Abstract
Simulation training plays a paramount role in medicine, especially when it comes to mastering surgical skills. By simulating, students gain not only confidence, but expertise, learning to apply theory in a safe environment. As the technological arsenal improved, virtual reality and physical simulators have developed and are now an important part of the Neurosurgery training curriculum. Based on deliberate practice in a controlled space, simulation allows psychomotor skills augment without putting neither patients nor students at risk. When compared to the master-apprentice ongoing model of teaching, simutation becomes even more appealing as it is time-efficient, shortening the learning curve and ultimately leading to error reduction, which is reflected by diminished health care costs in the long run. In this chapter we will discuss the current state of neurosurgery simulation, highlight the potential benefits of this approach, assessing specific training methods and making considerations towards the future of neurosurgical simulation.
Keywords: Medical education, neurological surgery, neurosurgery, neurosurgical education, simulation
Introduction
The master-apprentice system has been the cornerstone of medical education for centuries, especially when it comes to teaching surgical skills. This method, however, has several flaws that pose as a growing issue as the society's pressure on the health-care facilities increases and surgical trainees are permitted fewer operating opportunities.[1,2,3] In this context, medical simulation emerges as a feasible and promising alternative.[1,4,5,6,7]
The concept of simulation as an effective way of learning became popular with the airline industries, which required commercial pilots to exhaustively train in flight simulators before allowing them to carry passengers.[1,6,7] Defined as “devices, life-like virtual environments, and contrived social situations that mimic problems, events or conditions that arise in professional (medical) encounters,”[4,8] medical simulation rose from the belief that simulation could be applied in the medical field, accelerating the acquisition, and the development of various skills.
Simulation not only helps trainees to gain confidence but also expertise as they can apply theory - and makes mistakes - in a safe environment, without putting neither patients nor themselves at risk. This is particularly important in the early stages of learning, but it remains valid even when it comes to more experienced students and doctors. After all, we expect even experts to keep honing their psychomotor skills and updating their techniques.[9]
The deliberate practice in a controlled space also eliminates the need to settle for the scenarios that casually appear, which neither guarantee quantity, quality nor variability.[1] On the contrary, this practice has the potential of unifying knowledge by presenting the same wide range of cases to different individuals.
Another exciting benefit is that by simulating, the students’ become no longer limited to the working hours and the learning curve is shortened, as the patient is not necessary.[10,11] The above-mentioned features, in addition to the simulation's time-efficiency, ultimately lead to error reduction and better overall care, which is reflected by diminished health costs in the long run.[1,4,5,6] Therefore, we can imply that simulation, when compared to the master-apprentice ongoing model of teaching, is probably more cost-effective.
In this context, neurosurgery is a particularly complex field, in which the number and the difficulty of the procedures continue to increase at the same time that little – or no – error is tolerated, for mistakes can dangerously threaten the patient's life or functionality.[9] Hence, any effort to improve neurosurgeons’ training without possibly jeopardizing the patients is valid.
In this article, we will discuss the current state of neurosurgery simulation, highlighting the potential benefits of this approach, as well as its drawbacks. We will also assess specific training methods, and make considerations towards the future of neurosurgical simulation.
Historical Background and Current Situation of Simulation Methods
The search for novel methods of teaching led to many advances, which must undergo extensive validation processes that assess efficacy, cost-effectiveness, validity, versatility, and others, to become the part of residency programs.[6,9,12] Coelho et al. divided the adjuvant surgical training options into four major subgroups: animal models, cadaver training, training with synthetic physical models and VR simulators.[6]
Historically, animal and cadaveric dissection has contributed to the medical field since the Antiquity, with some reports dating the era of the Greek philosopher Alcmaeon of Croton, during the 6th century BC.[6,13,14] The Greek physician Galenus described numerous anatomical structures, which guided medical practice until Leonardo da Vinci's drawings.[15,16] The father of modern anatomy, however, was a title given to the Belgium Andreas Vesalius due to his most famous work “De humani corporis fabrica.”[15]
While this approach continues to be the most prevalent outside operating rooms, it presents significant limitations such as the availability of fresh cadavers and the social – and religious – stigma.[17] The presence of anatomical variations and comorbidities are other shortcomings that often hinder the students from experiencing the same scenarios.[6,13]
Synthetic physical models are another well-established facet of medical training. First developed in the 18th century, they were, in the beginning, solely represented by waxwork replicas. No advance in such category occurred until the advent of “Resusci-Anne,” a mannequin made by the toy manufacturer Asmund Laerdal, for cardiopulmonary resuscitation in the 1950s.[18,19] These models have quickly become popular since then, especially for rehearsing anesthesia and trauma-related minor procedures (such as chest tube placement and cricothyroidotomy). Nonetheless, they do not offer the students the level of reality proximity as the VR simulators do.[17,20]
These machines, allocated in the last subgroup, appear to address all the aforementioned problems, as the technological arsenal continuously improves and VR accuracy becomes closer to the real scenarios by the second, allowing students to practice in a riskless standardized environment that can be objectively evaluated and incremented.[6,9] Moreover, the technological advances enable the patient's specific anatomy – obtained through image examination – to be fully reproduced in three-dimensional models, allowing physicians to practice different approaches for a complex surgery before actually facing the patient.[21] As surgical simulation is a promising research field, a paradigm shift has been noticed especially in the past decades, with the increasing use of simulators.
In neurosurgery, nevertheless, the simulation platforms development is somewhat slower than in the other medical areas. This is partially explained by the institutions and professionals’ skepticism and by their resistance to change. Despite that fact, over the last 20–25 years, multiple virtual simulators have been created for many procedures, among which neuroendoscopy, percutaneous rhizotomy, endovascular stenting and coiling, third ventriculostomy, cranial microsurgery, and pedicle screw and external ventricular drain placement stand out.[21,22,23,24,25,26,27,28,29,30,31,32]
Physical Simulation
The physical simulation includes cadaveric training, animal and synthetic models. Despite being able to achieve significant anatomical accuracy and being nowadays considered the first line of practice, physical simulation has noteworthy disadvantages.[13,22]
Phantom models, also known as physical synthetic models, are artificial structures used to mimic body parts, allowing medical training at a reasonable cost and without the availability, storage, and ethical issues that surround cadavers.[33] However, even when maintenance is not required, these models’ duration is limited due to repetitive use, resulting in need of purchasing new devices, which are often very expensive.[34,35] Moreover, these models are generally mass-produced, leading to few or no anatomic variations’ representations.[34] VR simulators, on the other hand, are not associated with these issues since they are digital programs that can mimic anatomic variances – including those of the real patient – and that do not suffer from repetition.[7,13,36,37,38]
Although they give the students a close notion to what they will face in the operation room regarding surgical anatomy, both cadaveric, the oldest reported form of medical simulation, and animal training fail to provide pathology.[39] Moreover, they involve critical ethical restrictions.[1,39,40]
Regarding animal training, the equipment and the specific preparation required to offset the initial lower cost.[33] As for the cadavers, there are not as many available as necessary to adequately address the needs of residency programs worldwide and the costs related to these bodies’ maintenance are daunting.[5,40,41,42] Furthermore, the quality of the cadaveric tissues relies on the adopted embalming regimen and feedback is not immediate.[42,43]
Studies have already demonstrated the efficacy of cadaveric models training for skull base tumor debulking,[44] aneurysm clipping,[44,45,46,47,48] cerebrospinal flow evaluation,[49] and internal carotid artery injury [Table 1].[50]
Table 1.
Key points, techniques, and messages of physical simulation
| Key points, techniques, and messages of physical simulation |
| Physical simulation, which remains the most common form of simulation, includes cadaveric training, animal and synthetic models |
| Ethical and legal quandaries regarding the use of animals and cadavers hinder these models from being fully implemented in residency programs |
| Repetitive use is a remarkable limitation for physical models |
| Associated with lower costs, when compared to virtual reality simulation, physical models are currently the best alternative for low-resource environments |
| Studies have already demonstrated the efficacy of cadaveric models training for skull base tumor debulking,[44] aneurysm clipping,[44,45,46,47,48] cerebrospinal flow evaluation,[49] and internal carotid artery injury[50] |
Virtual Reality Simulation
VR simulation relies on different levels of immersion.[22] Based on the movement illusion that results from the visuospatial input and the vestibular system stimulation by acceleration and angulation,[17] it has three fundamental components – graphic rendering, tissue deformation, and haptic feedback.[51] The goal is to be as realistic as possible, aiming to ensure the best surgical training. Validity and feasibility are indispensable, and a suitable method for errors’ identification and measurement ought to be employed to guarantee the device's effectiveness.
There are three types of VR.[51,52] In the first, called “nonimmersive VR” and exemplified by the “Visual Human Project,”[53] the user stays an observer, with restricted interaction via computer, as a visual representation is constructed by the integration of standardized data, cadaveric dissections, and intraoperative images.[54]
As the name implies, in the second one, the “immersive VR,” the user is fully immersed in an artificial three-dimensional computer-generated scenario, much like an underwater diver. There are five immersive VR neurostimulators of significant importance: NeuroTouch, ImmersiveTouch, Temposurg, Dextroscope, and Robo Sim.[13,22,51]
The main problem regarding these simulators is the large capital investment that their purchase and maintenance required.[13,22,51] This still prevents the majority of the residency programs from incorporating them, especially outside the United States and Europe.
Indirect simulators such as video games and web-based surgical devices also show potential, particularly when integrated to kinetic technology.[22] A retrospective cohort assessed the surgeons that performed laparoscopic procedures revealed that those who played video games for over 3 h/week in the past were a 25% more efficient and made fewer mistakes.[52] Unfortunately, when it comes to neurosurgery, the small consumer market and the substantial funding necessary to develop these products discourage the related industries.[13,22,51]
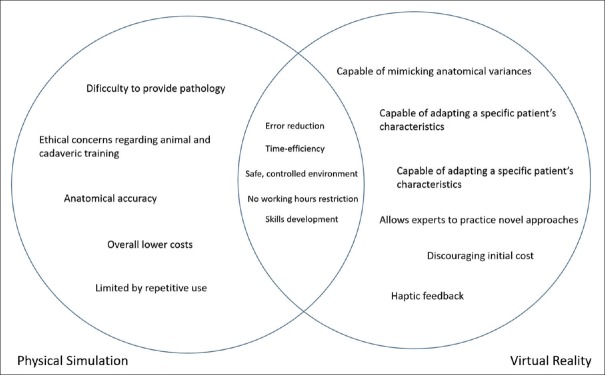
Particularly, interesting when it comes to minimally invasive neurosurgery, due to the limited haptic feedback associated to the procedures, VR obviates the need for physical models, and the odds are that this type of simulation will soon become the first-line of practice outside the operating room.[13,51] However, despite the investment in VR, synthetic physical simulators are still considered more reliable, mainly due to their reasonable cost and efficiency, with several studies demonstrating that the abilities acquired on such simulators are directly transformed into performance improvement at operations. The differences and similarities between physical models and VR are further demonstrated in Figure 1 and Table 2.
Figure 1.
Venn diagram demonstrating the relation between physical simulation and virtual reality
Table 2.
Key points, techniques, and messages of virtual reality
| Key points, techniques, and messages of virtual reality |
| VR, which is based on graphic rendering, tissue deformation, and haptic feedback, relies on different levels of immersion to provide the trainee with the most realistic experience possible[17,51] |
| Despite its several advantages, VR simulators are hardly incorporated in residency programs due to their association with a significant initial cost[13,22,51] |
| VR simulators are often able to incorporate a patient’s specific anatomy, offering the trainee a chance to perform particular approaches[13] |
| NeuroTouch, ImmersiveTouch, Temporsurg, Dextroscope, and Robo Sim are examples of virtual reality neurostimulators associated with good outcomes[13,22,51] |
VR – Virtual reality
Our contribution
Our group has published a work that presents the development and assessment of an interactive and stereoscopic resource for teaching neuroanatomy. Figure 2 illustrates this VR simulator.[53] The authors concluded that the method had a positive impact on students’ knowledge, encouraging significantly higher learning when compared with traditional teaching models.[53]
Figure 2.
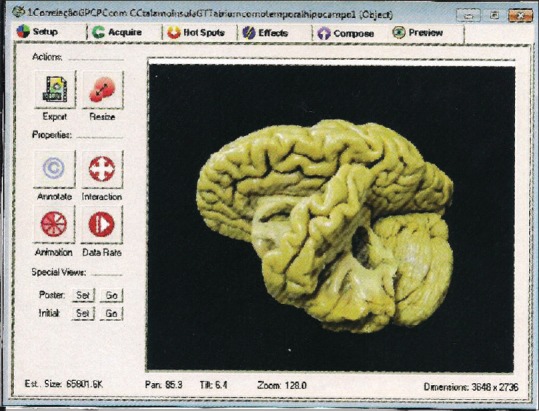
One of the many photographs of the brain obtained in a study oriented by professor Eberval Gadelha Figueiredo, at the University of São Paulo, Brazil. This image, along the others, was processed in the VR Worx 2.6 software (VR Toolbox, Inc.) – a resource that allowed them to be seen as a continuum, giving the impression that the brain was moving, and revealing its anatomical particularities[53]
Assessment Tools
Every training method must be validated before becoming fully integrated into the residency curriculum – and simulation devices are not an exception. To achieve the desired impact, not only an appropriate training program but also an extensive and thorough evaluation is mandatory. In clinical skills’ simulation training, the education validity of the simulator is often questioned. Therefore, objective assessment tools, capable of measuring the trainee's performance, are vital.
Knowledge (knows), performance (shows how), competence (knows how), and action (does) are the four pillars of the clinical competence pyramidal framework developed by Miller [Figure 3].[54] The evaluation of the first three stages trains the resident to act in a professional setting properly.[4]
Figure 3.

A representation of Miller's four-stage framework for clinical competence: action, performance, competence itself, and knowledge[54]
When it comes to simulation, the simulator itself can be considered a measurement tool, once it usually generates a report on performance based on specific measurement and preestablished goals. In this section, we will briefly discuss some of the most used evaluation tools in simulation training, considering both its advantages and disadvantages.[4]
Surveys
Participants’ overall satisfaction toward the experience, self-efficacy, comfort, and confidence can be estimated through a subjective tool, which consists either in open-ended answers or in rating scales analysis. The quality of the given feedback, as well as the training relevance and educational value, can be measured.[55]
Procedural checklists
Based on a binary or a rating scale, this is an objective method of evaluation, despite its subjective design. Reliable and widely used in the medical field, this tool is a good option, once it also allows post hoc assessment of videotaped exercises. The checklists neither comprise the sequence of actions nor the timing evaluation.[54,55,56,57,58,59]
Pre- and post-tests
Aiming to evaluate not only knowledge but also decision-making and clinical reasoning, these – usually – multiple choice tests are administered both before training and after training. The tests can be either different or not, depending on the situation and the assessor. Results often demonstrate knowledge improvement after the evaluated experiences, as disclosed by greater posttests scores on a study by Picard et al., which analyzed the impact of simulation courses and refresher lectures.[54,56,60]
As a further consideration, one need to keep in mind that cognitive science is essential whenever developing new training equipment and assessment methods, which despite the advances are yet scarce. In light of this, the cognitive load (CL) theory must be taken into consideration.[61,62]
CL is a limited mental effort that refers to the amount of information imposed on working memory. This limitation reflects on how the information is ultimately stored. For instance, too much information or too complicated, unstructured tasks can determine a cognitive overload, jeopardizing the learning process. The inherent, immutable difficulty of a task is known as its intrinsic load. On the other hand, the way in which such task is designed is the extraneous load, and the germane load is the schemas’ automation and construction.[61,62] It is thought that repetitive, well-structured practice can positively affect one's CL.[62]
In turn, the situation awareness (SA) is another relevant concept. SA, defined as a person's understanding of their dynamic environment, can be divided into three levels. The first is the perception of the environment; the second, the comprehension of such information, and the third is the projection of future actions and events. These levels’ assessment can provide significant information regarding the trainees’ perceptual processes and CL [Table 3].[63]
Table 3.
Key points, techniques and messages of assessment tools
| Key points, techniques, and messages of assessment tools |
| In order to be integrated into the residency curriculum, simulation methods must be thoroughly assessed and validated |
| The four pillars of clinical competence - knowledge, performance, competence, and action – have to be addressed in complete training programs[54] |
| Surveys, procedural checklists, pre- and post-tests, global rating scales, error analysis, time-action analysis, and simulation metrics are some of the evaluation tools that can be used in simulation training[4] |
| Regarding validation methods’ assessment, cognitive load, and situation awareness are two connected concepts that must be taken into consideration.[61,62] The former is defined as the amount of information imposed on working memory. This amount is limited, and such limitation affects the way information is finally stored.[61,62] |
| The latter, on the other hand, refers to a person’s understanding of their dynamic environment. Situation awareness is divided into three levels - the perception of the environment; the comprehension of such information; and the projection of future actions and events[62] |
Conclusion
Neurosurgery is one of the most demanding medical areas, requiring an extreme level of expertise, as even the smallest error might have dire consequences. With increasing time, ethical, and medico-legal constraints currently in place, as well as fewer operation opportunities, it is of vital importance to find alternative teaching methods. Even though nothing can perfectly replace the experience of being in an operating room with an actual patient, simulation – in its many forms – allows students to become more confident and skilled, in a controlled environment, where there are no restrictions regarding working hours. Moreover, it allows experts to practice novel approaches to enhance the patients’ safety and improve outcomes.
Up to this moment, physical models, largely represented by cadaveric training, which is the oldest form of simulation, remain the “golden standard” worldwide despite its many limitations. Nonetheless, the current situation is on the verge of change: the rapidly emerging researches and technological advances are allowing VR simulators to gain space. The cost remains the most prominent obstacle for VR simulators to become commercially available and for a bigger assessment of these devices’ beneficial effects to be made. The diversity and number of neurosurgical procedures, as well as the different types of tissues found – which offer specific resistance and have particular densities – are also significant hurdles, as the industries keep trying to mimic the exact tactile sensation of operating. Overall, the simulation has a pivotal role in medicine and neurosurgery is not an exception. Already proved feasible and effective, the different types of simulation should be implemented in the residency educational programs aiming to address the trainees’ needs, ultimately helping them to become experienced professionals and to better serve the community.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Akhtar KS, Chen A, Standfield NJ, Gupte CM. The role of simulation in developing surgical skills. Curr Rev Musculoskelet Med. 2014;7:155–60. doi: 10.1007/s12178-014-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chikwe J, de Souza AC, Pepper JR. No time to train the surgeons. BMJ. 2004;328:418–9. doi: 10.1136/bmj.328.7437.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonnadara RR, Van Vliet A, Safir O, Alman B, Ferguson P, Kraemer W, et al. Orthopedic boot camp: Examining the effectiveness of an intensive surgical skills course. Surgery. 2011;149:745–9. doi: 10.1016/j.surg.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Mirza S, Athreya S. Review of simulation training in interventional radiology. Acad Radiol. 2018;25:529–39. doi: 10.1016/j.acra.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Gasco J, Holbrook TJ, Patel A, Smith A, Paulson D, Muns A, et al. Neurosurgery simulation in residency training: Feasibility, cost, and educational benefit. Neurosurgery. 2013;73(Suppl 1):39–45. doi: 10.1227/NEU.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 6.Coelho G, Zanon N, Warf B. The role of simulation in neurosurgery. Childs Nerv Syst. 2014;30:1997–2000. doi: 10.1007/s00381-014-2548-7. [DOI] [PubMed] [Google Scholar]
- 7.Zanello M, Zerah M, Sainte-Rose C, Di Rocco F. Virtual simulation in neurosurgery: A comparison between pediatric and general neurosurgeons. Acta Neurochir (Wien) 2014;156:2215–6. doi: 10.1007/s00701-014-2214-6. [DOI] [PubMed] [Google Scholar]
- 8.Issenberg SB. The scope of simulation-based healthcare education. Simul Healthc. 2006;1:203–8. doi: 10.1097/01.SIH.0000246607.36504.5a. [DOI] [PubMed] [Google Scholar]
- 9.Chan S, Conti F, Salisbury K, Blevins NH. Virtual reality simulation in neurosurgery: Technologies and evolution. Neurosurgery. 2013;72(Suppl 1):154–64. doi: 10.1227/NEU.0b013e3182750d26. [DOI] [PubMed] [Google Scholar]
- 10.Seymour NE, Gallagher AG, Roman SA, O’Brien MK, Bansal VK, Andersen DK, et al. Virtual reality training improves operating room performance: Results of a randomized, double-blinded study. Ann Surg. 2002;236:458–63. doi: 10.1097/00000658-200210000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlberg G, Enochsson L, Gallagher AG, Hedman L, Hogman C, McClusky DA, 3rd, et al. Proficiency-based virtual reality training significantly reduces the error rate for residents during their first 10 laparoscopic cholecystectomies. Am J Surg. 2007;193:797–804. doi: 10.1016/j.amjsurg.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 12.Bova FJ, Rajon DA, Friedman WA, Murad GJ, Hoh DJ, Jacob RP, et al. Mixed-reality simulation for neurosurgical procedures. Neurosurgery. 2013;73(Suppl 1):138–45. doi: 10.1227/NEU.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 13.Suri A, Patra DP, Meena RK. Simulation in neurosurgery: Past, present, and future. Neurol India. 2016;64:387–95. doi: 10.4103/0028-3886.181556. [DOI] [PubMed] [Google Scholar]
- 14.Persaud TV. Illinois, Springfield: Charles C Thomas Publisher; 1997. A History of Anatomy: The Post-Vesalian Era. [Google Scholar]
- 15.Kunkler K. The role of medical simulation: An overview. Int J Med Robot. 2006;2:203–10. doi: 10.1002/rcs.101. [DOI] [PubMed] [Google Scholar]
- 16.Limbrick DD, Jr, Dacey RG., Jr Simulation in neurosurgery: Possibilities and practicalities: Foreword. Neurosurgery. 2013;73(Suppl 1):1–3. doi: 10.1227/NEU.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 17.Robison RA, Liu CY, Apuzzo ML. Man, mind, and machine: The past and future of virtual reality simulation in neurologic surgery. World Neurosurg. 2011;76:419–30. doi: 10.1016/j.wneu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Singh H, Kalani M, Acosta-Torres S, El Ahmadieh TY, Loya J, Ganju A, et al. History of simulation in medicine: From Resusci Annie to the Ann Myers medical center. Neurosurgery. 2013;73(Suppl 1):9–14. doi: 10.1227/NEU.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 19.Fahey DG. The self-inflating resuscitator – Evolution of an idea. Anaesth Intensive Care. 2010;38(Suppl 1):10–5. doi: 10.1177/0310057X100380S102. [DOI] [PubMed] [Google Scholar]
- 20.Cooper JB, Taqueti VR. A brief history of the development of mannequin simulators for clinical education and training. Qual Saf Health Care. 2004;13(Suppl 1):i11–8. doi: 10.1136/qshc.2004.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willaert WI, Aggarwal R, Van Herzeele I, Cheshire NJ, Vermassen FE. Recent advancements in medical simulation: Patient-specific virtual reality simulation. World J Surg. 2012;36:1703–12. doi: 10.1007/s00268-012-1489-0. [DOI] [PubMed] [Google Scholar]
- 22.Cobb MI, Taekman JM, Zomorodi AR, Gonzalez LF, Turner DA. Simulation in neurosurgery – A brief review and commentary. World Neurosurg. 2016;89:583–6. doi: 10.1016/j.wneu.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 23.Auer LM, Auer DP. Virtual endoscopy for planning and simulation of minimally invasive neurosurgery. Neurosurgery. 1998;43:529–37. doi: 10.1097/00006123-199809000-00072. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Brodlie K, Phillips N. Web-based VR training simulator for percutaneous rhizotomy. Stud Health Technol Inform. 2000;70:175–81. [PubMed] [Google Scholar]
- 25.Dias LA, Gebhard H, Mtui E, Anand VK, Schwartz TH. The use of an ultraportable universal serial bus endoscope for education and training in neuroendoscopy. World Neurosurg. 2013;79:337–40. doi: 10.1016/j.wneu.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Delorme S, Laroche D, DiRaddo R, Del Maestro RF. NeuroTouch: A physics-based virtual simulator for cranial microneurosurgery training. Neurosurgery. 2012;71:32–42. doi: 10.1227/NEU.0b013e318249c744. [DOI] [PubMed] [Google Scholar]
- 27.Spiotta AM, Rasmussen PA, Masaryk TJ, Benzel EC, Schlenk R. Simulated diagnostic cerebral angiography in neurosurgical training: A pilot program. J Neurointerv Surg. 2013;5:376–81. doi: 10.1136/neurintsurg-2012-010319. [DOI] [PubMed] [Google Scholar]
- 28.Fargen KM, Siddiqui AH, Veznedaroglu E, Turner RD, Ringer AJ, Mocco J, et al. Simulator based angiography education in neurosurgery: Results of a pilot educational program. J Neurointerv Surg. 2012;4:438–41. doi: 10.1136/neurintsurg-2011-010128. [DOI] [PubMed] [Google Scholar]
- 29.Hsu JH, Younan D, Pandalai S, Gillespie BT, Jain RA, Schippert DW, et al. Use of computer simulation for determining endovascular skill levels in a carotid stenting model. J Vasc Surg. 2004;40:1118–25. doi: 10.1016/j.jvs.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Eftekhar B, Ghodsi M, Ketabchi E, Rasaee S. Surgical simulation software for insertion of pedicle screws. Neurosurgery. 2002;50:222–3. doi: 10.1097/00006123-200201000-00038. [DOI] [PubMed] [Google Scholar]
- 31.Alaraj A, Lemole MG, Finkle JH, Yudkowsky R, Wallace A, Luciano C, et al. Virtual reality training in neurosurgery: Review of current status and future applications. Surg Neurol Int. 2011;2:52. doi: 10.4103/2152-7806.80117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beier F, Sismanidis E, Stadie A, Schmieder K, Männer R. An aneurysm clipping training module for the neurosurgical training simulator NeuroSim. Stud Health Technol Inform. 2012;173:42–7. [PubMed] [Google Scholar]
- 33.Leong JJ, Leff DR, Das A, Aggarwal R, Reilly P, Atkinson HD, et al. Validation of orthopaedic bench models for trauma surgery. J Bone Joint Surg Br. 2008;90:958–65. doi: 10.1302/0301-620X.90B7.20230. [DOI] [PubMed] [Google Scholar]
- 34.Konakondla S, Fong R, Schirmer CM. Simulation training in neurosurgery: Advances in education and practice. Adv Med Educ Pract. 2017;8:465–73. doi: 10.2147/AMEP.S113565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grechenig W, Fellinger M, Fankhauser F, Weiglein AH. The Graz learning and training model for arthroscopic surgery. Surg Radiol Anat. 1999;21:347–50. doi: 10.1007/BF01631337. [DOI] [PubMed] [Google Scholar]
- 36.Gasco J, Patel A, Ortega-Barnett J, Branch D, Desai S, Kuo YF, et al. Virtual reality spine surgery simulation: An empirical study of its usefulness. Neurol Res. 2014;36:968–73. doi: 10.1179/1743132814Y.0000000388. [DOI] [PubMed] [Google Scholar]
- 37.Malone HR, Syed ON, Downes MS, D’Ambrosio AL, Quest DO, Kaiser MG. Simulation in neurosurgery: A review of computer-based simulation environments and their surgical applications. Neurosurgery. 2010;67:1105–16. doi: 10.1227/NEU.0b013e3181ee46d0. [DOI] [PubMed] [Google Scholar]
- 38.Doyle WK. Low end interactive image-directed neurosurgery. Update on rudimentary augmented reality used in epilepsy surgery. Stud Health Technol Inform. 1996;29:1–1. [PubMed] [Google Scholar]
- 39.Gnanakumar S, Kostusiak M, Budohoski KP, Barone D, Pizzuti V, Kirollos R, et al. Effectiveness of cadaveric simulation in neurosurgical training: A review of the literature. World Neurosurg. 2018;118:88–96. doi: 10.1016/j.wneu.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Duguid A, Arundell M, Bainbridge L. How to set up and run a cadaveric surgical simulation programme. Bulletin. 2016;98:358–61. [Google Scholar]
- 41.Why there is a Shortage of Cadavers. [Last accessed on 2017 Aug 28]. Available from: https://www.economist.com/blogs/economist-explains/2014/01/economist-explains-10 .
- 42.Gomoll AH, O’Toole RV, Czarnecki J, Warner JJ. Surgical experience correlates with performance on a virtual reality simulator for shoulder arthroscopy. Am J Sports Med. 2007;35:883–8. doi: 10.1177/0363546506296521. [DOI] [PubMed] [Google Scholar]
- 43.Jaung R, Cook P, Blyth P. A comparison of embalming fluids for use in surgical workshops. Clin Anat. 2011;24:155–61. doi: 10.1002/ca.21118. [DOI] [PubMed] [Google Scholar]
- 44.Gragnaniello C, Nader R, van Doormaal T, Kamel M, Voormolen EH, Lasio G, et al. Skull base tumor model. J Neurosurg. 2010;113:1106–11. doi: 10.3171/2010.3.JNS09513. [DOI] [PubMed] [Google Scholar]
- 45.Payner TD, Melamed I, Ansari S, Leipzig TJ, Scott JA, Denardo AJ, et al. Trends over time in the management of 2253 patients with cerebral aneurysms: A single practice experience. Surg Neurol Int. 2011;2:110. doi: 10.4103/2152-7806.83728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawton MT, Du R. Effect of the neurosurgeon's surgical experience on outcomes from intraoperative aneurysmal rupture. Neurosurgery. 2005;57:9–15. doi: 10.1227/01.neu.0000163082.20941.ef. [DOI] [PubMed] [Google Scholar]
- 47.Aboud E, Aboud G, Al-Mefty O, Aboud T, Rammos S, Abolfotoh M, et al. “Live cadavers” for training in the management of intraoperative aneurysmal rupture. J Neurosurg. 2015;123:1339–46. doi: 10.3171/2014.12.JNS141551. [DOI] [PubMed] [Google Scholar]
- 48.de Oliveira MM, Ferrarez CE, Ramos TM, Malheiros JA, Nicolato A, Machado CJ, et al. Learning brain aneurysm microsurgical skills in a human placenta model: Predictive validity. J Neurosurg. 2018;128:846–52. doi: 10.3171/2016.10.JNS162083. [DOI] [PubMed] [Google Scholar]
- 49.Winer JL, Kramer DR, Robison RA, Ohiorhenuan I, Minneti M, Giannotta S, et al. Cerebrospinal fluid reconstitution via a perfusion-based cadaveric model: Feasibility study demonstrating surgical simulation of neuroendoscopic procedures. J Neurosurg. 2015;123:1316–21. doi: 10.3171/2014.10.JNS1497. [DOI] [PubMed] [Google Scholar]
- 50.Ciporen JN, Lucke-Wold B, Mendez G, Cameron WE, McCartney S. Endoscopic management of cavernous carotid surgical complications: Evaluation of a simulated perfusion model. World Neurosurg. 2017;98:388–96. doi: 10.1016/j.wneu.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen AR, Lohani S, Manjila S, Natsupakpong S, Brown N, Cavusoglu MC, et al. Virtual reality simulation: Basic concepts and use in endoscopic neurosurgery training. Childs Nerv Syst. 2013;29:1235–44. doi: 10.1007/s00381-013-2139-z. [DOI] [PubMed] [Google Scholar]
- 52.Rosser JC, Jr, Lynch PJ, Cuddihy L, Gentile DA, Klonsky J, Merrell R. The impact of video games on training surgeons in the 21st century. Arch Surg. 2007;142:181–6. doi: 10.1001/archsurg.142.2.181. [DOI] [PubMed] [Google Scholar]
- 53.de Faria JW, Teixeira MJ, de Moura Sousa Júnior L, Otoch JP, Figueiredo EG. Virtual and stereoscopic anatomy: When virtual reality meets medical education. J Neurosurg. 2016;125:1105–11. doi: 10.3171/2015.8.JNS141563. [DOI] [PubMed] [Google Scholar]
- 54.Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65:S63–7. doi: 10.1097/00001888-199009000-00045. [DOI] [PubMed] [Google Scholar]
- 55.Seagull FJ, Rooney DM. Filling a void: Developing a standard subjective assessment tool for surgical simulation through focused review of current practices. Surgery. 2014;156:718–22. doi: 10.1016/j.surg.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 56.Boulet JR, Murray D. Review article: Assessment in anesthesiology education. Can J Anaesth. 2012;59:182–92. doi: 10.1007/s12630-011-9637-9. [DOI] [PubMed] [Google Scholar]
- 57.Berry M, Lystig T, Beard J, Klingestierna H, Reznick R, Lönn L, et al. Porcine transfer study: Virtual reality simulator training compared with porcine training in endovascular novices. Cardiovasc Intervent Radiol. 2007;30:455–61. doi: 10.1007/s00270-006-0161-1. [DOI] [PubMed] [Google Scholar]
- 58.Bagai A, O’Brien S, Al Lawati H, Goyal P, Ball W, Grantcharov T, et al. Mentored simulation training improves procedural skills in cardiac catheterization: A randomized, controlled pilot study. Circ Cardiovasc Interv. 2012;5:672–9. doi: 10.1161/CIRCINTERVENTIONS.112.970772. [DOI] [PubMed] [Google Scholar]
- 59.Hseino H, Nugent E, Lee MJ, Hill AD, Neary P, Tierney S, et al. Skills transfer after proficiency-based simulation training in superficial femoral artery angioplasty. Simul Healthc. 2012;7:274–81. doi: 10.1097/SIH.0b013e31825b6308. [DOI] [PubMed] [Google Scholar]
- 60.Picard M, Curry N, Collins H, Soma L, Hill J. Comparison of high-fidelity simulation versus didactic instruction as a reinforcement intervention in a comprehensive curriculum for radiology trainees in learning contrast reaction management: Does it matter how we refresh? Acad Radiol. 2015;22:1268–76. doi: 10.1016/j.acra.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Luboz V, Zhang Y, Johnson S, Song Y, Kilkenny C, Hunt C, et al. ImaGiNe seldinger:First simulator for seldinger technique and angiography training. Comput Methods Programs Biomed. 2013;111:419–34. doi: 10.1016/j.cmpb.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Reedy G. Using cognitive load theory to inform simulation design and practice. Clin Simul Nurs. 2015;11:355–60. [Google Scholar]
- 63.Wright MC, Taekman JM, Endsley MR. Objective measures of situation awareness in a simulated medical environment. Qual Saf Health Care. 2004;13(Suppl 1):i65–71. doi: 10.1136/qshc.2004.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]