Abstract
Choroid plexus papilloma is a low-frequency entity in both the adult and pediatric populations. Its clinical presentation is very variable as it depends on its location and length. We must always do the differential diagnosis between papilloma and other intraventricular pathologies. This article is about a case report of a pediatric patient with a Choroid plexus papilloma located in the fourth ventricle, a location that is atypical for the pediatric population.
Keywords: Choroid plexus papilloma, fourth ventricle, pediatric
Introduction
Choroid plexus papilloma constitutes from 0.4% to 1% of all intracranial tumors, and in children, they rise from 1.5% to 6%.[1] Choroid plexus papilloma is localized in adults usually infratentorial, and about 70% can be found in the fourth ventricle, whereas in the pediatric population the most common site is supratentorial and located in the lateral ventricles.[2] Although it can appear at any age, 70% of the cases have been reported in patients younger than 2 years old,[1,3,4] extraventricular locations have also been described.[3,5,6]
In this report, we describe a rare case of choroid plexus papilloma located in the fourth ventricle, in a pediatric patient.
Case Report
A 13 year old female patient is referred to an emergency service with a mostly bilateral retroocular headache after 2 years of evolution. the pain increased in frequency and intensity for two months which caused the patient to woke up in the mornings. Due to this, in the remission site, a cranial computed tomography (CT) scan was performed. It showed an infratentorial lesion, so the initial emergency service physician sends us the clinical record and the patient for integral management. When the patient came, a cranial nuclear magnetic resonance imaging (MRI) was performed [Figures 1–3].
Figure 1.
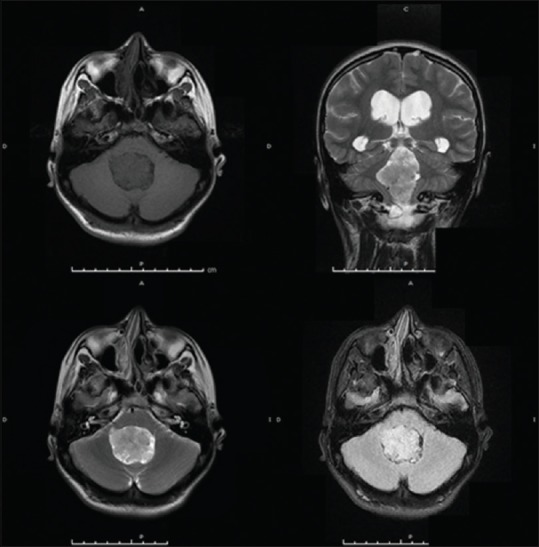
Simple magnetic resonance image, sequences weighted in T1 axial cut. Intraventricular mass hypointense. Coronal and axial cut T2: hyperintense lesion with irregular edges. The fluid-attenuated image recovery in which can observe that lesion has signal intensity relatively greater than the parenchyma
Figure 3.
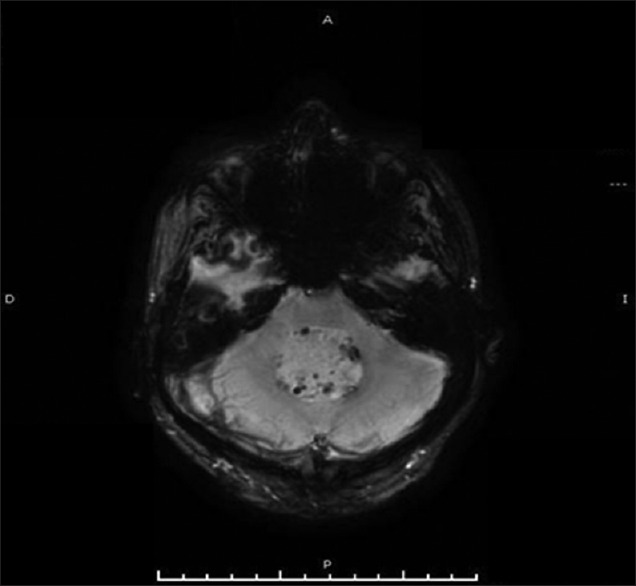
Susceptibility-weighted magnetic resonance sequences in which can observe multiple foci of small hemorrhages in the periphery of the lesion
Figure 2.
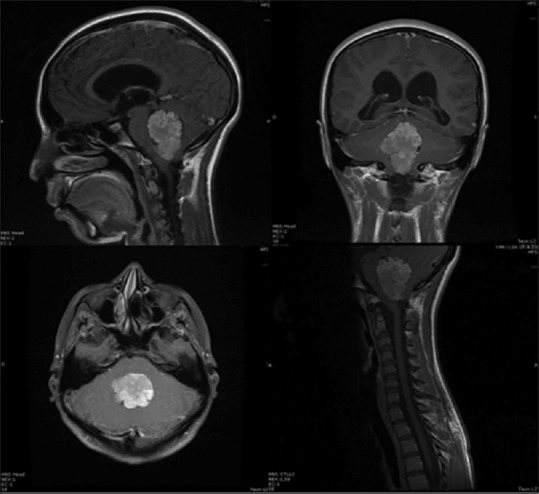
Magnetic resonance image sequence T1 contrasted with all patient cuts
A large infratentorial mass with robust enhancement, heterogeneous dilation of the lateral ventricles, and other signs of hydrocephalus such as horizontalization of the temporal horns was found. Taking into account the patient's findings and symptoms, it was considered necessary to perform a tumor resection through occipital craniectomy. During the procedure, we did not have any complications [Figure 4]. Furthermore, considering the high risk of hydrocephalus, we performed a system of the fifth ventricle. The patient was sent to the pediatric intensive care unit.
Figure 4.
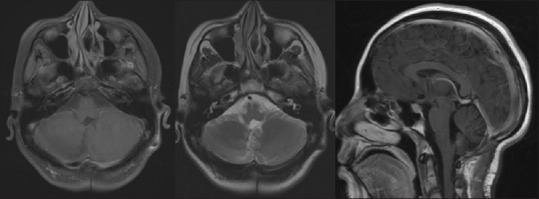
Simple and contrast magnetic resonance image, sequences weighted in T1 axial cut, T2 axial cut, and sequences contrast sagittal where no tumor residue is identified in the posterior fossa
The outcome of the 1st day became torpid, with a high rate of production of xanthochromic cerebrospinal fluid (CSF), for which it was considered necessary to perform a ventriculoperitoneal shunt.
After several days of satisfactory evolution, the patient became drowsy again, for that reason it was considered necessary to perform a new cranial CT scan [Figure 5], in which significant ventricular dilatation was evidenced. With a diagnosis of hydrocephalus due to shunt failure, the patient was taken back to surgery.
Figure 5.
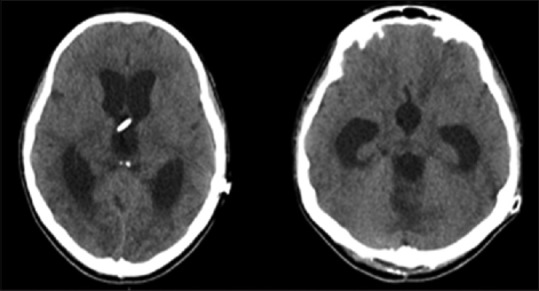
Emergency cranial computed tomography scan: dilatation of the lateral ventricles, horizontalization of temporal horns, and bulging of the third ventricle
In the surgery, it was found a high quantity of detritus in the porosities of the proximal catheter shunt, so a review of its functionality and a cleaning was performed. Two hours after the procedure, the patient was awake without neurological deficit. A control CT scan was performed on the 3rd day [Figure 6].
Figure 6.
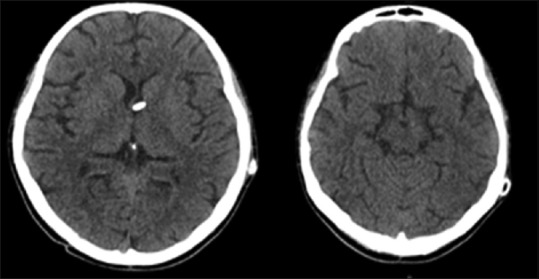
Postoperative control computed tomography scan: ventriculoperitoneal shunt in place. Normal ventricular size
Fifteen days postoperatively, the pathology reported choroid plexus papilloma, and after a follow-up at 3 months, 6 months, and a year later, the patient did not present any clinical deterioration or complication.
Discussion
Choroid plexus papilloma is the most frequent ventricular tumor, but it is not common in the third ventricle and cerebellopontine angle. In pediatric population, 80% of this kind of tumor can be found in the lateral ventricles, 16% in the fourth ventricle, and 4% in the third ventricle.[7,8,9] Just a few cases of choroid plexus papilloma of the fourth ventricle in pediatric patients have been reported, with an approximated incidence of 10%–20%.[2,10]
Choroid plexus papilloma is benign in 80% of the cases and have a good prognosis. The World Health Organization classification includes: (i) low grade, (ii) atypical, (iii) carcinoma.[11] The choroid plexus papilloma shows an annual incidence of 0.3/1,000,000 people, with a male-to-female ratio of 1.2:1.[12] Furthermore, it is more frequent in the 1st year of life, being the 10%–20% of the brain tumors at this age, with the mean of time for its diagnosis of 3.5 years.[13]
As mentioned before, in the pediatric population, this kind of tumors are usually found in the lateral ventricles, more frequent in the left ventricle,[12] and it has been reported a mean diagnosis age that varies with the localization of the tumor; 1.5 years for lateral and third ventricle, 22.5 years for fourth ventricle, and 35.5 years for cerebellopontine angle.[12]
The clinical manifestations of this entity, as any other central nervous system tumor, change in function of the location. Usually, the patient with a choroid plexus papilloma of the posterior fossa can have gait disturbance, dizziness, papilledema, cranial nerves palsy, convulsions, hydrocephalus, and retardation of the psychomotor functions.[13,14] However, in this kind of tumor, the symptoms are directly linked to hydrocephalus, that develops due to overproduction of CSF by the tumor cells, obstruction of the ventricular system, or by resorption dysfunction.[13,14]
Differential diagnosis of the intraventricular tumors in children includes wide giant subependymal astrocytoma, low-grade astrocytoma, meningiomas, ependymomas, medulloblastomas, metastases, and colloid cysts, between others. However, it is also important to consider other possible differential diagnoses, such as physiological enlargement of the choroid plexuses and xanthogranulomas of the choroid plexuses.[15,16] Some cases have been reported in siblings and in association with Li-Fraumeni, Aicardi, and Von Hippel-Lindau syndromes.[13,17]
The choroid plexus papilloma in MRI has an hyperdense or isodense signal, and it could have calcifications in 25% of the cases.[12,18] Hydrocephalus can be also found. In the MRI, this entity appears as a “cauliflower,” with vascularization, contrast-enhanced lesion, is rare but it could have a cystic form too.[18] In the T1 sequence, they appear well defined, isodense, or hypointense, whereas in the T2-weighted sequence, it is observed as an isodense or hyperintense signal. Whenever we perform the imaging evaluation of these patients, it is necessary to rule out dissemination by CSF[15] and a complete central nervous system study has to be made.
Spectroscopy is also very useful. This kind of tumor is described as low or none N-Methyl D-Aspartate, small peaks of choline and lactate, and elevation of myoinositol, which will help us to distinguish it from the carcinoma of choroid plexuses,[15] the carcinoma has a lower survival rate and requires adjuvant treatment with chemotherapy and sometimes radiotherapy.[19,20]
Other neuroimaging tools include angiography, which is useful for preoperative planning and visualization of the vascular supply for the tumor, as we mentioned before, choroid plexus papilloma has good vascularization, and the main mortality in the procedures is bleeding.[13,21] Preoperative embolization should be considered too, but the small vessels of the tumor may not be reachable.[21,22]
Surgical resection of the tumor is the cornerstone of the treatment, but hydrocephalus management highly important and is an open topic of discussion. Some surgeons/authors report that the hydrocephalus should be resolved before the tumor resection with an external ventricular drain, and after the resection, it could be retired without any other management.[23,24,25] Meanwhile, other authors say that is safer to put a ventriculoperitoneal shunt intraoperative, as some hydrocephalus symptoms may not resolve after tumor resection due to the possible development of CSF resorption dysfunction.[26]
Hence, as in our case, despite that the tumor was resected in approximately 100%, the patient evolution was torpid because of hydrocephalus, so a ventriculoperitoneal shunt was performed, but after 5 days, it became dysfunctional because of mechanical obstruction caused by detritus and hemorrhage, and a new intervention was performed to clean the valve, with later complete recovery of the patient.
About chemotherapy in pediatric patients, some reports say that 14% of the patients were declared tumor free.[26] Some papers report too that it could be useful for metastatic disease and recurrent disease.[13] On the other hand, radiotherapy is usually used to treat residual tumors by subtotal resection, and some authors say that it should be reserved to treat malignant lesions, recurrent or disseminated, and is not recommended for patients younger than 3 years old.[13]
In the past, the tumor survival rate was approximately 50%, but with the improvements in postoperative care, surgical approaches, and neuroimaging, the rate nowadays is close to 100%.[13] In a meta-analysis, the authors report that survival at 1 year was 90%, 5 years 81%, and 10 years 77%.[12] Surgical removal is the main factor associated with survival and there is not enough evidence yet to recommend other kind of therapy for a patient with choroid plexus papilloma.[12]
Conclusion
Choroid plexus papilloma of the fourth ventricle is an infrequent form of presentation of this entity in the pediatric population. Surgical resection is the cornerstone of the management as there is no enough evidence for neoadjuvant therapy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Boyd MC, Steinbok P. Choroid plexus tumors: Problems in diagnosis and management. J Neurosurg. 1987;66:800–5. doi: 10.3171/jns.1987.66.6.0800. [DOI] [PubMed] [Google Scholar]
- 2.Ellenbogen RG, Winston KR, Kupsky WJ. Tumors of the choroid plexus in children. Neurosurgery. 1989;25:327–35. doi: 10.1097/00006123-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Tacconi L, Delfini R, Cantore G. Choroid plexus papillomas: Consideration of a surgical series of 33 cases. Acta Neurochir (Wien) 1996;138:802–10. doi: 10.1007/BF01411257. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy AW, Galloway M, Revesz T, Kitchen ND. Metastatic choroid plexus papilloma: A case report. J Neurooncol. 2002;56:241–6. doi: 10.1023/a:1015044012574. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Singh S. Childhood choroid plexus papillomas: Operative complications. Childs Nerv Syst. 2005;21:138–43. doi: 10.1007/s00381-004-0993-4. [DOI] [PubMed] [Google Scholar]
- 6.Mortazavi MM, Griessenauer CJ, Adeeb N, Deep A, Bavarsad Shahripour R, Loukas M, et al. The choroid plexus: A comprehensive review of its history, anatomy, function, histology, embryology, and surgical considerations. Childs Nerv Syst. 2014;30:205–14. doi: 10.1007/s00381-013-2326-y. [DOI] [PubMed] [Google Scholar]
- 7.Karim A, Fowler M, McLaren B, Cardenas R, Patwardhan R, Nanda A, et al. Concomitant choroid plexus papillomas involving the third and fourth ventricles: A case report and review of the literature. Clin Neurol Neurosurg. 2006;108:586–9. doi: 10.1016/j.clineuro.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Carson BS, Weingart JD, Guarnieri M, Fisher PG. Third ventricular choroid plexus papilloma with psychosis. Case report. J Neurosurg. 1997;87:103–5. doi: 10.3171/jns.1997.87.1.0103. [DOI] [PubMed] [Google Scholar]
- 9.Kroppenstedt SN, Golfinos J, Sonntag VK, Spetzler RF. Pineal region lesion masquerading choroid plexus papilloma: Case report. Surg Neurol. 2003;59:124–7. doi: 10.1016/s0090-3019(02)00988-6. [DOI] [PubMed] [Google Scholar]
- 10.Prasad GL, Mahapatra AK. Case series of choroid plexus papilloma in children at uncommon locations and review of the literature. Surg Neurol Int. 2015;6:151. doi: 10.4103/2152-7806.166167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar C, Sharma MC, Gaikwad S, Sharma C, Singh VP. Choroid plexus papilloma: A clinicopathological study of 23 cases. Surg Neurol. 1999;52:37–9. doi: 10.1016/s0090-3019(99)00049-x. [DOI] [PubMed] [Google Scholar]
- 12.Wolff JE, Sajedi M, Brant R, Coppes MJ, Egeler RM. Choroid plexus tumours. Br J Cancer. 2002;87:1086–91. doi: 10.1038/sj.bjc.6600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safaee M, Oh MC, Bloch O, Sun MZ, Kaur G, Auguste KI, et al. Choroid plexus papillomas: Advances in molecular biology and understanding of tumorigenesis. Neuro Oncol. 2013;15:255–67. doi: 10.1093/neuonc/nos289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Swieten JC, Thomeer RT, Vielvoye GJ, Bots GT. Choroid plexus papilloma in the posterior fossa. Surg Neurol. 1987;28:129–34. doi: 10.1016/0090-3019(87)90086-3. [DOI] [PubMed] [Google Scholar]
- 15.Osborn AG, Salzman KL, Jhaveri MD, Barkovich AJ. 3rd ed. Ch. 137. Elsevier; 2016. Diagnostic Imaging: Brain. [Google Scholar]
- 16.Koeller KK, Sandberg GD. Armed Forces Institute of Pathology. From the archives of the AFIP. Cerebral intraventricular neoplasms: Radiologic-pathologic correlation. Radiographics. 2002;22:1473–505. doi: 10.1148/rg.226025118. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez E, Ball WS, Jr, Prenger EC, Castellote A, Crone KR. Magnetic resonance imaging of fourth ventricular choroid plexus neoplasms in childhood. A report of two cases. Pediatr Neurosurg. 1991;17:48–52. doi: 10.1159/000120567. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Savolaine ER. Imaging of atypical choroid plexus papillomas. Clin Imaging. 1996;20:85–90. doi: 10.1016/0899-7071(94)00078-6. [DOI] [PubMed] [Google Scholar]
- 19.Due-Tønnessen B, Helseth E, Skullerud K, Lundar T. Choroid plexus tumors in children and young adults: Report of 16 consecutive cases. Childs Nerv Syst. 2001;17:252–6. doi: 10.1007/pl00013728. [DOI] [PubMed] [Google Scholar]
- 20.Erman T, Göçer AI, Erdoǧan S, Tuna M, Ildan F, Zorludemir S, et al. Choroid plexus papilloma of bilateral lateral ventricle. Acta Neurochir (Wien) 2003;145:139–43. doi: 10.1007/s00701-002-1047-x. [DOI] [PubMed] [Google Scholar]
- 21.Keating RF, Goodrich JT, Packer RJ. 1st ed. New York: Thieme; 2001. Tumors of the Pediatric Central Nervous System. [Google Scholar]
- 22.Levy ML, Goldfarb A, Hyder DJ, Gonzales-Gomez I, Nelson M, Gilles FH, et al. Choroid plexus tumors in children: Significance of stromal invasion. Neurosurgery. 2001;48:303–9. doi: 10.1097/00006123-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal AK, Jaiswal S, Sahu RN, Das KB, Jain VK, Behari S, et al. Choroid plexus papilloma in children: Diagnostic and surgical considerations. J Pediatr Neurosci. 2009;4:10–6. doi: 10.4103/1817-1745.49100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawar SJ, Sharma RR, Mahapatra AK, Lad SD, Musa MM. Choroid plexus papilloma of the posterior third ventricle during infancy & childhood: Report of two cases with management morbidities. Neurol India. 2003;51:379–82. [PubMed] [Google Scholar]
- 25.Talacchi A, De Micheli E, Lombardo C, Turazzi S, Bricolo A. Choroid plexus papilloma of the cerebellopontine angle: A twelve patient series. Surg Neurol. 1999;51:621–9. doi: 10.1016/s0090-3019(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 26.Wrede B, Hasselblatt M, Peters O, Thall PF, Kutluk T, Moghrabi A, et al. Atypical choroid plexus papilloma: Clinical experience in the CPT-SIOP-2000 study. J Neurooncol. 2009;95:383–92. doi: 10.1007/s11060-009-9936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


