean | NR | None |
| 23 | 38 | SCS system implanted previously | Leads: cervical level, IPG: AAW | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Cesarean | NR | None | |||
| 33 | 35 | SCS system implanted previously and turn off during pregnancy | Leads: cervical level, IPG: Right flank | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Cesarean | NR | None | |||
| 34 | 35 | SCS system implanted previously and turn off during pregnancy | Leads: cervical and thoracic levels, IPG: Left flank | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Preterm baby, type: Cesarean | NR | None | |||
| 30 | 33 | SCS system implanted previously | Leads: thoracic level, IPG: Left flank | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Preterm baby, type: Cesarean | NR | None | |||
| 30 | 39 | SCS system implanted previously | Leads: cervical level, IPG: LUB | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Vaginal | NR | None | |||
| 31 | 34 | SCS system implanted previously | Leads: cervical level, IPG: RUB | Pain relief higher than 50% of baseline | None | Baby developed foot drop | Healthy | Healthy | Full-term baby, type: Vaginal | NR | None | |||
| Moussa et al., 2016[44] | 1 | 25 | NR | SCS system implanted previously | CRPS | Leads: T9 thoracic level, IPG: NR | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Vaginal | NR | None |
| Ahmed et al., 2016[26] | 1 | 26 | 26 | 1 | CRPS | Leads: C3-C5 cervical level, IPG: LUB | Pain relief higher than 50% of baseline | None | None | Healthy | Healthy | Full-term baby, type: Vaginal | 36 months | None |
| Pablo et al., 2017[45] | 1 | 25 | 26 | SCS system implanted previously | CRPS | Leads: NR, IPG: NR | Pain relief higher than 50% of baseline | GDM and preeclampsia***** | None | Healthy | Healthy | Full-term baby, type: Vaginal | 2 months | None |
| Ortín et al., 2017[46] | 1 | 25 | 26 | SCS system implanted previously | CRPS | Leads: NR, IPG: NR | Pain relief higher than 50% of baseline | GDM and preeclampsia****** | None | Healthy | Healthy | Full-term baby, type: Vaginal | 2 months | None |
*Patient underwent to two cesarean delivery with SCS system implanted and absence of prenatal and postnatal complications; **Patient underwent to three vaginal deliveries with SCS system implanted and absence of prenatal and postnatal complications; ***Patient underwent to two cesarean deliveries with SCS system implanted; ****After the implantation of the SCS, the patient underwent two further chemical abortions of natural concept uses, at the ages of 35 and 37 and at the age of 39, she underwent another abortion in the 2nd month of a pregnancy resulting from artificial insemination; *****Patient had a miscarriage after 2 weeks of SCS system turn off (8 weeks pregnancy), while after 1 year, patient did not turn off the system and it has been showed the absence of complications; ******The patient had a obstetric history of hypertension, mild preeclampsia, and DMG during a previous pregnancy. n – Number of patients; AAW – Anterior abdominal wall; ASSI – At SCS system implantation (years); AP – At pregnancy (years); CRPS – Complex regional pain syndrome; FBSS – Failed back pain syndrome; GDM – Gestational diabetes; LUB – Left upper buttock; IUGR – Intrauterine growth restriction; IPG – Implantable pulse generator; NR – Not reported; SCS – Spinal cord stimulation; TTO - Treatment associated to SCS
Table 2.
Comparative results of clinical features and prognostic of spinal cord stimulation in pregnant patients affected by neuropathic pain syndromes
| Number of essays | n | Number of pregnancies | Patient age* | Gestational age* | Etiology | Location of SCS system | Results in pain control | Complications | Outcome | Follow- up* | Treatment associated | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASSI (years) | AP (years) | Prenatal | Postnatal | Pregnant | Neonate | Delivery | |||||||||
| 18 | 25 | 32 | 30±4.04 | 33.5±4.0 | SCS system implanted previously: 92% (n=23/25) 1 week: 4% (n=1/25) 8 weeks: 4% (n=1/25) |
CRPS: 72% (n=18/25) FBSS: 24% (n=6/25) Neuritis by unknown infection: 4% (n=1/25) |
Leads** Cervical: 40% (n=10/25) Thoracic: 60% (n=15/25) NR: 8% (n=2/25) IPG UB: 36% (n=9/25) AAW: 24% (n=6/25) Flank: 12% (n=3/25) NR: 28% (n=7/25) |
Pain relief higher than 50% of baseline: 84% (n=21/25) Higher than 75%: 12% (n=3/25) None effect: 4% (n=1/25) |
Absence: 70% (n=22/32) IUGR: 3% (n=1/32) Hardware malfunction: 3% (n=1/32) Hypertension: 9% (n=3/32)*** Aborted: 9% (n=3/32)**** DMG: 6% (n=2/32)*** |
Absence: 94% (n=30/32) Lead breakage: 3% (n=1/32)**** Foot drop: 3% (n=1/32) |
Healthy: 100% (n=32/32) | Healthy: 88% (n=28/33) Aborted: 9% (n=3/33)*** NR: 3% (n=1/33) |
Vaginal: 31% (n=10/32) Caesarean: 12% (n=14/32) NR: 15% (n=5/32) Full term: 66% (n=21/32) Preterm: 15% (n=5/32) NR: 9% (n=3/32) |
28.1±21 months NR: 52% (n=13) |
None: 76% (n=19) Used pharmacological treatment associated: 24% (n=6) |
*Adopted measurements of mean tendency, **Two cases with cervical and thoracic leads simultaneously, ***Patients with previous clinical conditions or history of complications, ****After third vaginal birth. n – Number of patients; AAW – Anterior abdominal wall; SCS – Spinal cord stimulation; ASSI – At SCS system implantation; AP – At pregnancy; CRPS – Complex regional pain syndrome; FBSS – Failed back pain syndrome; UB – Upper buttock; IUGR – Intrauterine growth restriction; IPG – Implantable pulse generator; NR – Not reported; DMG – Diabetes mellitus gestation
Etiologically, the patients were affected by complex regional pain syndrome (CRPS) in 72% (n = 18/25) and failed back pain syndrome (FBSS) in 24% (n = 6/25) of patients, and in 4% (n = 1/25) of patients, the diagnosis adopted by the authors was a neuritis secondary to an unknown infection.
In the technical evaluation of SCS system implantation, by the way of topography of diseases, the leads were positioned in the cervical spine in 40% (n = 10/25) of patients and thoracic spine in 60% (n = 15/25) of cases, stressing that 8% (n = 2/25) of cases were underwent to cervical and thoracic leads implantation simultaneously. Regarding the implantable pulse generator (IPG), they were positioned in the upper buttock (UB) region in 36% (n = 9/25), anterior abdominal wall (AAW) in 24% (n = 6/25), and flank region in 12% (n = 3/25) of cases, and 28% (n = 7/25) of cases had been described without the data of IPG location of implantation. Finally, although this systematic review did not evaluate the parameter of stimulation, the authors showed that 79% (n = 26/33) of pregnancies were conducted with normal stimulation by system, while 21% (n = 7/33) of pregnancies were conducted with the system turn off during pregnancy.
The pain relief evaluation of cases was performed based on three criteria as follows: (1) complete pain relief, (2) pain relief higher than 50% of baseline, and (3) no pain relief. Although a complete pain relief was not shown in these cases, the pain relief is higher than 50% of baseline in 84% (n = 21/25) of patients and none effect in 4% (n = 1/25) of pregnant patients. Stressing that, 12% (n = 3/25) of these cases reported the reduction of pain intensity higher than 75% when compared with baseline.
During the prenatal and postnatal period, the absence of complications was shown in 70% (n = 22/32) and 94% (n = 30/32) of pregnancies, respectively. In the prenatal period, the intrauterine growth restriction (IUGR) was shown in 3% (n = 1/32), hardware malfunction in 3% (n = 1/32), systemic arterial hypertension (HAS) in 9% (n = 3/32), abortion in 9% (n = 3/32), and gestational diabetes (DMG) in 6% (n = 2/32) of pregnancies. Although the cases of abortion, DMG, and HAS presented these above-mentioned clinical conditions or history of complications during pregnancy, the development of foot drop and lead breakage in 3% (n = 1/32) of different pregnancies was presented as complication in the postnatal period.
In the analysis of delivery conditions, the pregnancies evolved to vaginal pathway is seen in 31% (n = 10/32) and cesarean in 12% (n = 14/32) of pregnants, although 15% (n = 5/32) of papers reported any data source. Regarding the neonate conditions, the full-term births were shown in 66% (n = 21/32) and the preterm births in 15% (n = 5/32) of pregnancies, although 9% (n = 3/32) of papers reported any of these data.
The outcome analysis showed maternal and neonate healthy in 100% (n = 32/32) and 88% (n = 28/33) of pregnancies, respectively. The authors observed, in the neonate outcome analysis, the presence of three miscarriages (9%, n = 3/33) and one report published without these data. Although the follow-up analyses showed a mean of 28.1 ± 21 months of evaluation, it was observed the absence of data in many of essays published in the literature (52%, n = 13).
Finally, the treatment associated was not adopted in 76% (n = 19) of cases, while 24% (n = 6) of patients adopted the pharmacological treatment associated to SCS. Regarding the epidural or oral pharmacological treatment adopted by these patients, the bupivacaine, fentanyl, epinephrine, bisoprolol, morphine, diazepam, tramadol, propranolol, mirtazapine, tramadol, buspirone, solifenacin succinate, mefenamic acid, ethyl loflazepate, sodium tianeptine, oxycodone, acetaminophen, and gabapentin were described in the literature for the treatment of these patients. Stressing that, 43% (n = 3/7) of SCS system disconnected adopted the pharmacological treatment as additional analgesic therapeutic.
Discussion
Based on the literature and author's experience, the evaluation of SCS effects during pregnancy of patients affected by neuropathic pain syndromes is still initial and controversial. The published essays had been based on individual descriptions of experiences [Figure 2], and therefore, there is not any significative cohort of patients evaluated with categorical variables evaluated standardized, such as pain relief rates and significative meantime in follow-up, published in the literature at the moment.
Figure 2.
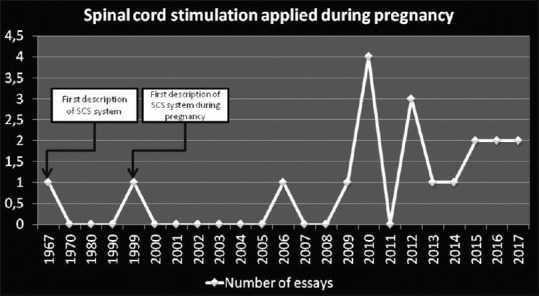
Essays correlating spinal cord stimulation and pregnancy published in the literature
In the literature, it has been described an increase in the number of patients affected by medically refractory neuropathic pain, whose actual prevalence has been estimated ranging from 6.9% to 8% in general population – stressing that 74% of these cases presented moderate-to-severe intensity and the neuropathic pain represents higher than 17% of patients’ complaint with pain.[1,2,7,8,16,17,18,19]
To determine the cause of a pain is essential to effective management of it.[10,18,19] Regarding the etiology of neuropathic pain, the use of SCS has been described in cases of central deafferentation, central pain, phantom limb pain, causalgia, myelopathic pain, oncologic pain, lumbosacral fibrosis, postherpetic neuralgia, FBSS, CRPS, reflex sympathetic dystrophy, spinal cord or brainstem or brain injury, and other etiologies.[18,28,29,30,31,32,33] There are many different kinds of neuropathic pain and there is no reason to believe that one procedure will be superior in all conditions.[18]
Based on this systematic review, the main etiologies that have been described in pregnant patients were the CRPS and FBSS. Nonpregnant and pregnant patients affected by CRPS and FBSS have demonstrated significant rates of success in pain control associated to a low rate of complications although the total pain control rarely was obtained through the use of SCS individually as therapy [Tables 3 and 4, adapted from Camporeze et al., 2017.[18,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] Furthermore, this procedure has been described associated to a significant cost-effectiveness rates when compared the conventional pharmacological pain management.[18,52,53,54,55,56,57,58]
Table 3.
Neuropathic pain control rates of spinal cord stimulation in nonpregnant and pregnant patients
| Obstetric status | Authors | Years | n | Etiology | Complete pain relief with SCS | Mean pain relief higher than 50% of baseline | Mean follow-up (months) |
|---|---|---|---|---|---|---|---|
| Nonpregnant | Kumar et al.[28] | 2007 | 100 | FBSS | 0% of patients | 88% of patients | 60 |
| Olsson et al.[29] | 2008 | 7 | CRPS | 71% of patients | 100% of patients | 96 | |
| Kemler et al.[30] | 2008 | 36 | CRPS | 63% of patients | 83% of patients | 60 | |
| Sears et al.[32] | 2011 | 35 | FBSS (n=17) CRPS (n=18) |
0% of patients 0% of patients | >50% of patients >50% of patients | 48 60 | |
| Geurts et al.[31] | 2013 | 84 | CRPS | 0% of patients | 64% of patients | 221 | |
| Kim et al.[33] | 2016 | 3 | CRPS | 0% of patients | 100% of patients | 12 | |
| Pregnant | Present study | 2018 | 25 | FBSS (n=6) CRPS (n=18) Undetermined (n=1) |
0% of patients | 96% of patients | 28.1±21 |
n – Number of patients; CRPS – Complex regional pain syndrome; FBSS – Failed back pain syndrome; SCS – Spinal cord stimulation
Table 4.
Technical and biological complications rates of spinal cord stimulation in nonpregnant and pregnant patients
| Nonpregnant | Pregnant patients | ||
|---|---|---|---|
| Rates of incidence (%) | n (1476 patients) | n (25 patients)** | |
| Absence of complications | 60.0 | 882 | - |
| Lead migration | 15.1 | 223 | - |
| Discomfort at the pulse generator | 7 | 103 | - |
| Hardware malfunction | 6.3 | 93 | 1 |
| Infection | 4 | 60 | - |
| Lead breakage | 4 | 60 | 1 |
| Hematomas | 1.2 | 18 | - |
| Cerebral fluid leak | 0.6 | 9 | - |
| Loss of therapeutic effect | 0.5 | 7 | - |
| Muscle spasms | 0.4 | 6 | - |
| Aseptic meningitis | 0.3 | 4 | - |
| Displacement of the pulse generator | 0.3 | 4 | - |
| Psychiatric distress | 0.2 | 3 | - |
| Rejection of the system | 0.1 | 2 | - |
| Paralysis | <0.001 | 1 | - |
| Seroma | <0.001 | 1 | - |
Although the IPG implantation has not been documented in around 30% (n = 7/25) of patients, the positions described in the literature were AAW, UB, and flanks. The authors suggest, based on the tabulated and analyzed descriptions in pregnant patients, that the side and implantation site did not show significant change in the rates of complications, pain relief, and both (maternal and neonate) outcomes. However, thinking in the esthetic appearance in postoperatory, the AWW implies in the higher visibility of SCS system components (IPG and extensor) with progression of pregnancy, and consequently, an esthetic discussion can be developed.
Although lasting complication rates SCS are very variable on this type of surgery [Table 4], the presence of electrode breakage, electrode migration, battery or pulse generator failures, hardware malfunction, change of amplitude of pulse by bodily movements, unwanted stimulation, unsatisfactory positioning of the electrode or generator, urinary disturbs, paresthesiae in other body parts, cerebrospinal fluid leakage, subcutaneous hematomas, epidural hematomas, deep or superficial infections, aseptic meningitis, paralysis, spinal cord injury, headache, asthenia, dizziness, muscle spasms, and pain located at the incision, electrode, or receiver site are risks to be considered during and after the surgical act.[10,18,30,31,47,48,49,50,51,52,53,54,55,56,57]
In 2004, Cameron[10] summarized 20 years about the SCS applications, and consequently, it included data obtained from 51 papers comprising 2972 patients overall. This essay listed the complications and concluded that essential literature had described complications related to technical or biological plots. The battery or pulse generator failures, as well as electrode breakage and migration, are the most frequently described technical complications, as the similar results showed in the pregnant population. Provided that the most frequently reported biological complications are as follows: cerebrospinal fluid leakage, infections, and pain located at the incision, electrode, or receiver site.[10] It ought to be stressed that this paper described the paralysis and the electrode migration as the most serious and the most common complications of SCS, respectively. Moreover, this assessment showed that the majority of the complications were not life-threatening and could usually be resolved by removing or correcting the device by another surgical approach, as shown in 8% (n = 2/25) of cases[35,41] described in this review.
Regarding the obstetric and nontechnical complications during the SCS, the authors summarized 10 complications: three cases of miscarriages and HAS, two cases of DMG, as well as one description of IUGR and foot drop each. The cases of patients affected by HAS, miscarriages, and DMG presented previous obstetric and clinical history of these complications such that they are not suggestive of secondary complications during SCS. The IUGR and one of the miscarriages, although there are not any description correlating these complications with SCS, the pharmacological treatment, with classes C and D based on the FDA classification [Table 5],[1,7,20,43,59,60,61] adopted by this patients [Table 1] can be justified this teratogenic effect. However, the foot drop diagnosis during the postnatal outcome is associated with the absence of any pharmacological treatment, technical, obstetric, and clinical complications; it is suggestive of idiopathic disturb although the literature has not been clear about this association, as well as the multiple miscarriages shown in these patients.
Table 5.
Food and Drug Administration classification about analgesia during pregnancy
| FDA classification (category) | FDA classification (description) | Pharmacological treatment adopted in analyzed cases |
|---|---|---|
| A | Controlled studies in women fail to demonstrate a risk to the fetus. The possibility of harm to the fetus appears remote | - |
| B | Either animal studies have not demonstrated a fetal risk, but there are no controlled human studies or animal studies have indicated an adverse effect that was not confirmed in controlled studies in women in the 1st trimester (and there is no evidence of risk in the later trimesters) | Acetaminophen Buspirone Oxycodone |
| C | Teratogenic or embryocidal risk indicated in animal studies, but controlled studies in women have not been done or there are no controlled studies in animals or humans | Fentanyl Mirtazapine Bisoprolol Morphine Gabapentin Propranolol Mefenamic acid Bupivacaine Tramadol |
| D | Positive evidence of fetal risk, but use in pregnant woman is acceptable since the maternal benefit outweighs the risk to the fetus | Diazepam |
| X | Animal and human studies demonstrate fetal abnormalities or there is evidence of fetal risk based on human experience or both; the risk outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant | - |
| - | - | Epidural analgesia Solifenacin succinate Epinephrine Sodium tianeptine Ethyl loflazepate |
Regarding the technical complications, during the SCS, although it is similar to nonpregnant patients [Table 4], the lead breakage after the third vaginal birth without other complications described by Takeshima et al.[38] can suggest the increase of mechanical stress in the system. The presence of multiple pregnancies did not show as a categorical variable for the contraindication of implantation of system and continuous stimulation during pregnancy, although the individual analyses of clinical and obstetric conditions of patients are need once the mechanical stress in the system by increase in abdominal pressure during pregnancy and delivery has been described for few authors.[11,26,38]
Another possible mechanism that can be associated to teratogenic effects and reduction in fertility is the chronic exposure to magnetism.[27,36,42,62,63] A well-structured prospective cohort study composed of 969 pregnant patients at <10 weeks of gestation was published by Li et al.,[62] in 2002 that did not show an association between miscarriage risk and the average magnetic field level; however, they observed a miscarriage risk increased with an increasing level of maximum magnetic field exposure with a threshold around 16 mG (relative risk [RR]: 2.9, 95%, confidence interval [CI]: 1.6–5.3). The association was stronger for early miscarriages (10 weeks of gestation) (RR: 5.7, 95% CI: 2.1–15.7) and among women with multiple prior fetal losses or subfertility (RR: 4.0, 95% CI: 1.4–11.5). Therefore, more studies will need to perform aiming to associated the stimulation parameters of SCS and the miscarriage or teratogenic rates in pregnant patients, and the current recommendation is deactivation once the diagnosis of pregnancy is performed.[36]
Conclusions
Based on literature and the authors’ experience, SCS is an initial and controversial procedure that it has been suggesting positive results in the treatment of patients with medically refractory neuropathic pain. Although the total control of pain through the SCS had not been described commonly in general and pregnant population, because of its nonpharmacologic nature, this therapy is devoid of the frequent adverse, interactive effects, as well as teratogenic risks present in analgesic drugs polypharmacy in the vulnerable pregnant population.
However, although many of adults and pregnant patients have already been implanted with SCS, the inclusion of heterogeneous patient populations within the isolated case reports and highly uncontrolled protocols of stimulation and pain relief evaluation made it very difficult to analyze and compare the results. Therefore, significative clinical cohorts evaluating SCS in patients during pregnancy what would be for future necessary for an important source of data about this topic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Díaz RR, Rivera AL. Management of non-obstetric pain during pregnancy. Review article. Rev Colomb Anestesiol. 2012;40:213–23. [Google Scholar]
- 2.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–7. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: An evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Edelbroek C, Terheggen M. High-frequency spinal cord stimulation and pregnancy: A Case report. Neuromodulation. 2015;18:757–8. doi: 10.1111/ner.12314. [DOI] [PubMed] [Google Scholar]
- 5.Ito S, Sugiura T, Azami T, Sasano H, Sobue K. Spinal cord stimulation for a woman with complex regional pain syndrome who wished to get pregnant. J Anesth. 2013;27:124–7. doi: 10.1007/s00540-012-1462-y. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Tong C, Eisenach JC. Pregnancy increases excitability of mechanosensitive afferents innervating the uterine cervix. Anesthesiology. 2008;108:1087–92. doi: 10.1097/ALN.0b013e31817302e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S, Banh ET, Koury K, Bhatia G, Nandi R, Gulur P, et al. Pain management in pregnancy: Multimodal approaches. Pain Res Treat. 2015;2015:987483. doi: 10.1155/2015/987483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vercauteren M, Waets P, Pitkänen M, Förster J. Neuraxial techniques in patients with pre-existing back impairment or prior spine interventions: A topical review with special reference to obstetrics. Acta Anaesthesiol Scand. 2011;55:910–7. doi: 10.1111/j.1399-6576.2011.02443.x. [DOI] [PubMed] [Google Scholar]
- 9.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg. 1967;46:489–91. [PubMed] [Google Scholar]
- 10.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: A 20-year literature review. J Neurosurg. 2004;100:254–67. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 11.Segal R. Spinal cord stimulation, conception, pregnancy, and labor: Case study in a complex regional pain syndrome patient. Neuromodulation. 1999;2:41–5. doi: 10.1046/j.1525-1403.1999.00041.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith MW, Marcus PS, Wurtz LD. Orthopedic issues in pregnancy. Obstet Gynecol Surv. 2008;63:103–11. doi: 10.1097/OGX.0b013e318160161c. [DOI] [PubMed] [Google Scholar]
- 13.Nappi RE, Albani F, Sances G, Terreno E, Brambilla E, Polatti F, et al. Headaches during pregnancy. Curr Pain Headache Rep. 2011;15:289–94. doi: 10.1007/s11916-011-0200-8. [DOI] [PubMed] [Google Scholar]
- 14.Pennick VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2007;18:CD001139. doi: 10.1002/14651858.CD001139.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Attal N, Cruccu G, Haanpää M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–69. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 16.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 18.Camporeze B, Simm RF, Estevão IA, Mathias-Junior LR, Aguiar PH, Carrondo-Cottin S. Spinal cord stimulation in the treatment of neuropathic pain: Current perspectives of indications, cost-effectiveness, complications and results. J Health Sci. 2017;1:1–35. [Google Scholar]
- 19.Teixeira MJ. Challenges in the treatment of neuropathic pain. Drugs Today (Barc) 2009;45(Suppl C):1–5. [PubMed] [Google Scholar]
- 20.Coluzzi F, Valensise H, Sacco M, Allegri M. Chronic pain management in pregnancy and lactation. Minerva Anestesiol. 2014;80:211–24. [PubMed] [Google Scholar]
- 21.Dupraz J, Graff V, Barasche J, Etter JF, Boulvain M. Tobacco and alcohol during pregnancy: Prevalence and determinants in Geneva in 2008. Swiss Med Wkly. 2013;143:w13795. doi: 10.4414/smw.2013.13795. [DOI] [PubMed] [Google Scholar]
- 22.Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL. Prescription of drugs during pregnancy in France. Lancet. 2000;356:1735–6. doi: 10.1016/s0140-6736(00)03209-8. [DOI] [PubMed] [Google Scholar]
- 24.Odalovic M, Vezmar Kovacevic S, Nordeng H, Ilic K, Sabo A, Tasic L, et al. Predictors of the use of medications before and during pregnancy. Int J Clin Pharm. 2013;35:408–16. doi: 10.1007/s11096-013-9750-7. [DOI] [PubMed] [Google Scholar]
- 25.Huggon IC, Ghi T, Cook AC, Zosmer N, Allan LD, Nicolaides KH, et al. Fetal cardiac abnormalities identified prior to 14 weeks’ gestation. Ultrasound Obstet Gynecol. 2002;20:22–9. doi: 10.1046/j.1469-0705.2002.00733.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed S, Lindsay JM, Snyder DI. Spinal cord stimulation for complex regional pain syndrome: A Case study of a pregnant female. Pain Physician. 2016;19:E487–93. [PubMed] [Google Scholar]
- 27.Yoo HS, Nahm FS, Yim KH, Moon JY, Kim YS, Lee PB, et al. Pregnancy in woman with spinal cord stimulator for complex regional pain syndrome: A case report and review of the literature. Korean J Pain. 2010;23:266–9. doi: 10.3344/kjp.2010.23.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–88. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Olsson GL, Meyerson BA, Linderoth B. Spinal cord stimulation in adolescents with complex regional pain syndrome type I (CRPS-I) Eur J Pain. 2008;12:53–9. doi: 10.1016/j.ejpain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: Five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292–8. doi: 10.3171/JNS/2008/108/2/0292. [DOI] [PubMed] [Google Scholar]
- 31.Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M, et al. Spinal cord stimulation for complex regional pain syndrome type I: A prospective cohort study with long-term follow-up. Neuromodulation. 2013;16:523–9. doi: 10.1111/ner.12024. [DOI] [PubMed] [Google Scholar]
- 32.Sears NC, Machado AG, Nagel SJ, Deogaonkar M, Stanton-Hicks M, Rezai AR, et al. Long-term outcomes of spinal cord stimulation with paddle leads in the treatment of complex regional pain syndrome and failed back surgery syndrome. Neuromodulation. 2011;14:312–8. doi: 10.1111/j.1525-1403.2011.00372.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim E, Roh M, Kim S, Jo D. Continuous thoracic sympathetic ganglion block in complex regional pain syndrome patients with spinal cord stimulation implantation. Pain Res Manag. 2016;2016:5461989. doi: 10.1155/2016/5461989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanson JL, Goodman EJ. Labor epidural placement in a woman with a cervical spinal cord stimulator. Int J Obstet Anesth. 2006;15:246–9. doi: 10.1016/j.ijoa.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Saxena A, Eljamel MS. Spinal cord stimulation in the first two trimesters of pregnancy: Case report and review of the literature. Neuromodulation. 2009;12:281–3. doi: 10.1111/j.1525-1403.2009.00240.x. [DOI] [PubMed] [Google Scholar]
- 36.Bernardini DJ, Pratt SD, Takoudes TC, Simopoulos TT. Spinal cord stimulation and the pregnant patient-specific considerations for management: A case series and review of the literature. Neuromodulation. 2010;13:270–4. doi: 10.1111/j.1525-1403.2010.00288.x. [DOI] [PubMed] [Google Scholar]
- 37.Sommerfield D, Hu P, O’Keeffe D, McKeating AK. Caesarean section in a parturient with a spinal cord stimulator. Int J Obstet Anesth. 2010;19:114–7. doi: 10.1016/j.ijoa.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Takeshima N, Okuda K, Takatanin J, Hagiwra S, Noguchi T. Trial spinal cord stimulator reimplantation following lead breakage after third birth. Pain Physician. 2010;13:523–6. [PubMed] [Google Scholar]
- 39.Fedoroff IC, Blackwell E, Malysh L, McDonald WN, Boyd M. Spinal cord stimulation in pregnancy: A literature review. Neuromodulation. 2012;15:537–41. doi: 10.1111/j.1525-1403.2012.00448.x. [DOI] [PubMed] [Google Scholar]
- 40.Gredilla E, Abejón D, Del Pozo C, Del Saz J, Gilsanz F. Failed back surgery, spinal cord stimulation and pregnancy: Presentation of a case. Rev Esp Anestesiol Reanim. 2012;59:511–4. doi: 10.1016/j.redar.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Domínguez Suárez E, Camblor Suárez E, Vázquez Del Valle S, López Rouco M, Rodríguez Vázquez ML. Epidural analgesia in a woman with spinal neurostimulation for complex regional pain syndrome type 1. A clinical case and a literature review. Rev Esp Anestesiol Reanim. 2012;59:515–6. doi: 10.1016/j.redar.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 42.Das B, McCrory C. Spinal cord stimulation in pregnancy with failed back surgery syndrome. Ir Med J. 2014;107:117–8. [PubMed] [Google Scholar]
- 43.Young AC, Lubenow TR, Buvanendran A. The parturient with implanted spinal cord stimulator: Management and review of the literature. Reg Anesth Pain Med. 2015;40:276–83. doi: 10.1097/AAP.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 44.Moussa AA, Glancz L, Das M, Basu S. Spinal cord stimulation during the antepartum and intrapartum periods: A case report showing no deleterious effect of conventional paraesthesia producing stimulation. Acta Neurochir (Wien) 2016;158:2365–7. doi: 10.1007/s00701-016-2954-6. [DOI] [PubMed] [Google Scholar]
- 45.Pablo LO, Elvira GR, Ángeles YC, Remedios GA, Cristina LV, Cristina LZ. Pregnancy in women with posterior cord neurostimulator for the treatment of complex chronic pain syndromes. Review of existing literature and recommendations: A case report. SM Pregnancy Care. 2017;1:1002. [Google Scholar]
- 46.Ortín PL, Ré ME, Carrillo AY, Andrés MR. Posterior spinal cord stimulation and pregnancy. Evidence and recommendations on the use of spinal cord stimulation during pregnancy and labour. Clin Invest Ginecol Obstet. 2017;44:42–4. [Google Scholar]
- 47.Franzini A, Ferroli P, Marras C, Broggi G. Huge epidural hematoma after surgery for spinal cord stimulation. Acta Neurochir (Wien) 2005;147:565–7. doi: 10.1007/s00701-004-0470-6. [DOI] [PubMed] [Google Scholar]
- 48.Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain. A 9-year experience. J Neurosurg. 1989;70:519–24. doi: 10.3171/jns.1989.70.4.0519. [DOI] [PubMed] [Google Scholar]
- 49.Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J, et al. Retrospective review of 707 cases of spinal cord stimulation: Indications and complications. Pain Pract. 2011;11:148–53. doi: 10.1111/j.1533-2500.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- 50.Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T, et al. Sustained effectiveness of 10 kHz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15:347–54. doi: 10.1111/pme.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigoard P, Jacques L, Delmotte A, Poon K, Munson R, Monlezun O, et al. An algorithmic programming approach for back pain symptoms in failed back surgery syndrome using spinal cord stimulation with a multicolumn surgically implanted epidural lead: A multicenter international prospective study. Pain Pract. 2015;15:195–207. doi: 10.1111/papr.12172. [DOI] [PubMed] [Google Scholar]
- 52.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: A systematic review of effectiveness and complications. Pain. 2004;108:137–47. doi: 10.1016/j.pain.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Hollingworth W, Turner JA, Welton NJ, Comstock BA, Deyo RA. Costs and cost-effectiveness of spinal cord stimulation (SCS) for failed back surgery syndrome: An observational study in a workers’ compensation population. Spine (Phila Pa 1976) 2011;36:2076–83. doi: 10.1097/BRS.0b013e31822a867c. [DOI] [PubMed] [Google Scholar]
- 54.Dario A, Fortini G, Bertollo D, Bacuzzi A, Grizzetti C, Cuffari S, et al. Treatment of failed back surgery syndrome. Neuromodulation. 2001;4:105–10. doi: 10.1046/j.1525-1403.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 55.Ohnmeiss DD, Rashbaum RF, Bogdanffy GM. Prospective outcome evaluation of spinal cord stimulation in patients with intractable leg pain. Spine (Phila Pa 1976) 1996;21:1344–50. doi: 10.1097/00007632-199606010-00013. [DOI] [PubMed] [Google Scholar]
- 56.Kemler MA, Furnée CA. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59:1203–9. doi: 10.1212/01.wnl.0000028686.74056.e3. [DOI] [PubMed] [Google Scholar]
- 57.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: A systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91–101. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Manca A, Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Quality of life, resource consumption and costs of spinal cord stimulation versus conventional medical management in neuropathic pain patients with failed back surgery syndrome (PROCESS trial) Eur J Pain. 2008;12:1047–58. doi: 10.1016/j.ejpain.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Pritham UA, McKay L. Safe management of chronic pain in pregnancy in an era of opioid misuse and abuse. J Obstet Gynecol Neonatal Nurs. 2014;43:554–67. doi: 10.1111/1552-6909.12487. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz EB, Postlethwaite DA, Hung YY, Armstrong MA. Documentation of contraception and pregnancy when prescribing potentially teratogenic medications for reproductive-age women. Ann Intern Med. 2007;147:370–6. doi: 10.7326/0003-4819-147-6-200709180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo WY, Friedman JM. Teratogenicity of recently introduced medications in human pregnancy. Obstet Gynecol. 2002;100:465–73. doi: 10.1016/s0029-7844(02)02122-1. [DOI] [PubMed] [Google Scholar]
- 62.Li DK, Odouli R, Wi S, Janevic T, Golditch I, Bracken TD, et al. A population-based prospective cohort study of personal exposure to magnetic fields during pregnancy and the risk of miscarriage. Epidemiology. 2002;13:9–20. doi: 10.1097/00001648-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Brent RL. Reproductive and teratologic effects of low-frequency electromagnetic fields: A review of in vivo and in vitro studies using animal models. Teratology. 1999;59:261–86. doi: 10.1002/(SICI)1096-9926(199904)59:4<261::AID-TERA12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]


