Abstract
Background
Maintaining care for ill persons in the community is heavily dependent on support from unpaid caregivers. Many caregivers, however, find themselves in a caring role for which they are ill prepared and may require professional support. The telephone is an easily accessible method of providing support irrespective of geographical location.
Objectives
The objective of this review was to evaluate the effectiveness of telephone support interventions, delivered by healthcare professionals, when compared to usual care or non‐telephone‐based support interventions for providing education and psychosocial support for informal caregivers of people with acute and chronic diagnosed illnesses, and to evaluate the cost‐effectiveness of telephone interventions in this population.
Search methods
We searched the following databases from inception to 16 November 2018: the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; Embase; PsycINFO; ProQuest Dissertations and Theses A&I; and CINAHL Complete. We also searched 11 caregiver‐specific websites, three conference links, and two clinical trial registries.
Selection criteria
We included randomised controlled trials (RCTs) (including cluster‐RCTs) and quasi‐RCTs. We excluded cross‐over trials because of the high risk of carry‐over effects from one intervention to another.
Data collection and analysis
Two authors independently screened citations against the review's inclusion criteria, extracted data, and assessed the included studies using the Cochrane 'Risk of bias' tool. The review's prespecified primary (quality of life and burden) and secondary outcomes (skill acquisition, psychological health, knowledge, health status and well‐being, family functioning, satisfaction, and economic outcomes), where reported, were assessed at the end of intervention delivery and at short‐term (≤ 3 months), medium‐term (> 3 to ≤ 6 months) and longer‐term time points (> 6 to 12 months) following the intervention. Where possible, meta‐analyses were conducted, otherwise results were reported narratively.
Main results
We included 21 randomised studies involving 1,690 caregivers; 19 studies compared telephone support interventions and usual care, of which 18 contributed data to the analyses. Two studies compared telephone and non‐telephone professional support interventions. Caregiver ages ranged from 19 years to 87 years across studies. The majority of participants were female (> 70.53%), with two trials including females only. Most caregivers were family members, educated beyond secondary or high school level or had the equivalent in years of education. All caregivers were based in the community. Overall risk of bias was high for most studies.
The results demonstrated that there is probably little or no difference between telephone support interventions and usual care for the primary outcome of quality of life at the end of intervention (SMD ‐0.02, 95% CI ‐0.24 to 0.19, 4 studies, 364 caregivers) (moderate‐certainty evidence) or burden at the end of intervention (SMD ‐0.11, 95% CI ‐0.30 to 0.07, 9 studies, 788 caregivers) (low‐certainty evidence). For one study where quality of life at the end of intervention was reported narratively, the findings indicated that a telephone support intervention may result in slightly higher quality of life, compared with usual care. Two further studies on caregiver burden were reported narratively; one reported that telephone support interventions may decrease burden, the other reported no change in the intervention group, compared with usual care.
We are uncertain about the effects of telephone support interventions on caregiver depression at the end of intervention (SMD ‐0.37, 95% CI ‐0.70 to ‐0.05, 9 studies, 792 caregivers) due to very low‐certainty evidence for this outcome. Depression was reported narratively for three studies. One reported that the intervention may reduce caregiver depression at the end of intervention, but this effect was not sustained at short‐term follow‐up. The other two studies reported there may be little or no difference between telephone support and usual care for depression at the end of intervention. Six studies measured satisfaction with the intervention but did not report comparative data. All six reported high satisfaction scores with the intervention. No adverse events, including suicide or suicide ideation, were measured or reported by any of the included studies.
Our analysis indicated that caregiver anxiety may be slightly reduced (MD ‐6.0, 95% CI ‐11.68 to ‐0.32, 1 study, 61 caregivers) and preparedness to care slightly improved (SMD 0.37, 95% CI 0.09 to 0.64, 2 studies, 208 caregivers) at the end of intervention, following telephone‐only support interventions compared to usual care. Findings indicated there may be little or no difference between telephone support interventions and usual care for all of the following outcomes at the end of intervention: problem‐solving, social activity, caregiver competence, coping, stress, knowledge, physical health, self‐efficacy, family functioning, and satisfaction with supports (practical or social). There may also be little or no effect of telephone support interventions for quality of life and burden at short‐term follow‐up or for burden and depression at medium‐term follow‐up.
Litttle or no difference was found between groups for any of the reported outcomes in studies comparing telephone and non‐telephone professional support interventions. We are uncertain as to the effects of telephone support interventions compared to non‐telephone support interventions for caregiver burden and depression at the end of intervention. No study reported on quality of life or satisfaction with the intervention and no adverse events were reported or noted in the two studies reporting on this comparison.
Authors' conclusions
Although our review indicated slight benefit may exist for telephone support interventions on some outcomes (e.g. anxiety and preparedness to care at the end of intervention), for most outcomes, including the primary outcomes, telephone‐only interventions may have little or no effect on caregiver outcomes compared to usual care. The findings of the review were mainly based on studies with overall high risk of bias, and few participants. Further high‐quality trials, with larger sample sizes are required.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Young Adult; Chronic Disease; Chronic Disease/psychology; Psychosocial Support Systems; Telephone; Adaptation, Psychological; Anxiety; Anxiety/psychology; Caregivers; Caregivers/psychology; Depression; Depression/psychology; Family; Mental Health; Quality of Life; Randomized Controlled Trials as Topic; Stress, Psychological; Stress, Psychological/psychology
Plain language summary
[Telephone interventions for providing education and psychosocial support to caregivers]
Background
Caregivers providing care to a family member, friend, or neighbour experience the role in differing ways. Some caregivers may find themselves in a caring role for which they are ill prepared and professional support is essential. This review examined whether telephone support interventions delivered by healthcare professionals had positive benefits on a range of outcomes including quality of life, burden (the experience of strain or load), skill acquisition (e.g. problem‐solving), psychological health (e.g. depression), knowledge, physical health, family functioning, satisfaction, or cost, for unpaid caregivers in the community. A telephone support intervention is one that is delivered via the telephone and designed to provide knowledge, advice, or help to caregivers to enable them to manage their own well‐being or that of the person they care for. It is an easily accessible method of providing support irrespective of geographical location. Studies that compared telephone support to usual care or to non‐telephone‐based professional support interventions were included.
Study characteristics
We included 21 studies involving 1,690 caregivers caring for persons with a range of diagnosed conditions. Caregiver ages ranged from 19 years to 87 years. Most were female and caring for a family member. The majority were spouses, in particular wives, except for one study that mainly focused on adult children. Most caregivers had greater than secondary school education. Eighteen studies reported funding from reputable sources.
Key results
Nineteen studies (18 studies contributing data) compared telephone support interventions and usual care. Telephone support interventions probably have little or no effect on caregiver quality of life (4 studies, 364 caregivers) and may have little effect on burden (9 studies, 788 caregivers) compared to usual care on completion of the intervention. Although anxiety may be slightly reduced and preparedness to care slightly improved following the intervention, we are uncertain about the effects on depression and overall, telephone interventions may have little or no effect on the outcomes assessed by this review. High satisfaction with the intervention was reported in six studies that measured this outcome, but no comparative data from usual care groups was reported.
Two studies compared telephone and non‐telephone‐based support interventions. There may be little or no evidence of an effect of telephone support when compared non‐telephone‐based support interventions for any reported outcome. No adverse events were measured or reported in any of the included studies.
Quality of evidence
The quality of the evidence was assessed as very low to moderate across outcomes, thus reducing confidence in the findings. Many of the results were based on data from single studies with few participants. Larger well‐designed studies are required to determine the effects of telephone support interventions.
Summary of findings
Summary of findings for the main comparison. Telephone support intervention compared to Usual care for providing education and psychosocial support for informal caregivers of adults with diagnosed illnesses.
| Telephone intervention compared to Usual care for providing education and psychosocial support for informal caregivers of adults with diagnosed illnesses | ||||
| Patient or population: Informal caregivers of adults with diagnosed illnesses Setting: Community Intervention: Education or psychosocial telephone support Comparison: Usual care | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Risk with Telephone intervention | ||||
|
Quality of Life End of intervention Assessed with: WHOQoL Brief instrument (26‐item), SF‐36 (0‐100 scale), SF‐12, and Adapted BKOS (15‐item, 0‐7 scale) For all scales, higher scores indicated higher QoL. |
The mean score for QoL in the intervention group was 0.02 standard deviations lower (0.24 lower to 0.19 higher) | 364 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1,2 | One further study reported that caregivers receiving a telephone intervention may have slightly higher QoL at end of intervention, compared with usual care. Overall, at the end of intervention, telephone interventions probably have little or no effect on caregiver QoL |
|
Burden End of intervention Assessed with: Revised Memory and Behaviour Problem Checklist (0‐24 range), 24‐ and 26‐item Caregiver Reaction Assessment, Family Appraisal of Caregiving Questionnaire (Caregiver Strain subscale – Palliative Care), Burden Interview (22‐ and 12‐item inventories; 0‐4 scale), Modified BKOS scale (22‐item, 5‐point scale) For all scales, higher scores indicated higher burden. |
The mean score for Burden in the telephone group was 0.11 standard deviations lower (0.3 lower to 0.07 higher) | 788 (9 RCTs) | ⊕⊕⊝⊝ LOW 2,3 | Two further studies reported caregiver burden. One reported that telephone interventions may decrease burden; the other reported no change in the intervention group, compared with usual care. Overall, at the end of intervention, telephone interventions may have little or no effect on caregiver burden. |
|
Psychological health: Depression End of intervention Assessed with: Center for Epidemiologic Studies Depression Scale 11‐item SF, 10‐item and, 20‐item measures (including German version) (0‐3 scales), Brief Symptom Inventory (18‐item, 5‐point scale), and the Geriatric Depression Scale (30‐item, score range 0‐30) For all scales, higher scores were associated with increased depression/symptoms of depression. |
The mean score for depression in the telephone group was 0.37 standard deviations lower (0.7 to 0.05 lower ) | 792 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 2,4 | Three further studies reported caregiver depression. One reported that telephone interventions may decrease depression; the other two reported no change in the intervention group, compared with usual care. Overall, we are uncertain of the effects of telephone interventions on caregiver depression at the end of intervention. |
|
Satisfaction with the intervention End of intervention |
See comment | ‐ | ‐ | No study was found that assessed this outcome comparatively. Six studies measured satisfaction with the intervention in the intervention group only. All six reported high levels of satisfaction with the intervention (i.e. 'mostly', 'very much so', 'good' or 'excellent'). |
| Adverse events including suicide and suicide ideation | See comment | ‐ | ‐ | No studies measured these outcomes. |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1 Although participant numbers were relatively few at n = 364, they were deemed sufficient for studies evaluating these types of intervention in a population of informal caregivers; we therefore did not downgrade on sample size (imprecision) for this reason.
2 Most information was from studies at low or unclear risk of bias on most items in the 'risk of bias' tool, although in some studies, one or two risk of bias criteria were assessed as having high risk of bias. We therefore downgraded by 1 level for plausible risk of bias that could seriously alter the results.
3 Some variation in the effect estimates and moderate heterogeneity; evidence downgraded by 1 level (serious inconsistency).
4 Variation in the effect estimates across studies and substantial heterogeneity; evidence downgraded by 2 levels (very serious inconsistency).
BKOS: Bakas Caregiver Outcomes Scale QoL: Quality of life SF: Short Form SF‐12: Short Form ‐12 items SF‐36: Short Form ‐ 36 items WHOQoL: World Healthcare Organisation Quality of Life
Summary of findings 2. Telephone support compared to non‐telephone support intervention for providing education and psychosocial support for informal caregivers of adults with diagnosed illnesses.
| Telephone compared to non‐telephone support intervention for providing education and psychosocial support for informal caregivers of adults with diagnosed illnesses | ||||
| Patient or population: Informal caregivers of adults with diagnosed illnesses Setting: Community Intervention: Education or psychosocial telephone support Comparison: Education or psychosocial non‐telephone support | ||||
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Risk with Telephone | ||||
| Quality of Life | No studies measured this outcome | ‐ | ‐ | |
|
Burden End of intervention Assessed with: the subjective burden subscale of the Caregiver Appraisal Inventory Higher scores indicated greater burden. |
The mean score for burden in the telephone group was 0.2 lower (0.74 lower to 0.34 higher) | 11 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | We are uncertain of the effects of telephone interventions on caregiver burden at the end of intervention. |
|
Psychological health: Depression: End of intervention Assessed with: Center for Epidemiological Studies Survey‐Depression scale (20‐item, 0‐3 scale) Higher scores indicated higher levels of depression. |
The mean score for depression in the telephone group was 4.3 lower (9.57 lower to 0.97 higher) | 11 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | We are uncertain of the effects of telephone interventions on caregiver depression at the end of intervention |
| Satisfaction with the intervention | No studies measured this outcome | ‐ | ‐ | |
| Adverse events including suicide and suicide ideation | No studies measured these outcomes | ‐ | ‐ | |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1 Most information from the 1 included study indicated low or unclear risk of bias on most items on the 'risk of bias' tool, although risk of bias was high for selective reporting. We therefore downgraded by 1 level for plausible risk of bias that could seriously alter the results.
2 Participant numbers were deemed insufficient at 11 and the upper and lower CI limits were > 0.5 from the effect size (downgraded by 2 levels for imprecision).
Background
Many people with diagnosed medical conditions are dependent upon family members and informal caregivers (that is, a caregiver who is not paid (Levine 2010)), to provide support and care, usually in the home of the person needing care (Care Alliance Ireland 2015; International Alliance of Carer Organisations 2016). The care provided varies according to individuals' needs, with care categories defined as low (mainly companionship, with some caring assistance), medium (instrumental care such as cooking and shopping), medium with personal assistance (such as washing and dressing) and high (including low and medium level caring when the person receiving care cannot provide much assistance to the caregiver) (Care Alliance Ireland 2015). In many instances, informal carers find themselves in a caring role for which they are ill prepared (Coleman 2015; Levine 2013; Nalder 2012). Providing care may impact negatively on the caregiver from an emotional, physical, social, and financial perspective (Care Alliance Ireland 2010; Glendinning 2009; OECD 2011). Internationally, the focus of health care is to have people cared for in the community for as long as is possible. The aim is to shift to community‐based and patient‐centred paradigms of care for the treatment of chronic diseases (WHO 2006); and, where possible, prevent admission to secondary healthcare facilities. Unpaid or informal caregivers have been described as the backbone of the healthcare system (Care Action Network 2013; Levine 2010; OECD 2013); and worldwide they play a key role in the provision of care, saving billions in healthcare expenditure (Levine 2010; Navine‐Waliser 2002).
Description of the condition
The international literature suggests that caregiving impacts similarly on caregivers irrespective of geographical location or of the illness being experienced by the care‐recipient. In a UK study, Golics 2013 reported that caregivers caring for family members with a range of illnesses experienced worry, frustration, anger, and guilt. For some, adjustment to the role is difficult and requires significant emotional and life changes. This is reflected across the world with national studies from Japan (Oshio 2015), South Korea (Do 2015) and Canada (Penning 2015) highlighting the negative effects of caregiving on informal caregivers.
Family members providing unpaid care have been described “…as a hidden 'patient' group…” (Golics 2013, p.795). The need for professional support for caregivers has been reported and highlighted across a range of acute (i.e. time‐bound and responsive to treatment) and chronic (i.e. not time‐bound, non‐curable and susceptible to remission and exacerbation) conditions (Murrow 1996). This includes support for caregivers of people surviving complex illnesses (Czerwonka 2015), patients with cancers ( Heese 2013; Merckaert 2013; Mosher 2013), mental health problems (Gavois 2006), stroke (Cameron 2013), Parkinson’s disease (Oguh 2013), dementia ( Lilly 2012; Van Mierlo 2012b; Zwaanswijk 2013) and multiple sclerosis (Corry 2009). Golics 2013 argues that having access to people with the knowledge and skill to provide support, in particular emotional support, may ease the burden of caring. Burden is a multidimensional concept that can be viewed objectively, in terms of externally observable phenomena, such as the financial impact of caring, or subjectively, in terms of how it is perceived by the individual (Buhse 2008). This may include the experience of strain, stress, or load as a result of the caring role (Buhse 2008).
Although the impact of caregiving may be similar, how it is experienced by caregivers differs. Within caregiver groups such as caregivers of older persons (Unson 2016), intensive care survivors (ICU) (Foster 2003), and people with schizophrenia (Roick 2007), researchers have noted that gender, relationship to the patient (Foster 2003; Roick 2007; Unson 2016) level of contact with the patient (Roick 2007; Unson 2016), younger age (Unson 2016) and unemployment (Roick 2007) all influence how caregivers experience burden. McCabe 2009 further reported lower mood and quality of life in caregivers of people with motor neurone disease and Huntington’s disease compared to caregivers of people with Parkinson's disease and multiple sclerosis. Adjusting to the role of caregiver has been described as a non–linear or oscillating process (Greenwood 2010; Robinson 2005) that is continual (O’Shaughnessy 2010), gradual and occurs over time (Hasson 2010; Robinson 2005), with the adjustment process differing for caregivers within and across conditions (Cameron 2016; Davidson 2012; Smith 2004).
Description of the intervention
Healthcare professionals commonly communicate with care‐recipients and their family members by telephone. A number of research studies have evaluated use of the telephone only (Bakas 2009; Hartke 2003; Van Mierlo 2012b), or the telephone as a component of an intervention (Borman 2009; Piamjariyakul 2013; Sepulveda 2008; Tremont 2008; Van Mierlo 2012b). Some of the interventions are delivered by healthcare professionals and others are delivered by peers (Goodman 1990), or co‐facilitated by befriending volunteers (Charlesworth 2008). In this review, the focus is on evaluating the telephone only, as a means of delivering a support intervention, by healthcare professionals for caregivers of people with acute and chronic conditions. In this review, a telephone intervention is defined as an intervention that enables healthcare professionals to verbally communicate remotely with caregivers. A healthcare professional is a trained healthcare person who has received specific healthcare education and training in the management and care of people with diagnosed conditions, their family members, significant others or caregivers (e.g. nurses, medical doctors, social workers, physiotherapists, occupational therapists, counsellors/psychologists, and dieticians/nutritionists).
How the intervention might work
Professional support
Healthcare professionals provide services to patients and families/caregivers that includes direct care to people with diagnosed illnesses and indirect care, in the form of supportive advice, professional information, and psychosocial/educational support. In general, the benefits of professional support are likely to be dependent on the issues being addressed (Rosland 2008), and the readiness and receptivity of the person receiving the support (Hogan 2002; Toseland 1989). Reinhard 2008 demonstrated that professional support selectively reduces caregiver burden for those caring for people with mental health problems. Specific types of professional support, such as practical advice in managing behaviours, were found to be helpful in reducing objective burden (family arguments, missing days at work, household disruptions) (Reinhard 2008). Deek 2016 also reported favourably on family‐centred self‐care interventions, delivered by trained personnel, for adults living with chronic conditions and concluded that appropriate education and support should be provided by healthcare professionals (Deek 2016). Professionals have the education and training to provide emotional support to caregivers, helping ease the social isolation and emotional demands of caregiving (Mittelman 1996).
Strategies to improve caregiver outcomes
Caregiver support programmes help promote caregiver health by providing psychological support, information, and education to caregivers, while taking cognisance of caregivers’ limited time and resources (Gendron 2013). These psychoeducational programmes help carers develop skills in identifying signs of distress, managing symptoms, coping strategies/skills, and provide help with finding and accessing social support services (Riess‐Sherwood 2002). The telephone has been described as a good means of exchanging information, providing health education and advice, managing symptoms, recognising complications early, giving reassurance, and providing quality service (Thompson 2007).
Strategies to improve caregiver outcomes include providing education or information, assisting carers with problem‐solving, learning coping skills/behaviours, effective use of resources, seeking out social support, and identification of signs of distress (Riess‐Sherwood 2002). Coping strategies have been effective in improving the psychological health of caregivers of people with dementia (Selwood 2007). Likewise preparedness to care was found to reduce caregiver burden for caregivers of older persons (Zwicker 2010), and those with cancer (Scherbring 2002; Zwicker 2010). It was also found to ameliorate some aspects of role strain (Archbold 1990), and was the strongest predictor for lowering caregiver stress in stroke caregivers (Ostwald 2009). Failure to help caregivers master the skills and ability to manage their own health and well‐being during the early phases of caregiving may lead to greater difficulty integrating strategies, such as coping strategies, into daily life in later stages of the caregiving process (Riess‐Sherwood 2002). All of these strategies are amenable to delivery via the telephone. Reinhard 2008 contends that "...even a simple one‐to‐one telephone call may be effective in helping the caregiver..." (p.345). In this review, any strategy involving education or psychosocial support, or a combination of these, that focused on improving caregiver outcomes (see 'Types of outcome measures' for further detail) was considered.
Barriers to supporting caregivers
Many factors mitigate against the delivery of strategies to provide support for caregivers. Professional support services in the community often lack funding and availability; and, when available, may be insufficient to meet the needs of people with chronic illnesses (Rosland 2010). The large numbers of caregivers means that face‐to‐face interventions are unlikely to be feasible (Wilz 2016), because of distance (Hartke 2003) or cost, time, and inconvenience (Hartke 2003; Wilz 2016).
Factors that help overcome barriers to supporting caregivers
When distance, inconvenience, being homebound, or reluctance to leave the care‐recipient hinder face‐to‐face interventions (Hartke 2003), telecommunications and other media can be used (Badr 2016). Wilz 2016 concluded that the telephone is highly acceptable to family carers and reported on two qualitative studies which indicated that such interventions may meet caregivers' needs in respect of information, guidance, professional, and emotional support. Badr 2016 also suggested that telecommunications and other media interventions would enable caregivers to manage their own feelings and promote their ability to care. These findings support earlier qualitative research which reported that telephone support was a convenient and trouble‐free means of providing support to caregivers of people with dementia (Salfi 2005).
Reported benefits of telephone support interventions for caregivers
Previous research indicated that caregiver telephone interventions lead to positive outcomes (Chi 2015; Topo 2009). In a systematic review of telehealth tools and interventions to support caregivers, 20 of the 65 included studies reported on telephone‐based interventions (Chi 2015). Detailed results from individual telephone‐based studies were not reported in the review, rather, a collective summary of the findings of all technology‐based interventions, such as videoconferencing, telemetry and remote monitoring were presented, with the authors stating that 62 of the 65 included studies (95%) reported that caregivers had significantly improved outcomes (Chi 2015). In a systematic review of social support interventions for caregivers of people with dementia, Dam 2016 reported mixed findings from telephone interventions, but further analysis of the included studies revealed that various research designs, including the 'pre‐test post‐test' design, were used, and, in some instances, the telephone was only a component of the intervention upon which the conclusions were formed.
The benefits of any intervention are dependent on timing, readiness of the recipient, and the nature of the issues that need to be addressed. Research indicated that support may only be effective when the recipient perceived a need for the support (Melrose 2015). In this sense, the appropriateness of professional support is likely to be dependent on the required effects. Although we could not find any studies that explicitly explored the differences between the effectiveness of professional and peer support for caregivers, a study by Rosland 2008 found that support from family and friends impacted on different self‐management behaviours for people with diabetes to those impacted upon by professionals. This suggests that, for some self‐management behaviours, family support may be required; but professional support is more appropriate for others and that the type of support offered should be guided by the desired outcomes. In general, professionals are more likely than non‐professionals to affect outcomes that require therapeutic intervention (e.g. psychological functioning and personal change), while non‐professionals are more likely to positively change participation in informal social support networks (Toseland 1989).
Why it is important to do this review
The number of caregivers internationally varies according to overall population with figures varying from 60,000 in Finland to 43.5 million in the USA (International Alliance of Carer Organisations 2016). It is estimated that across the Organisation for Economic Co‐Operation and Development (OECD) countries, more than one in 10 adults provide informal care. Across the European Union (EU), 19 million people provide care of which 9.6 million provide at least 35 hours' care a week (Glendinning 2009). This number is expected to grow by 2030 (Glendinning 2009). While the financial contribution of informal caregivers to international reduction in healthcare expenditure is unknown, it is estimated that informal caregivers contribute an annual estimated national reduction in healthcare expenditure varying from EUR 20 billion in Sweden to USD 470 billion in the USA (International Alliance of Carer Organisations 2016). This is likely to reflect the contribution of estimated care hours provided by informal caregivers.
The contribution of family members is being increasingly recognised as important to the provision and management of care in chronic illness (Rosland 2010), and across the spectrum of illnesses (Coleman 2015; Haines 2015). However, uptake of the support provided may not be feasible for caregivers owing to geographical location, time, and cost. A report on a survey of eight European countries highlighted that, while the availability of support for caregivers of people with dementia was high, uptake was low, and utilisation may depend on the degree of accessibility of the support and caregivers' ability to perceive, seek, reach out, pay, and engage with the services (Lethin 2016). The telephone provides a mode of intervention delivery that has the potential to increase accessibility and affordability of support programmes.
Distribution of caregivers and telephone availability
As caregivers live in the community, are regionally and nationally dispersed, and are often in paid employment in addition to their unpaid caregiving role (International Alliance of Carer Organisations 2016; OECD 2011), face‐to‐face contact with people who can provide emotional support and advice is not always feasible. Attendance‐based interventions can be time‐consuming and expensive for the caregiver (Kaltenbaugh 2015; Ravenson 2016). Telephone communication is widely available internationally, with almost everyone having some form of access to a telephone including individuals living in remote settings (Lavender 2013). Pew Research Centre 2015 reported a median of 84% mobile phone ownership in emerging and developing countries with mobile phone ownership rates ranging from 47% to 97% in Pakistan and China, respectively. In 2011, of the 5.3 billion users of mobile phones worldwide, 3.5 billion were from developing countries (Shozi 2013), and it is projected that 70% of the world population will use smartphones by 2020 (Williams 2015), which will equate to more than 6.1 billion users (Lunden 2015). However, 10% of the world's population do not have access to mobile phones, with the majority of these from the rural areas of Asia and sub‐Saharan Africa (Consumer Technology Association 2015). Seventeen percent of people in sub‐Saharan Africa do not own a mobile phone but more than half of those people have, at times, access to a fixed line phone (Pew Research Centre 2015). Despite this, the mobile market growth rate in sub‐Saharan Africa is one of the highest worldwide (Deloitte 2012); and the growth in mobile phone networks has transformed communications in sub‐Saharan Africa, an area with the highest disease burden (Vos 2015).
Feasibility of technology‐based interventions
Research studies, in particular studies in stroke, dementia, and human immunodeficiency virus, indicate that technology‐based interventions can be feasibly implemented for caregivers of people with many different conditions (Brereton 2007; Herman 2006). Integrating telephone/mobile technology into current healthcare strategies provides a potential means for new ways for healthcare professionals to deliver care to patients and their caregivers (Deloitte 2014). Finkel 2007 argued that "...technology offers a cost effective and practical method for delivering interventions to caregivers” (p.443). Despite this assertion, there is little evidence currently of economic advantage (an aspect explored in this review) other than the suggestion that the need for healthcare professionals and caregivers to travel is eliminated, and caregiver access to existing resources and programmes is enhanced (Finkel 2007).
Factors that mitigate against implementation of findings to date
A number of factors mitigate against the usefulness of the findings from existing literature reviews and individual studies that included a telephone component. For example, in a literature review on technology studies to meet the needs of people with dementia and their caregivers, in which 15 of the included studies focused on caregiver interventions (Topo 2009), most of the interventions were complex interventions with the telephone as one component. As outcomes from the specific components of the intervention were not isolated or presented individually, the benefit of the telephone alone was difficult to determine. Failure to isolate or present findings from individual components of a multicomponent intervention can limit the application of such interventions. If the benefits from a multicomponent intervention could be realised with the application of any one component of the intervention, this needs to be highlighted so that healthcare resources are applied in an efficient and effective manner. Likewise, the potential benefits of telephone‐only support interventions, delivered by healthcare professionals to individuals or groups, need to be established. There is little empirical evidence to support the effectiveness of group interventions over interventions delivered to participants individually (Toseland 1989). While studies evaluated the effects of different modes of delivering interventions to groups, e.g. telephone versus face‐to‐face, we were unable to find any studies that evaluated the effects of a telephone group versus telephone one‐to‐one approach to intervention delivery, although these studies may be conducted in the future.
No Cochrane review was found that focused on telephone‐only support interventions for informal caregivers across a range of medical conditions. We found one Cochrane review that used the telephone for delivering a counselling intervention by healthcare professionals to caregivers of people with dementia only (Lins 2014). In a meta‐analysis of three trials in this review, depressive symptoms from telephone counselling alone were reduced and potential positive effects of other outcomes, including distress, burden, anxiety, quality of life, self‐efficacy, satisfaction, and social support, were also suggested. While the studies included in Lins 2014 were likely to be included in this review, we planned to analyse them along with telephone support interventions for a range of conditions, so improving our knowledge on the telephone's effectiveness as a means of delivering psychosocial support or education to caregivers of people across a broad spectrum of conditions. This Cochrane review differs from other Cochrane reviews on caregiver interventions (Aubin 2012; Chan 2011; Ellis 2010; Forster 2012; Legg 2011; Vernooij‐Dassen 2011), as, unlike these reviews, the main objective of our review was to determine whether or not the telephone alone as a mode of delivering a support intervention to caregivers of diagnosed illnesses was effective. Other Cochrane reviews that differ from our review include those by Candy 2011 and Lavender 2013. Candy 2011, who evaluated peer‐support interventions for caregivers, did not report any findings specific to the telephone. Lavender 2013 concluded that there was insufficient evidence to recommend routine telephone support for women accessing maternity services.
Two Cochrane protocols where telephone interventions were likely to be included as part of the review were identified (González‐Fraile 2015; Santin 2012). González‐Fraile 2015 focused on the provision of information, support, and training for informal caregivers of people with dementia and indicated that the telephone is a potential format for administering the intervention. Santin 2012 focused on psychosocial interventions for informal caregivers of people living with cancer, stating that interventions that included telephone counselling would be included. Although there may be some overlap between these two reviews and our review, the overall scope of this review is broader and has a specific focus on the telephone only as the mode of intervention delivery across a range of conditions.
In summary, the need for professional support for caregivers across a range of conditions is well established. As difficulties for caregivers attending face‐to‐face interventions have been highlighted (Badr 2016; Wilz 2016), telephone‐based interventions across caregiver groups provide a potentially important alternative. To date, there is no Cochrane review on the effectiveness of telephone support interventions alone, delivered by healthcare professionals, for caregivers across a range of medical conditions. It is therefore important to determine whether or not support interventions delivered by telephone are effective so that healthcare professionals can make informed decisions about whether or how to use the telephone in providing support to caregivers, should it be shown to be effective. Consequently, this review set out to determine the effectiveness of education or psychosocial support interventions, or a combination of both, delivered exclusively by telephone and by healthcare professionals, for informal caregivers of people with diagnosed illness. The results of this review have the potential to inform strategy on the use of the telephone as an easily accessible, low‐cost method to provide high‐quality care with the potential to benefit hundreds of thousands of informal caregivers worldwide. It can also contribute to the primary care agenda by delivering healthcare to caregivers and patients in remote and rural areas. In addition, the findings will assist with research, resource allocation, and future planning for the promotion and optimisation of the health and well‐being of informal caregivers.
Objectives
To evaluate the effectiveness of telephone support interventions, delivered by healthcare professionals, when compared to usual care or non‐telephone‐based support interventions for providing education and psychosocial support to informal carers of people with acute and chronic diagnosed illnesses, on these carers' quality of life, psychosocial, and physical well‐being. We aim, additionally, to evaluate the cost‐effectiveness of telephone interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) (including cluster‐RCTs) and quasi‐RCTs. We included multi‐arm studies that used a pairwise comparison of groups that otherwise met the inclusion criteria for this review (Higgins 2011), and where data specific to the telephone component of the intervention could be extracted in isolation. We excluded studies where the caregivers and care‐recipients received the intervention together or caregivers were only included if they chose to take part in the intervention which was offered to the care‐recipient. We also excluded cross‐over trials as there is a high risk of carry‐over effects from one intervention to another (Higgins 2011).
Types of participants
We included informal adult caregivers, defined as persons aged 18 years or over, caring for adult individuals with a diagnosed illness and in receipt of telephone intervention support from a healthcare professional. For the purpose of this review, a caregiver was defined as a person (family member, friend, or significant other) who provides personal help (support or care) for a person with an acute or chronic illness, and was not a paid healthcare provider. Caregivers are also commonly referred to as 'carers' in the literature, and are used interchangeably in this review. An acute illness was defined as a diagnosed condition lasting less than six months and a chronic illness was defined as a diagnosed condition lasting for six months or more. We included caregivers of people with both acute and chronic illnesses because categorisation of conditions can be difficult. Acute conditions can become chronic and chronic conditions can have acute episodes of illness. Many patients have multiple conditions and may have an acute condition superimposed upon a previously diagnosed chronic condition. In this context, separation of diseases into acute and chronic categories, or exclusion of one category from the review, did not seem appropriate, as the experiences and needs of caregivers were not likely to be neatly divided along these lines. The inclusion of both acute and chronic conditions therefore enabled us to capture and consider studies across the range of illnesses impacting on caregivers in the community.
We included telephone support interventions delivered by healthcare professionals to caregivers of people with a range of diagnosed illnesses who were living in a hospital, residential care, or in the community. The following provides an indicative list of examples, based on author familiarity with the subject area and referenced sources, as available. These examples of condition categories are intended to be illustrative rather than exhaustive.
Complex critical illness survivors (i.e. people who need caregivers on the path to recover from the intensive care unit to the home environment).
Mental health: severe mental illnesses (e.g. schizophrenia, depression, bipolar affective disorders) (Vermeulen 2015).
Neurological conditions (e.g. dementia, epilepsy, multiple sclerosis, Parkinson's disease, stroke, traumatic brain injuries, Huntington's disease, headache disorders, neuro‐infections, pain associated with neurological disorders) (WHO 2006).
Respiratory conditions (e.g. asthma, chronic obstructive airways disease).
Cardiac conditions (e.g. congestive heart failure, myocardial infarction).
Renal conditions (e.g. renal failure).
Orthopaedic conditions (e.g. hip fractures, spinal injuries).
Musculoskeletal (e.g. degenerative osteoarthritis).
Infections (e.g. HIV/AIDS).
Haematological conditions (e.g. post‐bone marrow transplant).
Endocrine (e.g. diabetes 1 and 2).
Alcohol, drug or substances issues/misuse.
Cancer: any category.
Terminal illness: due to any of the above conditions.
Older persons: frail older persons or older persons with any of the above conditions.
People with comorbidity or multimorbidity.
Types of interventions
We included all telephone support interventions delivered by healthcare professionals that provided education or psychosocial support or a combination of these for informal caregivers. Telephone interventions where the first session was an introductory session either delivered by telephone or face‐to‐face and where all remaining sessions were delivered by telephone were included. Accordingly, we excluded all caregiver interventions that were not telephone‐based, telephone interventions delivered by non‐healthcare professionals and telephone interventions targeted towards paid caregivers, patients, people living in the community who were not informal caregivers, and healthcare professionals. Neither did we include interventions that included the telephone as a component of a multicomponent intervention where the findings for the telephone component of the interventions could not be isolated. Telephone interventions with more than one face‐to‐face session or where the first face‐to‐face session followed an overall introductory session to the intervention were also excluded.
We included trials that compared a telephone support intervention delivered by a healthcare professional with either ‘usual’ care (as defined by the study's authors, and described in the Characteristics of included studies table), or a support intervention delivered by a healthcare professional that was not telephone‐based (for example, online or face‐to‐face delivery at the individual or group level), analysing these comparisons separately. Educational, psychosocial, and combined psychosocial educational interventions were included. The term 'psychosocial interventions' refers to the cognitive, behavioural and/or social mechanisms of action, e.g. counselling, psychoeducation, behavioural and cognitive intervention and social support, that aim to improve the psychosocial and physical well‐being of carers of people with chronic conditions.
Educational interventions, which in many instances include information provision, are often more difficult to pin down and define. For the purposes of this review, we categorised an education intervention as one in which information was provided for the purpose of increasing the carer's factual knowledge, as well as interventions that included a component that ensured that the carer understood the information given and could put it into action (Mahan 1963), and/or where the intervention was defined or described as an education intervention by the trial/study authors. The following operational definitions were used to identify papers for inclusion in our review:
A healthcare professional was defined as a registered healthcare practitioner, who might or might not be a member of the wider clinical team, who had received an education or training qualification and who provided telephone education and psychosocial support to caregivers. This included nurses, social workers, medical doctors, counsellors, psychologists, and other related allied healthcare professionals.
A telephone intervention referred to any intervention, delivered via the telephone, with an education or psychosocial (mental, emotional, social, or spiritual) focus, or a combination of these, that was designed to provide knowledge, advice, or help to caregivers in order to enable them to manage their own well‐being or that of the person they cared for. This support could be provided individually or in group format. For the purpose of this review, telephone interventions included calls from any device that enabled audio communication between healthcare professionals and caregivers, including calls made using landlines, mobile phone devices, and devices that enabled the use of Skype or other applications that facilitated verbal communication between healthcare professionals and caregivers. Telehealth interventions that provided online education or interventions other than telephone calls between healthcare professionals and caregivers were excluded.
Types of outcome measures
The following outcomes, where reported, were assessed at several time points, reflecting the possible changes in caregiver outcomes over time. All outcomes were assessed at the end of intervention delivery and at short‐term (≤ 3 months), medium‐term (> 3 to ≤ 6 months) and longer‐term time points (> 6 to 12 months) following intervention delivery.
Primary outcomes
Caregiver quality of life (QoL) as measured by the trial/study authors or using a standardised/validated measurement instrument (e.g. SF 36, WHOQoL or caregiver QoL index).
Caregiver burden as measured by the trial/study authors or using a standardised/validated measurement instrument (e.g. Caregiver Reaction Assessment, Carer Burden Inventory, or Caregiver Strain Index).
Secondary outcomes
The following secondary outcomes, where reported, were measured.
Skill acquisition (preparedness to care, caregiver competence, problem‐solving).
Psychological health (depression, anxiety, stress, coping).
Knowledge and understanding (knowledge).
Health status and well‐being (physical health, self‐efficacy, social activity).
Family functioning.
Satisfaction (satisfaction with the intervention, perceived satisfaction with practical or other supports such as technical aids, peer support, or self‐help groups).
Economic outcome data as reported from cost benefit analysis, cost‐effectiveness analysis, cost utility analysis.
Unintended outcomes that could be attributed to the intervention were considered adverse events. These included any worsening of the above outcomes in the intervention group, as reported by the study authors or as evident in worsening at the end of intervention from baseline (pre‐intervention) measurement, where provided in the included studies, in particular, anxiety and depression. Reported incidents of suicide ideation and suicide were also considered adverse events.
Outcomes reported in the included studies were categorised to the groupings above by two authors working independently. Had any differences in categorisation occurred, they would have been resolved by involvement of a third author, but this was not necessary.
The results of the following outcomes, where reported are presented in a 'Summary of findings' table (Table 1 and Table 2).
Caregiver quality of life.
Caregiver burden.
Psychological health (depression, anxiety, stress, coping).
Satisfaction (satisfaction with the intervention).
Suicide ideation and suicide.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases using a combination of appropriate keywords and controlled vocabulary terms.
The Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (searched on 3 April 2017, updated 16 November 2018);
MEDLINE [Ovid] (1946 to 3 April 2017, updated 16 November 2018);
Embase [Ovid] (1947 to 3 April 2017, updated 16 November 2018);
PsycINFO [Ovid] (1597 to 3 April 2017, updated 16 November 2018);
ProQuest Dissertations and Theses A&I [ProQuest] (1743 to 18 April 2017, updated 16 November 2018);
CINAHL Complete [Ebsco] (1937 to 3 April 2017, updated 16 November 2018).
The strategy for MEDLINE [Ovid] is presented in Appendix 1. This strategy was tailored to the other databases, as appropriate, and provided in Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; and Appendix 8. No language or date restrictions were applied.
Searching other resources
To identify any further potentially eligible studies that might not have been captured in our search of the electronic databases, we searched the grey literature database Open Grey and manually searched the reference lists of the studies included in our review. We also searched online trial registers, including the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), searched on 18 April 2017, and ClinicalTrials.gov, searched on 6 June 2017, for ongoing and recently completed studies. We contacted experts in the field and authors of included studies for advice as to other potentially relevant studies. In addition, we searched the following websites: Grey Matters, primarily for details of international Health Technology Assessment agencies; RIAN, for Irish open access research; various caregiver associations (Care Alliance Ireland, Canadian Caregiver Coalition, Carers UK, Carers Friends UK, Crossroads, Eurocarers, Family Caregiver Alliance (US), New Zealand Carers, Patient View, The Princess Royal Trust for Carers, International Caregivers Association); and conference links (Dementia Care Events ‐ International Caregivers Association, Caregiver Intervention database – The Rosalynn Carter Institute for Caregiving and the US Department of Veteran Affairs, Health Services Research & Development).
Data collection and analysis
Data collection was conducted in accordance with the published protocol (Corry 2017). Due to the small number of studies identified for each intervention type, duration, and caregiver group, data were analysed by outcome at each outcome time point following intervention delivery (end of intervention, short‐term (≤ 3 months), medium‐term (> 3 to ≤ 6 months) and longer‐term time points (> 6 to 12 months)).
Selection of studies
All database search results were merged using reference management software EndNote and duplicate citations were removed. Two pairs of two review authors (MC & KN and MC & SB) screened the titles and abstracts identified from the searches to determine those that met the inclusion criteria. Each pair independently screened half of the selected titles and abstracts, with MC screening all citations. We retrieved the full text of any papers identified as potentially relevant by at least one author. The same pairs of reviewers independently screened full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion and by consulting a third reviewer (VS) as was necessary, to reach consensus. Studies were not excluded on the basis of non‐measurement/reporting of our reviews’ prespecified outcomes, where all other inclusion criteria were fulfilled. All potentially relevant papers excluded from the review at this stage are listed, with reason(s) for exclusion, in the 'Characteristics of excluded studies' table. We also provided citation details and any available information about ongoing studies, and collated and reported details of duplicate publications, as each study (rather than each report) was the unit of interest in the review. We reported the screening and selection process in an adapted PRISMA flow chart (Mohler 2009) (Figure 1).
1.
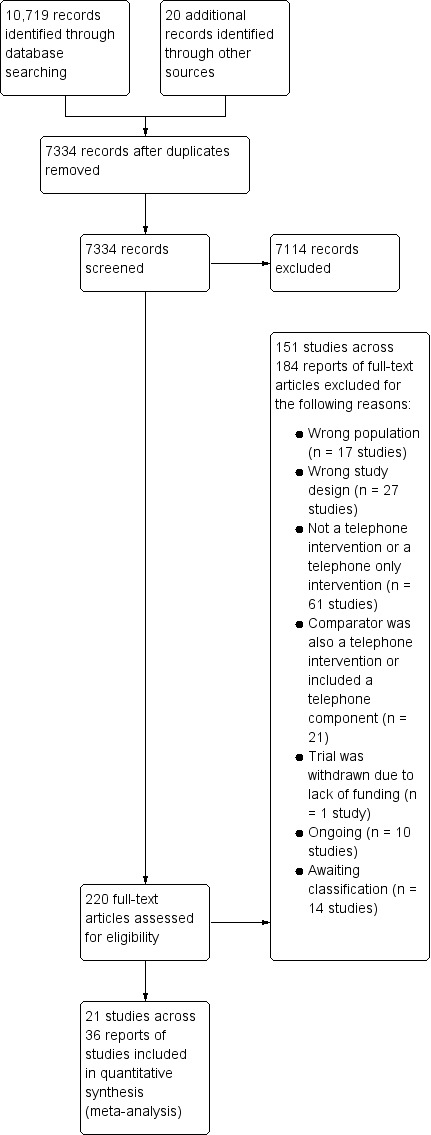
Study flow diagram.
Data extraction and management
The same pairs of review authors extracted data independently from the included studies. For the included study conducted by one of the review authors (Corry 2015), data extraction was undertaken by other review authors. Clear decision rules based on participants, interventions, comparators, and outcomes were developed to assist the reviewers prior to commencing data extraction. Any discrepancies were resolved by discussion until consensus was reached, or through consultation with a third reviewer (VS) as was necessary. If disagreements had remained unresolved, the study authors would have been contacted for study details that would lead to a resolution of the disagreement; however, this was not necessary.
We developed and piloted a data extraction form using the Cochrane Consumers and Communication Review Group Data Extraction Template (available at: cccrg.cochrane.org/author‐resources). We extracted the following data: aim of study, location, study design and methods, medical condition of care‐recipient, intervention type and detail, comparison, number and detail of participants, ethical approval, risk of bias, outcomes of interest, data and results, and funding sources.
As recommended by Herbert 2005, we noted and recorded any reported quality descriptions or rating by the study authors. We modified Section 5 of the data extraction form to ensure that we extracted data that allowed us to evaluate the quality of the intervention in terms of the framework used to develop the intervention, stated aim/goal of the intervention, match between intervention and stated goal, intensity of the intervention in terms of frequency of delivery/receipt (weekly, bi‐weekly, two‐weekly, monthly) and duration (in months), and fidelity to the intervention in terms of the extent to which it was delivered in a consistent manner (Bellg 2004; Mars 2013), and in accordance with the intervention trial protocol (Gearing 2011; Mars 2013). The extent to which contamination was minimised and monitored, the selection and standardisation of training the interventionists, standardisation and monitoring the delivery of the intervention, monitoring receipt of the intervention and the ability of participants to use the skills are all important aspects of fidelity which were evaluated (Bellg 2004; Mars 2013; Resnick 2005). We devised and piloted a quality‐assessment instrument based on Section 5 of the data extraction form, which enabled us to categorise the interventions as low‐, medium‐, or high‐quality based on the extent to which it was developed and delivered in accordance with best practice guidelines (Bellg 2004; Corry 2010; Gearing 2011; Mars 2013; MRC 2008).
One review author (MC) entered all extracted data into Review Manager 5 (RevMan 2014), and a second review author (VS) working independently, checked it for accuracy against the data extraction sheets.
Assessment of risk of bias in included studies
We assessed and reported on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) and the guidelines of the Cochrane Consumers and Communication Group (Ryan 2013), which recommend the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data; selective outcome reporting; and other sources of bias such as unbalanced groups and risk of contamination. We considered the 'risk of bias' domains separately for the different outcomes, and judged each outcome as high, low, or unclear risk of bias using the guidance provided by Higgins 2011a, and provided a quote or used information from the study reports to support our judgements for each domain provided in the 'Risk of bias' tables.
Studies were deemed to have the highest risk of bias if they were scored as high or unclear risk of bias on both sequence generation and allocation concealment and high or unclear on either risk of contamination, selective outcome reporting, or attrition bias domains, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011a). Blinding is not always possible at the point of intervention delivery and receipt due to the nature of the intervention, and, for this reason, lack of blinding of participants and personnel was not considered as a critical source of bias in this review and this domain was not rated as at high risk of bias.
In all cases, two review authors (MC & KN or MC & SB) independently assessed the risk of bias of included studies, with any disagreements resolved by discussion to reach consensus. For the lead review author's included study (Corry 2015), two other reviewers (KN & SB) assessed the study's risk of bias. We contacted study authors for additional information about the included studies, or for clarification of the study methods, as required. Had quasi‐RCTs been included in the review, we would have assessed and reported quasi‐RCTs as being at high risk of bias on random sequence generation; this was not necessary, however, as no quasi‐RCTs were included. If cluster‐RCTs had been included in the review, we would have assessed and reported the risk of bias associated with an additional domain: selective recruitment of cluster participants; this was not necessary, however, as no cluster‐RCTs were included in the review. For the one multi‐arm trial included in the review (Vazquez 2016), had the outcomes not been reported for each arm of the trial separately, we would have evaluated the risk of selective reporting of comparisons of intervention arms; this, however, was not necessary. 'Risk of bias' judgements for the included studies are presented in Figure 2 and Figure 3.
2.
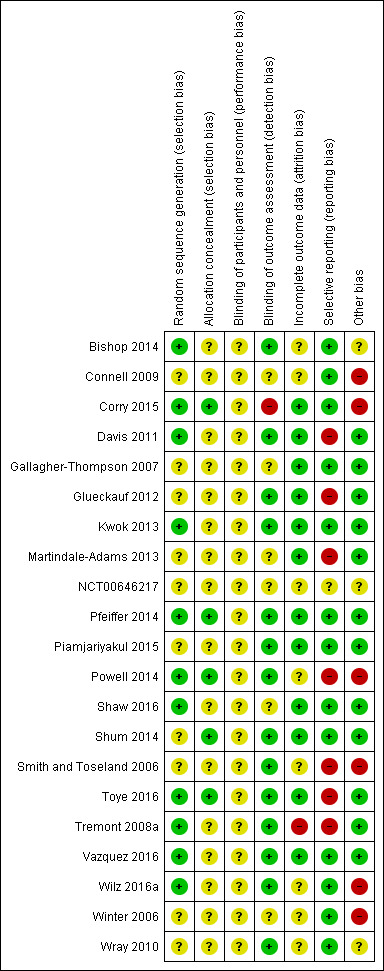
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
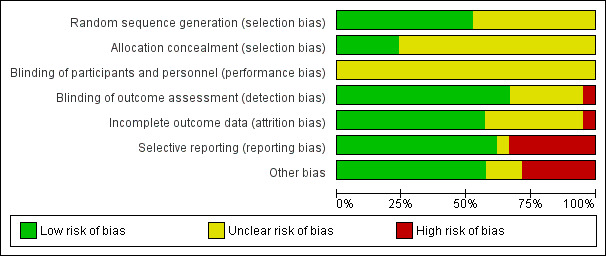
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
For dichotomous outcomes, such as those that may have been reported on the Caregiver Strain Index (CSI)/Caregiver Burden Scale (CBS‐M), we had planned to analyse data based on the number of events and the number of people assessed in the intervention and comparison groups, and use these to calculate the risk ratio (RR) and 95% confidence interval (CI). As none of the included studies reported any of the review's prespecified outcomes in this way, RRs were not reported in the review. For continuous measures, we analysed data based on the mean, standard deviation (SD), and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI. If the MD was reported without individual group data, we used this to report the study results. Where more than one study measured the same outcome but used a different measurement scale, we calculated the standardised mean difference (SMD) and 95% CI using the inverse variance method in RevMan 2014.
Where a study reported on more than one outcome from an outcome category, and the outcomes were included in a meta‐analysis, we selected the outcome that the study authors had identified as being their primary outcome. Where no primary outcome had been identified, we selected the one specified in the study's sample size calculation. If there were no sample size calculations, we ranked the effect estimates of the outcomes (as presented in the study’s results) and selected the median effect estimate. Where there was an even number of outcomes, the outcome whose effect estimate was ranked n/2, where n is the number of outcomes, was selected. Results, where feasible, were reported at different follow‐up times: end of intervention, short‐term (following end of intervention to ≤ 3 months), medium‐term (> 3 to ≤ 6 months) and long‐term (> 6 to 12 months).
Unit of analysis issues
For multi‐arm trials, we extracted data from comparisons relevant to our review; i.e. we extracted data from study arms that compared the effects of telephone‐only interventions delivered by healthcare professionals to usual care or a support intervention delivered by healthcare professionals that was not telephone‐based, for caregivers of persons with diagnosed acute illness who were living in a hospital, residential care, or the community. To avoid a unit of analysis error, in accordance with Higgins 2011a guidelines, in the one multi‐arm trial included in the review (Vazquez 2016), because the intervention groups' data were analysed separately, we divided the numbers for the comparator group by half prior to analysis to avoid over‐counting of data. Although no cluster‐RCTs were included in the review, for future updates, where cluster‐RCTs are included, we will check for unit of analysis errors. If errors are found, and sufficient information is available, we will re‐analyse the data using the appropriate unit of analysis, by taking account of the intracluster correlation (ICC). We will obtain estimates of the ICC by contacting authors of included studies, or impute the ICC using estimates from external sources. If it is not possible to obtain sufficient information to re‐analyse the data, we will report effect estimates and annotate unit of analysis errors. If necessary, we will seek further expert statistical advice when analysing data from cluster trials in any future update of this review.
Dealing with missing data
We contacted study authors to obtain missing data (participant, outcome, or summary data), where it was necessary and appropriate to do so. For participant data, where possible, we conducted analyses on an intention‐to‐treat basis; otherwise, data were analysed as reported and noted as a potential source of bias in our 'Risk of bias' assessments. Studies of telephone interventions for caregivers are likely to have high loss to follow‐up, with attrition rates of up to 45% reported in intervention groups (Tremont 2008) and 65% for control groups (Glueckauf 2007). We reported on the levels of loss to follow‐up and assessed this as a source of potential bias where more than 40% loss to follow‐up on primary outcomes was reported and considered this high risk of bias. Following attempts to contact study authors, where we failed to obtain missing outcome data, the denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing; that is, we used the numbers as reported in the included study. For continuous data, where measures of central tendency and variance, for example, medians and standard errors, were sufficiently provided in a study report, we converted these to means and SDs where possible, using the appropriate formulae, and inputted these values accordingly. If means only were available, we used the SD from another study in the review for the same outcome, where it was appropriate to do so (Higgins 2008).
Assessment of heterogeneity
The included studies were analysed by outcome, irrespective of care‐recipients' condition or duration of the intervention, and by follow‐up time‐frames (end of intervention, short‐term follow‐up to ≤ 3 months, medium‐term > 3 to ≤ 6 months and long‐term > 6 to 12 months). Where studies were considered similar enough in terms of populations, intervention, outcome measures, and timing of outcome assessment to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots and by examining the Chi² test for heterogeneity. Heterogeneity was quantified using the I² statistic. An I² value of 50% or more was considered to represent substantial levels of heterogeneity, but this value was interpreted in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2011a). Where there are few trials included in a meta‐analysis, the Chi² test has little power to detect heterogeneity. In such instances, a non‐significant result was interpreted with care and was not taken as evidence of no heterogeneity. Where we detected substantial clinical, methodological or statistical heterogeneity across included studies, we did not report pooled results from meta‐analysis but, instead, used a narrative approach to data synthesis. In this event, we attempted to minimise clinical or methodological heterogeneity by grouping studies that were similar in terms of populations, intervention features, and timing of outcome assessment in the analyses.
Assessment of reporting biases
We assessed reporting bias qualitatively based on the characteristics of the included studies (e.g. if only small studies that indicated positive findings were identified for inclusion), and if information that we obtained from contacting experts and authors of studies suggested that there were relevant unpublished studies. Had we identified sufficient studies (at least 10) for inclusion in a meta‐analysis for an outcome, we would have constructed a funnel plot to investigate small‐study effects, which may indicate the presence of publication bias; this, however, was not required. Had it been necessary, we would have formally tested for funnel plot asymmetry, with the choice of test made based on advice in Sterne 2011 and bearing in mind, when interpreting the results, that there may be several reasons for funnel plot asymmetry; we will consider this in any future updates, as necessary.
Data synthesis
Decisions on whether to meta‐analyse data were based on whether the included studies were similar enough in terms of populations, intervention, outcome measures, and timing of outcome assessment to ensure meaningful conclusions from a statistically pooled result. Owing to the observed variability in the caregiver groups, intervention types, duration of intervention delivery, and timing of outcome measurements, we used a random‐effects model for the meta‐analyses. Within the data categories, the main comparisons of the review were telephone support interventions delivered by healthcare professionals versus usual care and telephone support interventions delivered by healthcare professionals versus an alternative support intervention delivered by a healthcare professional that was not telephone‐based, for persons caring for adults with diagnosed illness. For outcomes that could not be meta‐analysed, we reported the results narratively according to timing of outcome assessment (end of intervention, short‐term completion of the intervention to ≤ 3 months; medium‐term > 3 to ≤ 6 months; and long‐term > 6 to 12 months).
Subgroup analysis and investigation of heterogeneity
Potential explanatory factors included type of condition (acute or chronic), caregiver group (diagnosis), intervention type (education or psychosocial support) and form of delivery (individual or group). There were insufficient included studies providing data for subgroup analyses; however, had there been sufficient studies, we would have conducted subgroup analyses separately on the primary outcomes for the following groups.
Intervention type (education, psychosocial, education and psychosocial combined).
Approach to telephone intervention delivery (group, one‐to‐one).
Caregiver characteristics (condition of the person being cared for grouped by category of condition (e.g. cardiac, cancer, or respiratory), gender, age (young/older caregivers), relationship to the care‐recipient).
Acute versus chronic illnesses.
Intervention duration (≤ 6 weeks, 7 to 12 weeks, 13 to 23 weeks, ≥ 24 weeks).
Sensitivity analysis
We had planned to examine the impact of studies that were categorised as high risk of bias on the outcomes in the overall meta‐analyses. However, most studies were rated as at high risk of bias overall, and, in many cases, meta‐analyses did not include a large enough number of studies to make such analysis meaningful. Similarly, we did not explore the influence of excluding unpublished studies and large studies on the overall effect size as planned, as this was not possible due to limited study numbers in meta‐analyses; we will, however, consider these methods for future updates.
‘Summary of findings’ table
We prepared a 'Summary of findings' table to present the results based on the methods described in chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and the guidelines of the Cochrane Consumers and Communication Group (Ryan 2016; Ryan 2016a). We presented the results for the major comparisons of the review, for each of the primary outcomes (quality of life and burden), psychological health (depression), satisfaction (with the intervention) and the potential harms/adverse events, as outlined in the Types of outcome measures section. Where more than one outcome was reported per category we used the methods described above to select outcomes for reporting in the ‘Summary of findings’ tables. We used the GRADE system to rank the quality of the evidence using the GRADEprofiler (GRADEpro) software (Schünemann 2011). In future updates, if necessary, we will provide a source and rationale for each assumed risk cited in the table(s), as needed. For outcomes where a meta‐analysis was not possible, we presented the results narratively.
Ensuring relevance to decisions in health care
The protocol and the review received feedback from at least one consumer referee in addition to a health professional as part of the Cochrane Consumers and Communication Group’s standard editorial process. During the development of the review, a caregiver provided comment; no changes were made to the review on receipt of the comments.
Results
Description of studies
See: Characteristics of included studies; Characteristics of ongoing studies; Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
Electronic searches generated a total of 10,719 citations, of which 3,405 were duplicate citations across databases and were removed, resulting in 7,314 records. Searching of additional sources identified twenty further records for potential inclusion, resulting in 7,334 records for assessing for relevance on title and abstract. Of these, 7,114 citations were excluded following title and abstract screening, resulting in 220 for full‐text screening. Following full‐text review, a further 184 were excluded primarily because the comparator also included the telephone or a component of the comparator was telephone‐based, the intervention was patient‐focused, both the caregivers and care‐recipients received the intervention together, or the study did not use a randomised design (see Characteristics of excluded studies). Fourteen citations are awaiting classification (Au 2014; Bass 2017a; Chodosh 2015a; Chwalisz 2017; Gitlin 2018; Mavandadi; NCT00031265; NCT00183781; NCT00416078; NCT00869739; NCT02152033; NCT02215187; NCT02505425; NCT03260608) and ten are classified as ongoing studies (Gitlin 2013; Gopinah 2017; Heckel 2015; Mavandadi 2017; Nasiriani 2017; NCT00646074; NCT02505737; NCT02806583; Soellner 2015; Wilz 2018).
Included studies
Twenty‐one studies involving 1,690 caregivers, across 36 citations, reporting on randomised trials of healthcare professional‐led telephone caregiver support, were included in the review (Bishop 2014; Connell 2009; Corry 2015; Davis 2011; Gallagher‐Thompson 2007; Glueckauf 2012; Kwok 2013; Martindale‐Adams 2013; NCT00646217; Pfeiffer 2014; Piamjariyakul 2015; Powell 2014; Shaw 2016; Shum 2014; Smith and Toseland 2006; Toye 2016; Tremont 2008a; Vazquez 2016; Wilz 2016a; Winter 2006; Wray 2010). Two studies compared the intervention to a non‐telephone professional‐led support intervention (Gallagher‐Thompson 2007; Glueckauf 2012), both self‐identified pilot studies. All others compared the intervention to a usual care/control group, of which three were self‐identified pilot studies (Bishop 2014; Piamjariyakul 2015; Vazquez 2016). One study did not contribute any data for analyses (NCT00646217): this was a completed trial, available only as a registered trial, with no available data following author contact. Thirteen studies were conducted in the USA (Bishop 2014; Connell 2009; Davis 2011; Gallagher‐Thompson 2007; Glueckauf 2012; Martindale‐Adams 2013; NCT00646217; Piamjariyakul 2015; Powell 2014; Smith and Toseland 2006; Tremont 2008a; Winter 2006; Wray 2010), two in Hong Kong (Kwok 2013; Shum 2014), two in Germany (Pfeiffer 2014; Wilz 2016a), two in Australia (Shaw 2016; Toye 2016), one in the Republic of Ireland (Corry 2015) and one in North West Spain (Vazquez 2016). Almost half of the studies (n = 10) were conducted with carers of persons with chronic conditions (Connell 2009; Corry 2015; Davis 2011; Glueckauf 2012; Martindale‐Adams 2013; Pfeiffer 2014; Piamjariyakul 2015; Tremont 2008a; Wilz 2016a; Winter 2006), two were conducted with caregivers of persons with acute conditions (Powell 2014; Shaw 2016), one for caregivers of persons with an acute condition which may have included persons with an acute exacerbation of a chronic condition (Toye 2016), and eight studies did not indicate if the care‐recipients were in the acute or chronic phase of the condition (Bishop 2014; Gallagher‐Thompson 2007; Kwok 2013; NCT00646217; Shum 2014; Smith and Toseland 2006; Vazquez 2016; Wray 2010). In one study, care‐recipients were admitted to a nursing home (Davis 2011); this study was included because the intervention was designed to help caregivers adjust to the new burdens and stresses of nursing home placement in the first few months after placement had occurred. Nursing homes are community‐based and many caregivers spend a considerable time and continue to provide much care to care‐recipients, in particular spousal caregivers in the initial few months of admission. Most interventions were delivered to caregivers individually (i.e. one‐to‐one) (n = 14), four were delivered solely by group, two used a combined group and individual delivery format, and one did not provide sufficient information to determine whether the intervention was delivered individually to caregivers or in group format. Total sample sizes across the included studies ranged from 11 (Glueckauf 2012) to 175 (Toye 2016).
Attempts were made to contact all authors to confirm study details or to request further details. Most authors provided some detail on the study and intervention. Only four provided missing outcome data for inclusion in this review (Corry 2015; Davis 2011; Toye 2016; Wilz 2016a). Contact could not be made with two study authors (Bishop 2014; Smith and Toseland 2006). All other study authors provided detail on the study for categorisation at the screening stage or the study and intervention details, or both. All authors were contacted for information on studies awaiting categorisation and, where necessary, ongoing studies.
Eighteen of the 21 included studies received funding from reputable sources (e.g. national organisations or funding bodies). Three study authors did not detail sources of funding (Kwok 2013; NCT00646217; Shum 2014). One study author declared a conflict of interest (Corry 2015), and seven declared no conflict of interest (Davis 2011; Piamjariyakul 2015; Powell 2014; Shaw 2016; Shum 2014; Toye 2016; Vazquez 2016). The remaining studies did not provide details on conflicts of interest.
Summary characteristics of informal caregivers
Ten studies focused on caregivers of people with dementia (Connell 2009; Davis 2011; Gallagher‐Thompson 2007; Glueckauf 2012; Kwok 2013; Martindale‐Adams 2013; Tremont 2008a; Wilz 2016a; Winter 2006; Wray 2010), three on caregivers of people with stroke (Bishop 2014; NCT00646217; Pfeiffer 2014), and one study each for the following conditions: colorectal cancer (Shum 2014), heart failure (Piamjariyakul 2015), traumatic brain injury (Powell 2014), gastrointestinal cancers (Shaw 2016), frail older persons (Smith and Toseland 2006), older people (Toye 2016), multiple sclerosis (Corry 2015), and people with various conditions (Vazquez 2016). The minimum and maximum mean age of the caregivers in the included studies was 49 years (Powell 2014) and 74 years (Wray 2010), respectively, with a reported age range of 19 years (Shum 2014) to 87 years (Martindale‐Adams 2013). The majority of participants in the individual studies were female (> 70.5%). Two trials included females only (Connell 2009; Winter 2006), and, for two trials, the gender of participants was not provided (NCT00646217; Wray 2010). Most studies comprised family member caregivers, with the majority being spousal caregivers, in particular wives, except one trial where most of the participants were non‐spousal family caregivers (Gallagher‐Thompson 2007). Other family members included adult children and, to a lesser extent, siblings and mothers, grandchild, sons‐in‐law, or daughters in‐law (see Characteristics of included studies). The majority of the studies reported that caregivers were educated beyond secondary or high school level or had the equivalent in years of education (i.e. over 12 years of education). Three reported that the majority had post‐secondary school education (Piamjariyakul 2015; Powell 2014; Winter 2006). One study reported that most of the participants were literate or had primary education (Vazquez 2016) while two studies included participants who were either illiterate (Shum 2014) or had no primary education (Shaw 2016), albeit the majority had secondary level education or above in both studies.
Category of interventions
Ten interventions were categorised as psychosocial interventions (Bishop 2014; Connell 2009; Gallagher‐Thompson 2007; Glueckauf 2012; Pfeiffer 2014; Shaw 2016; Toye 2016; Vazquez 2016; Wilz 2016a; Winter 2006), 10 as combined psychosocial education interventions (Corry 2015; Davis 2011; Kwok 2013; Martindale‐Adams 2013; Piamjariyakul 2015; Powell 2014; Shum 2014; Smith and Toseland 2006; Tremont 2008a; Wray 2010), and one was not classified due to insufficient detail to enable an accurate classification (NCT00646217). No study evaluated an intervention that was exclusively educational (see Characteristics of included studies).
Two interventions were rated as high‐ (Bishop 2014; Glueckauf 2012), 16 as medium‐ (Connell 2009; Corry 2015; Davis 2011; Martindale‐Adams 2013; Pfeiffer 2014; Piamjariyakul 2015; Powell 2014; Shaw 2016; Shum 2014; Smith and Toseland 2006; Toye 2016; Tremont 2008a; Vazquez 2016; Wilz 2016a; Winter 2006; Wray 2010) and two as low‐quality interventions (Gallagher‐Thompson 2007; Kwok 2013). One intervention was not assessed as there was insufficient information available (NCT00646217) (Table 3).
1. Summary of quality ratings for interventions in included studies.
| Y=YES, PY=Partly YES, N=NO | |||||||||||||||||||||||
| ITEM | 1 | 2* | 3 | 4 | 5* | 6* | 7 | 8* | 9 | 10 | 11 | 12* | 13 | 14 | 15 | 16 | 17 | 18 | 19* | 20 | 21 | 22 | Overall rating |
| Bishop 2014 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | PY | N | Y | PY | Y | Y | Y | N | N/A | High |
| Connell 2009 | PY | PY | PY | Y | PY | PY | Y | PY | N | Y | N | N | N | N | N/A | Y | N | Y | Y | N | N | N/A | Medium |
| Corry 2015 | Y | Y | Y | Y | Y | Y | Y | N/A | Y | Y | N | Y | PY | PY | N/A | Y | N | Y | Y | Y | Y | PY | Medium |
| Davis 2011 | Y | Y | PY | Y | Y | PY | Y | N | N/A | N/A | Y | N | PY | N | Y | PY | N | Y | Y | Y | PY | N | Medium |
| Gallagher‐Thompson 2007 | Y | PY | PY | N | PY | PY | N/A | N | N | N | N | N | N | N | N/A | N | N | N | Y | N | N | N/A | Low |
| Glueckauf 2012 | Y | Y | Y | Y | Y | Y | PY | Y | PY | Y | N | N | N | PY | N/A | Y | Y | Y | Y | Y | N | N/A | High |
| Kwok 2013 | Y | PY | Y | PY | PY | PY | N/A | N | N | N | N/A | N | N | Y | N | N | N | N | N | N/A | N | N/A | Low |
| Martindale‐Adams 2013 | Y | PY | Y | PY | PY | PY | Y | N | N | Y | N | N | N | N | Y | N | N | Y | N | N/A | N | N/A | Medium |
| NCT00646217 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | No information |
| Shum 2014 | PY | PY | Y | N | PY | PY | PY | PY | N | N | PY | N | N | N | PY | N | N | N | PY | N | N | N | Medium |
| Pfeiffer 2014 | Y | Y | Y | Y | Y | PY | Y | PY | N/A | PY | Y | N | PY | N | N/A | PY | Y | PY | Y | N | N | N/A | Medium |
| Piamjariyakul 2015 | Y | PY | Y | PY | PY | PY | Y | N | N/A | N/A | Y | N | N | N | Y | PY | Y | Y | PY | Y | PY | N | Medium |
| Powell 2014 | Y | PY | Y | Y | Y | PY | Y | Y | N/A | N/A | Y | N | N | N | PY | Y | N | N | Y | N | N | N/A | Medium |
| Shaw 2016 | Y | PY | Y | N | PY | PY | Y | PY | N/A | PY | Y | N | N | N | Y | PY | PY | PY | Y | N | PY | N | Medium |
| Smith and Toseland 2006 | Y | PY | Y | PY | PY | Y | Y | PY | N/A | N/A | N/A | N | PY | N | Y | PY | N | Y | Y | PY | PY | N | Medium |
| Toye 2016 | Y | PY | Y | PY | Y | PY | Y | Y | N/A | N/A | PY | N | N | N | Y | PY | PY | Y | Y | N | PY | PY | Medium |
| Tremont 2008a | Y | Y | Y | PY | Y | PY | PY | PY | N | Y | NA | N | N | N | Y | Y | Y | Y | Y | Y | N | N/A | Medium |
| Vazquez 2016 | Y | Y | Y | PY | Y | PY | N/A | PY | Y | PY | PY | N | N | PY | Y | Y | Y | Y | Y | N | N | N/A | Medium |
| Wilz 2016a | Y | PY | Y | PY | Y | PY | PY | PY | Y | Y | N/A | N | N | PY | PY | Y | Y | Y | Y | Y | N | N/A | Medium |
| Winter 2006 | Y | Y | Y | N | PY | Y | N/A | PY | N/A | N | N/A | N | N | N | N/A | N | N | N | N | N/A | N | N/A | Medium |
| Wray 2010 | Y | Y | Y | N | Y | Y | PY | PY | PY | N | PY | N | N | N | N | PY | N | N | N | N/A | N | N/A | Medium |
| Items assessed (Inclusion of detail for all Items with a * was essential for a high rating) 1. Did the researchers/authors provide a clear definition of the intervention so it could be replicated?* (this should include type, overview of content but very in‐depth details such as the manual do not have to be included) 2. Were the aims/goal of the intervention clearly stated?* (the aim/goal of the intervention may be the same/similar to the goal of the study) 3. Did the researchers/authors provide clear rationale for the intervention? 4. Did the researchers/authors provide an overview of the theory underpinning the intervention/framework used to develop the intervention? 5. Was the content of the intervention consistent with the stated aim/goal of the intervention?* 6. Was a clear description provided of how (method) the intervention was delivered? (e.g. phoned using mobile phone, skype, landline)* 7. If appropriate, did the researchers/authors provide an overview of other materials used e.g. Guidebook, information sent by post, etc? 8. Did the researchers/authors justify the selection of interventionists?* (e.g. appropriateness in terms of professional background/education of the person delivering the intervention) 9. If relevant, did the researchers/authors provide appropriate justification for the selection of co‐interventionists? 10. If more than one interventionist was involved in delivery of the intervention, did the researchers/authors indicate how delivery of the intervention was standardised across interventionists?* 11. Did the researchers/authors indicate that the intervention was delivered at an appropriate time period for the caregivers, which was in accordance to the overall goal? (e.g. if the goal is to support caregivers who are new to the role then it should be delivered during the early stages of caregiving) 12. Was there any potential risk of intervention contamination across the study groups?* 13. Did the researchers/authors justify the intensity of the intervention (in terms of frequency of delivery and duration of each session)? 14. Duration: Did the researchers/authors indicate that the complete intervention was delivered to the participants i.e. 100% of the intervention was delivered? 15. If the intervention was tailored/modified/adapted, did the researchers/authors indicate why? what? and how? 16. Did the researchers assess consistency in intervention delivery? 17. Did the researchers/authors state that the intervention was delivered in accordance with the trial protocol? 18. Was interventionist training standardised? 19. Did the authors indicate that intervention delivery was monitored?* 20. If yes/PY to item 19, was intervention delivery monitored using an objective measure? 21. Did the authors indicate that caregiver receipt of the intervention was monitored? (i.e. Caregivers’ understanding of and use of the intervention) 22. If ‘yes/PY’ to item 21, was caregiver receipt of the intervention monitored using an objective measure? | |||||||||||||||||||||||
N/A: not applicable
Comparison groups
Thirteen of the included studies indicated that the comparison group was usual care, standard medical follow‐up or no intervention with very little or no additional explanation (Bishop 2014; Connell 2009; Corry 2015; Davis 2011; Powell 2014; Shaw 2016; Smith and Toseland 2006; Toye 2016; Tremont 2008a; Vazquez 2016; Wilz 2016a; Winter 2006; Wray 2010). Five studies described the comparator as no formal intervention other than resource or education information, or both, which may or may not have been part of standard care (Davis 2011; Kwok 2013; Pfeiffer 2014; Piamjariyakul 2015; Shum 2014) and one study included a telephone help line number (Shum 2014) (Characteristics of included studies).
The two non‐telephone support intervention studies indicated that the comparator comprised six modules(Gallagher‐Thompson 2007) or that the structure and content of the programme were the same as the intervention group (Glueckauf 2012) and provided an explanation of the content and duration and mode of delivery (Characteristics of included studies).
Excluded studies
Studies were excluded because the intervention was for patients, caregivers and care‐recipients who received the intervention together or caregivers were only included if they chose to take part in the intervention which was offered to the care‐recipient (Achie 2015; Bell 2005; Hori 2009; Mendyk 2018; NCT00067171; NCT00131092; NCT00247000; NCT00271739; NCT00288132; NCT00483522; NCT00693563; NCT00829361; NCT02094846; NCT02483494; NCT03164239; Porter 2011; Samus 2014), the study design was not a randomised trial (Aguirrezabal 2013; Bailey 1997; Bauman 2015; Bauman 2018; Brown 1999; Cox 2012; Demiris 2011; Erten‐Lyons 2017; Gilliss 1992; Greaves 2016; Hirsch 2014; Lindauer 2016; Morgan 2015; NCT03177447; Nichols 2011; Piamjariyakul 2012; Piamjariyakul 2013; Pirrraglia 2005; Richardson 2007; Schinköthe 2014; Shanley 2008; Stewart 2001; Teel 2005; Tompkins 2009; Tsai 2005; Uphold 2015; Van Mierlo 2012a), the intervention was not a telephone intervention or a telephone‐only intervention (Badr 2015; Barclay 2016; Belle 2006; Berwig 2017; Callahan 2006; Chang 2004; Czaja 2013; Dellasega 2002; Demiris 2012; Duncan 2017; Elliott 2009; Finkel 2007a; Garand 2002; Gaugler 2008; Gitlin 2003; Gitlin 2010; Gitlin 2010a; Gitlin 2016; Gonyea 2016; Graham‐Philips 2016; Grant 1999; Grant 2002; Hasan 2015; Hicken 2017; Huang 2013; Hudson 2015; Johnson 2018; Kozachik 2001; Kuo 2017; Kwok 2012; Linton 2018; Martín‐Carrasco 2009; Mazanec 2017; McCann 2015a; NCT00721383; NCT02036294; NCT02347202; NCT02364505; NCT02475954; NCT02703532; NCT03127930; NCT03142841; NCT03506945; Nobili 2004; Penner 2016; Piette 2015; Prick 2015; Radziewicz 2009; Reeves 2018; Rivera 2008; Schure 2006; Schwarz 2008; Sherrod 2013; Silveira 2016; Sneed 1997; Uphold 2014; Valeberg 2013; Van Knippenberg 2016; Williams 2010; Yamada 2011; Yan 2016), the comparator was also a telephone intervention or included a telephone component (Badger 2007; Bakas 2009a; Bakas 2015; Blumenthal 2009; Chambers 2014; Chodosh 2015; Gant 2007a; Livingston 2013; McCann 2015; McLennon 2016; Mosher 2018; NCT00052104; NCT00822510; NCT01993550; NCT03378050; NCT03635151; Sherwood 2012; Tremont 2014; Tremont 2015; Tremont 2017; Wilder ongoing), or the trial was withdrawn due to lack of funding (ACTRN12616000467437).
Risk of bias in included studies
The 'risk of bias' assessment across the domains for each reported outcome was assessed and 'risk of bias' judgements summarised for each included study (see Characteristics of included studies). The summary results are presented in 'risk of bias' tables and illustrated in Figure 2 and Figure 3. Due to the nature of the intervention, blinding of participants and personnel was highly unlikely or possible and was therefore judged to be unclear for all included studies; this domain was not considered critical in assessing the overall risk of bias of each included study. Only one study included in this review was assessed as having an overall low risk of bias (Pfeiffer 2014), with the remainder receiving an overall rating of high risk of bias. Table 4 and Table 5 present the 'risk of bias' assessments for each included study for each 'risk of bias' domain by outcome, for each comparator, respectively.
2. Comparator 1: Summary of Risk of Bias by outcome for telephone‐only versus usual care.
3. Comparator 2: Summary of risk of bias by outcome for telephone‐only versus non‐telephone professional support intervention.
Allocation
Of the included studies, 11 reported adequate randomisation methods and were rated as having low risk of bias (Bishop 2014; Corry 2015; Davis 2011; Kwok 2013; Pfeiffer 2014; Powell 2014; Shaw 2016; Toye 2016; Tremont 2008a; Vazquez 2016; Wilz 2016a). All remaining studies were rated as having unclear risk for sequence generation (Figure 2).
Only 5 of the included studies (Corry 2015; Powell 2014; Pfeiffer 2014; Shum 2014; Toye 2016) reported adequate methods to ensure that allocation to groups was concealed and were rated as having low risk of bias. The remaining studies provided insufficient information to be able to judge the likelihood of allocation concealment bias and were therefore rated as having unclear risk of bias (Figure 2).
Blinding
All 21 included studies were judged as having unclear risk of bias for blinding of participants and personnel. Fourteen reported adequate blinding of outcome assessments (Bishop 2014; Davis 2011; Glueckauf 2012; Kwok 2013; Pfeiffer 2014; Piamjariyakul 2015; Powell 2014; Shum 2014; Smith and Toseland 2006; Toye 2016; Tremont 2008a; Vazquez 2016; Wilz 2016a; Wray 2010), six were unclear due to insufficient information to assess (Connell 2009; Gallagher‐Thompson 2007; Martindale‐Adams 2013; NCT00646217; Shaw 2016; Winter 2006), and one was rated as having high risk of bias due to non‐blinding (Corry 2015) of outcome assessment (Figure 2).
Incomplete outcome data
Of the included studies, 12 were judged as having low risk of bias for incomplete outcome data reporting (Corry 2015; Davis 2011; Gallagher‐Thompson 2007; Glueckauf 2012; Kwok 2013; Martindale‐Adams 2013; Pfeiffer 2014; Piamjariyakul 2015; Shaw 2016; Shum 2014; Toye 2016; Vazquez 2016), eight as having unclear risk of bias, of which five were due to insufficient information to assess (NCT00646217; Smith and Toseland 2006; Wilz 2016a; Winter 2006; Wray 2010), one due to non‐reporting of attrition by group (Bishop 2014), and two due to lack of adequate rationale for imbalance in attrition across the groups (Connell 2009; Powell 2014). One was judged as having high risk of bias due to greater than 40% loss to follow‐up in both groups (Tremont 2008a) overall (Figure 2). 'Risk of bias' assessments by outcome are presented in Table 4 and Table 5.
Selective reporting
Of the included studies, 13 were assessed as having low risk of bias (Bishop 2014; Connell 2009; Corry 2015; Gallagher‐Thompson 2007; Kwok 2013; Pfeiffer 2014; Piamjariyakul 2015; Shaw 2016; Shum 2014; Vazquez 2016; Wilz 2016a; Winter 2006; Wray 2010), one as unclear due to insufficient information to assess(NCT00646217), seven as high, of which six were due to non‐reporting of one or more prespecified outcomes(Davis 2011; Glueckauf 2012; Martindale‐Adams 2013; Powell 2014; Toye 2016; Tremont 2008a) and one due to reporting outcomes for a subsample of the included participants(Smith and Toseland 2006) (Figure 2).'Risk of bias' assessment by outcome is presented in Table 4 and Table 5.
Other potential sources of bias
Of the included studies, 12 were assessed as having an overall low risk of bias (Davis 2011; Gallagher‐Thompson 2007; Glueckauf 2012; Kwok 2013; Martindale‐Adams 2013; Pfeiffer 2014; Piamjariyakul 2015; Shaw 2016; Shum 2014; Toye 2016; Tremont 2008a; Vazquez 2016) for this domain and three were assessed as unclear, two due to insufficient information to assess (NCT00646217; Wray 2010), and one due to non‐reporting of caregiver characteristics separately for each group (Bishop 2014). Five were assessed as having high risk of bias due to baseline imbalances (Connell 2009; Powell 2014; Smith and Toseland 2006; Wilz 2016a; Winter 2006) and one high risk of bias due to risk of contamination (Corry 2015) (Figure 2). 'Risk of bias' assessment by outcome is presented in Table 4 and Table 5.
Effects of interventions
All outcomes reported for comparator 1 and 2 below refer to caregivers.
Comparator 1: Telephone intervention versus usual care
Quality of Life
Of the five studies that reported on caregiver quality of life (QoL), one evaluated a psychosocial intervention (Shaw 2016) and the remainder evaluated combined psychosocial‐education interventions (Corry 2015; Davis 2011; Powell 2014; Shum 2014). The duration of the interventions varied from ≤ 6 weeks (Shum 2014), to from 7 to 12 weeks (Corry 2015; Davis 2011; Shaw 2016) and 13 to 23 weeks (Powell 2014). Mode of intervention delivery was individual. The care‐recipients across the studies included people with cancer (Shaw 2016; Shum 2014), dementia (Davis 2011), multiple sclerosis (MS) (Corry 2015) and traumatic brain injury (TBI) (Powell 2014). The conditions were categorised as acute in two studies (Powell 2014; Shaw 2016), chronic in two studies (Corry 2015; Davis 2011), and, for one study, it was unclear if the condition was acute or chronic (Shum 2014). No study reporting QoL evaluated an education‐only intervention. All five studies reported on caregiver QoL at the end of intervention, and three studies also reported QoL at short‐term follow‐up (≤ 3 months) (Corry 2015; Shaw 2016; Shum 2014).
There is probably little or no difference between telephone support interventions and usual care for QoL at the end of intervention (SMD ‐0.02, 95% CI ‐0.24 to 0.19, 4 studies, 364 carers) (moderate‐certainty evidence) (Analysis 1.1) and at short‐term follow‐up (≤ 3 months) (MD 0.00, 95% CI ‐4.43 to 4.43, 1 study, 128 carers) (Analysis 1.2). In the fifth study, for caregivers of people with colorectal cancer, mean QoL was marginally higher (mean score 67.87) in the intervention group than in the control group (mean score 67.42) at the end of intervention and at short‐term follow‐up (mean 73.25 versus 70.84, intervention versus control group) using the WHOQoL BREF (Hong Kong) subscale scores (Shum 2014) (see Table 6). Due to clinical heterogeneity, it was not possible to impute SD data for this study from another study, and no response was received for these data from contacting the author.
1.1. Analysis.
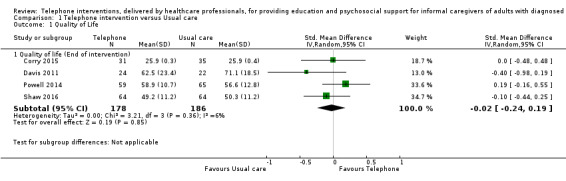
Comparison 1 Telephone intervention versus Usual care, Outcome 1 Quality of Life.
1.2. Analysis.
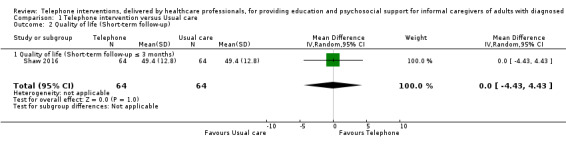
Comparison 1 Telephone intervention versus Usual care, Outcome 2 Quality of life (Short‐term follow‐up).
4. Comparator 1: Telephone Intervention versus Usual Care (Results as reported by study authors).
| Outcome and time point | Study | Result (as presented by study authors) | Notes/comments |
|
QoL End of intervention |
Shum 2014 | Intervention group mean 67.87, n = 70, versus control group mean 67.42, n = 69 | No data available for each group SD or 95% CI |
| Short‐term | Shum 2014 | Intervention group mean 73.25 versus control group 70.84, N = 140 | Reported numbers assessed by group, n = 68 intervention group and n = 67 control group. No data available for each group SD or 95% CI |
|
Burden End of intervention |
Shum 2014 | Intervention group mean 17.37, n = 70 versus control group mean 26.26, n = 69, P < 0.001 | No data available for each group SD. Reported mean change data with 95% CI |
| Winter 2006 | Mean and SD: Intervention group mean 31.7, SD 15.2 versus control group mean 31.7, SD 17.3, N = 81, P = 0.49 |
No data available for the numbers by study group. No response from author contact | |
| Short‐term | Shum 2014 | Intervention group mean 8.6 versus control group mean 17.34, N = 140, P < 0.001 |
Reported numbers assessed by group, n = 68 intervention group and n = 67 control group. No data available for each group SD |
|
Skill Acquisition: Competence End of intervention |
Winter 2006 | Intervention group mean 13.52, SD 2.85 versus control mean 14.17, SD 2.57, total N = 94, P = 0.932 | No data available for the numbers by study group. No response from author contact |
|
Psychological Health: Caregiver Depression End of intervention |
Winter 2006 | Intervention group mean 18.17, SD 7.19 versus control group 20.2, SD 7.2, N = 94, P = 0.121 | No data available for the numbers by study group. No response from author contact |
| Shum 2014 | Intervention group mean 4.57, n = 70 versus control group mean 7.45, n = 69, P = 0.013 | No data available for each group SD | |
| Bishop 2014 | intervention mean ‐0.16, SD 2.6 versus control mean ‐1.22, SD 3.1, P > 0.05 | Mean change data from baseline only provided. No mean difference for each arm available. No data available for the numbers in each group, SD or 95% CI | |
|
Psychological Health: Caregiver Depression Short‐term |
Shum 2014 | Intervention group mean 2.41, versus control group mean 4.21, N = 70, P = 0.144 | Reported numbers assessed by group, n = 68 intervention group and n = 67 control group. No data available for each group SD |
|
Psychological Health: Caregiver Anxiety End of intervention |
Shum 2014 | Intervention group mean 3.97, n = 70 versus control group 6.41, n = 69 | No data available for each group SD or 95% CI |
| Short‐term | Shum 2014 | Intervention group mean 1.15 versus control group 2.90, N = 140 | Reported numbers assessed by group, n = 68 intervention group and n = 67 control group. No data available for each group SD or 95% CI |
|
Psychological Health: Caregiver Stress End of intervention |
Shum 2014 | Intervention group mean 9.06, n =70 versus control group 12.45, n = 69 | Reported numbers assessed by group, n = 68 intervention group and n = 67 control group. No SD or 95% CI |
| Short‐term | Shum 2014 | Intervention group mean 3.71 versus control group 7.79, N = 140 | Reported numbers assessed by group, n = 68 intervention group and n = 67 control group. No data available for each group SD or 95% CI |
|
Health Status and Well‐Being: Physical Health End of intervention |
Bishop 2014 | Mean change ‐0.84, SD 4.5 intervention and mean change 1.74, SD 3.8 control group; P < 0.10 | Mean change data only provided. No participant number, means or standard deviation score reported. No response from author contact |
|
Family Functioning End of intervention |
Bishop 2014 | Mean change scores from baseline of 2.7, SD 6.4 and ‐2.8, SD 4.0: P < 0.05 | Mean change data from baseline only provided. No mean difference for each arm available. No participant number, means or standard deviation score reported. No response from author contact |
|
Cost End of intervention |
Toye 2016 | Intervention mean 352.53 Australian Dollars, SD = 81.5, n = 62 versus control mean 15.89 Australian Dollars, SD = N/A, n = 69 | Reported figures for total acute care costs which included hospital admissions, ED presentations, and ambulance services, and were not isolated to the intervention costs. Communication with the author resulted in retrieving further cost data specific to intervention costs (e.g. nurses time, cost, of training and telephone charges, etc). |
| Short‐term | Wray 2010 | Mean (SD) and study numbers (US Dollars): Intervention mean 7008.3 SD 9226.2, n = 83 versus control mean 8831.4 SD 13,245.8, n = 75 |
Cost utility analysis, mean, SD and study group numbers provided by the authors |
| Medium‐term | Wray 2010 | Mean (SD) and study numbers (US Dollars): Intervention mean 6783.9, SD 7767, n = 83 versus control mean 5648, SD 6353.4, n = 75 |
CI:Confidence interval ED: Emergency department N/A: Not applicable SD: Standard deviation
Overall, telephone interventions compared with usual care probably have little or no effect on QoL (moderate‐certainty evidence) at the end of intervention or at short‐term follow‐up.
Caregiver Burden
Of the 12 studies that reported on caregiver burden, four evaluated psychosocial interventions (Connell 2009; Shaw 2016; Toye 2016; Winter 2006) and eight evaluated combined psychosocial‐education interventions (Corry 2015; Davis 2011; Kwok 2013; Martindale‐Adams 2013; Piamjariyakul 2015; Shum 2014; Smith and Toseland 2006; Tremont 2008a). Eleven studies reported burden at the end of intervention. Two also reported burden at short‐term follow‐up (≤ 3 months) and two at medium‐term follow‐up (> 3 to ≤ 6 months). Mode of intervention delivery was individual except for two studies which used a group format (Martindale‐Adams 2013; Winter 2006). The duration of the interventions varied from ≤ 6 weeks (Piamjariyakul 2015; Shum 2014; Toye 2016), 7 to 12 weeks (Corry 2015; Davis 2011; Kwok 2013; Smith and Toseland 2006) and ≥ 24 weeks (Connell 2009; Martindale‐Adams 2013; Tremont 2008a; Winter 2006). Care‐recipient conditions were dementia (Connell 2009; Davis 2011; Kwok 2013; Martindale‐Adams 2013; Tremont 2008a; Winter 2006), MS (Corry 2015), colorectal cancer (Shum 2014), heart failure (Piamjariyakul 2015) GI cancer (Shaw 2016), frail older persons (Smith and Toseland 2006), and older persons with mixed conditions (Toye 2016). None of the included studies evaluated an education‐only intervention.
There may be little or no difference between telephone support interventions and usual care for burden at the end of intervention (SMD ‐0.11, 95% CI ‐0.30 to 0.07, 9 studies, 788 carers) (low‐certainty evidence) (Analysis 1.3), at short‐term follow‐up (≤ 3 months) (MD ‐0.20, 95% CI ‐0.75 to 0.35, 1 study, 128 carers) (Analysis 1.4), and at medium‐term follow‐up (> 3 to ≤ 6 months) (SMD ‐0.00, 95% CI ‐0.32 to 0.33, 2 studies, 147 carers), (Analysis 1.3). As the numbers analysed were not reported by group and the authors did not report use of an intention‐to‐treat approach to analysis, the results of one study (Winter 2006) were not included in the meta‐analysis.
1.3. Analysis.
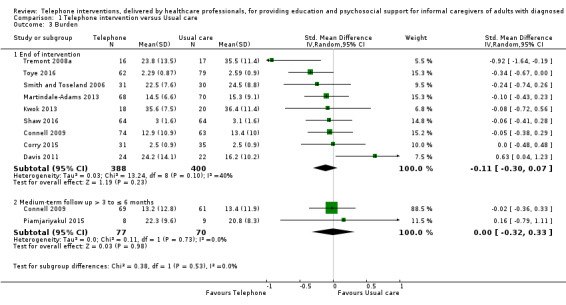
Comparison 1 Telephone intervention versus Usual care, Outcome 3 Burden.
1.4. Analysis.
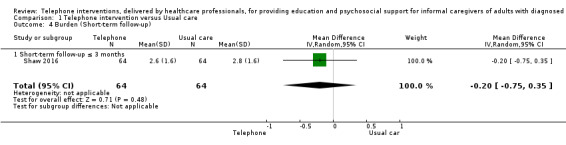
Comparison 1 Telephone intervention versus Usual care, Outcome 4 Burden (Short‐term follow‐up).
Shum 2014, reported reduced burden in the intervention group at the end of intervention (mean 17.37) compared to the control group (mean 26.26) (P < 0.001), and at short‐term follow‐up in the intervention group (mean 8.6), in the control group (mean 17.34) (P < 0.001). In comparison, Winter 2006 found no difference between the intervention (mean 31.7; SD 15.2) and usual care (mean 31.7; SD 17.3) for caregiver burden at the end of intervention (Table 6).
Overall, telephone support interventions, compared with usual care, may have little or no effect on burden (low‐certainty evidence) at the end of intervention or at short‐ or medium‐term follow‐up.
Skill Acquisition: Problem‐Solving
Of three studies that reported on caregiver problem‐solving, two evaluated a combined intervention of 7 to 12 weeks duration (Corry 2015; Smith and Toseland 2006) and one evaluated a psychosocial intervention, also of 7 to 12 weeks duration (Pfeiffer 2014). All three studies reported effects at the end of intervention.
For problem‐solving, there may be little or no difference between telephone support interventions and usual care at the end of intervention (SMD 0.25, 95% CI ‐0.21 to 0.71, 3 studies, 236 carers) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Telephone intervention versus Usual care, Outcome 5 Skill acquisition: Problem‐Solving.
Overall, telephone interventions, compared with usual care, may have little or no effect on problem‐solving at the end of intervention.
Skill Acquisition: Preparedness to Care
Of the three studies that reported on caregiver preparedness to care, one evaluated a psychosocial intervention (Toye 2016) and two evaluated combined interventions (Corry 2015; Piamjariyakul 2015). Interventions were of ≤ 6 weeks (Piamjariyakul 2015; Toye 2016) and 7 to 12 weeks (Corry 2015) duration.
There may be some evidence of a small benefit in favour of the telephone support intervention for preparedness to care at the end of intervention (SMD 0.37, 95% CI 0.09 to 0.64, 2 studies, 208 carers) (Analysis 1.6), but little or no difference at the medium‐term follow‐up (> 3 months to ≤ 6 months) (MD ‐0.30, 96% CI ‐1.02 to 0.42, 1 study, 17 carers) (Analysis 1.7). Overall, telephone interventions may have some small benefit in terms of caregiver preparedness to care when compared with usual care at the end of intervention but not at medium‐term follow‐up.
1.6. Analysis.
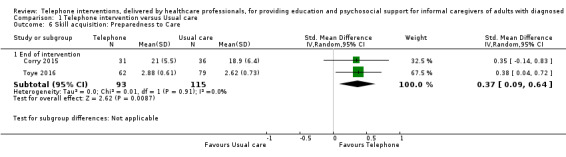
Comparison 1 Telephone intervention versus Usual care, Outcome 6 Skill acquisition: Preparedness to Care.
1.7. Analysis.

Comparison 1 Telephone intervention versus Usual care, Outcome 7 Skill acquisition: Preparedness to Care (medium‐term follow‐up).
Skill Acquisition: Competence
Two studies evaluated psychosocial interventions, one with a duration of 7 to 12 weeks (Pfeiffer 2014) and one with a duration of ≥ 24 weeks (Winter 2006), on caregiver competence at the end of intervention in carers of people with stroke and dementia, respectively.
The results demonstrated that when compared to usual care, telephone support interventions may have little or no effect on caregiver competence scores at the end of intervention (MD 4.10, 95% CI ‐2.19 to 10.39, 1 study, 107 carers) (Analysis 1.8). Similarly, for Winter 2006, there was little or no effect in favour of telephone support interventions; mean caregiver competence in the intervention group (mean 13.52; SD 2.85) compared to the control group (mean 14.17; SD 2.57) (P = 0.932) using the 6‐item scale adapted from Kaye’s Gain Through Group Involvement Scale to assess perceived personal gains over the past few months (Table 6).
1.8. Analysis.
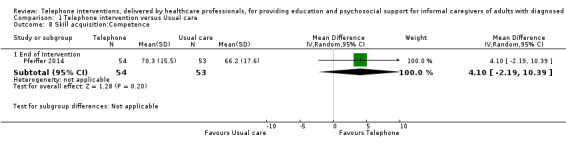
Comparison 1 Telephone intervention versus Usual care, Outcome 8 Skill acquisition:Competence.
Overall, telephone support interventions, compared with usual care, may have little or no effect on caregiver competence at the end of intervention or at short‐term follow‐up.
Psychological Health: Caregiver Depression
Of the 13 studies that reported on caregiver depression, five evaluated psychosocial interventions, one of ≤ 6 weeks duration (Vazquez 2016), one of 7 to 12 weeks duration (Pfeiffer 2014), one of 13 to 23 weeks duration (Wilz 2016a), and two of ≥ 24 weeks duration (Connell 2009; Winter 2006). The remaining eight evaluated combined interventions, two that were of ≤ 6 weeks duration (Piamjariyakul 2015; Shum 2014), two of 7 to 12 weeks duration (Davis 2011; Smith and Toseland 2006), two of 13 to 23 weeks duration (Bishop 2014; Powell 2014), and two of ≥ 24 weeks duration (Martindale‐Adams 2013; Tremont 2008a).
The effects of telephone interventions on depression at the end of intervention were uncertain (SMD ‐0.37, 95% CI ‐0.70 to ‐0.05, 9 studies, 792 carers) (very low‐certainty evidence). Telephone interventions may have little or no effect at medium‐term follow‐up (> 3 to ≤ 6 months) (SMD ‐0.05, 95% CI ‐0.56 to 0.45, 3 studies, 227 carers) (Analysis 1.9). Three studies were not included in meta‐analysis. At the end of intervention, Shum 2014 reported reduced depression in the intervention group (intervention group mean 4.57 versus control group mean 7.45, P = 0.013). Winter 2006, however, found no difference in depression scores between the groups (intervention mean 18.172; SD 7.19 versus control mean 20.2; SD 7.2, P = 0.121, total N = 94). Likewise, Bishop 2014 found no differences between the groups in depression at the end of intervention (intervention mean ‐0.16, SD 2.6 versus control mean ‐1.22, SD 3.1, P > 0.05), nor did Shum 2014 at the short‐term follow‐up (intervention group mean 2.41 versus control group mean 4.21, P = 0.144) (Table 6).
1.9. Analysis.
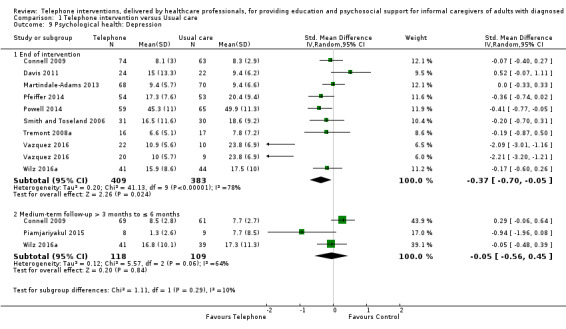
Comparison 1 Telephone intervention versus Usual care, Outcome 9 Psychological health: Depression.
Overall, we are uncertain of the effects of telephone interventions compared with usual care for caregiver depression.
Psychological Health: Caregiver Anxiety
Two studies reported on caregiver anxiety, one evaluating a combined intervention of ≤ 6 weeks duration (Shum 2014) and the second a combined intervention of 7 to 12 weeks duration (Smith and Toseland 2006). For Smith and Toseland 2006, the results demonstrated that telephone support interventions may slightly decrease anxiety at the end of intervention (MD ‐6.0, 95% CI ‐11.68 to ‐0.32, 1 study; 61 carers) (Analysis 1.10). Similarly Shum 2014 reported lower mean anxiety scores for the intervention versus control group (3.97 versus 6.41, respectively) at the end of intervention and at short‐term follow‐up (1.15 versus 2.90, respectively) using the DASS‐21 (Shum 2014) (Table 6).
1.10. Analysis.
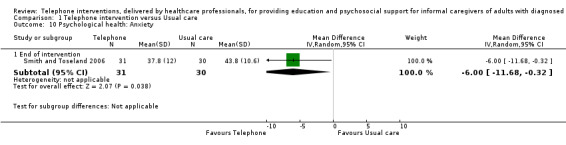
Comparison 1 Telephone intervention versus Usual care, Outcome 10 Psychological health: Anxiety.
Overall, telephone interventions compared with usual care may slightly decrease anxiety levels at the end of intervention and short‐term follow‐up; the quality of this evidence (GRADE) was not formally assessed, but both studies contributing data for this outcome had methodological limitations that may reduce certainty in the findings.
Psychological Health: Caregiver Coping
One study reported on caregiver coping at the end of intervention (Powell 2014). The results showed that telephone support interventions may have little or no effect on caregiver coping, when compared to usual care (MD 1.00, 95% CI ‐0.45 to 2.45, 1 study, 121 carers) (Analysis 1.11).
1.11. Analysis.
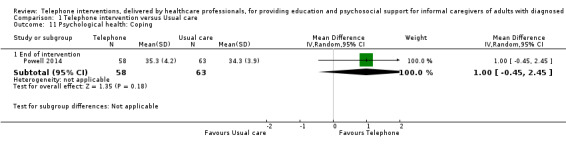
Comparison 1 Telephone intervention versus Usual care, Outcome 11 Psychological health: Coping.
Psychological Health: Caregiver Stress
Of the two studies that reported caregiver stress, one evaluated a psychosocial intervention of ≥ 24 weeks duration (Connell 2009) and one a combined intervention of ≤ 6 weeks duration (Shum 2014).
Telepone support interventions compared to usual care may have little or no effect on caregiver stress at the end of intervention (MD ‐0.10, 95% CI ‐0.30 to 0.10, 1 study, 137 carers) (Analysis 1.12) or at medium‐term follow‐up (MD 0.10, 95% CI ‐0.11 to 0.31, 1 study, 130 carers) (Analysis 1.12). Shum 2014 provided mean data only, and not SDs at the end of intervention and at short‐term follow‐up; the reported means for the intervention and control groups, respectively, were 9.06 and 12.45 at the end of intervention, and 3.71 and 7.79 at short‐term follow‐up, using DASS‐21 (Table 6).
1.12. Analysis.
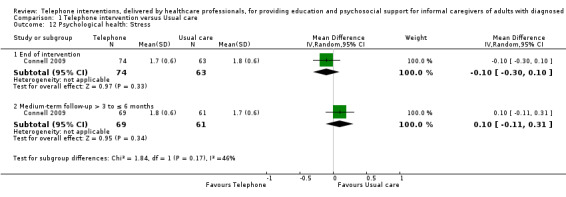
Comparison 1 Telephone intervention versus Usual care, Outcome 12 Psychological health: Stress.
Overall, telephone support interventions may have little or no benefit over usual care for caregiver stress at the end of intervention or at short‐term follow‐up.
Knowledge and Understanding: Knowledge
Three studies prespecified the outcome knowledge (Powell 2014; Smith and Toseland 2006; Tremont 2008a) but only one study reported data for this outcome (Smith and Toseland 2006) at the end of intervention. Telephone support interventions may have little or no effect on overall knowledge scores (i.e. knowledge of services and on how to access them combined) (MD 1.90; 95% CI ‐0.63 to 4.43, 1 study, 61 carers) (Analysis 1.13).
1.13. Analysis.
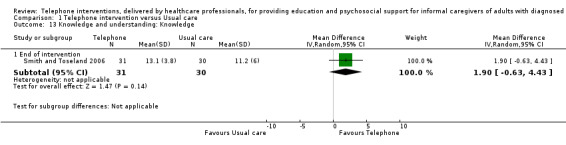
Comparison 1 Telephone intervention versus Usual care, Outcome 13 Knowledge and understanding: Knowledge.
Health Status and Well‐Being: Physical Health
Three studies reported on caregiver physical health, of which two evaluated a psychosocial intervention, one of ≤ 6 weeks duration (Toye 2016) and one of 7 to 12 weeks duration (Pfeiffer 2014) and one a combined intervention with 13 to 23 weeks duration (Bishop 2014),
Telephone support interventions, when compared to usual care may have little or no effect on caregiver physical health at the end of intervention (SMD ‐0.09, 95% CI ‐0.35 to 0.17, 2 studies, 248 carers) (Analysis 1.14). In the third study (Bishop 2014), mean change was reported (‐0.84 (SD 4.5) and 1.74 (SD 3.8), P < 0.10 for intervention and control groups) following the intervention as measured by the Frenchay Activity Index; no difference between the groups was noted (Table 6).
1.14. Analysis.
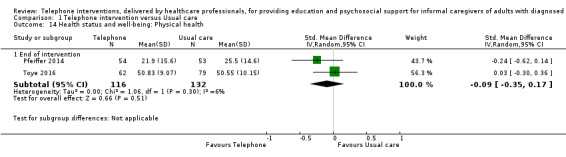
Comparison 1 Telephone intervention versus Usual care, Outcome 14 Health status and well‐being: Physical health.
Overall, telephone support interventions, compared with usual care, may have little or no effect on physical health at the end of intervention.
Health Status and Well‐Being: Self‐efficacy
Of the two studies that reported on caregiver self‐efficacy, one evaluated a psychosocial intervention of ≥ 24 weeks duration (Connell 2009) and one a combined intervention of 7 to 12 weeks duration (Kwok 2013).
There may be little or no effect of a telephone intervention on caregiver self‐efficacy at the end or intervention (SMD 0.04, 95% CI ‐0.26 to 0.33, 2 studies, 175 carers) (Analysis 1.15) or at medium‐term follow‐up (MD 0.00, 95% CI ‐0.29 to 0.29, 1 study, 130 carers) (Analysis 1.16).
1.15. Analysis.

Comparison 1 Telephone intervention versus Usual care, Outcome 15 Health status and well‐being: Self‐efficacy.
1.16. Analysis.
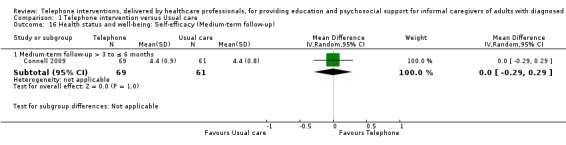
Comparison 1 Telephone intervention versus Usual care, Outcome 16 Health status and well‐being: Self‐efficacy (Medium‐term follow‐up).
Overall, telephone support interventions, compared with usual care, may have little or no effect on caregiver self‐efficacy at the end of intervention.
Health Status and Well‐Being: Social Activity
One study only reported on this outcome (Powell 2014). The study evaluated a combined intervention of 12 to 23 weeks duration, at the end of intervention in carers of people with TBI. The results demonstrated that, when compared to usual care, telephone support interventions may have little or no effect on caregiver social activity (MD 0.04, 95% CI ‐0.10 to 0.18, 1 study, 121 carers) (Analysis 1.17).
1.17. Analysis.
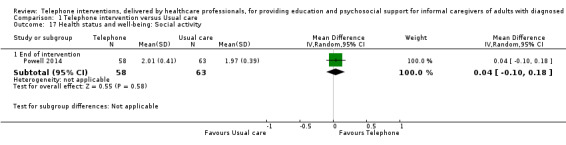
Comparison 1 Telephone intervention versus Usual care, Outcome 17 Health status and well‐being: Social activity.
Family Functioning
Two studies reported on caregiver family functioning, one evaluating a psychosocial intervention of ≤ 6 weeks duration for carers of older persons with acute conditions (Toye 2016) and one a combined psychoeducation intervention of 13 to 23 weeks in duration for carers of people with stroke (Bishop 2014).
The results demonstrated there may be little or no effect of telephone support interventions, when compared to usual care, for caregiver family functioning at the end of intervention (MD 0.20, 95% CI ‐0.04 to 0.44, 1 study, 141 carers) (Analysis 1.19). Bishop 2014, reporting on a combined psychoeducation intervention of 13 to 23 weeks duration for carers of people with stroke, reported mean change scores from baseline of 2.7 (SD 6.4) and ‐2.8 (SD 4.0) (P < 0.05), for intervention and control groups, respectively (Table 6).
1.19. Analysis.
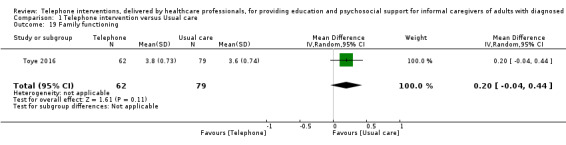
Comparison 1 Telephone intervention versus Usual care, Outcome 19 Family functioning.
Overall, telephone support interventions may have little or no effect on family functioning at the end of intervention, compared with usual care.
Perceived satisfaction with practical or other supports
Three studies reported on perceived satisfaction with supports; one evaluating a psychosocial intervention of 7 to 12 weeks duration (Pfeiffer 2014) and the other two combined interventions, one of 7 to 12 weeks duration (Davis 2011) and the other of ≥ 24 weeks duration (Martindale‐Adams 2013).
The finding suggested that there may be little or no effect of telephone support interventions compared to usual care at the end of intervention (SMD 0.10, 95% CI ‐0.24 to 0.44 3 studies, 291 carers) (Analysis 1.18).
1.18. Analysis.
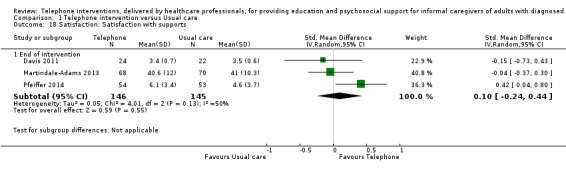
Comparison 1 Telephone intervention versus Usual care, Outcome 18 Satisfaction: Satisfaction with supports.
Overall, telephone support interventions may have little or no benefit over usual care for caregiver satisfaction with supports at the end of intervention.
Satisfaction with the intervention
Satisfaction with the intervention was evaluated in six studies for the intervention arm of the study only, thus results for this outcome are indicative and not comparative. Satisfaction was reported for carers of people with MS (Corry 2015), dementia (Davis 2011; Martindale‐Adams 2013; Tremont 2008a; Wilz 2016a) and mixed conditions (Vazquez 2016). All six studies reported high satisfaction scores following the intervention; that is 'mostly', 'very much so', 'good' or 'excellent' to specific questions according to the descriptors used for and within the scales (see Characteristics of included studies). Similarly, most reported that their needs were met (Corry 2015; Davis 2011; Wilz 2016a) or would recommend the service to friends or others who had similar needs (Corry 2015; Davis 2011; Wilz 2016a) or return to the service or seek similar treatment again, if required (Corry 2015; Davis 2011Tremont 2008a).
Cost
Two included studies reported cost data, one evaluating a psychosocial intervention of ≤ 6 weeks duration in older persons of mixed conditions (Toye 2016) and the other a combined intervention of 7 to 12 weeks duration in people with dementia (Wray 2010).
Toye 2016 reported figures for total acute care costs (in Australian Dollars) at the end of intervention, with higher costs associated with the intervention, (intervention mean 352.53, SD 81.5 (n = 62) and control group mean 15.89, SD N/A (n = 69)). There were no differences in total costs (in US Dollars) between the groups at short‐term assessment (intervention group mean 7,008.3, SD 9,226.2 (n = 83) and control group mean 8,831.4, SD 13,245.8 (n= 75)), with a reported mean difference of MD ‐1823.10 (95% CI ‐5418.41 to 1772.21, 1 study, 158 carers) or at medium‐term follow‐up (intervention group mean 6,784, SD 7,767 (n = 83) and control group mean 5,648, SD 6,353 (n = 75)) with a reported mean difference of MD 1135.90, 95% CI ‐1068.54 to 3340.34, 1 study, 158 carers) (Wray 2010), (Table 6).
Overall, the evidence to suggest that telephone interventions are more or less costly than usual care at the end of intervention is inconclusive.
Adverse events: Worsening of outcome following intervention
None identified or reported in any of the included studies.
Adverse events: Suicide ideation and suicide
Not measured or reported in any of the included studies.
Comparator 2: Telephone Intervention versus non‐telephone professional support intervention
The outcomes of quality of life, skill acquisition (problem‐solving, preparedness to care, competence), psychological health (caregiver anxiety, caregiver coping), knowledge and understanding, health status and well‐being (self‐efficacy, family functioning, social activity), satisfaction, and adverse events were not measured or reported for this comparison.
Burden
One study evaluated a psychosocial intervention of 7 to 12 weeks duration for carers of people with dementia (Glueckauf 2012). We are uncertain of the effects of a telephone support intervention, compared with non‐telephone support intervention, on caregiver burden at the end of intervention (MD ‐0.20, 95% CI ‐0.74 to 0.34, 1 study, 11 carers) (very low‐certainty evidence) (Analysis 2.1).
2.1. Analysis.
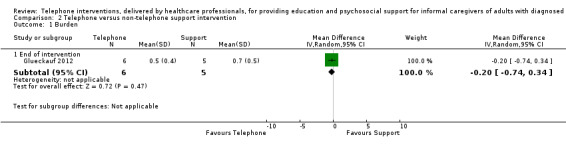
Comparison 2 Telephone versus non‐telephone support intervention, Outcome 1 Burden.
Health Status and Well‐Being: Depression
Two studies evaluated psychosocial interventions of 7 to 12 weeks duration on depression in carers of people with dementia (Gallagher‐Thompson 2007; Glueckauf 2012). We are uncertain of the effects of telephone support interventions compared with non‐telephone professional support interventions, at the end of intervention (MD ‐4.30, 95% CI ‐9.57 to 0.97, 1 study, 11 carers) (very low‐certainty evidence) (Analysis 2.2) and at an unknown time point post‐intervention (MD 1.20, 95% CI ‐5.35 to 7.75, 1 study; 45 carers) (Analysis 2.2).
2.2. Analysis.
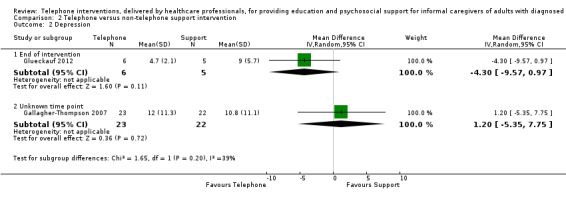
Comparison 2 Telephone versus non‐telephone support intervention, Outcome 2 Depression.
Overall, we are uncertain of the effects of telephone support when compared to non‐telephone support interventions for caregiver depression at the end of intervention.
Psychological Health: Caregiver stress
Gallagher‐Thompson 2007 evaluated a psychosocial intervention of 7 to 12 weeks duration and reported on caregiver stress at an unknown time point post‐intervention. We are uncertain of the effects of telephone support compared to non‐telephone support interventions on stress (MD ‐0.6, 95% CI ‐3.17 to 1.97, 1 study, 45 carers) (Analysis 2.3).
2.3. Analysis.
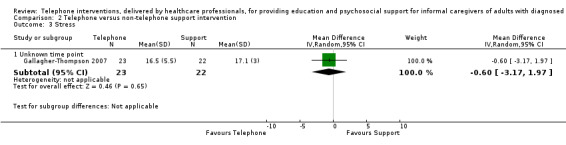
Comparison 2 Telephone versus non‐telephone support intervention, Outcome 3 Stress.
Health Status and Well‐Being: Physical Health
One study (Glueckauf 2012) evaluated a psychosocial intervention of 7 to 12 weeks duration and reported on carers' physical health at the end of intervention for carers of people with dementia . We are uncertain of the effects of telephone support compared to non‐telephone support interventions on physical health (MD 1.9, 95% CI ‐0.65 to 4.45 1 study; 11 carers) (Analysis 2.4).
2.4. Analysis.
Comparison 2 Telephone versus non‐telephone support intervention, Outcome 4 Physical Health.
Discussion
Summary of main results
The objective of this review was to evaluate the effectiveness of telephone‐only support interventions, delivered by healthcare professionals, when compared to usual care or non‐telephone‐based support interventions for educating and psychosocially supporting informal caregivers of people with diagnosed illnesses, on these caregivers' quality of life, psychosocial, and physical well‐being. In addition, the aim was to evaluate the cost‐effectiveness of telephone support interventions.
The review's prespecified primary and secondary outcomes, where reported, were assessed at the end of intervention delivery and at short‐term (≤ 3 months), medium‐term (> 3 to ≤ 6 months) and longer‐term follow‐up time points (> 6 to 12 months) following the intervention. The quality of evidence, assessed using GRADE Table 1; Table 2, indicated evidence ranging from very low‐ to moderate‐quality for three important caregiver outcomes (burden, depression, and quality of life).
Eighteen included studies reported on the comparison of telephone‐only support versus usual care; two reported on the comparison of telephone‐only with a non‐telephone support intervention (Gallagher‐Thompson 2007; Glueckauf 2012). No adverse effects were reported or noted in the included studies.
For the review's primary outcomes of quality of life and burden, there is probably little or no difference between telephone‐only support interventions and usual care. The quality of the evidence for quality of life was moderate and of low certainty for burden. Of the secondary outcomes, telephone‐only support interventions may slightly reduce anxiety and improve preparedness to care to a small degree at the end of intervention. The quality of the evidence (GRADE) for the outcome of depression, however, was very low: we are therefore uncertain of the effects on this outcome. Results for anxiety and preparedness to care were based on small studies with methodological limitations, which may also reduce our confidence in these results. For all of the remaining secondary outcomes in our overall comparison of telephone support interventions compared to usual care, we found that the intervention may have little no effect at any follow‐up time point.
For the review's second comparison of telephone support interventions versus a non‐telephone support intervention, only two studies were included. Of these studies, one reported our prespecified outcomes of burden, depression, and physical health at the end of intervention (Glueckauf 2012) and the second reported depression and stress at an unknown time point (Gallagher‐Thompson 2007). There may be little or no difference between telephone‐only support interventions and non‐telephone support interventions on any outcome measured at all follow‐up time points, based on these two studies.
No evidence of adverse effects was found for worsening of any of the outcomes sought. The adverse outcome of suicide or suicide ideation was not reported in any of the included studies.
Overall completeness and applicability of evidence
Using the evaluation method developed for this review, the quality of the interventions varied with the majority considered to be of medium quality. Two were of low quality, and two were of high quality (Table 3). There was considerable variation in intervention content and duration across the included studies, with few studies providing justification for the intervention duration or intensity. Although most studies provided a reasonably comprehensive description of the intervention, the theory underpinning the intervention was not always explicit; five studies mentioned a theory, while seven did not specify what theory, if any, was used. The effectiveness of interventions can also be influenced by the intensity and duration of the intervention. As only five of the included studies made an attempt to provide some justification for the duration or intensity of the intervention, outcome results may have differed if the impact of intervention intensity and duration on planned outcomes were considered. There is, however, a dearth of methodological studies exploring the impact of intervention intensity and duration on outcomes and no study examining this potential influencing factor was found for including in this review. Despite this, intervention intensity has been identified as an important but complicated aspect of intervention testing (Yoder 2012) with studies on maternal well‐being highlighting its importance on the impact of an outcome (Schwichtenberg 2007). Likewise, fidelity to the intervention may impact on outcomes. Although in this review a number of studies indicated that intervention delivery and fidelity were monitored, most did not describe the results of these assessments. Such limitations in the conduct or reporting of intervention studies limit the applicability of the results, as confidence in these is reduced and it is unclear as to whether effectiveness or non‐effectiveness may be due to deficits in the intensity, duration, or delivery of the intervention rather than the intervention itself.
The considerable heterogeneity across the studies in terms of health conditions, intervention duration, intervention type, and typically small numbers of studies included in meta‐analyses precluded performing subgroup meta‐analyses by intervention duration and care‐recipient condition for the review's prespecified outcomes. Categorising conditions as acute and chronic was also difficult due to non‐reporting of time since diagnosis by some authors, the different categorisation systems used across studies, and the unpredictable nature of some conditions. For example, for one study(Toye 2016), the author described the care‐recipients as having acute medical conditions but later clarified that some care‐recipients may have had an acute episode linked to a chronic condition. Only three studies with very different care‐recipient diagnoses fulfilled our definition for acute conditions. The lack of necessary data prevented subgroup analyses, and outcome data which was derived from a single study for a number of outcomes, many with small and under powered sample sizes, leads to uncertainty regarding the findings for these interventions. Similarly, even where a greater number of studies contributed data to an outcome, (such as for caregiver burden and depression), the findings need to be interpreted with caution as the findings show there may be little or no effect of telephone support interventions over usual care, and there is uncertainty even about these results because of underlying limitations in the quality of the evidence that contributed to these outcomes. This means that with further studies the results are likely to change. While some slight benefit may exist for anxiety for telephone support interventions compared to usual care, the difference was small and the single study assessing this outcome was assessed as having high risk of bias (Smith and Toseland 2006), with a very small and underpowered sample size (n = 61). Likewise, for preparedness to care at the end of intervention, a small difference may exist in favour of the telephone support intervention when compared to usual care from two studies assessed as having high risk of bias. The amount of heterogeneity in the included studies may be due to the range of factors that can impact on caregiver outcomes, such as their own health and well‐being, age, gender, and relationship to care‐recipient. Variability of the comparator group may also impact on outcomes as some comparators included educational information beyond 'standard' usual care. The one study with caregivers of persons transitioning to a nursing home setting may also have increased heterogeneity.
The non‐reporting of the prespecified outcome of QoL (Davis 2011), stress (Glueckauf 2012), knowledge (Powell 2014), physical health (Martindale‐Adams 2013; Toye 2016), mental health (Toye 2016), and incomplete reporting of outcome data (Tremont 2008a) may also have impacted on the outcome of this review, as the inclusion of such data in the analysis may impact on the reported results. In addition to being unable to obtain data for some of these outcomes from the study authors, other outcomes, such as adverse event outcomes were not reported and, for the second comparison, only very limited outcomes were reported.
Quality of the evidence
Only one study included in this review was assessed as having an overall low risk of bias (Pfeiffer 2014), with the remaining studies receiving either a high or unclear rating overall, due to high risk or unclear judgements for any one of the domains of sequence generation, allocation concealment, selective outcome reporting, or attrition bias. The quality of the evidence for the outcomes assessed using GRADE were found to vary from very low‐ to low‐quality evidence, with the exception of QoL which was of moderate‐quality evidence (Table 1; Table 2). In particular, for the main comparison of telephone interventions versus usual care, the quality of the evidence for the primary outcomes of QoL and burden was of moderate‐ and low‐certainty evidence respectively, reducing our confidence in these results. For the outcome of depression, although the pooled results indicated reduced depression with the intervention, the quality of the evidence was of very low‐certainty, meaning that we are uncertain about the effects of telephone support interventions on this outcome. Downgrading the evidence was primarily based on methodological limitations reflected by the risk of bias assessments, inconsistency (statistical heterogeneity) and imprecision (few participants). These findings suggested that more studies of high quality within a programme of caregiver telephone research are required in order to conduct meaningful analyses and such studies are likely to change the conclusions of the review or our certainty, or both, in the findings.
Potential biases in the review process
We believe that the potential for bias in the review process is low. In accordance with the protocol, a broad search of the literature with no language restrictions, including grey literature to minimise the possible influence of publication bias, was conducted. Missing data were requested from study authors. All author conflict of interests were reported and, for the included study conducted by one of the review authors (Corry 2015), data extraction and 'risk of bias' assessment was undertaken by other review authors.
Agreements and disagreements with other studies or reviews
Although a number of other reviews included telephone interventions, these reviews differed from our review. One review focused exclusively on the effectiveness of telephone counselling interventions for caregivers of people with dementia (Lins 2014). A second review looked at telephone and computer interventions, also focusing on benefit for caregivers of people with dementia (Waller 2017), with findings reported separately for computer and telephone‐based interventions.
In the Lins 2014 review, the inclusion criteria for telephone interventions were broader than those in our review. All telephone counselling interventions, for example, those that included video series were included providing there was no face‐to‐face component to the intervention. The authors did, however, conduct separate analysis for those studies categorised as ‘without an additional intervention' (n = 6) (Davis 2011; Finkel 2007a; Glueckauf 2012; Tremont 2008a; Wilz 2016a; Winter 2006) and for telephone interventions that included video–series (n = 3) (Chang 1999; Gant 2007; Steffen 2000). As in our review, Lins 2014 did not separate out the studies by intervention duration and conducted a meta‐analysis on three studies of differing intervention duration; three months (Wilz 2016a), six months (Finkel 2007a) and one year, respectively (Tremont 2008a). Five of the six studies included in Lins 2014 were included in this review. One study (Finkel 2007) did not fulfil our inclusion criteria as the intervention included both text and voice, which makes comparing findings difficult. Despite this, however, the findings of the review were consistent with our review's findings, as reduced depressive symptoms were reported for telephone counselling delivered without additional interventions. Likewise, as in our review, there was little or no difference reported between telephone support interventions and usual care on the studies meta‐analysed on the outcome of burden.
Similarly, in a non‐Cochrane review by Waller 2017, which assessed the scope, volume, and quality of research on the acceptability, utilisation, and effectiveness of telephone‐ and computer‐delivered interventions for caregivers of people living with dementia, the inclusion criteria were broad. The review authors included all interventions that included the telephone either alone or as a component of the intervention. Although the review authors categorised the telephone interventions as telephone interventions alone separate to those with video and respite, the findings were not separated for the telephone interventions alone. The interventions termed ‘telephone interventions alone’ differed from the inclusion criteria used in our review as we excluded telephone intervention studies that also had a telephone component in the usual care group.
A number of other reviews included telephone support interventions and although the effectiveness of interventions delivered by telephone were reported, the effectiveness of the telephone‐only as the mode of intervention delivery was not the focus of the review or specifically evaluated within reviews. Broader telephone intervention inclusion criteria were used such as telephone plus video, more than one face‐to‐face contact, or use of the telephone as a follow‐up method after face‐to‐face sessions (Aubin 2012; Ellis 2010; Forster 2012; Legg 2011; Vernooij‐Dassen 2011), therefore telephone‐only interventions as per our inclusion criterion were not reported separately making it impossible to compare our findings with the findings from these reviews.
Authors' conclusions
Implications for practice.
Overall, the effects of telephone‐only support interventions when compared to usual care are unclear. For the majority of caregiver outcomes, including the primary outcomes of caregiver quality of life and burden, there may be little or no difference between telephone interventions and usual care when considering both meta‐analysed and narratively reported data. There may be small beneficial effects of telephone interventions over usual care on caregiver anxiety and preparedness to care, but there is some degree of uncertainty with these findings because of limitations in the quality of evidence. Consequently, we cannot conclude with certainty that telephone‐only support interventions, as defined in this study, infer greater benefit to caregivers in practice, compared to usual care or non‐telephone professional support interventions.
Implications for research.
Our review shows that telephone‐only interventions have been evaluated across a range of conditions, are of varying duration and quality, but are typically evaluated in studies with relatively small sample sizes. The variation in usual care and other comparators is a source of heterogeneity that is difficult to control for and adds to the complexity when assessing interventions of this type. Our findings, however, are mainly from studies conducted in the USA, of overall high risk of bias, with interventions of low to medium quality. Only two interventions were assessed as high quality. The GRADE assessments indicated very low‐ to moderate‐quality evidence suggesting that most findings are likely to change with the inclusion of more studies. Consequently, there is scope for the refinement and further testing of the interventions included in this review across a range of conditions. However, the methodological limitations of the included studies indicate the need for more robust testing of telephone‐only support interventions with greater emphasis on the reporting of the theoretical underpinnings of the interventions along with findings from the evaluations on fidelity to the intervention and adherence to intervention protocol. A wider range of outcomes relevant to caregivers, such as those sought by this review, need to be routinely considered in future research. In addition, studies of sufficient power to detect differences between groups and to allow the conduct of subgroup analysis are required. More emphasis needs to be placed on the use of the criteria for reporting the development and evaluation of complex interventions in healthcare (CREDICI) guidelines for the conduct and reporting of telephone‐only interventions (Möhler 2012). More studies testing telephone‐only support interventions need to be conducted internationally and future evaluations need to focus on uncovering the most effective intervention intensity and duration, group versus individual approaches and creating a body of high quality evidence both within and across health conditions. Adverse event outcomes and outcomes specific to measuring cost should also be prespecified and reported upon in all future studies.
Notes
This review is based on standard text and guidance provided by Cochrane Consumers and Communication (CCCRG 2016).
Acknowledgements
We thank the editors and staff of the Cochrane Consumers and Communication Group, particularly our managing editor Megan Prictor and co‐ordinating editors Sophie Hill and Rebecca Ryan, for their input to this protocol. We thank the Health Research Board Ireland for awarding a Cochrane research fellowship to MC for the conduct of this systematic review.
We wish to thank an anonymous caregiver who has commented on the review, in particular, the outcomes and discussion sections.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1. exp family/
2. (family or families or parent$2 or relative? or spous$2 or partner? or husband? or wife or wives or child or children or grandchild* or son? or daughter? or sibling? or brother? or sister? or mother? or father?).tw.
3. friends/
4. (friend? or significant other?).ti,ab,kw.
5. 2 or 4
6. (care* or caring).ti,ab,kw.
7. 5 and 6
8. caregivers/
9. (carer* or caregiv* or care giv*).ti,ab,kw.
10. exp home nursing/
11. or/1,3,7‐10
12. exp telephone/
13. (telephon* or phone? or phoning or calls or callback* or call* back* or cellphone? or smartphone? or iphone? or skype).ti,ab,kw.
14. mobile applications/
15. (mobile device* or mobiles or mhealth or m‐health or (portable adj2 app*)).ti,ab,kw.
16. exp telemedicine/
17. telenursing/
18. (telemedicine or tele‐medicine or telecare or tele‐care or telehealth* or tele‐health* or telenursing or ehealth or e‐health).ti,ab,kw.
19. hotlines/
20. (hotline* or help line* or helpline*).ti,ab,kw.
21. or/12‐20
22. 11 and 21
23. exp health personnel/
24. ((health* or medical or paramedical or nurs* or hospital or operating‐room or psychiatric or pharmac*) adj2 (personnel or provider* or professional* or practitioner* or worker* or aide* or assistant* or staff or officer* or specialist* or consultant*)).ti,ab,kw.
25. (doctor* or physician* or general practitioner* or gp or gps or nurse* or clinician* or dentist* or pharmacist* or an?esthetist* or hospitalist* or surgeon* or obstetrician* or gyn?ecologist* or geriatrician* or gerontologist* or therapist* or physiotherapist* or audiologist* or dietitian* or nutritionist* or psychologist* or psychiatrist* or psychotherapist* or counselor* or counsellor* or social worker* or welfare worker*).ti,ab,kw.
26. or/23‐25
27. 22 and 26
28. randomised controlled trial.pt.
29. controlled clinical trial.pt.
30. randomized.ab.
31. placebo.ab.
32. drug therapy.fs.
33. randomly.ab.
34. trial.ab.
35. groups.ab.
36. or/28‐35
37. 27 and 36
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
1. MeSH descriptor: [Family] explode all trees
2. (family OR families OR parent* OR relative* OR spous* OR partner* OR husband* OR wife OR wives OR child OR children OR grandchild* OR son OR sons OR daughter* OR sibling* OR brother* OR sister* OR mother* OR father*):ti,ab,kw
3. MeSH descriptor: [Friends]
4. (friend* OR “significant other*”):ti,ab,kw
5. #2 OR #4
6. (care* OR caring):ti,ab,kw
7. #5 AND #6
8. MeSH descriptor: [Caregivers]
9. (carer* OR caregiv* OR “care giv*”):ti,ab,kw
10. MeSH descriptor: [Home Nursing] explode all trees
11. {OR #1, #3, #7‐#10}
12. MeSH descriptor: [Telephone] explode all trees
13. (telephon* OR phone* OR phoning OR calls OR callback* OR “call* back*” OR cellphone* OR smartphone* OR iphone* OR skype):ti,ab,kw
14. MeSH descriptor: [Mobile Applications]
15. (“mobile device*” OR mobiles OR mhealth OR “m‐health” OR (portable near/2 app*)):ti,ab,kw
16. MeSH descriptor: [Telemedicine] explode all trees
17. MeSH descriptor: [Telenursing]
18. (telemedicine OR “tele‐medicine” OR telecare OR “tele‐care” OR telehealth* OR “tele‐health*” OR telenursing OR ehealth OR “e‐health”):ti,ab,kw
19. MeSH descriptor: [Hotlines]
20. (hotline* OR “help line*” OR helpline*):ti,ab,kw
21. {OR #12‐#20}
22. #11 AND #21
23. MeSH descriptor: [Health Personnel] explode all trees
24. ((health* OR medical OR paramedical OR nurs* OR hospital OR “operating‐room” OR psychiatric OR pharmac*) near/2 (personnel OR provider* OR professional* OR practitioner* OR worker* OR aide* OR assistant* OR staff OR officer* OR specialist* OR consultant*)):ti,ab,kw
25. (doctor* OR physician* OR “general practitioner*” OR gp OR gps OR nurse* OR clinician* OR dentist* OR pharmacist* OR anaesthetist* OR anesthetist* OR hospitalist* OR surgeon* OR obstetrician* OR gynaecologist* OR gynecologist* OR geriatrician* OR gerontologist* OR therapist* OR physiotherapist* OR audiologist* OR dietitian* OR nutritionist* OR psychologist* OR psychiatrist* OR psychotherapist* OR counselor* OR counsellor* OR “social worker*” OR “welfare worker*”):ti,ab,kw
26. {OR #23‐#25}
27. #22 AND #26
17. telenursing/
18. (telemedicine or tele‐medicine or telecare or tele‐care or telehealth* or tele‐health* or telenursing or ehealth or e‐health).ti,ab,kw.
19. hotlines/
20. (hotline* or help line* or helpline*).ti,ab,kw.
21. or/12‐20
22. 11 and 21
23. exp health personnel/
24. ((health* or medical or paramedical or nurs* or hospital or operating‐room or psychiatric or pharmac*) adj2 (personnel or provider* or professional* or practitioner* or worker* or aide* or assistant* or staff or officer* or specialist* or consultant*)).ti,ab,kw.
25. (doctor* or physician* or general practitioner* or gp or gps or nurse* or clinician* or dentist* or pharmacist* or an?esthetist* or hospitalist* or surgeon* or obstetrician* or gyn?ecologist* or geriatrician* or gerontologist* or therapist* or physiotherapist* or audiologist* or dietitian* or nutritionist* or psychologist* or psychiatrist* or psychotherapist* or counselor* or counsellor* or social worker* or welfare worker*).ti,ab,kw.
26. or/23‐25
27. 22 and 26
28. randomised controlled trial.pt.
29. controlled clinical trial.pt.
30. randomized.ab.
31. placebo.ab.
32. drug therapy.fs.
33. randomly.ab.
34. trial.ab.
35. groups.ab.
36. or/28‐35
37. 27 and 36
Appendix 3. Embase (Ovid) search strategy
1. exp family/
2. (family or families or parent$2 or relative? or spous$2 or partner? or husband? or wife or wives or child or children or grandchild* or son? or daughter? or sibling? or brother? or sister? or mother? or father?).tw.
3. friend/
4. (friend? or significant other?).ti,ab,kw.
5. 2 or 4
6. (care* or caring).ti,ab,kw.
7. 5 and 6
8. caregiver/ or caregiver burden/ or caregiver support/
9. (carer* or caregiv* or care giv*).ti,ab,kw.
10. exp home care/
11. or/1,3,7‐10
12. telephone/ or exp mobile phone/
13. (telephon* or phone? or phoning or calls or callback* or call* back* or cellphone? or smartphone? or iphone? or skype).ti,ab,kw.
14. mobile application/
15. (mobile device* or mobiles or mhealth or m‐health or (portable adj2 app*)).ti,ab,kw.
16. exp telemedicine/
17. telenursing/
18. (telemedicine or tele‐medicine or telecare or tele‐care or telehealth* or tele‐health* or telenursing or ehealth or e‐health).ti,ab,kw.
19. (hotline* or help line* or helpline*).ti,ab,kw.
20. or/12‐19
21. 11 and 20
22. exp health care personnel/
23. ((health* or medical or paramedical or nurs* or hospital or operating‐room or psychiatric or pharmac*) adj2 (personnel or provider* or professional* or practitioner* or worker* or aide* or assistant* or staff or officer* or specialist* or consultant*)).ti,ab,kw.
24. (doctor* or physician* or general practitioner* or gp or gps or nurse* or clinician* or dentist* or pharmacist* or an?esthetist* or hospitalist* or surgeon* or obstetrician* or gyn?ecologist* or geriatrician* or gerontologist* or therapist* or physiotherapist* or audiologist* or dietitian* or nutritionist* or psychologist* or psychiatrist* or psychotherapist* or counselor* or counsellor* or social worker* or welfare worker*).ti,ab,kw.
25. or/22‐24
26. 21 and 25
27. randomised controlled trial/
28. controlled clinical trial/
29. single blind procedure/ or double blind procedure/
30. crossover procedure/
31. random*.tw.
32. placebo*.tw.
33. ((singl* or doubl*) adj (blind* or mask*)).tw.
34. (crossover or cross over or factorial* or latin square).tw.
35. (assign* or allocat* or volunteer*).tw.
36. or/27‐35
37. 26 and 36
38. limit 37 to embase
Appendix 4. PsycINFO (Ovid) search strategy
1. exp family/ or exp family members/
2. (family or families or parent$2 or relative? or spous$2 or partner? or husband? or wife or wives or child or children or grandchild* or son? or daughter? or sibling? or brother? or sister? or mother? or father?).ti,ab,hw,id.
3. significant others/
4. (friend? or significant other?).ti,ab,hw,id.
5. 2 or 4
6. (care* or caring).ti,ab,hw,id.
7. 5 and 6
8. caregivers/ or caregiver burden/ or respite care/
9. (carer* or caregiv* or care giv*).ti,ab,hw,id.
10. home care/
11. or/1,3,7‐10
12. exp telephone systems/
13. (telephon* or phone? or phoning or calls or callback* or call* back* or cellphone? or smartphone? or iphone? or skype).ti,ab,hw,id.
14. exp mobile devices/
15. (mobile device* or mobiles or mhealth or m‐health or (portable adj2 app*)).ti,ab,hw,id.
16. telemedicine/
17. (telemedicine or tele‐medicine or telecare or tele‐care or telehealth* or tele‐health* or telenursing or ehealth or e‐health).ti,ab,hw,id.
18. hot line services/
19. (hotline* or help line* or helpline*).ti,ab,hw,id.
20. or/12‐19
21. 11 and 20
22. exp health personnel/
23. ((health* or medical or paramedical or nurs* or hospital or operating‐room or psychiatric or pharmac*) adj2 (personnel or provider* or professional* or practitioner* or worker* or aide* or assistant* or staff or officer* or specialist* or consultant*)).ti,ab,hw,id.
24. (doctor* or physician* or general practitioner* or gp or gps or nurse* or clinician* or dentist* or pharmacist* or an?esthetist* or hospitalist* or surgeon* or obstetrician* or gyn?ecologist* or geriatrician* or gerontologist* or therapist* or physiotherapist* or audiologist* or dietitian* or nutritionist* or psychologist* or psychiatrist* or psychotherapist* or counselor* or counsellor* or social worker* or welfare worker*).ti,ab,hw,id.
25. or/22‐24
26. 21 and 25
27. random*.ti,ab,hw,id.
28. trial*.ti,ab,hw,id.
29. controlled stud*.ti,ab,hw,id.
30. placebo*.ti,ab,hw,id.
31. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
32. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
33. (assign* or allocat* or volunteer*).ti,ab,hw,id.
34. treatment effectiveness evaluation/
35. mental health program evaluation/
36. exp experimental design/
37. "2000".md.
38. or/27‐37
39. 26 and 38
Appendix 5. ProQuest Dissertations and Theses A&I (ProQuest) search strategy
ti(telephone OR telemedicine OR telenursing OR telehealth OR phone) AND all(random*)
in: Anywhere but Full text
Appendix 6. CINAHL Complete (Ebsco) search strategy
1. MH Family+
2. family OR families OR parent OR parents OR relative OR relatives OR spous* OR partner OR partners OR husband* OR wife OR wives OR child OR children OR grandchild* OR son OR sons OR daughter* OR sibling* OR brother* OR sister* OR mother* OR father*
3. MH Social Networks
4. AB (friend OR friends OR “significant other*”) OR TI (friend OR friends OR “significant other*”)
5. S2 OR S4
6. AB (care* OR caring) OR TI (care* OR caring)
7. S5 AND S6
8. MH Caregivers OR MH Caregiver Support OR MH Caregiver Burden OR MH Caregiver Strain Index
9. AB (carer* OR caregiv* OR “care giv*”) OR TI (carer* OR caregiv* OR “care giv*”)
10. MH Home Nursing
11. S1 OR S3 OR S7 OR S8 OR S9 OR S10
12. MH Telephone OR MH Cellular Phone OR MH Smartphone
13. AB (telephone OR telephones OR phone* OR phoning OR calls OR callback* OR “call* back*” OR cellphone* OR smartphone* OR iphone* OR skype) OR TI (telephone OR telephones OR phone* OR phoning OR calls OR callback* OR “call* back*” OR cellphone* OR smartphone* OR iphone* OR skype)
14. MH Mobile Applications
15. AB (“mobile device*” OR mobiles OR mhealth OR m‐health OR (portable N2 app*)) OR TI (“mobile device*” OR mobiles OR mhealth OR m‐health OR (portable N2 app*))
16. MH Telehealth+
17. AB (telemedicine OR tele‐medicine OR telecare OR tele‐care OR telehealth* OR tele‐health* OR telenursing OR ehealth OR e‐health) OR TI (telemedicine OR tele‐medicine OR telecare OR tele‐care OR telehealth* OR tele‐health* OR telenursing OR ehealth OR e‐health)
18. MH Telephone Information Services
19. AB (hotline* OR “help line*” OR helpline*) OR TI (hotline* OR “help line*” OR helpline*)
20. S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19
21. S11 AND S20
22. MH Health Personnel+
23. (AB (health* OR medical OR paramedical OR nurs* OR hospital OR operating‐room OR psychiatric OR pharmac*) N2 AB (personnel OR provider* OR professional* OR practitioner* OR worker* OR aide* OR assistant* OR staff OR officer* OR specialist* OR consultant*)) OR (TI (health* OR medical OR paramedical OR nurs* OR hospital OR operating‐room OR psychiatric OR pharmac*) N2 TI (personnel OR provider* OR professional* OR practitioner* OR worker* OR aide* OR assistant* OR staff OR officer* OR specialist* OR consultant*))
24. AB (doctor* OR physician* OR general practitioner* OR gp OR gps OR nurse* OR clinician* OR dentist* OR pharmacist* OR anesthetist* OR anaesthetist* OR hospitalist* OR surgeon* OR obstetrician* OR gynecologist* OR gynaecologist* OR geriatrician* OR gerontologist* OR therapist* OR physiotherapist* OR audiologist* OR dietitian* OR nutritionist* OR psychologist* OR psychiatrist* OR psychotherapist* OR counselor* OR counsellor* OR “social worker*” OR “welfare worker*”) OR TI (doctor* OR physician* OR general practitioner* OR gp OR gps OR nurse* OR clinician* OR dentist* OR pharmacist* OR anesthetist* OR anaesthetist* OR hospitalist* OR surgeon* OR obstetrician* OR gynecologist* OR gynaecologist* OR geriatrician* OR gerontologist* OR therapist* OR physiotherapist* OR audiologist* OR dietitian* OR nutritionist* OR psychologist* OR psychiatrist* OR psychotherapist* OR counselor* OR counsellor* OR “social worker*” OR “welfare worker*”)
25. S22 OR S23 OR S24
Appendix 7. International Clinical Trials Registry Platform (ICTRP) search strategy
telephone OR telemedicine OR telenursing OR telehealth OR phone
in: Title
Appendix 8. ClinicalTrials.gov search strategy
telephone OR telemedicine OR telenursing OR telehealth OR phone
in: Title
Data and analyses
Comparison 1. Telephone intervention versus Usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of Life | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Quality of life (End of intervention) | 4 | 364 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.24, 0.19] |
| 2 Quality of life (Short‐term follow‐up) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐4.43, 4.43] |
| 2.1 Quality of life (Short‐term follow‐up ≤ 3 months) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐4.43, 4.43] |
| 3 Burden | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 End of intervention | 9 | 788 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.07] |
| 3.2 Medium‐term follow up > 3 to ≤ 6 months | 2 | 147 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.32, 0.33] |
| 4 Burden (Short‐term follow‐up) | 1 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.75, 0.35] |
| 4.1 Short‐term follow‐up ≤ 3 months | 1 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.75, 0.35] |
| 5 Skill acquisition: Problem‐Solving | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 End of intervention | 3 | 236 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.21, 0.71] |
| 6 Skill acquisition: Preparedness to Care | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 End of intervention | 2 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.09, 0.64] |
| 7 Skill acquisition: Preparedness to Care (medium‐term follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Medium‐term follow‐up > 3 months to ≤ 6 months | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.02, 0.42] |
| 8 Skill acquisition:Competence | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 End of intervention | 1 | 107 | Mean Difference (IV, Random, 95% CI) | 4.10 [‐2.19, 10.39] |
| 9 Psychological health: Depression | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 End of intervention | 9 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.70, ‐0.05] |
| 9.2 Medium‐term follow‐up > 3 months to ≤ 6 months | 3 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.56, 0.45] |
| 10 Psychological health: Anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 End of intervention | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐11.68, ‐0.32] |
| 11 Psychological health: Coping | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 End of intervention | 1 | 121 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.45, 2.45] |
| 12 Psychological health: Stress | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 End of intervention | 1 | 137 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.10] |
| 12.2 Medium‐term follow‐up > 3 to ≤ 6 months | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.11, 0.31] |
| 13 Knowledge and understanding: Knowledge | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 End of intervention | 1 | 61 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐0.63, 4.43] |
| 14 Health status and well‐being: Physical health | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 End of intervention | 2 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.35, 0.17] |
| 15 Health status and well‐being: Self‐efficacy | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 End of intervention | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.26, 0.33] |
| 16 Health status and well‐being: Self‐efficacy (Medium‐term follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Medium‐term follow‐up > 3 to ≤ 6 months | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.29, 0.29] |
| 17 Health status and well‐being: Social activity | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 End of intervention | 1 | 121 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.18] |
| 18 Satisfaction: Satisfaction with supports | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 End of intervention | 3 | 291 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.24, 0.44] |
| 19 Family functioning | 1 | 141 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
Comparison 2. Telephone versus non‐telephone support intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Burden | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 End of intervention | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.74, 0.34] |
| 2 Depression | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End of intervention | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐4.3 [‐9.57, 0.97] |
| 2.2 Unknown time point | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐5.35, 7.75] |
| 3 Stress | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Unknown time point | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.17, 1.97] |
| 4 Physical Health | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End of intervention | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 1.9 [‐0.65, 4.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bishop 2014.
| Methods | Randomised trial (pilot study) (grant dates April 1st 1994‐March 31 1998) | |
| Participants | Caregivers of stroke survivors recruited from a university medical centre in Rhode Island USA. Caregiver age ranged from 21‐86 years with a mean of 56.8 (16.4). The majority were female (65.3%). Caregivers were spouses (45%), daughters (33%), sons (10%) or other (e.g. sister, partner, mother) (2%). Forty‐nine percent had an annual household income of $15,000‐29,999, 26.5% of $30,000‐49,000, 18.3% of $0‐14,999 and 6.1% had an annual income ≥ $50,000. Care‐recipients: Stroke survivors were required to be fully oriented and able to follow a 3‐step command and had either evidence of stroke on neuroimaging or were hemiparetic. Stroke survivors were mainly female (65.3%) with an age range of 44‐87 years (mean (SD) 70.1 (11.6)). Fifty‐one percent were married, 42.9% divorced/separated/widowed and 6.1% were single. Annual household income was $0‐14,999 (32.6%), $15,000‐29,999 (46.9%), or $30,000‐49,000 (20.4%). |
|
| Interventions | Intervention: Family Intervention: Telephone Tracking (FITT) plus usual care (n = 23) Aim: The primary goal of FITT is to assist stroke survivors and their caregivers in identifying problems during their transition back home. It consists of two main components: psychoeducation and follow‐up. Interventionist(s): Four individuals with prior clinical experience of either family therapy or stroke (a psychiatric resident, family therapy graduate students, a stoke rehabilitation nurse, a master’s level family therapist). Mode of delivery: Telephone Duration: 6 months: Weekly for 6 weeks, biweekly for next 2 months, for a total of 13 calls to each individual (26 calls per dyad). Content: FITT focuses on 5 key areas: (1) family functioning, (2) mood, (3) neurocognitive functioning, (4) functional independence, and (5) physical health. Expectations and transitional challenges within each of these areas are discussed. To reinforce attention to these areas, during the calls, participants were asked to rate themselves and their partner in the 5 areas (worse, same, better) using a structured grid. Telephone contacts were designed to identify and address problems in these key areas, provide psychoeducation, facilitate the dyad’s problem‐solving, and provide follow‐up support. No direct treatment of psychiatric or family problems was given, but participants were supported in seeking referrals for special assessment or treatment as required. Standardisation: All interventionists received didactic instruction, familiarization with the FITT manual, role playing, and group supervision. Therapist adherence and competence was monitored and found to be acceptable. Comparison group: Standard medical follow‐up (n = 26) |
|
| Outcomes | 1. Psychological health (depression): 13‐item Geriatric Depression Scale (GDS) Short Form which uses yes/no responses. Higher scores indicate higher levels of depression. 2. Health status and well‐being:
3. Family functioning: The Family Assessment Device (FAD) and the Perceived Criticism Scale (PCS). Higher scores indicate better family functioning. Outcome data were collected at 3 and 6 months post‐stroke (end of intervention is 6 month time point). |
|
| Notes | Unpublished information requested via email but no response received from the contact author. Professor Ivan Miller, a named author on the paper did provide some additional information via email to enable categorisation of the paper. For the outcome physical health, the Frenchay Activity Index mean change scores as reported were used in this review. For family functioning, the global family functioning score was used in the review and reported in Additional Table 4. Funding source: National Institute for Mental Health (NIMH) grant 1 R21 MH54182‐01 (p.S72). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation (S64) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Data collectors were blinded to group assignment” (S66). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 caregivers left for reasons including stroke survivor death (n = 2), caregiver death (n = 1), self‐withdrawal/repeated failures to return calls (n = 3), permanent nursing home placement of stroke survivor (n = 3), and refusal to complete assessments (n = 2); not differentiated by intervention or control groups (S67). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported (S69). |
| Other bias | Unclear risk | Caregiver characteristics were not reported separately for each group. "...an urn randomisation procedure was used and this should have balanced our imbalances" (S64). |
Connell 2009.
| Methods | Randomised controlled trial (study dates not reported) | |
| Participants | Caregivers were wives of people with dementia recruited from the Michigan Alzheimer’s Disease Research Center (MADRC) and local chapters of the Alzheimer’s Association (AA) in Michigan and Ohio. The average age of the sample was 66.8 years (SD = 9.4), the majority (65.7%) had at least some education beyond high school and described themselves as white/caucasian (92.7%). About one‐fifth (21.9%) were employed part‐ or full‐time. | |
| Interventions | Intervention: Health First (n = 74) Aim: To assess whether compared with baseline, participants in the Health First showed greater improvements in selected outcomes than the control group Interventionist(s): Trained behaviour change counsellors who were current or retired health or social service professionals (confirmed by author via email) Mode of delivery: Telephone Duration: 14 telephone calls over a 6‐month period (weekly for 2 months, biweekly for 2 months, monthly for 2 months). Content: During the first two calls, caregivers were directed to complete daily activity logs (to establish baseline levels of physical activity) and to set a realistic long‐term exercise goal that specified the type of exercise as well as duration and frequency. They were encouraged to set a goal that consisted of a minimum of 30 minutes of low to moderate intensity aerobic exercise at least 3 times a week, supplemented with stretching and strength training. During subsequent calls, participants set short‐term goals for exercise and a problem‐solving process was used to address barriers to goal attainment. They also received a Health First video featuring spouse caregivers discussing strategies for fitting physical activity into their daily routine as a way to model desired behaviour, a choice of exercise videos, a copy of the booklet “Pep up our Life”, and a Health First workbook that explains each step of the program. Standardisation: Counsellors participated in a day‐long training session to address program fidelity that included opportunities for role play and performance feedback to promote appropriate and accurate delivery of the program. A project manager monitored several calls made by each counsellor to confirm that the intervention was being delivered correctly and uniformly. Comparison group: No intervention/usual care (n = 63). |
|
| Outcomes | 1. Burden:
2. Psychological health (depression): The 11‐item Iowa short form of the Center for Epidemiologic Studies Depression Scale (CES‐D). Participants were asked the frequency with which they experienced symptoms in the past week (hardly ever or never, some of the time, or much or most of the time). Possible scores range from 0 to 22; higher scores were associated with more symptoms. 3. Psychological health (stress): Perceived stress was measured with the 14‐item Cohen Perceived Stress Scale. Participants rate the degree to which events in the past month were perceived as stressful using a 5‐point scale ranging from never (0) to very often (4); higher scores indicating higher levels of perceived stress. 4. Health status and well‐being (self‐efficacy):
All outcomes were collected using follow‐up interviews administered at the end of intervention short‐term approximately 6 months and approximately 12 months from baseline. |
|
| Notes | For the outcome burden, data from the subjective burden questionnaire was used in our analysis. For the outcome self‐efficacy, the data for self‐efficacy for self‐care was used. Funding source: A grant from the National institute on Aging to the Michigan Alzheimer’s Disease Research Center (p.172). Unpublished data sought via email, author responded and all available data were provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15.9% overall (n = 27) (17 intervention versus 9 control) (p.179) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | High risk | At baseline, there was a difference between the groups noted in depression scores (intervention 9.4 SD 2.9; control 7.9, SD 2.8) (p.181). |
Corry 2015.
| Methods | Randomised controlled trial (pilot study) (September 2009‐September 2015) | |
| Participants | Caregivers of people with multiple sclerosis (PwMS) recruited from 3 neurological sites (n = 70) in the Republic of Ireland. Caregiver mean age was 51.3 years (SD 13.4), range 22‐84 years; 47.1% were male and 52.9% were female. The majority had at least secondary level education (51.5%) or tertiary education (41.4%). The remaining 7.1% had primary education. Twelve (17.1%) were single, 50 (71.4%) were married and 2 (2.9%) were living as married. Three (4.3%) were separated or divorced and three (4.3%) widowed. An average of 8.8 hours (SD 9.04) were spent caring in a 24‐hour period and the average number of years caring for the PwMS was 11 (SD 7.69). Most (60%) were living with the care‐recipient. Twenty‐five (35.3%) were in paid employment with hours ranging from 6 to 90 hours per week. Thirty‐six (51.4%) were not employed. | |
| Interventions | Intervention: Nurse‐led pro‐active telephone support (n = 33) Aim: To enable nurse specialists in multiple sclerosis (NSMS) help family members and caregivers of PwMS learn problem management skills in order to be better prepared for their role in supporting a person with MS Interventionist(s): Three NSMS who had completed a postgraduate diploma in clinical practice along with a certificate in MS nursing Mode of delivery: Telephone Duration:3 months (four calls; two in month one, one in month two and one in month three) Content: In advance of receiving the calls, the support persons received the support person guidebook. During the calls, the NSMS referred to the guidebook. The support person guidebook was designed to facilitate the process and enable nominated support persons prepare for the calls from the nurse specialists. Scripted interviews were designed to provide a focus for the telephone contacts and help the NSMS and support persons structure their interaction using a problem management approach. Standardisation: Standardisation of interventionist training was maximised through the inclusion of a training section in the intervention manual. Training for delivery of the intervention was provided in accordance with the intervention manual. Each NSMS received a minimum of two hours one‐to‐one training. Comparison group: Usual care (n = 38). |
|
| Outcomes | 1. Quality of life: WHOQoL BREF instrument ‐ 26 questions covering four domains (physical, psychological, social relations, and environmental). Higher scores indicated better quality of life. 2. Burden: Caregiver Reaction Assessment (CRA), a 24‐item scale, which assesses how caregivers react to caring for an ill person in 5 domains: how caring affects caregivers health, daily schedule (schedule disruption), finances, their sense of self‐worth (self‐esteem), and their family. Higher scores indicated greater burden. 3. Skill acquisition (preparedness to care): using the support person preparedness scale, an 8‐item subscale with a five point rating scale developed as part of The Family Care Inventory in the early 1980s (Archbold 1990). Higher scores indicated greater perceived preparedness to care. 4. Health status and well‐being (self‐efficacy): Self‐Efficacy for Problem‐solving scale, a 4‐item caregiver self‐efficacy in problem management scale and Self‐Efficacy for Obtaining Respite subscale. Higher scores indicated greater self‐efficacy. 5. Satisfaction with the intervention: Client Satisfaction Questionnaire‐8 (CSQ‐8), an 8‐item questionnaire with a four option response ranging from 1‐4, with higher scores indicating greater satisfaction. Data were collected at the 4‐week time point which was prior to completion of the intervention, and at the 3‐month time point (approximately week 12), which was the end of the intervention time point. |
|
| Notes | The term support person (SP) was used for caregivers. Standard deviation data for burden and preparedness to care were obtained from the author. Following email communication with the originators of the burden instrument (CRA), the subscale result for 'schedule disruption' was used for 'Burden' in the review. Funding source: Fellowship from the Health Research Board Ireland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random list of numbers for the control and intervention groups at each site was generated by a statistician independent of the study (p.174). |
| Allocation concealment (selection bias) | Low risk | Allocation held by a person independent of the trial and sent via email on enrolment to the trial (p.174) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition minimal (2 per group) and reasons provided (p.238) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | High risk | The interventionists who delivered the intervention delivered care to both groups. |
Davis 2011.
| Methods | Randomised controlled trial (study dates not reported) | |
| Participants | Informal caregivers of people with dementia whose care‐recipient was admitted to a nursing home in Rhode Island, USA. The mean age of the caregivers (data provided by the author) was 60.26 years (SD = 11.42) for the entire group. The majority of caregivers were adult children, 83% in the intervention group and 72% in the control group. The majority of caregivers were female (87% data provided by author) for the entire group. The mean years of education was 15.17 (SD = 3.03) in the intervention group and 14.82 (SD = 3.54) in the control group. The mean duration in months of caregiving for the intervention group was 49.23 (SD = 37.59), and for the control group 46.30 (SD = 38.11). Twenty‐seven caregivers received the intervention and 26 caregivers received standard care. Care‐recipients: mean care recipient age in the intervention group was 82.54 (SD = 5.48) and in the control group 82.73 (SD = 9.05). The mean length of time since dementia diagnosis (months) for the intervention group was 41.14 (SD = 30.15) and control group 42.05 (SD = 33.01). Care‐recipients nursing home placement (weeks) for the intervention group was 6.58 (SD = 3.88) and control group 5.50 (SD = 3.64). |
|
| Interventions | Intervention: Family Intervention: Telephone Tracking‐Nursing Home (FITT‐NH) plus a resource pack containing local resources and educational material (n = 27) Aim: The intervention was designed to help caregivers adjust to the new burdens and stresses of nursing home placement in the first few months after placement has occurred. Interventionist(s): A trained Master’s level therapist (counsellor – confirmed by author via email) Mode of delivery: Telephone Duration: 3 months (initial call, followed by 7 weekly follow‐up calls, and 2 biweekly termination calls over the third month. Initial contact lasted 60 minutes and follow‐up and termination calls lasted 35‐45 minutes. Content: The FITT‐NH was delivered by a standardised method based on a detailed treatment manual that included sample dialogue, a behavioural problems guide to generate solutions with the caregiver, and a specific interventions guide matched to specific caregiver situations. The FITT model assesses caregiver and care‐recipient functioning in key areas (i.e. the caregiver’s emotional functioning, health, social support, family functioning, and communication with staff; care‐recipient’s emotional adjustment, behaviour, and cognition). These key areas are repeatedly assessed throughout the treatment, and particular interventions are applied based on these assessments. Specific interventions include supportive approaches (i.e. empathy, giving permission, normalising, validation, or venting) and active strategies (i.e. bibliotherapy, interpretation, positive reframing, problem‐solving, reference to resource packet, referral, and setting task directive). In the first contact, caregivers are provided with a rationale for the FITT, description of future telephone contacts, an introduction to resource materials, and an assessment of key areas thought to be instrumental in addressing caregiver coping and adjustment. The psychoeducation component reviews information about dementia, specialty care units, and common psychological and physical effects of caregiving. Scheduled telephone contacts identify new problems, discuss positive and negative changes, provide psychoeducation, and caregiver problem‐solving is assisted. The final two calls (biweekly) address termination by anticipating FITT contacts coming to an end and fostering reliance on the support network established in FITT‐NH. This phase reviews caregiver progress and reinforces success, coping strategies, and positive change. The therapist summarised these sessions in a post‐treatment letter sent to the caregiver. Standardisation: States that it was delivered in a standardised way. The intervention was administered according to the written manual procedures (confirmed by author via email). Comparison group: Control group (no formal intervention, received a resource pack containing local resources and educational material) (n = 26) |
|
| Outcomes | 1. Quality of life: SF‐36. Higher scores indicated better quality of life. 2. Burden: Burden Interview (ZBI) a 22‐item inventory assessed caregivers’ subjective feelings of the impact of caregiving on emotional and physical health functioning, social life, and financial status. Higher scores reflected greater burden. 3.Psychological Health (depression): Center for Epidemiology Studies Depression Scale, a 20‐item measure of depressive symptoms. Higher scores reflected higher level of depression. 4. Satisfaction:
Outcome data were collected using face‐to‐face assessments with the caregivers at their home or nursing home at the end of intervention (3 months from baseline). |
|
| Notes | Outcome data on quality of life and Information on interventionist training was provided by the author via email. Funding source: Grant from the National Institute on Aging (AG026122; J.Davis, PI) (p.387). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomised to balance groups to gender relationships and facility type (p.381) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A trained research assistant, blind to group membership, conducted face‐to‐face assessments with the caregivers at their home or nursing home at baseline and at the end of the intervention (p.382). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition low (13%) and balanced between groups (3 and 4); reasons for attrition provided (death n = 5; discharge from nursing home n = 1, and withdrawal n = 1) (p.384). |
| Selective reporting (reporting bias) | High risk | Quality of life was not reported (Table 3, p.385). |
| Other bias | Low risk | Groups did not differ in caregiver age, education, gender, relationship (spouse versus adult child), and length of caregiving, length of dementia diagnosis or time since placement.(Table 2, p.384, table 3, p.385). |
Gallagher‐Thompson 2007.
| Methods | Randomised clinical trial (Study dates not reported) | |
| Participants | Chinese family caregivers of people with dementia in the San Francisco Bay area, United States of America (USA). All caregivers were female (n = 55, 100%) and were included in the study if they were at least 21 years of age, caring for a family member with significant memory loss or deterioration in cognitive abilities, spending at least 8 hours/week caregiving for at least 6 months, owned a phone, planning to remain in the area for 6 months, and agreed to random assignment to both conditions. Care‐recipients were required to have a score of 23 or less on the Mini‐Mental State Examination (MMSE) and be unable to perform one or more activities of daily living (ADLs) and two or more Instrumental ADL (IADLs), or have a documented dementia diagnosis. The mean age of the caregivers who completed the study was 59.3 (SD 12.23), were in the USA for 31.13 years (SD ‐20.93), and had a mean number of years in education of 13.42 (SD 4.10). Most were non‐spousal family relationships (mean spouse caregiver in telephone support condition (TSC) (7, SD 30.4), in‐home behavioural management program (IHBMP) (7, SD 31.8); mean non‐spouse caregiver TSC (16, SD 69.6) IHBMP (15, SD 68.2). Average duration of caregiving was roughly four years (TSC (41.26 months, SD 29.77), IHBMP group (48.32 months, SD 42.86)). More than 75% of caregivers were married: TSC (single (n = 2, 8.7%) , married (n = 18, 78.3%), widowed (n = 1 4.3%), divorced (n = 2, 8.7%)); IHBMP group (single (n = 4, 18.2%), married (n = 17, 77.3%), widowed (n = 1, 4.5), divorced (0)). About 80% of them had children: TSC: (n = 18, 78.3%), IHBMP (n = 18, 81.8%)). At least 75% reported that they had some help with caregiving (n = 20, 87% of the TSC and n = 6, 27.3% of the IHBMP group). About 30% said they were having financial difficulties and over 30% said that they assumed the primary caregiver role because no one else was available. |
|
| Interventions | Intervention: Telephone support groups (n = 28) Aim: To evaluate the efficacy of an in‐home intervention, based on cognitive behaviour therapy principles, to relieve stress and depression in female Chinese‐American caregivers Interventionists: Advanced doctoral students in psychology from a local university program Mode of delivery: Telephone Duration: 12 weeks (six phone calls at two week intervals) Content: Calls began with a general inquiry as to caregiver and care‐recipient well‐being, then one or more problems were identified for discussion. Common themes were incontinence, incessant questioning, temper outbursts, and nocturnal awakenings. The interventionist remained empathic and supportive, and at a comfortable moment, indicated that written information was available to help. Consumer‐friendly materials (in Chinese or English) were mailed if requested. The next phone call was scheduled and the session ended with expressions of concern for the welfare of caregiver and care‐recipient. Standardisation: No detail provided Comparison group: IHBMP is comprised of six modules that focus on learning new skills to help the caregivers cope with caregiving stress. Each module required one or more 90‐minute sessions; one additional session was used for any module requiring extra time (n = 27). |
|
| Outcomes | 1. Psychological health (depression): 20‐item Center for Epidemiological Studies Depression scale (CES‐D). Higher scores indicated higher level of depression. 2. Psychological health (stress): The 10‐item Perceived Stress Scale and the Conditional Bother Subscale (CBS) is derived from the Revised Memory and Behavior Problems Checklist (RMBPC). Higher scores indicated higher level of stress. 3. Health status and well‐being (self‐efficacy): The revised self‐efficacy scale (SE). Higher scores indicated better perceived self‐efficacy. Time point for data collection not stated |
|
| Notes | For the outcome 'stress', the results from the 10‐item Perceived Stress Scale were used in the analysis for this review. Funding source: Research grant from the National office of the Alzheimer’s Association, Chicago – grant IIRG‐01‐3157 to DGT (p.433). Unpublished data sought via email but not received; author did provide information to enable categorisation of the study during the data screening process. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Ten of the 55 (18%) participants dropped out either before or in the early stages of treatment — five from each group (p.427). |
| Selective reporting (reporting bias) | Low risk | Reports on all prespecified outcomes (p.431). |
| Other bias | Low risk | “The two groups were statistically equivalent at baseline, although the IHBMP appears to be higher than the TSC on CES‐D" (p.431). |
Glueckauf 2012.
| Methods | Randomised pilot trial (Study start date: October 2008, end date was not reported but final data collection date for primary outcome was February 2012) | |
| Participants | Informal caregivers of people with dementia recruited from 2 memory disorder clinics, the local Alzheimer’s caregiver organisations, local newspapers, and self‐referral based on information from a friend in Jacksonville‐Tallahassee, USA. Caregivers (CGs) were included if they were 18 years of age and older, provided direct care to their care‐recipients (CR) for a minimum of 6 hours per week for at least 6 months, reported specific caregiving problems amenable to change within a 12‐week intervention frame (e.g. increasing CG social and recreational activities and managing effectively CR agitation and aggressive behaviours), scored a minimum of 10 on the Patient Health Questionnaire‐9 indicating a moderate level of depression, and reported no difficulties in hearing over the phone.The caregivers consisted of husbands (n = 1), wives (n = 5), daughters (n = 4), granddaughter (n = 1) of the care‐recipients. Five of the 7 caregivers randomised to the intervention group completed the intervention and 6 of the 7 randomised to the control group completed the study. The mean age of the caregivers who completed the study was 58.09 (SD = 10.11) years and 1 was male and 10 female. All caregivers had an average of 14.18 (SD = 1.78) years education. Care‐recipients: All care‐recipients were African‐Americans with mean age in years 76.73 (SD = 6.60) and education (years) 12.27 (SD = 3.80), independence in activity of daily living mean score of 2.64 (SD = 1.91) and independence in instrumental activities of daily living mean score of 20.36 (SD = 2.46). Care‐recipients were required to have a medical diagnosis of possible Alzheimer’s disease or other type of progressive dementia verified by a physician at a memory disorder clinic approved by the Alzheimer’s Disease Initiative and at least one limitation in basic activities of daily living and 2 dependencies in IADL. |
|
| Interventions | Intervention: A cognitive behavioural program (CBT) (group and individual format) (n = 7) Aim: To assess CGs appraisal of the intervention process and its impact on daily caregiving experiences and to conduct a preliminary analysis of the effects of face‐to‐face and telephone‐based CBT on changes in subjective burden, assistance support, depression, and health status in African‐American dementia CGs. Interventionist(s): Four African American counsellors, 3 females and 1 male, and were randomly assigned to the groups. All counsellors had a master’s degree in a counselling related profession and at least 1 year of group intervention experience. All 4 regularly used CBT in their practices but none had participated in a formal CBT workshop prior to the study. Average age of counsellors (66 years, SD = 9.2), average years of education (21.5, SD = 1.29), average years in professional practice (30.75, SD = 13.38). Mode of delivery: Telephone Duration: Twelve, 1‐hr, weekly sessions Content: Telephone‐based CBT took place at the CGs’ homes mediated by either a Florida State University or Mayo Clinic Jacksonville teleconferencing system. The intervention program consisted of seven group and five individual CG goal‐setting and implementation sessions. The small group format was used to encourage discussion and clarification about the rationale for and application of fundamental, cognitive–behavioural skills (e.g. assertiveness and effective thinking), as well as to enhance social support among participants. Individual sessions concentrated on the development of problem‐solving skills, beginning with the identification of key caregiving problems and the performance of focused problem histories, followed by goal setting, rehearsal of goal‐related behaviours, goal implementation, and monitoring change over time. Acquisition of such skills was time‐intensive and required tailoring of the intervention to the specific circumstances, characteristics and preferences of the CG, thus necessitating a one‐on‐one format. All participants received a CBT guidebook and a copy of The 36 Hour Day, and information about local dementia care resources prior to the first training session. Standardisation: Interventionist training consisted of two 6‐hour training workshops performed over a period of 2 months by two of the authors who were doctoral‐level licensed clinicians. The authors concluded that overall findings of treatment fidelity analysis suggested that pilot counsellors adhered to implementation guidelines in conducting the CBT program. Comparison group: Face‐to‐face CBT was performed at a university‐based conference room or in a private, soundproof room at a public library. The structure and contents of the programme were the same as that for the telephone intervention (n = 7). |
|
| Outcomes | 1. Burden: The subjective burden scale of the Caregiver Appraisal Inventory – a subscale of the Caregiver Appraisal Inventory (CAI). Higher scores indicated greater burden. 2. Psychological health (depression): Center for Epidemiological Studies Survey‐Depression scale (CES‐D) is a 20‐item self‐report scale that assesses depression in non‐clinical community populations. Respondents rate the frequency of a variety of depressive symptoms they have experienced over the past week on a 0 to 3 scale. A total score ranging from 0 to 60 is derived by summing the item scores. Individuals scoring 16 or higher on the CES‐D are generally considered to be at risk for developing clinical depression. 3. Psychological health (stress): The Revised Memory and Behaviour Problem Checklist (RMBPC) ‐ The disruptive behaviour and depression subscales (17 items) measure CG distress associated with CR disruptive behaviours and CR difficulties with depression. Higher scores indicated higher levels of stress. 4. Health status and well‐being (physical health): Physical symptoms subscale of the modified Caregiver Health and Behaviour inventory (CHHB). The modified CG Health and Health Behaviors inventory (CHHB) is a 42‐item scale to assess dementia CG perceived health, sleep quality, unhealthy behaviours, chronic health conditions, and physical symptoms. Two items ask respondents to rate their general health; two items measure quality of sleep; four items assess involvement in unhealthy behaviours such as smoking and drinking alcohol to excess; 15 items assess the presence of CG health problems, such as high blood pressure, diabetes, cancer, and arthritis; and 21 items measure physical symptoms, such as headaches, shortness of breath, heartburn, and sore throat. Higher scores indicated greater physical ill‐health. Outcome data were collected via the telephone at the end of intervention, approximately 1 week after the 12 week CBT program. |
|
| Notes | Funding source: Grants from the National Institute of Mental Health (R34MH078999) Florida State University College of Medicine, and University of South Florida Health Byrd Alzheimer’s Institute (p.124). Unpublished data sought via email but not received, author did provide information to enable categorisation of the study during the data screening process. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Post treatment assessments were also administered over the telephone by an independent interviewer..." (p.130). "The interviewer was unaware of assignment to treatment condition" (p.130) . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition (3:1 intervention and control) and reasons provided, p.133 |
| Selective reporting (reporting bias) | High risk | Results for stress not reported (p.134) |
| Other bias | Low risk | Groups fairly balanced on all baseline characteristics and measures, although Jacksonville CRs had a significantly greater number of years of education than their Tallahassee counterparts (p.134). |
Kwok 2013.
| Methods | A single‐blinded randomised controlled trial (recruitment February 2011‐March 2012) | |
| Participants | Family caregivers of persons with dementia (PWD) in Hong Kong. Caregivers were the primary caregivers of care‐recipients who had a clinical diagnosis of dementia. The majority of caregivers were in the mean age category of between 41‐50 years (n = 21, 55.2%). The remaining study participant ages in years were 31‐40 (n = 3, 7.89%), 51‐60 (8, 21.05%), 61‐70 (n = 3, 7.89%), 71‐80 (n = 1, 2.63%) and > 80 (n = 2, 5.62%). The majority were female (n = 24, 63.15%) and males accounted for 28.94% (n = 11). The caregivers were children of the care‐recipients (n = 30, 78.9%), spouses (n = 4, 10.52%), grandchild (n = 1, 2.63%), son/daughter in‐law (n = 3, 7.89%). Education ranged from secondary education (n = 27, 71.05%), tertiary (n = 8, 21.05%), primary (n = 2, 5.26%) or Illiterate (n = 1, 2.63%). In the intervention group, caregiver monthly income ranged from $10,000 or less, n = 3 (16.7%), $ 10,001‐$20.000, n = 9 (50%), $20.001‐$30,000, n = 2 (11.1%), $30,001‐$40,000, n = 2 (11.1%), $40,001‐$50,000, n = 2 (11.1%). In the control group, monthly income was $10,000 or less, n = 5 (25%), $10,001‐$20.000, n = 9 (45%), $20.001‐$30,000, n = 2 (10%), $30,001‐$40,000, n = 2 (10%), $40,001‐$50,000, n = 1 (5%), and more than $50,000, n = 1 (5%). Caregivers spent between 1‐9 hours caregiving in the intervention group (1 hour (n = 3, 16.7%), 1‐3 hours (n = 2, 11.1%), 4‐6 hours (n = 6, 33.3%), 7‐9 hours (n = 1, 5.6%), 9 hours (n = 6, 33.3%)). In the control group, caregivers also spent between 1 and 9 hours caregiving (1 hour (n = 1, 5.3%), 1‐3 hours (n = 4, 21.1%), 4‐6 hours (n = 9, 47.7%), 7‐9 hours (n = 1, 5.3%), 9 hours (n = 4, 21.1%)). | |
| Interventions | Intervention: A psychoeducation intervention plus a DVD that contained educational information about dementia caregiving (n = 20; of whom 18 received the intervention) Aim: To investigate the effectiveness of a telephone‐delivered psychoeducational intervention in supporting dementia caregivers in the community Interventionist(s): Registered social workers Mode of delivery: Telephone Duration:12 weeks (approximately 30 minutes per session, one session per week; the day and time of phone calls were flexible to the agreement between the participants and the social workers). Content: Participants in the intervention group were educated and given advice on topics related to dementia caregiving, including knowledge of dementia, skills of communicating with the patient, management of behavioural and psychological symptoms of dementia (BPSD), caregivers’ own emotional issues, resources available in the community, and long‐term care plan. The topics covered and the schedule of presentation were similar to typical psychoeducation interventions held 'on site' at community centres. The focus was on providing emotional support; directing caregivers to appropriate resources; encouraging them to attend to their own physical, emotional, and social needs; and educating them on strategies to cope with ongoing problems. Standardisation: no detail provided Comparison: Caregivers in the control group were given a DVD containing educational information about dementia caregiving (n = 22, of whom 20 remained as control). |
|
| Outcomes | 1. Burden: Zarit Burden Interview Chinese version (ZBI) which consisted of 22 items pertaining to dementia caregiving in areas of perceived physical and psychological well‐being, social life, and finances. The participants indicated, on a 5‐point scale (0 = not at all to 4 = nearly always) during pretest and post‐test, how often they experienced distress resulting from caring for a relative with dementia. Higher scores indicated greater burden. 2. Health status and well‐being (self‐efficacy): Chinese version of The Revised Scale for Care giving Self Efficacy: Obtaining respite (SE‐OR), Responding to Disturbing Behaviours (SE‐RDB), Controlling Upsetting Thoughts (SE‐CUT). Higher scores indicated greater self‐efficacy. Outcome data were collected at the end of intervention i.e. approximately 3 months after the pretest. |
|
| Notes | For the outcome self‐efficacy the results for the sub‐scale 'Responding to Disturbing Behaviours (SE‐RDB)' were used in the analysis. Funding source: none stated Unpublished information requested and received at the data screening stage |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "computerised randomisation program" was used (p.1192). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...research assistant blind to group assignment" (p.1192) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition (for each group; with reasons) very low, and balanced (p.1194) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (p.1195, table 4) |
| Other bias | Low risk | No significant differences at baseline on demographic variables and baseline measures (p.1194, table 2) |
Martindale‐Adams 2013.
| Methods | Randomised controlled trial (February 2005‐June 2007 (Final data collection date for primary outcome)) | |
| Participants | Caregivers of people with dementia (n = 154), Memphis, USA. Caregiver age in years ranged from 37.9‐86.5 (mean age 65.6, SD 12.4). The sample consisted of 83.3% (n = 129) females and 16.2% (n = 25) males of which n = 38 (24.7%) were employed and n = 132 (85.7%) were married. The majority of the caregivers were white (n = 108, 70.1%), black (n = 45, 29.2%), other (n = 1, 0.6%). Most were married (n = 132, 85.7%); n = 38 (24.7%) were employed and mean years of education were 12.8 (SD = 2.0) (i.e. almost a year beyond high school). | |
| Interventions | Intervention: CONNECT (individual and group delivery, 5‐6 caregivers/group; 15 groups) (n = 77) Aim: To determine if telephone support groups for dementia caregivers have an effect on bother with patient behaviours, burden, depression, and general well‐being Interventionist(s): 3 group leaders each with a case load (one with an MSc in divinity, one an MSc in psychology, one an MA in Sociology) Mode of delivery: Telephone Duration: One year (biweekly for 2 months and monthly thereafter for a year, for a total of 14 hour‐long sessions) Content: Content and structure of the intervention were based on the 6‐month REACH II intervention of 12 individual in‐home and by‐telephone sessions and five telephone support group sessions. Session materials consisted of a Caregiver Notebook and commercially available pamphlets. The Notebook, initially developed for a primary care intervention comprised behaviour management chapters and 17 caregiver stress/coping chapters. Each participant received a one‐on‐one introductory telephone call. Like REACH, the multicomponent intervention targeted caregiving risks, including risks associated with emotional and physical well‐being, safety, burden, social support, and patient behaviour management. Standardisation: Training and certification helped to ensure consistency across group leaders. During the final certifying role play, each prospective Group Leader provided the entire first session and two additional educational presentations. Study investigators evaluated behaviourally anchored ratings of specific procedural techniques (e.g. correct use of forms) and clinical skills (e.g. active listening). Comparison group: Caregivers received pamphlets on dementia and safety as well as telephone numbers for local resources. At the end of the study, they received the Caregiver Notebook and a workshop focusing on knowledge, safety, health, well‐being, behaviour management, and stress (n = 77). |
|
| Outcomes | 1. Burden: The 12‐item Zarit Burden Interview (ZBI) assessed caregiver burden. Scoring was 0 (never) to 4 (nearly always); a higher score indicated greater burden. 2. Psychological health (depression): The 10‐item Center for Epidemiological Studies Depression Scale (CES‐D) assessed depressive symptoms within the past week. Scoring was 0 (rarely, none of the time) to 3 (most, almost all the time), for a score of 0 to 30; higher scores indicated greater symptoms. 3. Satisfaction:
Outcome data were collected at the end of intervention which was the 12‐month post‐discharge time point. |
|
| Notes | For the outcome 'satisfaction', the satisfaction scores from the 19‐item social support items were used in the analysis for this review. The author provided additional information on the interventionist training and outcome data for satisfaction with supports. Funding source: This work was supported by the Veterans Health Administration, Health Services Research and Development Service, US Department of Veterans Affairs with additional support from the Memphis Veterans Affairs Medical Center (p.47). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition low, n = 15 (9.7%), Reasons for attrition similar for both groups: Intervention group, N = 8 (refused contact, n = 3; not interested n = 2; illness, n = 1; other reasons n = 2); control group, N = 7 (refused contact, n = 2; illness, n = 3; other reasons n = 2) (p.38 figure 1 and p.39). |
| Selective reporting (reporting bias) | High risk | Physical health outcomes not analysed |
| Other bias | Low risk | No significant group differences reported at baseline and baseline data on outcome measures was similar for both groups (p.40 table 1 and p.41 table 2). |
NCT00646217.
| Methods | A randomised, treatment/comparison, repeated‐measures experimental design (July 2005‐February 2010) | |
| Participants | Spouse/partner caregivers of stroke survivors, Kansas, USA. Caregivers were included if they were aged 55 years or older, married or married equivalent, living with and caring for a spouse or/partner surviving a first‐ever stroke occurring 6‐36 months before enrolment, could participate by telephone and spoke English. | |
| Interventions | Intervention: Self‐Care TALK (detail on number not available) Mode of delivery: Telephone Aim: To test the effectiveness of a self‐care intervention for older, spouse caregivers of persons with stroke in reducing caregiving strain, promoting caregiver health and well‐being, self‐efficacy related to health, and in reducing depressive symptoms Comparison: No intervention (detail on numbers not available) |
|
| Outcomes | 1. Burden: M‐CSI: modified (caregiver strain) 2. Psychological health (depression): CED‐D (depression) 3. Health status and well‐being (physical health): SF‐36 v2, PCS (perceived physical health) 4. Health status and well‐being (self‐efficacy): SRAHP (self‐efficacy for health) No detail available on the scoring system for any of the outcomes Data collection time points: 2 and 6 months post‐enrolment |
|
| Notes | The principal investigator Cynthia Teel, University of Kansas School of Nursing, confirmed via email on 27 August 2017 that the trial registration was the only publication for this trial. Study data requested; author replied that no study data were available for inclusion in this review. Funding sources: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail available |
| Allocation concealment (selection bias) | Unclear risk | No detail available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail available |
| Selective reporting (reporting bias) | Unclear risk | No detail available |
| Other bias | Unclear risk | No detail available |
Pfeiffer 2014.
| Methods | Randomised controlled trial (recruitment March 2007‐October 2009) | |
| Participants | Caregivers of persons with stroke at two large rehabilitation facilities in the greater metropolitan area of Stuttgart Germany, and from a statutory health insurance program. Consenting participants included 27 men (22.1%, mean age 69.78 years, SD 9.09) and 95 women (77.9%, mean age 65.13, SD 10.01). The sample comprised native Germans (n = 100, 82.0%), ethnic German repatriates from Eastern European states (n = 10, 8.2%), and individuals with various European migration backgrounds (n = 12, 9.8%). At enrolment, participants, n = 23 (18.9%), had worked while providing care. The caregivers were spouses or partners (n= 106, 86.9%), children (n= 15, 12.3%), or grandchildren (n= 1, 0.8%) of the care‐recipient and had been providing care for a mean period of 28 months (SD 33). During the 3 months before enrolment, they provided care and support in activities of daily living for 1.98 hr (SD 1.70) per day on average, additional support (e.g. preparing meals, buying goods, doing the laundry, providing outdoor assistance) for 3.72 hr (SD 2.32), and supervision due to cognitive impairment (e.g. disorientation, impaired memory, poor judgment) for 1.75 hr (SD 3.62). Fifteen caregivers (12.3%) were also responsible for the care of a second person who had not been enrolled in the study. Care‐recipients included 84 men (68.9%, mean age in years 73.05, SD 7.33) and 38 women (31.1%, mean age in years 73.37, SD 7.89). Thirty‐five stroke survivors (28.7%) had already experienced more than one stroke in the past. Forty‐one care‐recipients (33.6%) had aphasia, 37 (30.3%) had dysphagia symptoms, and 71 (58.2%) were incontinent. |
|
| Interventions | Intervention: Problem‐Solving Intervention (PSI) and usual support (n = 60) Aim: To examine the effectiveness of a problem‐solving intervention (PSI) for stroke caregivers who provided care for at least 6 months and who experienced significant strain in their role Interventionist(s): Two clinical psychologists experienced in providing cognitive behavioural interventions with older persons conducted the PSI. Mode of delivery: Telephone Duration: 12 months (an initial in‐home visit, five weekly (month 1), and four biweekly (months 2 and 3) telephone sessions. In the following maintenance period (months 4–12), the second component consisted of another in‐home visit (month 4) and nine monthly telephone sessions. Each call was 60 minutes. Content: During the initial in‐home face‐to‐face session, the interventionist explained the purpose of the intervention in detail and gave a short introduction into the principles of problem‐solving and the written problem‐solving guide. The intervention started with capturing the facts and identifying specific burdensome issues the caregiver was willing to change as a basis for a shared agenda. A card sorting task was used to facilitate problem identification. At the end of the card‐sorting task, the caregiver was asked to select and prioritise the burdensome problems that needed immediate attention. The caregiver was instructed to seek all available facts related to the selected problem and was then assisted in articulating a specific, realistic goal to overcome the identified problem and in determining possible obstacles to meeting the established goal. In the following step, the caregiver was instructed to think of as many possible solutions or obstacles to the problem and to write them on a worksheet. Various techniques were offered to increase the number of alternative solutions. After completing a comprehensive list of possible solutions, caregivers were encouraged to consider the potential outcomes of the chosen solutions and weigh the perceived benefit and feasibility of each on a 5‐point rating scale. The final phase in the problem‐solving process was the act of implementing the chosen and carefully planned solution. The PSI group also received the same monthly information leaflets like the information‐only control group. Standardisation: The therapists were supervised every 6‐8 weeks for 3‐4 hr by the third author, who had access to the protocols of the sessions. During these contacts, all participants in the PSI group were discussed on the basis of the interventionists’ records and in regard to study protocol and adherence, intervention progress, and possible difficulties. If needed, telephone‐based supervision was possible at each point in time. Comparison group: An information‐only control group and usual support. Participants assigned to this group received monthly information letters with care‐specific topics like relaxation, pain, depression, and nutrition, as well as addresses for supporting services or groups in the region corresponding to available written material offered by health insurances or local information centres. They also received the usual support that was regulated by law and the various benefits provided by the compulsory long‐term care insurance (n = 62). |
|
| Outcomes | 1. Psychological health (depression): The 20‐item Center for Epidemiological Studies–Depression Scale (CES–D). Total scores range from 0 to 60. A score of 16 or higher was used as an indicator of clinical severity. 2. Skill acquisition (competence): The Sense of Competence Questionnaire (SCQ). The SCQ contains 27 items, each rated on a 4‐point scale. The three domains of the SCQ — satisfaction with the stroke patient as a recipient of care, satisfaction with one’s own performance as a caregiver, and consequences of involvement in care for the personal life of the caregiver — have been confirmed for informal caregivers of older adults with diagnosed stroke. A higher total score indicated a greater sense of competence or with a reversed scaling, a higher burden. Total scores ranged from 27 to 135. 3. Skill acquisition (problem‐solving): The short version of the Social Problem‐Solving Inventory–Revised (SPSI ‐R). The SPSI–R:S has 25 items that are rated on a 5‐point scale ranging from 0 (not very true of me) to 4 (extremely true of me). The total score ranges from 0 to 100. Two constructive dimensions (positive problem orientation (PPO), rational problem‐solving (RPS)) and three dysfunctional dimensions (negative problem orientation (NPO), impulsivity/carelessness style (ICS), and avoidance style (AS)) can be differentiated. The total score serves as a global index of problem‐solving ability. Higher scores indicated better problem‐solving abilities. 4. Health status and well‐being (physical health): Physical complaints were assessed with the Giessen Subjective Complaints List. The intensity of each complaint is rated on a 5‐point scale, ranging from 0 (not existing) to 4 (strong). The scores of the 24 items are summed to a total score (from 0 to 96). Higher scores indicated greater physical ill‐health. 5. Satisfaction:
Outcome data were assessed at baseline (T0), following the intensive intervention period at 3 months (end of intervention) and after the maintenance period at 12 months. |
|
| Notes | We used the 3‐month outcome data because the maintenance period included a second in‐home visit at month 4 which is not consistent with the review's inclusion criteria. Funding source: Grants of the GKV‐Spitzenverband (National Association of Statutory Health Insurance Funds) Berlin, Germany (p.628) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated – remote location (p.631) |
| Allocation concealment (selection bias) | Low risk | Remote randomisation centre provided by an "...independent randomisation center at the University of Tübingen" (p.631) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome "Assessors were trained research assistants who were blind to the treatment condition ..." (p.631) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition low: intervention n = 2 (death of care‐recipient, n = 1, and moved outside the region, n = 1); control n = 4 (death of care‐recipient, n = 1 and discontinued participation, n = 3) (Figure 1, p.630) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported (Table 2, p.637) |
| Other bias | Low risk | "At pretreatment, PSI and control groups evidenced no significant differences (P > .05) on demographic characteristics or primary and secondary outcomes" (see Table 2) (p.636). "More caregivers in the control condition than the PSI group received ambulant therapies like physiotherapy, massage or sports therapy ...and had relatives, friends or neighbours who assisted care‐recipients in ADL‐related tasks ... there was a trend for a greater use of home care services for ADL assistance and a higher rate of aphasia among the care‐recipients in the PSI group ..." (p.636). |
Piamjariyakul 2015.
| Methods | A mixed‐method design with random assignment (pilot study) (Study dates not reported) | |
| Participants | African‐American caregivers of people with heart failure recruited from an outpatient cardiology HF follow‐up clinic in a Midwestern Medical Centre, USA. Caregivers were spouses (65%, N = 13) of patients or were other family members (35%, N = 7), i.e. sister, parent, daughter or granddaughter. Of 20 dyads, 15 (75%) lived in the same household, while 25% (5 dyads) lived separately. Ten caregivers were assigned to the intervention group and 10 to the standard care group. Caregiver age ranged from 40‐78 years with a mean age of 61.4 years (SD 10.0). The majority, n = 17 (85%), were female, 8 (40%) high school or lower, 12 (60%) vocational, college or more. The majority were married, 14 (70%), and employed, 12 (60%). Seven caregivers in the intervention group had vocational or higher education versus 5 in the standard care group. Caregivers reported their chronic health conditions: hypertension (n = 11), myocardial infarction or cardiovascular disease (n = 4), diabetes mellitus (n = 4), osteoarthritis/pain (n = 4), and one caregiver each reported the conditions of depression, thyroid problems, asthma, and HIV. | |
| Interventions | Intervention: The adapted FamHFcare coaching intervention plus standard care (n = 10). Aim: To test whether a culturally‐sensitive telephone coaching intervention could reduce patients’ HF‐related re‐hospitalisation and family caregiver burden and depression, and increase family caregiver confidence, social support, and preparedness to care Interventionist(s): Experienced nurse interventionist Mode of delivery: Telephone Duration: 4 weeks (weekly calls 60‐90 minutes depending on caregiver questions and need for reinforcement). Content: FamHFcare includes 4 weeks of post‐hospital coaching via telephone on specific HF home care skills using teach‐back strategies. FamHFcare aligns with all ACCF/AHA clinical guideline based instructions for daily sodium/fluid restrictions, medication adherence, and symptom monitoring and reporting. Prior to the first telephone session, each family received the coaching program materials by mail: (1) two AHA home caregiving guides (symptoms checklist and staying healthy guidelines for caregivers); (2) a list of local support organizations; (3) the national award winning book Comfort of Home for Chronic Heart Failure: A Guide for Caregivers; (4) low‐sodium booklet, and (5) a plastic daily pill organiser. The nurse interventionist engaged each dyad in four weekly FamHFcare coaching sessions scheduled at their convenience. Standardisation: no details provided Comparison group: Standard care. This included the education and materials routinely given to all HF patients through hospital discharge planning. The standard medical and nursing clinical care in both groups was not changed for this study. Standard care information is not specific to the needs of African‐Americans or to caregivers (n = 10). |
|
| Outcomes | 1. Burden: a 17‐item five‐point Likert‐type scale in which higher scores indicated more burden or difficulty in providing caregiving. Response options were: 1 = providing caregiving but the task was not difficult to 5 = extremely difficult. Option “N/A = not applicable” was provided and selected by caregivers who did not provide a specific caregiving task. Higher scores indicated greater perceived burden. 2. Psychological health (depression): The Center for Epidemiologic Studies Depression Scale (CES‐D). A higher score indicated higher level of depression. 3. Skill acquisition (preparedness to care): A one‐item Likert type scale (1 = not at all, 4 = very well prepared); higher scores indicating caregivers felt better prepared. Outcome data were collected at 6 months (medium‐term follow‐up > 3 to ≤ 6 month time point). |
|
| Notes | Author provided detail via email, which was used in the evaluation of the quality of the intervention, for example, detail on monitoring of delivery of the intervention and adherence to trial protocol. Funding source: Award from Kansas City Life Science Institute, Blue Cross Blue Shield, Kansas City, Kansas (p.466). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make assessment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make assessment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collectors were trained research nurses who were blinded to random assignment" (p.468) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition (Intervention: 2 withdrew after completing and evaluating the first two intervention sessions (one was too ill to continue and the other had a busy work schedule); Control group: 1 patient died) (p.470) |
| Selective reporting (reporting bias) | Low risk | None evident – all caregiver outcomes reported in Table 3 (p.471) |
| Other bias | Low risk | No statistically significant differences were found for caregivers or patients (p.469). |
Powell 2014.
| Methods | A randomised 2‐group design (June 2008‐April 2013) | |
| Participants | Caregivers of adult patients with traumatic brain injury (TBI) from an inpatient rehabilitation unit of a level I trauma centre, Washington, USA. Caregiver age ranged from 19 to 89 years (mean 49.7; SD 13.5). The sample comprised of 82% female, 18% male, of which 79% were of a white non‐Hispanic race and 69% were married. The majority of caregivers had post‐high school education (75%), with employment ranging from working full‐time at time of injury (49%); working part‐time (18%); student (not working) (1%); unemployed (5%) and not in workforce or other (27%). Most were spouses or partners (54%) of the care‐recipient and 35% were parents. | |
| Interventions | Intervention: An individualised education and mentored problem‐solving intervention plus usual care (n = 77). Aim: To investigate the effect of a solely telephone‐based, individualised, combined education and problem‐solving intervention on the quality of life (QoL) and emotional well‐being of caregivers of persons with moderate to severe TBI. Interventionist(s): A master’s level social worker with experience in prior studies of TBI and problem‐solving treatment approaches. Mode of delivery: Telephone Duration: Planned maximum 10 calls (20 weeks), with a target of 8 calls and 2 additional calls at the caregiver’s discretion. Content: The experimental intervention combined education and mentored problem‐solving for topics relating to caregiving and TBI recovery and management (in addition to usual care). The focus of the intervention was on self‐management of issues by the caregivers through applied problem‐solving rather than the provision of solutions or direction to resources, or both, by study personnel. The study interventionist began each call by asking open‐ended questions to ascertain what, if any, issues had arisen or had been resolved since the last call. The caregiver was then asked to identify the concern that he or she wished to address on the call. The final choice of the concern(s) to be targeted on each call was left up to the caregiver, with no requirement to address a new issue or a previously targeted one with action plans in progress. The interventionist then mentored the caregiver in a problem‐solving approach aimed at addressing the concern. Standardisation: No details provided, only one interventionist Comparison group: Usual care (n = 76) |
|
| Outcomes | 1. Quality of life: Adapted Bakas Caregiving Outcomes Scale (BCOS), a 15‐item, 7‐point scale that measures change in social functioning, emotional well‐being, and physical health related to caregiving. Higher scores reflected better quality of life. 2. Psychological health (depression): Brief Symptom Inventory (BSI‐18), an 18‐item instrument designed to quantify symptoms of somatisation, depression, and anxiety. Respondents use a 5‐point Likert scale to indicate the extent to which each symptom bothered them over the preceding 2 weeks. Higher scores indicated greater symptoms of depression. 3. Psychological health (coping): Modified Caregiver Appraisal (MCA); higher scores indicated better coping. 4. Health Status and Well‐Being (social activity): The PART‐O (Participation Assessment with Recombined Tools–Objective) as a measure of community participation; higher scores indicated greater social activity. 5. Knowledge and understanding (knowledge): No instrument specified, stated structured interview and Likert ratings; no detail provided on the scoring system Data were collected at the end of intervention which was 6 months after discharge of the survivor to the community. |
|
| Notes | Funding source: Funded by the Department of Education, National Institute on Disability and Rehabilitation Research, TBI Model Systems: University of Washington Traumatic Brain Injury Model System (H133A070032) (p.180). Author confirmed that the abstract was linked to the study. Additonal information requested from the author but not provided at the time of submission of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (p.182) |
| Allocation concealment (selection bias) | Low risk | Password‐protected database, "The study coordinator entered identifying information into the database and was given the group assignment (p.182). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An examiner blinded to the group allocation conducted the follow‐up assessments..." (p.182). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size estimates allowed for a 10% loss to follow‐up. Loss to follow‐up, however, was greater, and there was an imbalance in loss to follow‐up across the groups (23% in the intervention group and 13% in the control group). Withdrawn from the intervention group (n = 4); withdrawn from the control group (n = 0) (Figure 1, p.185). |
| Selective reporting (reporting bias) | High risk | Unclear how or if the prespecified secondary outcome of knowledge was reported; satisfaction not assessed due to insufficient responses at follow‐up (Table 4, p.187) |
| Other bias | High risk | Fewer caregivers in the intervention group providing direct financial support which could potentially influence outcome measures of QoL and emotional well‐being (p.185) |
Shaw 2016.
| Methods | A parallel randomised trial (April 2010‐March 2013) | |
| Participants | Caregivers of consenting people with gastrointestinal cancers receiving treatment at one of four metropolitan hospitals in Sydney, Australia, for a newly diagnosed or recurrent primary upper GI cancer, metastatic liver disease, or stage 4 colorectal cancer. Caregivers were recruited either during the patient’s hospital admission or within 2 weeks of patient discharge. Mean caregiver age in years was 54.18 (SD 13.5) and most were female (n = 93, 73%); male (n = 35, 27%). Relationship to care‐recipient was spouse or partner (n = 89, 69.5%), child (n = 29, 22.6%), parent (n = 3, 2.3%), sibling (n = 3, 2.3%), other family member (n = 1, < 1%), and friend (n = 3, 2.3%). Education ranged from none or primary (n = 6, 4.7%), intermediate certificate year 10 (n = 24, 19%), leaving certificate or year 12 (n = 20, 15.6%), technical certificate or diploma (n = 33, 25.8%), tertiary (n = 45, 35.1%). Most were employed full‐time (n = 56, 43.7%), part‐time (n = 21, 16.4%), retired (n = 28, 21.9%), unemployed (n = 5, 3.9%), or engaged in home duties (n = 18, 14.1%). | |
| Interventions | Intervention: The Family Connect intervention (n = 64) Aim: To investigate the effectiveness of a standardised, telephone‐based intervention to improve caregivers’ QoL in the first 3 months following a patient’s discharge from hospital. Secondary aims included evaluating the interventions effectiveness in reducing caregivers’ unmet supportive care needs, caregiver burden, and distress. The study also aimed to establish whether a caregiver‐focused intervention could also indirectly reduce patient distress, unmet need, and unplanned hospital presentations to improve overall patient QoL. Interventionist(s): Experienced healthcare professionals (clinical psychologists with training in clinical aspects of cancer care) Mode of delivery: Telephone Duration: 10 weeks (biweekly for the first 3 calls and one month later the final call, mean call length ranged from 32 minutes at 2 weeks to 17 minutes at 10 weeks) Content: The intervention involved a manualised, standardised assessment of caregiver need across the domains of patient care, maintaining family relationships and emotional and physical self‐care, as well as an assessment of information and practical needs. Within each of these domains, the manual provided a list of resources and strategies that might address identified needs, to guide the health professionals delivering the intervention. The resources provided and the level of discussion that was related to management strategies were tailored to individual caregiver needs. Strategies were based on published evidence and clinical experience. Standardisation: All intervention calls were recorded. Recordings were used during regular sessions to provide support and further training to intervention staff and for quality assurance purposes to confirm intervention fidelity. The intervention fidelity was assessed throughout the study and remained high Comparison group: Usual care (n = 64) |
|
| Outcomes | 1. Quality of life: The Short Form (SF)‐12 v2, a 12‐item QoL questionnaire with two subscales that assesses physical and mental well‐being; higher scores indicated better quality of life. 2. Burden: Caregiver Reaction Assessment (CRA), a 26‐item questionnaire which comprises five subscales (disrupted schedule, financial problems, lack of family support, health impact, and impact on self‐esteem). Higher scores indicated greater burden. Outcome data were assessed at 3 (end of intervention) and 6 months post‐hospital discharge (short‐term time point ≤ 3 months) using self‐administered mailed questionnaires. |
|
| Notes | The study did not specify that the intervention group also received usual care. Usual care was not defined. Following email communication with the originators of the burden instrument (CRA), the subscale result for 'schedule disruption' was used in the analysis for this review. For the QoL outcome data, the result from the physical health subscale was used. The authors used a substitution method to impute the data for the entire sample and that is why the number 64 was used (confirmed via email November 2017). Authors confirmed the study outcome data collection time point and provided further detail on the study and intervention. Funding source: The study was funded by the National Health Medical Research (NHMRC) Project Grant 632645 (p.594). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... computer generated randomisation list" (p.587) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups (19% versus 17% at 6 mths; and reasons given (Figure 1, p.589) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (Table 3, p.592) |
| Other bias | Low risk | Baseline characteristics were similar for both groups. Demographic and clinical characteristics for participating and non‐participating patients were similar, although non‐participating patients were slightly older (p.588 and Table 1, p.590), table 2, p.591). |
Shum 2014.
| Methods | A randomised controlled trial design (recruitment May 2011‐May 2012) | |
| Participants | Caregivers of people with colorectal cancer recruited from the colorectal cancer clinic of Queen Mary Hospital in Hong Kong. Caregivers were caring for a family member diagnosed with colorectal cancer in the preceding four weeks, were at least 18 years old and spoke Cantonese. Domestic helpers, those who were cognitively impaired, and those who did not speak Cantonese were excluded. Caregivers age ranged from 19‐86 years; mean age in years was 54 (SD = 14.6). Most were females (n = 103, 74%) and 37 men comprised 26% of the sample. Education ranged from Illiterate (n = 14, 10%), primary (n = 37, 26%), secondary (n = 69, 49%), tertiary (n = 19, 14%), doctorates (n = 1, < 1%). Monthly family income in Hong Kong dollars (£) for the entire sample (n = 140) ranged from, less than 10,000 (769) (n = 55, 39.28%), 10,001‐20,000 (770‐1,539) (n = 42, 30%), 20,001‐30,000 (1,540‐2,307) (n = 26, 18.57%), 30,001‐40,000 (2,308‐3,079) (n = 8, 5.71%), 40,001‐50,000 (3,080‐3,846) (n = 9, 6.4%). | |
| Interventions | Intervention: Nurse‐led, telephone, psychoeducation programme plus usual care (n = 70) Aim: To evaluate the efficacy of the programme in reducing depression, anxiety, stress and burden of care among caregivers of patient with colorectal cancer Interventionist(s): Colorectal nurse specialist Mode of delivery: Telephone Duration: Five weeks (three structured telephone calls to the caregivers at 1, 3, and 5 weeks after discharge. Each call lasted no longer than 45 minutes). Content: The calls sought to understand the caregivers’ situation and identify their problems so that information, as well as education and psychosocial support, could be provided. The interval between telephone calls and the content of the intervention followed a telecare protocol called individual support condition (Taylor 2008). Each call began with an enquiry about the patient’s and carer’s general condition. Specific caring problems or psychological issues were identified, and related information or psychological support was given to caregivers. The nurse also provided education to caregivers according to the patient’s needs at different recovery stages. Before the end of the call, the nurse asked about any additional problems and ensured that caregivers’ needs had been met. Standardisation: The content of the telephone checklists and field notes were reviewed regularly to ensure accuracy and consistency and conversations were documented. Comparison group: Caregivers received routine education on home care on discharge using an information sheet. In addition, a telephone help line number was provided (n = 70). |
|
| Outcomes | 1. Quality of life: The World Health Organization Quality of Life Measure‐BREF (WHOQoLBREF) Hong Kong (HK) was used to assess quality of life and consists of 28 items covering four domains: physical health, psychological health, social relationships and environment. Higher scores indicated better quality of life. 2. Burden: The Chinese version of the Zarit Burden Scale is a 22‐item, self‐report inventory that measures carer burden. Each question was scored on a five‐point Likert scale, ranging from 0 for ‘never’ to four for ‘nearly always present’. The total score ranged from 0 to 60, with a higher score indicating greater burden. 3. Psychological health (depression, anxiety and stress): The Chinese version of the Depression, Anxiety and Stress Scale‐21 (DASS‐21) (a self‐report instrument that measures a patient’s state over the preceding week). It consists of 21 items, spread equally across three scales: depression, anxiety, and stress. Each item uses a four‐point Likert scale, ranging from 0 (‘did not apply to me at all’) to three (‘applied to me very much, or most of the time’). For depression, a score less than nine was regarded as normal, 10‐13 as mild, 14‐20 as moderate, 21‐27 as severe, and higher than 28 as extremely severe. For anxiety, a score less than seven was regarded as normal, 8‐9 as mild, 10‐14 as moderate, 15‐19 as severe, and higher than 20 as extremely severe. For stress, a score less than 14 was regarded as normal, 15‐18 as mild, 19‐25 as moderate, 26‐33 as severe, and higher than 37 as extremely severe. Outcome data were collected at week 2 (end of intervention), 4, and 8 weeks (short‐term time point) after the intervention. |
|
| Notes | Funding sources: none stated For the QoL outcome, the physical health subscale result was used in the analysis for this review. Additional information requested on the published registered trial. The author responded to the queries and emailed the linked published paper. Additional data were requested in October 2018 but these data have not been provided by the author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A person not involved in participant recruitment generated the randomisation schedule ..." (p.32) ‐ detail of method not provided. |
| Allocation concealment (selection bias) | Low risk | "...sequentially numbered, sealed, opaque envelopes..." (p.32) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The research nurses responsible for carrying out the interviews were masked to the treatment assignment" (p.32). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details not provided in the paper, figure 1 (p.33); attrition minimal and accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported upon (Page 8, table 2&3) |
| Other bias | Low risk | No significant differences in baseline characteristics of the caregivers (p.4 and 5, table 1) |
Smith and Toseland 2006.
| Methods | A randomised study (study dates not reported) | |
| Participants | Ninety‐seven caregivers of frail older persons, adult child caregivers (n = 61) and spouse caregivers (n= 36) from a 16‐county area that included urban, suburban, and rural settings, New York, USA. Participants were recruited via newspaper advertisements, direct mailings, appearances before civic and religious organisations, radio announcements and referrals from geriatrics professionals. Caregivers were included if they had a minimum score of 7 or higher on the Caregiver Strain Index. Care‐recipients had to exhibit two or more activities of daily living (ADL)/instrumental activities of daily living (IADL) impairments as reported by the caregiver. Thirty‐one adult child caregivers and 33 spouses received the intervention. The mean adult child caregiver age in years in the intervention group was 54 and in the control group was 54.9. Years spent in education was 14.3 for the intervention group and 15 for the control group. Most were female (77.4% in the intervention group and 96.7% in the control group). Male caregivers accounted for 22.6% in the intervention group and 3.3% in the control group. Most were of white race or ethnicity (87.7% in the intervention group and 96.7% in the control group); black race or ethnicity accounted for 12.9% of the intervention group and 3.3% of the control group. Relationship status ranged from married (intervention group 35.3%, control group 46.2%), single/never married (intervention group 17.6%, control group 23.1%), divorced (intervention group 29.4%, control group 30.8%), separated (intervention group 11.8%, control group 0%) and widowed (intervention group 5.9%, control group 0%). The mean spouse caregiver age in years in the intervention group was 70.2 and in the control group was 66.2. Years spent in education was 14.1 for the intervention group and 14.3 for the control group. Most were female (86.4% in the intervention group and 92.9% in the control group). Male caregivers accounted for 13.6% in the intervention group and 7.1% in the control group. Most were of white race or ethnicity (90.5% in the intervention group and 85.7% of the control group); black race/ethnicity accounted for 9.5% of the intervention group and 7.1% of the control group. Relationship status ranged from married (intervention group 95.2%, control group 100%), single/never married, divorced, separated, and widowed (0% across groups) and 'other' accounted for 4.8% of the intervention group and 0% of the control group). |
|
| Interventions | Intervention: The telephone support group (TSG) intervention (group‐delivered) (n = 31) Aim: To evaluate the effectiveness of a telephone support Interventionist(s): License Master’s prepared social worker (who had several years of clinical geriatric social work experience) led all groups. Mode of delivery: Telephone Duration: Weekly for 12 weeks. Each weekly session lasted 90 minutes (15 minutes for hook‐up and 75 for group meeting). Content: A multicomponent intervention that includes education about the effects of chronic illness and about emotion‐focused coping strategies, problem‐solving, and support. A leader’s manual and a participant workbook was developed. The leader’s manual was used to train the group leader and a workbook was given to each member in the TSG arm of the study. The leader instructed members to turn to the appropriate pages in the workbook each week during telephone meetings and to follow along using the structured agendas and the educational materials provided. The first half of each weekly meeting began with conference call connections using a voice‐over internet provider. After the initial period in which the leader called each member in turn, the group leader gave a brief overview of the previous meeting. Following this was a ‘‘check‐in’’ with group members regarding their progress on target goals between meetings. In order to help group members develop supportive relationships beyond the TSG program, the leader asked each of them to select a telephone buddy to call between group meetings. Conversations between telephone buddies were to focus on caregiving issues and the coping and problem‐solving skills that participants were learning. Emotion‐focused coping strategies were taught and practiced during the first half of each weekly TSG meeting. The group leader introduced problem‐focused coping skills during the second half of each meeting and practised them with the members. Standardisation: Delivery was monitored, with one interventionist for all groups, and a leaders manual was used to train the group leader. Comparison group: Usual services offered by the senior services centre (n = 30) |
|
| Outcomes | 1. Burden: Zarit Burden Interview (ZBI), a 22‐item Likert‐type scale that measures the total strain, role strain, and personal strain that caregivers experience as a result of the impact of the patient’s disabilities on their life. For each item, caregivers indicate how often they have felt a certain way: (0) never, (1) rarely, (2) sometimes, (3) quite frequently, or (4) nearly always. Higher scores signified greater burden. 2. Psychological health (depression): Center for Epidemiologic Studies–Depression Scale (CES‐D). Respondents were asked how frequently they had experienced 20 different events in the past 7 days. These events were indicative of depression. Each event had a score of 0 (happened rarely or not at all) to 3 (most or all of the time). Higher scores indicated more depression. 3. Psychological health (anxiety): State–Trait Anxiety Inventory (STAI) —This scale measures anxiety for caregivers. It presents 20 statements that people use to describe themselves and asks caregivers the extent to which they agree (4) or disagree (1) with each statement. The final score is a summary of the answers to the 20 statements. Higher scores indicated more anxiety. 4. Skill acquisition (problem‐solving): Pressing Problems Index (PPI). Researchers developed the 18‐item PPI in order to assess the extent to which participants’ health and social service problems were being addressed. The PPI contains a list of problems that caregivers frequently encounter when caring for a family member with a chronic illness. For each problem, we asked the caregiver how stressful the problem was, from (0) not at all to (4) extremely; how much their stress had changed, from ‐3 (much worse) to 3 (completely better); how effective they had been in dealing with this problem, from 0 (not at all effective) to 4 (extremely effective); and how much their effectiveness had changed from ‐3 (much worse) to 3 (completely better). Higher scores indicated better problem‐solving. 5. Knowledge and understanding (knowledge): the 'Community Services Inventory' subscales (of services and how to access them); higher scores indicated greater knowledge. Outcome data were collected at the end of intervention and within 2 weeks of completing the intervention. |
|
| Notes | For the outcome 'problem‐solving', the reported results for 'how effective' were used in the analysis for this review. Mean scores for the two subscales of the 'Community Services Inventory' subscales were used for the analysis. Unpublished data was requested; the author replied on 11 October 2017 stating that the data was no longer available. Funding source: Project supported in part by United States Administration on Aging Grant (p.620) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Interviewers remained blind to the participants’ assigned condition ..." (p.622). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information on post‐test data to assess |
| Selective reporting (reporting bias) | High risk | Adult children subsample only, reported as this group ‘drove the overall effects’ (p.625) |
| Other bias | High risk | Adult child demographics showed significant differences between groups in terms of education with the control group having more years of education than intervention group (table 3, p.626). |
Toye 2016.
| Methods | Parallel group, single‐blind, randomised controlled trial (recruitment April 2015‐November 2015) | |
| Participants | Caregivers of older people discharged from the medical assessment unit within a metropolitan tertiary hospital in Western Australia with over 600 beds. Caregivers were recruited in the hospital at the time of patient discharge. A family caregiver was defined as a family member or friend who provides unpaid personal care, support, and assistance. Inclusion criteria for dyads were that they comprised a patient aged 70 years or older being discharged to their home or the home of their family caregiver, plus a family caregiver who could speak and read English. Caregiver mean age in years for the intervention group was 63.1 (12.6 SD) and the control group 61.3 (13.4 SD). Females comprised 74% (n = 104) and males 26% (n = 37). Relationship to care‐recipient was husband (n = 13), wife (n = 29), son (n = 62), daughter (n = 14) and other (n = 104). | |
| Interventions | Intervention: Further Enabling Care at Home (FECH) program and usual discharge care (n = 86). Aim: The FECH intervention is intended to identify family caregivers of older patients during the hospital admission, help ensure their understanding of discharge information that has implications for the caregiving role, prompt reflection by the caregiver on this role and what is needed to help sustain this, and guide the caregiver as they identify and address prioritised needs for support (information provided by author via email). Interventionist(s): A nurse with acute care knowledge relevant to the care of older people in poor health, knowledge of how to access local services, understanding of the family caregiver role, the capacity to work flexible hours to fit in with caregivers’ needs, and the skills to support the caregiver during the process of reflection and self‐assessment. Mode of delivery: Telephone Duration: Up to 40 days (planned calls were delayed). Planned calls were weekly to biweekly (call 1: within a week post‐discharge, call 2: 7‐10 days post‐discharge, call 3: 14 days after discharge). Actual call delivery: call 1 within and up to 9 (instead of 7), contact 2 within 24 (instead of 10) and contact 3 within 40 (instead of 14), days post‐discharge. Mean and standard deviation call contact time in minutes was contact 1: 15.4 (9.6), contact 2: 59.7 (24.1) and contact 3: 28.3 (17.7). Content: The Further Enabling Care at Home program involved the implementation of a strict telephone protocol by the specially trained nurse, using the Carer Support Needs Assessment Tool, which has fourteen items covering: (a) support that enables the caregiver to care for the patient at home, and (b) support for the caregiver in their caring role. There were three, sequential, telephone contacts. Contact 1 was planned to take place within a week post‐discharge. Contact 2 was designated to occur from 7 to 10 days post‐discharge. Contact 3 was planned to follow within 14 days of the discharge. Standardisation: The delivery of the intervention was monitored at regular meetings with investigators responsible for this issue. Field notes were taken by the FECH nurse during intervention contacts to provide a record that allowed the discussion of cases during these meetings so that fidelity could be assured. Using this process, intervention delivery was as planned and consistent, except with respect to the planned time of the contacts, which were delayed in some instances because of the busy schedules of the caregivers. The selection and preparation of the FECH nurse, plus the use of a pre‐prepared resource manual, also helped to ensure the standardised quality of the intervention (information provided by author via email). Comparison group: Usual discharge care (n = 89). |
|
| Outcomes | 1. Burden: Caregiver strain subscale of the Family Appraisal of Caregiving Questionnaire – Palliative Care; higher scores indicated greater burden. 2. Skill acquisition (preparedness to care): Preparedness for Caregiving Scale from the Family Care Inventory; higher scores indicated better perceived preparedness to care. 2. Family functioning: Family Well‐Being subscale of the Family Appraisal of Caregiving Questionnaire – Palliative Care; higher scores indicated better family functioning. 3. Health status and well‐being (physical health): SF‐12 v2 Health Survey used for assessing physical health and not as a QoL outcome (caregiver ratings of their own health and well‐being). Higher scores indicated better physical health. Cost: No specific instrument (intervention costs recorded include (i) nurse time for the duration of each contact; (ii) nurse time to implement and organise resources; (iii) nurse time to write notes following each contact for each patient‐carer dyad; (iv) cost of training the FECH nurse; (v) telephone charges; and (vi) stationary and postage costs. Costs in the control group were estimates of nurse time for usual discharge procedures). Higher scores indicated greater cost. Outcome data were collected at Time 1 (within 4 days of discharge), Time 2 (15–21 days after discharge) and Time 3 (end of intervention time point, six weeks after discharge). |
|
| Notes | Unpublished mean and standard deviations along with details of the cost data collected were obtained from authors via email. Author confirmed via email that care‐recipients may have included those with an exacerbation of a chronic condition or an additional acute illness or both. Funding source: A Department of Health Western Australia, SHRAC Research Translation Project grant (p.40). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list of random allocations prepared prior to the study commencing, using a permuted random blocks strategy (p.35) |
| Allocation concealment (selection bias) | Low risk | Allocation schedule held by researcher not involved in recruitment (p.35) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All researchers involved in quantitative data collection were blinded to the allocation schedule and actual group assignment (p.35). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition Intervention group 19.5%; control group 8.1% (p.37). “From the 12 dyads withdrawing after randomisation without providing data, most failed to provide a reason but three caregivers had concerns about, or difficulties with, the planned telephone data collection and one experienced a bereavement (p.37)”. |
| Selective reporting (reporting bias) | High risk | Physical and mental health outcomes not reported |
| Other bias | Low risk | No significant difference in caregiver characteristics between the groups (p.37) No significant differences in baseline measures for outcomes (Table 2, p.38; table 3 page 39) |
Tremont 2008a.
| Methods | A randomised controlled trial (study dates not reported) | |
| Participants | Caregivers of people with dementia recruited from memory disorder clinics, support groups, and newspaper or television advertisements in the Southern New England region of the United States of America. Caregivers were aged 21 years or older; lived with a relative with dementia in the community; and provided a minimum of four hours of supervision or direct care per day for at least six months prior to enrolment. Sixty caregivers were enrolled in the study at baseline, with 32 assigned to the treatment condition and 28 assigned to standard care. By the 12‐month assessment point, 33 caregivers had data for analysis, with 16 caregivers in the FITT‐D group and 17 caregivers in standard care. There were 20 spousal caregivers and 13 adult child caregivers. Caregiver age ranged from 41‐87 years with an overall group mean of 63.30 years (SD 11.836). The majority were female (n = 26) and male (n = 7). Both groups were similar in terms of years of education; mean caregiver years of education in the intervention group was 14.22 (3.41) and in the control group 15.88 (2.14). | |
| Interventions | Intervention: Family Intervention: Telephone Tracking – Dementia (FITT‐D) plus a binder containing local resource information (e.g. list of support groups, adult day care centres) and educational material from the Alzheimer’s Association (n = 32) Aim: To examine the preliminary efficacy of FITT‐D, a multicomponent intervention that is delivered in 23 telephone contacts over 12 months Interventionist(s): Master’s level therapists (counsellors, nurses, social workers – confirmed by author email) Mode of delivery: Telephone Duration: One year (one initial call, then weekly for 6 weeks, 12 additional contacts every 2 weeks and 4 monthly termination calls. Initial contacts lasted 60 minutes, follow‐up contact 15‐30 minutes giving approximately 12 hours of contact between the therapist and caregiver). Content: The calls focused on providing emotional support, directing caregivers to appropriate resources, encouraging caregivers to attend to their own physical, emotional and social needs, and teaching caregivers strategies to cope with ongoing problems. The intervention addressed a broad range of issues and problems related to caregiving. The FITT method consists of two stages. The initial stage, orientation and psychoeducation, involved providing caregivers with a rationale for the FITT, an introduction to educational and resource materials, a description of what would happen during future phone contacts and an assessment of key areas thought to be instrumental in addressing caregiver burden and mental health (i.e. caregivers’ health, functioning, mood, thinking, and family life). The psychoeducation component of this initial stage involved reviewing information about dementia and common psychological, emotional, psychosocial, and medical effects of caregiving. The second stage, follow‐up, involved weekly and biweekly contacts in which new problems were identified, positive and negative changes in caregivers or care‐recipients were discussed, and psychoeducational information was reviewed and applied for particular situations. The initial and follow‐up calls were structured around assessment of key areas of functioning in both the caregiver and care‐recipient. Specific interventions were applied at therapists’ discretion, including supportive approaches (i.e. empathy, giving permission, normalising, provision of information, validation, or venting) or more active strategies (i.e. bibliotherapy, interpretation, positive reframing, problem‐solving, reference to resource packet, referral, and setting task directives). The final four follow‐up calls (monthly) addressed issues of termination by allowing caregivers to anticipate FITT contacts coming to an end and to foster reliance on the support network established during the intervention. Standardisation: The two therapists were trained in the FITT‐D procedure and were required to achieve at least 80% correct on a 50‐item multiple choice test about dementia and the FITT treatment manual prior to initiating treatment. Doctoral staff supervised therapists weekly to ensure adherence to the protocol and minimise drift. Comparison group: No telephone intervention. They received a binder containing local resource information e.g. list of support groups, adult day care centres, and educational material from the Alzheimer’s Association (n = 28). |
|
| Outcomes | 1. Quality of life: SF‐36 General Health; higher scores indicated better quality of life. 2. Burden:
3. Psychological health (depression) Geriatric Depression Scale (GDS). The GDS is a 30‐item self‐report yes/no measure that is designed specifically for older adults by excluding somatic signs and symptoms of depression. Total scores range from 0 to 30. Higher scores indicated depression. 4. Knowledge and understanding: Alzheimer’s Disease Knowledge Test; higher scores indicated greater knowledge. 5. Health status and well‐being (self‐efficacy): Self‐Efficacy Scale; higher scores indicated greater self‐efficacy. 6. Satisfaction with the intervention: Treatment satisfaction, caregivers in the FITT‐D group completed a 12‐item treatment satisfaction questionnaire. Higher scores indicated greater satisfaction. Date were collected at 12 months (end of intervention) via face‐to‐face assessments with caregivers at their homes. |
|
| Notes | For the outcome burden, the results from the Revised Memory and Behavior Problem Checklist (RMBPC) are used in this review. Funding source: Grant from National Institute of Mental Health (MH62561; G.Tremont, PI) (p.516). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation (p.507) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants were blinded to group membership (p.507). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: n = 15 (47%) (n = 11 due to death of care‐recipient); control group: n = 12 (43%) (n = 4 due to death of care‐recipient) (p.511) |
| Selective reporting (reporting bias) | High risk | Provided results on the main outcomes (burden and depression) and reported on the additional measure to address secondary goal of the intervention but did not report actual statistics for QoL, self‐efficacy, knowledge, and satisfaction with the intervention (p.513). |
| Other bias | Low risk | No baseline imbalances (Table 1, p.511). Analysis of differences between those who completed and did not complete the 12‐month assessment and whose care‐recipients had died showed that care‐recipient age was the only statistically significant difference between the groups (p.511). |
Vazquez 2016.
| Methods | Randomised controlled trial (pilot study) (November 2014 ‐December 2015) | |
| Participants | Non‐professional caregivers of people with various conditions recruited from an official register of caregivers maintained by the Ministry of Labor and Welfare of the Government of the Autonomous Community of Galicia to North West Spain. Conditions included: diseases of the musculoskeletal system, connective tissue, cardiovascular and respiratory (19.7%, n = 12), chromosomal, congenital and perinatal abnormalities (23.0%, n = 14), mental disorders, neurological diseases, brain damage (18.0%, n = 11), dementia (39.3%, n = 24). Caregiver mean age was 58.4 (SD 8.0, range 42‐75 years). The majority (93.4%, n = 57) were female and 6.6% (n = 4) were male. Caregivers were caring for either father or mother (n = 21, 34.5%), son or daughter (n = 24, 39.3%) or other (n = 16, 26.2%) and had been involved in caregiving for an average of 12.3 years (SD 5.7) providing an average of 17.1 hours of care per day (SD 2.1). Forty caregivers (65.6%) were couples (married or had partners), 50.8% (n = 31) were of low or low middle social class, 49.2% (n = 30) were from middle, middle high or high social class. The majority (65.6%, n = 40) were literate or had a primary education; 34.4% (n = 21) had high school or university education. Most (63.9%, n = 39) had responsibility for housework and 36.1% (n = 22) were retired, employed or unemployed. | |
| Interventions | Intervention: A cognitive behavioural intervention via group conference call (CBC) (n = 20) and a behavioural activation intervention through group conference call (BAC) (n = 22) (group delivered conference calls, approximately 5/group) Aim: To assess the feasibility/acceptability of a preventive cognitive‐behavioural intervention implemented via conference call for caregivers, and to conduct a preliminary assessment of the efficacy of the behavioural activation component alone compared to the complete cognitive behavioural intervention Interventionist(s): Four psychologists Mode of delivery: Telephone Duration: 5 weeks (weekly 90‐minute sessions) Content: Prior to the study, CBC and BAC intervention protocols were developed. The CBC intervention was based on a multifactorial integrative model of depression and was adapted from a proven indicated prevention program for depression previously implemented as a face‐to‐face group format. The BAC intervention was also adapted from prior work but in this case the intervention focused solely on the behavioural activation component. Standardisation: Training consisted of 35 hours for each of the interventions including contents, viewing videos, and role‐playing exercises and was administered by two clinical experts in both therapies, each with over 20 years of experience. Each intervention session was audio‐taped and protocol adherence was evaluated by one of two experienced clinicians. These clinicians also provided weekly therapist supervision. Therapist protocol adherence was 93% for CBC and 95% for BAC, indicating that the primary elements of the protocol were all administered. Comparison group: No intervention (n = 19) |
|
| Outcomes | 1. Psychological health (depression)
2. Satisfaction with the intervention: Client Satisfaction Questionnaire [CSQ‐8]. The CSQ‐8 is an 8‐item scale with 4 response options and a total score ranging from 8 to 32, with a higher score indicating greater satisfaction with the service received. Outcome data were collected at the end of intervention. |
|
| Notes | For the outcome depression, the reported results for 'The Center for Epidemiologic Studies Depression Scale (CES‐D‐Spanish version)' were used in the analysis for this review. On behalf of the principal investigator, Prof. Fernando L. Vázquez , Patricia Otero, PhD advised via email on the 6th August 2017 that at the time of this review a doctoral thesis was being finalised in which the efficacy of the clinical trial was being analysed. The pilot study for the trial which was published is included in this review for analysis. Patricia Otero PhD advised that the findings of the doctoral thesis are in the line with the pilot study. Details of the pilot study in terms of characteristics of the care‐recipients, the adaptation of the intervention, and intervention monitoring for the pilot study linked to this registered trial were provided by the study authors. Funding source: Work supported by the Ministry of Economy and Competitiveness of Spain (2012‐PN162 (PSI2012‐37396)) (p.594). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician randomly assigned participants to groups using the table of random numbers (p.939). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All pre‐ and post‐treatment assessments were conducted face‐to‐face by trained interviewers not directly involved in the research study and who were blind to the group to which each participant had been assigned" (p.940). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition (2:2:1); balanced across groups and reasons provided (Figure 1, p.939) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported (p.943) |
| Other bias | Low risk | No remarkable or clinically relevant baseline differences, suggesting that randomisation had resulted in a balanced pilot study (p.940) |
Wilz 2016a.
| Methods | Randomised controlled trial (October 20018‐July 2010) | |
| Participants | Informal caregivers of people with dementia recruited mainly from the areas of Berlin/Brandenburg and Thuringia, Germany. Most of the caregivers were recruited via print media (80%). Some participants learned about the study via the internet (6%), cooperating institutions (5%), relatives and friends (4%), practitioners (2%), television (2%), or radio (1%). Caregivers were included if they had the main responsibility for caregiving for a patient with a diagnosis of Alzheimer’s disease and a Global Deterioration Score > 3 as rated by the screening person based on the caregiver’s report. Caregivers were also required to have no simultaneous psychotherapy, no obvious cognitive impairment (estimated in the comprehensive screening procedure through assessor’s evaluation), and no severe acute mental and/or physical illness. Mean caregiver age was 62.01 years (SD = 9.33) and females comprised 82.2% (n = 157) of the sample. Most were spouses or partners (n = 76, 39.8%) and daughters or daughters‐in law (n = 75, 39.3%) of the care‐recipients. Of the male participants, more partners (n = 21, 11%) than sons or sons‐in‐law (n = 12, 6.3%) were included. |
|
| Interventions | Intervention: CBT (n = 50) Aim: To analyse whether caregivers of the intervention group reported better well‐being and health post‐treatment than caregivers of an untreated control group and an attention control group (treated with progressive muscle relaxation (PMR)), and whether these benefits were maintained at 6‐month follow‐up Interventionist(s): Six experienced clinical therapists (Master’s Degree) Mode of delivery: Telephone Duration: 3 months Content: This is a multicomponent intervention focused on managing the behaviour problems and personality changes of the care‐recipient, reduction of social isolation, assisting in utilization of professional and informal support, stress reduction, emotion regulation, reinforcement of positive activities, and supporting acceptance of role change and loss. The intervention included a therapeutic manual consisting of five CBT intervention modules, matched to the needs of the caregivers of people with dementia. Standardisation: Interventionists attended intensive pre‐intervention training with twice‐monthly supervision during the delivery of the intervention, which was carefully monitored based on intervention documentation (date, duration, content) and audiotaping of each session. Comparison group: Untreated control group (n = 50) |
|
| Outcomes | 1. Psychological health (depression) German version of the 20‐item Center for Epidemiologic Studies Depression Scale (CES‐D). Higher scores indicated greater symptoms of depression. 2. Satisfaction with the intervention: A 5‐point Likert scale (where 1 = very good, 2 = good, 3 = average, 4 = below average, 5 = unsatisfactory). Lower scores indicated greater satisfaction. Data were collected at the end of intervention and at 6 months, the medium‐term follow‐up time point. |
|
| Notes | In this study, a second 'selected' non‐randomised experimental group was created where all sessions were delivered by telephone. This non‐randomised arm of the study did not fulfil our inclusion criteria. The study was deemed as meeting our inclusion criteria because the findings from the randomised experimental group were provided by the author. The attention‐only arm was excluded from our review as it was administered over the phone. Funding source: The study was supported by a grant from the German Federal Ministry of Health (LTDEMENZ‐44‐092) (p.43). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent data management and biometry centre was involved to ensure blinded randomisation. The random number generator Random.org was used for randomisation (p.30). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An independent data management and biometry centre was involved for blinded assessment (p.30). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The experimental group to which the reported loss to follow‐up related was not specified. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes for this review were reported (p.38 and 39). |
| Other bias | High risk | Significant differences between untreated control group and experimental group in terms of perceived health (p.31) |
Winter 2006.
| Methods | A randomised, controlled, 2‐group design (study dates not reported) | |
| Participants | Female caregivers of community‐dwelling individuals with dementia from Philadelphia in United States of America. Caregivers were included in the study if they were female, 50 years of age or older, providing care for a minimum of 6 months to a relative with a physician’s diagnosis of Alzheimer’s disease or related disorders (ADRD), and having weekly access to a telephone for at least 1 hour. Caregiver mean age was 66.6 years (SD = 9.1; range, 51‐86); 68.3% were white, and the remaining caregivers were African‐American. Most were educated beyond high school (51.0%), 35.6% were high school graduates, and 10.6% had less than 12 years of education. Wives constituted 57.7% of the sample. | |
| Interventions | Intervention: Telesupport groups (group delivered teleconference, approximately 5/group) (n = 58) Aim: To enhance caregiver ability to manage daily stressors by providing emotional support and validation Duration: 6 months (weekly for one hour) Interventionist(s): Trained social workers Mode of delivery: Telephone Content: Caregivers used their own telephones with no charge. Initially, facilitators focus on developing group cohesion. As groups progress, disclosure of intimate problems and personal conflicts emerge. Caregivers express emotions and share coping strategies including cognitive reframing and practical approaches to organising care routines. They also assist each other in problem‐solving and share educational resources. The mutual support and validation provided by group members normalise experiences and provide a supportive social network, core to the service model. Standardisation: No detail provided Comparison group: Usual care (n = 45) |
|
| Outcomes | 1. Burden: The 22‐item Zarit Burden scale. Caregivers report the extent of agreement on a scale ranging from 0 (never/not at all) to 4 (always/extremely) in accordance with the scale item. Responses were summed to produce a total score ranging from 0 to 88, with high scores indicating greater burden. 2. Psychological health (depression): The 20‐item Centers for Epidemiological Studies–Depression Scale (CES‐D). The response format for each item is 0 (never or rarely) to 4 (always). Scores were summed, with higher scores indicating greater depression and a score of 16 or higher indicative of depressive symptoms. 3. Skill acquisition (competence): The 6‐item scale adapted from Kaye’s Gain Through Group Involvement Scale to assess the extent to which caregivers perceive personal gains over the past few months in new friendships, knowing what to do when lonely, how to handle the blues, how to handle stress, how to find health care or other resources, and ability to deal with family relationships. Responses to each item were not at all (1), a little (2), or a great deal (3). The sum of the 6 items was calculated, yielding a possible range from 6 to 18. The actual range was 7. Higher scores indicated greater competence. Outcome data were collected at 6 months from baseline i.e. the end of intervention. |
|
| Notes | Funding source: Funds from the Alzheimer’s Association awarded to Laura N. Gitlin, PhD (p.391). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to assess |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Just stated that 94 (91.3%) were available for the 6‐month telephone interviews. Among these, 81 were still caregiving at home; the remaining had placed their relative in nursing home facilities or were bereaved (p.393). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (p.394 table 2) |
| Other bias | High risk | Those randomised to the experimental group were significantly older than those in the control group. Control group subjects scored slightly higher than the treatment group on gains (p.393 and table 1). |
Wray 2010.
| Methods | A prospective 2 × 3 randomised control group design (September 2005‐April 2007) | |
| Participants | Spouse or partner caregivers of veterans with moderate‐to‐severe dementia identified via (a) the Veterans Information System Technology Architecture Patient Care database activity indicating an encounter coded for a dementia diagnosis, (b) clinician referral, (c) self or family referral in response to information and publicity about the study. The study was conducted in New York, United States of America. Caregivers were primary caregivers who lived with the person for at least one year, and exhibited at least a moderate level of caregiving strain as defined by a score of 7 or more. Caregivers mean age was 73.94 years (SD not reported). Mean years of caregivers education in the intervention group was 12.69 (SD 3.04) and in the control group 12.34 (SD 2.40). Mean monthly income (US dollars) was similar across the two groups; intervention group (2784.22, SD 1351.47) and control group (2420.75, SD 1376.32). | |
| Interventions | Intervention: The Telehealth Education Program (TEP) (group‐delivered, up to 8/group) (n = 83) Aim: To address major areas that can be problematic for caregivers who want to continue to take care of the veteran with dementia at home: (a) verbal and nonverbal communication, (b) effective structuring of caregiver–patient interactions, (c) management of challenging behavioural problems, and (d) accessing resources and planning for the future. Interventionist(s): Four trained group leaders (three masters‐prepared social workers and one nurse dementia care manager) with expertise in geriatrics led the support groups. Mode of delivery: Telephone Duration: 10 weeks (group format in groups of up to 8, 1 hour telephone meetings) Content: A TEP participant workbook and leader manual were developed for the project. Caregiver participants followed a TEP participant workbook at each of the sessions and weekly homework assignments were included. The TEP group intervention protocol included three primary components: (a) education about dementia and its symptoms and about caregiving skills and resources to address these symptoms, (b) emotion‐focused (such as relaxation and self‐care strategies) and problem‐focused coping strategies (such as problem‐solving and caregiving skills), and (c) group support. TEP content was designed to address major areas that can be problematic for caregivers who want to continue to take care of the veteran with dementia at home: (a) verbal and nonverbal communication, (b) effective structuring of caregiver–patient interactions, (c) management of challenging behavioural problems, and (d) accessing resources and planning for the future. Standardisation: No detail provided Comparison group: Usual care (n = 75) |
|
| Outcomes | Cost: No specific instruments. Veteran health care cost and utilisation data were collected from national abstracts of the VA’s Decision Support System (DSS) and the fee basis files hosted at the VA Austin Automation Center (AAC). For each participant, all cost and utilisation data were summed over 6‐month time intervals, resulting in a total value for each of three data collection periods: baseline (0–6 months before the intervention), short‐term follow‐up time point (from intervention start to 6 months following the start of the intervention), and medium‐term follow‐up time point (from 6 to 12 months after the start of the intervention). |
|
| Notes | The information reported was from a paper linked to the registered trial. VA and VMCA were not explained but they appear to be the names or linked to the name of the health care centres/organisations. Additional unpublished results requested via email in October 2017; results not received at the time of submission of this review Funding source: Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (IIR 03‐076‐01) (p.631) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated that “…data were extracted by one of the investigators (Jian Gao) who was blind to the participants’ group membership” (p.626) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to assess |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Insufficient information to assess. Stated “no statistically significant differences between participants in the two conditions at baseline” (p.627). This referred to caregivers only but results were based on patient hospitalisation and this may have impacted on outcomes. |
AA: Alzheimer's association AAC: Austin Automation Center ADL: Activities of daily living ADRD: Alzheimer's disease or related disorders AHA: American Heart Association BAC: Behavioural activation intervention through group conference call BCOS: Bakas caregiver outcomes scale BPSD: Behavioural and psychological dimensions of depression BSI‐18: Brief symptom inventory ‐ 18 CAI: Caregiver appraisal instrument CBC: Cognitive behavioural intervention via group conference call CBS: Conditional bother scale CBT: Cognitive behavioural therapy CED‐D: Definition unable to be found CES‐D: Center for epidemiological depression scale CGs: Caregivers CHHB: Caregiver health and behaviour inventory CONNECT: Definition unable to be found, may not be an acronym CR: Care recipient CRA: Caregiver reaction assessment CSQ‐8: Client satisfaction questionnaire ‐ 8 DASS‐21: Depression, anxiety and stress scale ‐ 21 DSM‐IV: Diagnostic and statistical of mental disorders ‐ IV DSS: Decision support system DVD: Digital video disc FAD: Family assessment devise FAI: Frenchley activities index FamHFcare: Family heart failure care FECH: Further enabling care at home FITT: Family intervention telephone tracking FITT‐D: Family intervention telephone tracking ‐ dementia FITT‐NH: Family intervention telephone tracking ‐ nursing home GDS: Geriatric depression scale GI: Gastrointestinal HF: Heart failure hr: hour IADL: Instrumental activities of living IHBMP: In‐home behavioural management program MA: Masters of Arts MADRC: Michigan Alzheimer’s Disease Research Center MCA: Modified caregiver appraisal M‐CSI: Modified caregiver strain index MMSE: Mini‐mental state examination MS: Multiple sclerosis MSc: Master of Science NHMRC: National health medical research NIMH: National institute for mental health NSMS: Nurse specialist in multiple sclerosis PART‐O:Participation assessment with recombined tools–objective PCS: Perceived criticism scale PMR: Progressive muscle relaxation PPI: Pressing problems index PPO:Positive problem orientation PSI: Problem solving intervention PWD: People with dementia PwMS: People with multiple sclerosis REACH:Resources for enhancing alzheimer's caregiver health RMBPC: Revised memory and behavior problem checklist RPS: Rational problem‐solving SCIDCV: Structured clinical Interview for disease and statistics manual ‐IV Axis I disorders clinician version SCQ: Sense of competence questionnaire SE: Self‐efficacy SE‐CUT: Self‐efficacy: controlling upsetting thought SE‐OR: Self‐eficacy: obtaining respite SE‐RDB: Self‐efficacy: responding to disturbing behaviours SF‐12: Short Form ‐ 12 SF‐36: Short Form ‐36 SP: Support person SPSI‐R: Social problem‐solving inventory–revised SPSI‐S: Short version of the social problem solving inventory – revised SRAHP: Self‐rated abilities for health practices scale STAI: State–trait anxiety inventory TALK: Defintion not able to be found, may not be an acronym TBI: Traumatic brain injury TEP:Telehealth education program TO: baseline TSC: Telephone support condition TSG: Telephone support group WHOQoLBREF: World Health Organisation Quality of Life Abbreviated Version ZBI: Zarit Burden Interview
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achie 2015 | Wrong population and Intervention: caregivers only received the intervention if they choose to join and they did not receive a telephone intervention (confirmed by authors via email 24 June 2017). |
| ACTRN12616000467437 | Trial withdrawn due to a lack of funding. |
| Aguirrezabal 2013 | Wrong design: not an randomised trial. |
| Badger 2007 | Wrong comparator: comparator also included the telephone for 'attention only' purposes. |
| Badr 2015 | Wrong intervention: care‐recipients and caregivers (dyads) received the intervention together. |
| Bailey 1997 | Wrong design: not an randomised trial. |
| Bakas 2009a | Wrong comparator: was also a telephone intervention. |
| Bakas 2015 | Wrong comparator: was also a telephone intervention. |
| Barclay 2016 | Wrong intervention: not a telephone‐only intervention (email from author 29 September 2017). |
| Bauman 2015 | Wrong design: not a randomised trial (confirmed by author via email 23 June 2017). |
| Bauman 2018 | Wrong design: not a randomised trial. |
| Bell 2005 | Wrong population: not caregivers. |
| Belle 2006 | Wrong intervention: not a telephone intervention. |
| Berwig 2017 | Wrong intervention: not a telephone‐only intervention. |
| Blumenthal 2009 | Wrong comparator: included telephone calls (confirmed via email by author on 26 June 2017). |
| Brown 1999 | Wrong design: participants were not randomised. |
| Callahan 2006 | Wrong intervention: not a telephone‐only intervention. |
| Chambers 2014 | Wrong comparator: comparator was also a telephone intervention. |
| Chang 2004 | Wrong intervention: not a telephone‐only intervention. |
| Chodosh 2015 | Wrong comparator: included a telephone component. |
| Cox 2012 | Wrong study design: not a randomised trial. |
| Czaja 2013 | Wrong intervention: not a telephone intervention. |
| Dellasega 2002 | Wrong intervention: in‐person delivery. |
| Demiris 2011 | Wrong study design: not a randomised design, study was a pre‐post test design. |
| Demiris 2012 | Wrong intervention (visuals introduced ‐ confirmed by author via email on 27 June 2017). |
| Duncan 2017 | Wrong intervention: intervention was patient‐focused. |
| Elliott 2009 | Wrong intervention: not a telephone‐only intervention. |
| Erten‐Lyons 2017 | Wrong design: not a randomised trial. |
| Finkel 2007a | Wrong Intervention: not a telephone‐only intervention (intervention included both text and voice). |
| Gant 2007a | Wrong comparator: comparator was also a telephone intervention. |
| Garand 2002 | Wrong intervention: not a telephone‐only intervention (results for telephone component not reported separately). |
| Gaugler 2008 | Wrong intervention: not a telephone‐only intervention (telephone component was responsive and ad hoc). |
| Gilliss 1992 | Wrong design: not a randomised design. |
| Gitlin 2003 | Wrong intervention: not a telephone‐only intervention (results for telephone component not reported separately). |
| Gitlin 2010 | Wrong intervention: not a telephone‐only intervention (results for telephone component not reported separately). |
| Gitlin 2010a | Wrong intervention: not a telephone‐only intervention (results for telephone component not reported separately). |
| Gitlin 2016 | Wrong intervention: both groups received the intervention face‐to‐face. |
| Gonyea 2016 | Wrong intervention: not a telephone‐only intervention (telephone component was a follow‐up to the face‐to‐face sessions). |
| Graham‐Philips 2016 | Wrong intervention: not a telephone‐only intervention. |
| Grant 1999 | Wrong intervention: first face‐to‐face session was more than an introductory session. |
| Grant 2002 | Wrong intervention: first face‐to‐face session was more than an introductory session. |
| Greaves 2016 | Wrong design: not a randomised study. |
| Hasan 2015 | Wrong intervention: not a telephone‐only intervention (confirmed by author via email on 19 July 2017). |
| Hicken 2017 | Wrong intervention: not a telephone‐only intervention. |
| Hirsch 2014 | Wrong design: participants were not randomised to the intervention and control groups. |
| Hori 2009 | Wrong population: intervention was given to the caregiver and patient together. |
| Huang 2013 | Wrong intervention: not a telephone‐only intervention (had more than one introductory session). |
| Hudson 2015 | Wrong intervention: not a telephone‐only intervention (had a home visit after an introductory telephone contact). |
| Johnson 2018 | Wrong intervention: intervention was patient‐focused. |
| Kozachik 2001 | Wrong intervention: not a telephone‐only intervention (more than one in‐person visit). |
| Kuo 2017 | Wrong intervention: not a telephone‐only intervention (more than one face‐to‐face before the telephone calls). |
| Kwok 2012 | Wrong intervention: not a telephone‐only intervention (DVD given to both groups). |
| Lindauer 2016 | Wrong study design: not a randomised trial. |
| Linton 2018 | Wrong intervention: both groups received telephone calls. |
| Livingston 2013 | Wrong comparator: the telephone was also used in the comparator (confirmed via email by author on 31 July 2017). |
| Martín‐Carrasco 2009 | Wrong intervention: not a telephone intervention. |
| Mazanec 2017 | Wrong intervention: both groups received the intervention. |
| McCann 2015 | Wrong comparator: comparator also received telephone calls (confirmed by the author via email on 30 July 2017). |
| McCann 2015a | Wrong intervention and comparator also received telephone calls (confirmed by the author via email on 30 July 2017). |
| McLennon 2016 | Wrong comparator: comparator also received telephone calls. |
| Mendyk 2018 | Wrong population: intervention focused on patients, not caregivers. |
| Morgan 2015 | Wrong study design: not a randomised design. |
| Mosher 2018 | Wrong comparator: comparator was also telephone‐based. |
| NCT00052104 | Wrong comparator: comparator was also telephone‐based. |
| NCT00067171 | Wrong population: patients not caregivers. |
| NCT00131092 | Wrong population: patients not caregivers. |
| NCT00247000 | Wrong population: patients not caregivers. |
| NCT00271739 | Wrong population: intervention was targeted to the patients not caregivers. |
| NCT00288132 | Wrong population: patients not caregivers. |
| NCT00483522 | Wrong population: patients not caregivers. |
| NCT00693563 | Wrong population: intervention was targeted to patients not caregivers (confirmed via email by authors on 29 July 2017). |
| NCT00721383 | Wrong intervention: intervention not a telephone‐only intervention. |
| NCT00822510 | Wrong comparator: comparator was also an active telephone intervention. |
| NCT00829361 | Wrong population: patients not caregivers. |
| NCT01993550 | Wrong comparator: comparator was also a telephone intervention. |
| NCT02036294 | Wrong intervention: not a telephone‐only intervention (confirmed by author via email on 17 November 2018) |
| NCT02094846 | Wrong population: patients not caregivers. |
| NCT02347202 | Wrong intervention: not a telephone‐only intervention. |
| NCT02364505 | Wrong intervention: not a telephone‐only intervention (included an online component), |
| NCT02475954 | Wrong intervention: not a telephone intervention (delivered via webcam using a computer ‐ confirmed by author via email on 1 August 2017). |
| NCT02483494 | Wrong population: patients not caregivers. |
| NCT02703532 | Wrong intervention: not a telephone intervention. |
| NCT03127930 | Wrong intervention: not a telephone‐only intervention (most caregivers received a minimum of 3 face‐to‐face contacts and mode of delivery was not used for analysis, confirmed by author via email on 8 November 2018) |
| NCT03142841 | Wrong intervention: not a telephone‐only intervention (telephone component was linked to the online component and not analysed separately ‐ confirmed by author via email on 5 August 2017). |
| NCT03164239 | Wrong population: healthy persons, not caregivers. |
| NCT03177447 | Wrong design: not a randomised controlled trial. |
| NCT03378050 | Wrong comparator: usual care group also received two brief calls. |
| NCT03506945 | Wrong intervention: not a telephone‐only intervention (web‐based and smart phones were used to complete homework, confirmed by author via email 9 November 2018) |
| NCT03635151 | Wrong comparator: comparator also delivered by telephone. |
| Nichols 2011 | Wrong design: not a randomised controlled trial. |
| Nobili 2004 | Wrong intervention: not a telephone intervention. |
| Penner 2016 | Wrong intervention: not a telephone‐only intervention (had two baseline home visits ‐ confirmed by author via email 3 August 2017). |
| Piamjariyakul 2012 | Wrong design: not a randomised design (one group feasibility study ‐ confirmed by author via email 3 August 2017). |
| Piamjariyakul 2013 | Wrong design: not a randomised study. |
| Piette 2015 | Wrong intervention: not a telephone intervention. |
| Pirrraglia 2005 | Wrong design: not a randomised trial. |
| Porter 2011 | Wrong population: care‐recipients and caregiver received the intervention together. |
| Prick 2015 | Wrong intervention: not a telephone intervention. |
| Radziewicz 2009 | Wrong intervention: paper focused on treatment fidelity of a caregiver intervention tested using a randomised trial but the intervention was not telephone‐only. |
| Reeves 2018 | Wrong intervention: neither of the two intervention groups were telephone‐only. |
| Richardson 2007 | Wrong design: not a randomised trial. |
| Rivera 2008 | Wrong intervention: not a telephone‐only intervention (comparator was telephone‐only but the intervention included in‐home visits plus telephone contacts). |
| Samus 2014 | Wrong population: patients not caregivers. |
| Schinköthe 2014 | Wrong design: not a randomised study. |
| Schure 2006 | Wrong intervention: not a telephone intervention. |
| Schwarz 2008 | Wrong intervention: not a telephone intervention. |
| Shanley 2008 | Wrong design: not a randomised controlled trial. |
| Sherrod 2013 | Wrong intervention: not a telephone‐only intervention. |
| Sherwood 2012 | Wrong comparator: comparator was telephone based (confirmed by principal investigators via email on 22 August 2017). |
| Silveira 2016 | Wrong intervention: intervention was an automated telephone system. |
| Sneed 1997 | Wrong intervention: not a telephone‐only intervention. |
| Stewart 2001 | Wrong design: not a randomised controlled trial. |
| Teel 2005 | Wrong design: not a randomised study (focus was on intervention development). |
| Tompkins 2009 | Wrong design: participants were not randomised to the groups. |
| Tremont 2014 | Wrong comparator: comparator was also telephone‐based. |
| Tremont 2015 | Wrong comparator: comparator was also telephone‐based. |
| Tremont 2017 | Wrong comparator: comparator was also telephone‐based. |
| Tsai 2005 | Wrong design: not a randomised controlled trial. |
| Uphold 2014 | Wrong intervention: not a telephone‐only intervention (combination of online and telephone). |
| Uphold 2015 | Wrong design: not a randomised study. |
| Valeberg 2013 | Wrong intervention: not a telephone‐only intervention. |
| Van Knippenberg 2016 | Wrong intervention: not a telephone intervention. |
| Van Mierlo 2012a | Wrong design: not a randomised trial for the informal caregiver component of the study (confirmed via email by author on 23 October 2017). |
| Wilder ongoing | Wrong comparator: comparator was also delivered by telephone. |
| Williams 2010 | Wrong intervention: not a telephone‐only intervention. |
| Yamada 2011 | Wrong intervention: not a telephone intervention. |
| Yan 2016 | Wrong intervention: not a telephone‐only intervention. |
DVD: Digital video disc
Characteristics of studies awaiting assessment [ordered by study ID]
Au 2014.
| Methods | Parallel randomised controlled trial |
| Participants | Informal caregivers of people with dementia where the caregiver was the primary full‐time carer (for at least 6 months), were aged 25 years and were able to read and speak Chinese/Cantonese. The caregivers consisted of spouses, daughters/sons, and daughter/son‐in‐laws of the patients.Thirty caregivers received the intervention and 30 caregivers received standard care. The mean age of caregivers who completed the study were: intervention group 58.1 (SD 12.4); control group 55.1 (SD 11.3). Gender (intervention group, male 6 (21.4%), female 22 (78.6%) and control group, male 7 (22.6%), female 24 (77.4%). In the intervention group, education ranged from none 2 (7.1%), primary/kindergarten 6 (21.4%), junior secondary 6 (21.4%), senior secondary 8 (28.6%), form 6‐7/vocational institutes 2 (0%), college sub‐degree 2 (7.1%), college, bachelor degree 4 (14.3%). In the control group, participants education levels were: none 1 (3.2%), primary/kindergarten 12 (41.9%), junior secondary 2 (6.5%), senior secondary 10 (32.2%), form 6‐7/vocational institutes 2 (6.5%), college sub‐degree 2 (6.5%), college, bachelor degree 1 (3.2%). The mean duration in years of caregiving for the intervention group was 3.2 ± 2, and for the control group 3.3 ± 2.3. Patients: Mean age in years for the intervention group was 80.1 ± 6 and for the control group 79.9 ± 8.6. Relationship to caregivers for the intervention group were spouse 12 (42.9%), children 15 (53.6%), children‐in‐laws 3 (3.6%) and for the control group, spouse 11 (35.5%), children 14 (45.2%), children‐in‐laws 4 (12.9%), relatives 1 (6.5%). The mean duration of dementia (in years) for the intervention group was 3.4 ± 2 and for the control group was 3.3 ± 2.2. Care‐recipients in the intervention group were in receipt of average hours of care per day of 8.3 ± 7; those in the control group received a mean of 7 9.1 ± 9.5 hours of care per day. |
| Interventions | Title of the intervention: Telephone‐assisted pleasant‐event scheduling (TAPES) Aim: To evaluate the effectiveness of TAPES on enhancing the psychological well‐being of community‐dwelling family caregivers.\ Interventionist(s): no details provided Duration: 4 weeks (2 calls for first two weeks and one call per week for weeks 3 and 4). Each call was 20 minutes in duration. Content: The intervention had three components. First, the project rationale of behavioural activation was introduced, and the Pleasant Event Schedule (revised from California Older Person’s Pleasant Events Schedule) was administered. An information package was distributed to advise on how to access social and psychological services in the community. Participants were then asked to decide on one or two activities that they would like to work on for the coming weeks. Second, six telephone calls were made. In the first phone call, participants were taught to schedule pleasant events according to the procedures of behavioural activation by working through the Pleasant Activity Planning Worksheet. To monitor individual progress, participants were asked to fill the Pleasant Event Tracking Form and the Daily Mood Record Form on a daily basis. Participants then mailed the completed progress charting forms back to the researcher. Third, concepts of adaptive coping were discussed from weeks 2 to 4: active coping, passive coping, and the goodness of fit between coping and situations, problem‐solving coping (e.g. making preparations), emotion‐regulation coping (e.g. distancing) and using situation‐appropriate strategies (e.g. stepping back and taking a break when no immediate solution was available). The compliance of treatment was closely monitored. Participants had to complete the preceding component first before moving on the next component. The completion of the tasks was recorded on the intervention protocol. Regular weekly meetings were carried out by the intervention team to review the progress of caregivers. Standardisation: no details provided Comparision group: Usual care (TAU) – treatment‐as‐usual (standard care provided by a psychogeriatric team with regular psychiatric follow‐up for the care‐recipients and support from social workers upon request). |
| Outcomes | 1. The Centre for Epidemiologic Studies Depression Scale (CES‐D) 2. Revised Scale for Caregiving Self‐Efficacy (SE) Data were collected pre‐intervention (1‐3 days before the first intervention call), post‐intervention (1‐3 days after the last intervention call), and at follow‐up (1 month after post‐intervention). |
| Notes | Professional status of the interventionists unknown (awaiting author response) |
Bass 2017a.
| Methods | Stated that three randomised trials were underway |
| Participants | Caregivers (one study for caregivers of people with dementia, one for caregivers of people with depression and one for people with multiple chronic conditions) |
| Interventions | Title of the intervention: Care Consultation Aim: To help caregivers and care receivers by providing information about health problems and available resources mobilising and facilitating the use of informal supports and formal services; and providing emotional support. Interventionist(s): Care consultants (social workers or nurses) Duration: no details provided Content: no details provided Standardisation: no details provided. Comparison group: usual care (no details provided) |
| Outcomes | Not stated |
| Notes | Reference was from the Rosalynn Carter Institute for Caregiving website which provided a brief outline of the study. Unclear as to whether the intervention focused on helping caregivers or primarily focused on patients. |
Chodosh 2015a.
| Methods | Randomised controlled trial |
| Participants | Dementia caregivers |
| Interventions | Title of the intervention: An evidence‐based dementia care management (DCM) Aim: To implement an evidence‐based dementia care management (DCM) program in a Medicare managed care plan and evaluate the program’s effectiveness and costs Interventionist(s): Care managers (social workers specially trained in evidence‐based dementia care) Duration: no details provided Content: no details provided Standardisation: no details provided Comparison group: usual care (no details provided) |
| Outcomes | No detail provided on specific outcome measurement instruments. Stated that caregiver surveys and medical records were used to estimate between‐group differences on measures of recommended dementia care within areas of 1) assessment, 2) treatment, 3) safety, and 4) education and support. The abstract indicated that the date of caregiver satisfaction, burden, social support, self‐efficacy, and healthcare utilisation costs were collected. Data were collected at 9 and 18 months. Method of data collection was not specified. |
| Notes | Abstract only available. It was unclear if the caregivers got the intervention separately to the patients. Author contacted and stated that the manuscript was in process and no further details are available at the time of contact. |
Chwalisz 2017.
| Methods | Unclear |
| Participants | Informal caregivers in a rural area |
| Interventions | Title of the intervention: Southern Illinois Rural Caregiver Telehealth Project Aim: To specifically meet the needs of informal caregivers in a rural area Interventionist(s): Masters level psychologist Duration: Eight‐session structured telephone intervention Content: Stated that caregiver knowledge, problem‐solving skills, help‐seeking behaviour, and affect were the major components addressed Standardisation: no details provided Comparison group: Call‐in helpline |
| Outcomes | No detail provided |
| Notes | Reference was from the Rosalynn Carter Institute for Caregiving website which provided a brief outline of the study. The study design was unclear. |
Gitlin 2018.
| Methods | Randomised trial |
| Participants | Family caregivers of veterans with dementia |
| Interventions | Title of the intervention: Information only Aim: This was an attention‐only comparator for the face‐to‐fact TAP‐VA Interventionist(s): Masters level team member Duration: no details provided Content: 8 telephone sessions with information on relevant topics (home, safety, dementia), with no discussion of activity or behavioural activity. Standardisation: no details provided Comparison group: TAP‐VA: 8 in‐home sessions delivered by occupational therapists |
| Outcomes | Caregiver assessment of function and upset scale (CAFU) |
| Notes | Study control group received the telephone intervention. We need to confirm if the individuals delivering the telephone calls were healthcare professionals and assess the intervention in greater detail for inclusion in the update of this review. |
Mavandadi.
| Methods | Randomised controlled design |
| Participants | Caregivers of veterans diagnosed with dementia |
| Interventions | Title of the intervention: Telephone Education program Aim: To facilitate resource connection and provide education, psychosocial support, and care management for individuals caring for veterans with dementia Interventionist(s): no details provided Duration: no details provided Content: Caregivers received education, continuous support, skills training and monitoring of veterans medication adherence, symptoms and service needs Standardisation: no details provided Comparison group: Participants were sent general material about VA and community resources for patients with dementia and their caregivers, as well as brochures for the caregivers. In addition, they received usual care and were free to seek medical, psychological, social support, and social services that are available through VAMCs or any other non‐VA/community resource. |
| Outcomes | Caregivers were asked to complete an assessment battery of standardised measures of care‐recipient and caregiver characteristics. |
| Notes | This is a brief summary of a study from the Health Services Research and Development website. The study needs to be further assessed prior to inclusion in the update of this review. |
NCT00031265.
| Methods | Randomised controlled trial |
| Participants | Caregivers of patients with stroke recruited from patient admissions to Rhode Island Hospital following an acute stroke. Caregivers were 18 years or over (confirmed by named principle investigator. Professior Ivan W. Miller). Patients inclusion criteria: age > 35 years, MRI or CAT scan proof of stroke or definitive hemiplegia, and competency to sign an informed consent form |
| Interventions | Title of the intervention: Family Intervention: Telephone Tracking (FITT) plus standard medical care Aim: To determine if a family intervention administered by telephone to stroke patients and their caregivers increases adaptation and functioning after stroke Interventionist(s): no details provided Duration: six‐month period (participants contacted by telephone every week for 6 weeks, then every 2 weeks for 2 months, and then monthly for 2 months) Content: no details provided Standardisation: no details provided Comparison group: no intervention plus standard medical care |
| Outcomes | During the trial, specially trained staff will carefully monitor the progress of the stroke patient and his/her family member, checking for changing in thinking, concentration, attention, memory, mood, and family functioning that sometimes occurs in stroke. The telephone calls will check on how the participants are doing after discharge and will assist with questions and concerns. |
| Notes | Unclear as to whether the interventionist was a healthcare professional or not |
NCT00183781.
| Methods | Randomised controlled trial |
| Participants | Family member or friend who was identified as the primary caregiver. Both recently diagnosed HIV‐infected individuals and primary caregiver were included. |
| Interventions | Title of the intervention: Family Intervention: Telephone Tracking (FITT) plus regular medical care Aim: To evaluate the effectiveness of FITT in improving family functioning, enhancing coping skills, and reducing depression in HIV‐infected individuals and their caregivers. Interventionist(s): no details provided Duration: 12 months Content: FITT is a telephone‐based intervention program that assists in identifying problems and resolving them through referrals to medical and community organisations that provide HIV‐related support and services. It is also an educational resource that provides information on the many medical and psychological aspects of HIV infection. Standardisation: no details provided Comparison group: an assessment‐only group that did not receive FITT but received regular medical care throughout the study |
| Outcomes | Outcome measurements were self‐assessments of depression, coping, and family functioning. In addition, participants receiving FITT were asked to evaluate the effectiveness of the telephone intervention. No details provided on outcome measures. All measurements were assessed at baseline, and months 3, 6, and 12. |
| Notes | This is a registered trial on the ClinicalTrials.gov website. The site indicates that the study has been completed. It is unclear if the age of participants refers to the caregiver of the person with HIV and if the caregivers received the intervention separately to the care‐recipient. |
NCT00416078.
| Methods | Randomised trial |
| Participants | Caregivers of people with Alzheimer's disease |
| Interventions | Title of the intervention: Customary care and monthly brief telephone calls Aim: Not stated Interventionist(s): Project staff Duration: Six months Content: Caregiver brief supportive telephone calls for 6 months embedded in one year of customary care Standardisation: No detail provided Comparison group: Customary care and access to an intensive, interactive online education and support website |
| Outcomes | 1. Burden: Zarit Short Burden Scale, a 12‐item instrument that utilises a Likert scale 1‐5 rating of frequency (range 12 (never) to 60 (nearly always)), higher scores were more indicative of caregiver burden. 2. Depression: Beck Depression Inventory. The Beck Depression Inventory is a 21‐item Likert scale instrument with a total range of 0 to 63. Higher scores indicated increased endorsement of depressive symptoms. 3. Frequency of Patient Problematic Behavior: Frequency of Problematic Behaviors on the Revised Memory and Behavior Problem Checklist (a 24‐item instrument that measures the frequency of a behaviour on a 0‐4 Likert scale (range 0‐96, higher numbers indicated greater frequency of problematic behaviour). 4. Caregiver Negative Reactions to Problematic Behavioural Patterns: Negative Reactions Scale from the Revised Memory and Behavior Problem Checklist. The scale measures the caregiver's level of reaction to a series of potential problematic behaviours on a 0‐4 Likert scale; higher numbers indicated a greater degree of distress. The range is 0‐96. Data were collected at 6 months (end of intervention). |
| Notes | This study is complete and to be evaluated for inclusion in the next update of this review. Unclear as to what 'customary care' refers to. Not stated if project staff were health care professionals. More detail is required on the telephone arm, which is the comparator arm of this study. |
NCT00869739.
| Methods | Parallel randomised trial |
| Participants | Partners of African‐American Prostate Cancer survivors |
| Interventions | Title of the intervention: PA‐CST intervention Aim: To help African‐American prostate cancer survivors and their partners cope with challenges after surgery for early‐stage prostate cancer Interventionist(s): African‐American doctoral clinical psychologists Duration: 8 weeks (weekly for six weeks) Content:· Partner‐assisted coping skills training (PA‐CST): Survivor/partner dyads underwent a telephone‐based culturally sensitive PA‐CST intervention relating to knowledge about prostate cancer. Participants were trained in a variety of cognitive and behavioural skills to manage symptom‐related distress and to improve their quality of life after prostate cancer treatment. The skills included strategies for communication (i.e. effective speaking and listening); behavioural coping methods (i.e. activity pacing, applied relaxation techniques, and goal setting to increase pleasant activities); and skills for managing negative mood and reducing emotional stress. They also received guidance in working co‐operatively with their partners to improve symptom management, including joint practicing of coping skills and problem‐solving strategies. Standardisation: Not stated Comparison group: Wait‐list control: Survivor/partner dyads received usual care and were placed on a wait‐list. After completing the study, survivors and their partners had the option of participating in either the CST or cancer education interventions. |
| Outcomes | 1. Burden: Caregiver Strain Index (CSI) 2. Depression: Profile of Mood States‐SF (POMS‐SF) 3. Self‐efficacy: Partners' self‐efficacy for symptom control as assessed by the Self‐Efficacy for Symptom Control Inventory; EPIC; and CSI at baseline, 2 months, and 5 months 4. Relationship quality: Dyadic Adjustment Scale and the Miller Social Intimacy Scale at baseline, 2 months, and 5 months 5. Coping: a measure of coping strategies Outcomes measured at baseline, 2 months, and 5 months |
| Notes | Trial recruitment completed. Trial registration site last updated February 25, 2013. Need to determine that caregivers and care‐recipients received the intervention separately. |
NCT02152033.
| Methods | Randomised controlled trial |
| Participants | Parents of young adults leaving residential substance abuse treatment |
| Interventions | Title of the intervention: Home‐based Continuing Care (HCC) Aim: To help parents support the recovery of their Young Adult (YA) child who was leaving residential substance abuse treatment Interventionist(s): Masters or doctoral level therapists in social work or psychology Duration: Not specified (5 individual sessions and 1 joint session with their child, each session was 45‐50 min). Content: All sessions occurred over the phone or Cisco WebEx meetings. Parents participated in 5 individual sessions and 1 joint session with their child (45‐50 minutes each). Young Adults (YAs) participated in 1‐3 individual meetings (30‐45 minutes each) and 1 joint session (45‐50 minutes). In addition, YAs were contacted weekly for the first 8 weeks of HCC, then every other week for the remaining 24 weeks (20 calls total). He or she were asked questions addressing risk and protective factors for relapse. Finally, parents were trained to collect and test their child's urine sample and deliver incentives to the YA contingent upon biologically‐verified abstinence and verified engagement in continuing service plan activities. Urine samples were collected regularly over a 32‐week period. Standardisation: No detail provided Comparison group: Continuing service plan recommended by the residential treatment program. Parents were told to support this and were sent information on continuing care developed by the Treatment Research Institute and the Partnership @ Drugfree.org (continuingcare.drugfree.org). No supplemental services were provided during the study. Parents were trained to collect urine samples for research purposes only. Parents and YAs were offered separate 4‐hour workshops after they had completed participation as an added study participation incentive for this group. |
| Outcomes | 1. Satisfaction: Parent Happiness with Youth 2. Parent and Young Adult Treatment Retention 3. Parent and Young Adult Treatment: Treatment Evaluation Inventory 4. Parent and Young Adult Engagement in HCC by number of calls completed 5. Parent and Young Adult Recruitment Rate by monthly recruitment rate Data were collected week 16 and 32 |
| Notes | Author confirmed that the interventionists were healthcare professionals. Results have yet to be submitted for publication. Author stated almost all of the sessions were conducted by phone ‐ full detail of the intervention and its delivery needs to be assessed prior to inclusion in this review. |
NCT02215187.
| Methods | Parallel randomised trial |
| Participants | Caregivers of people with traumatic brain injury (TBI) Inclusion Criteria: age ≥ 19 years old, meets study project definition of a military caregiver, documentation or determination of an Operation Iraqi Freedom/Operation Enduring Freedom (OIF/OEF) deployment‐related TBI Service member will have presented to a Veterans Administration Medical Center (VAMC) or military medical centre, English‐speaking, has access to a telephone for the administration of measures and/or intervention calls, has no significant cognitive or communication problems that might significantly interfere with adequately understanding information or talking on the telephone which will be determined by the clinical judgment of the person consenting the participant. |
| Interventions | Title of the intervention: Problem‐Solving Training (PST) Aim: To evaluate the impact of a telehealth‐based, cognitive behavioural therapy (CBT) intervention (problem‐solving training: PST) for adult informal military family/friend caregivers of OIF/OEF service members with a deployment‐related TBI Interventionist(s): no details provided Duration: 6 weeks (one hour per week) Content: Problem‐Solving Training (PST) is a cognitive‐behavioural intervention. PST consists of education related to problem‐solving skills/problem‐solving model and application to caregiving and managing caregiver‐related problems. Standardisation: no details provided Comparison group: Sham Comparator: Attention control/social contact control. Health education (non‐skill focused). |
| Outcomes | Patient Health Questionnaire‐9 (PHQ‐9) Data collection time points are baseline and post‐program (3 months follow‐up). |
| Notes | The type of interventionist i.e. professional/non‐professional was not stated. The study is ongoing and the final data collection date for the primary outcome measure was September 2017. |
NCT02505425.
| Methods | Randomised controlled trial |
| Participants | Caregivers of people with heart failure |
| Interventions | Title of the intervention: ENABLE CHF‐PC Aim: The primary aim is to encourage participant empowerment; however, occasionally the nurse coaches may provide feedback directly to the HF teams (or palliative care teams) about specific issues (e.g. unrelieved pain) or make referrals to other resources Interventionist(s): Nurses Duration: 48 weeks or until patient death Content: This includes an in‐person comprehensive Palliative Care team (PCT) consultation as soon as possible after enrolment and a Palliative Care Nurse Coach (PNC) embedded within HF teams with phone based 4‐session caregiver manualised curriculum titled Charting Your Course (CYC), followed by monthly phone‐based supportive care. Standardisation: No detail provided Comparison group: Usual care, this includes any available supportive care resources and heart failure patient medical management based on national HF guidelines. |
| Outcomes | 1. Quality of life: Bakas Caregiving Outcomes Scale (BCOS) 2. Burden: Montgomery Borgatta Caregiver Burden Scale (MBCB) 3. Depression: the Hospital Anxiety and Depression Scale (HADS) 4. Health status: PROMIS SF Global Health Data were collected 8 and 16 weeks following baseline |
| Notes | Need to determine if caregivers received the intervention separate to the care‐recipients |
NCT03260608.
| Methods | Randomised controlled trial |
| Participants | Caregivers of patients with dementia |
| Interventions | Title of the intervention: Telesupport psychoeducation and support Aim: No detail provided Interventionist(s): No detail provided Duration: Eight weeks Content: In addition to usual primary health care, participants will receive psychoeducational guidelines and support in the management of their relatives with dementia. They will have access to a number of phones to spontaneously contact specific guidelines during the eight weeks of intervention. Standardisation: No detail provided Comparison group: Control group with usual follow‐up at primary health care |
| Outcomes | 1. Quality of life: WHOQoL 2. Burden: Zarit Burden Interview 3. Depression: Beck Depression Inventory 4. Anxiety: Beck Anxiety Inventory Data will be collected at week 9 and 18. |
| Notes | More detail is required on the guidelines that participants in the intervention group will have access to during the intervention. Interventionists need to be identified. |
BCOS: Bakas caregiver outcomes scale CAFU: Caregiver assessment of function and upset scale CAT: Computerised tomography scan CBT: Cognitive behavioural therapy CES‐D: Center for epidemiological depression scale CHF‐PC: Comprehensive heartcare for patients and caregivers CSI: Caregiver strain index CYC:Charting your course DCM: Dementia care management ENABLE: Educate, nurture, advise, before life ends, comprehensive heartcare for patients and caregivers EPIC: Expanded prostate cancer index composite scale FITT: Family Intervention: telephone tracking HADS: Hospital anxiety and depression scale HCC: Home‐based continuing care HF: Heart failure HIV: Human immunodeficiency virus MBCB: Montgomery Borgatta caregiver burden scale MRI: Magnetic resonance imaging PA‐CST: Partner‐assisted coping skills training PCT: Palliative care team PHQ‐9: Patient health questionnaire‐9 PNC: Palliative care nurse coach POMs‐SF:Profile of mood states‐ short form PROMIS SF: Patient reported outcomes measurement information system short form PST:Problem‐solving training OEF:Operation enduring freedom OIF:Operation Iraqi freedom SE: Self‐efficacy TAPES: Telephone‐assisted pleasant‐event scheduling TAP‐VA: Tailored activity program ‐ Veterans Affairs TAU: Treatment as usual TBI: Traumatic brain injury VA: Veterans Affairs VAMC:Veterans Administration Medical Center YA: Youth adult WHOQoL: World health organisation quality of life
Characteristics of ongoing studies [ordered by study ID]
Gitlin 2013.
| Trial name or title | A non‐pharmacologic approach to address challenging behaviours of veterans with dementia: description of the tailored activity program‐VA randomised trial |
| Methods | Randomised controlled trial |
| Participants | Caregivers of people with dementia |
| Interventions | Title of intervention: Telephone attention control Aim: The telephone component of the study is the attention control and serves three purposes: 1) creates clinical equipoise, ensuring that ethical treatment is provided to all study participants; 2) controls for the one‐on‐one attention to caregivers in the Tailored Activity treatment group to rule out potential effects of professional contact; and 3) serves as a retention tool to keep control group caregivers meaningfully connected to the trial. Interventionist(s): Trained healthcare professional Mode of delivery: Telephone Duration: Sixteen weeks (biweekly telephone contact (up to 8 contacts), each contact is approximately 30 minutes in length) Content: In each session, caregivers are provided with important information about dementia and strategies for managing the disease at home. Each telephone contact begins with a brief overview of the specific purpose of the session, followed by a description of the key facts about the session topic, and concludes with a question and answer period. Standardisation: Not stated Comparison group: The Tailored Activity Program – Veterans Administration (TAP‐VA) provides an assessment of the veterans home environment and provides caregivers with the requisite knowledge and skills to use activities. Caregivers are instructed in specific skills such as ways to simplify activities, the environment and their communication, and how to help the veteran initiate and follow a sequence. The overall goal is to provide predictability, familiarity, and structure in the daily life of the veteran and establish a level of environmental stimulation appropriate to that person’s abilities. |
| Outcomes | 1. Burden: Zarit Short Form Burden Scale 2. Depression: The Center for Epidemiologic Studies Depression Scale (CES‐D) 3. Cost: Cost‐effectiveness is measured as the cost of achieving one additional unit of benefit as defined by caregiver hours per day “doing things” and hours per day “being on duty.” |
| Starting date | August 2012 |
| Contact information | Laura N Gitlin, Johns Hopkins University, 525 N. Wolfe Street, Baltimore, MD 21205, USA Email: lgitlin1@jhu.edu |
| Notes | This is a protocol for a registered trial (ClinicalTrials.gov Identifier NCT01357564). The registered trial primary completion date was June 2016 with an estimated study completion date of August 2018. The ClinicalTrials.gov site indicates that the study is not yet recruiting. |
Gopinah 2017.
| Trial name or title | Implementing a multi‐modal support service model for the family caregivers of persons with age‐related macular degeneration: a study protocol for a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Caregivers of persons with late age‐related macular degeneration (AMD) |
| Interventions | Title: Mail‐delivered cognitive behavioural therapy (M‐CBT) and Telephone‐delivered group counselling sessions Aim: To empower family caregivers by improving their coping strategies, enhancing hopeful feelings such as self‐efficacy and helping them make the most of available sources of social and financial support. Interventionists: Practising dietician (confirmed by author via email on 22 November 2018) Mode of delivery: Telephone Duration: 10 weeks Content: The intervention group will receive a multi‐modal support service programme consisting of a brief mail‐delivered (M‐CBT) treatment delivered fortnightly as five individual modules and five Talk‐Link group counselling sessions. The Talk‐Link Counselling and M‐CBT will occur weekly on an alternating basis. M‐CBT of fortnightly modules formatted in Microsoft PowerPoint, with additional homework worksheets and accompanying templates for practising acquired skills, will be mailed to participants in the intervention group. Each module will target a specific stressor and/or train a new adaptive coping method and will be supported by targeted homework assignments for the caregiver to practice between sessions. Standardisation: Not stated Comparator group: Active wait‐list control group will receive reading materials concerning AMD and caring for persons diagnosed with the condition. All control participants will be offered the opportunity to receive the multicomponent intervention after the study ends (12–18 months after inclusion). |
| Outcomes | 1. Quality of life: EUROQoL ‐ EQ‐5D 5‐level scale 2. Burden: Caregiver Burden Scale (CBS) 3. Depression: Centre for Epidemiologic Studies Depression Scale. 4. Cost effectiveness of intervention: conducted from the perspective of the national health provider in Australia and will determine the cost per person to deliver the support service model and the cost‐effectiveness compared with usual care. Data on health‐related quality of life will be collected during 12 months post‐treatment using the EQ‐5D‐5L questionnaire. EQ‐5D scores will be converted into utility values using a valuation algorithm for the Australian population. Data will be collected at baseline and 12‐month follow‐up. |
| Starting date | 25 January 2017 |
| Contact information | Dr Bamini Gopinath; bamini.gopinath@sydney.edu.au |
| Notes | Dr. Gopinath confirmed via email on 22 November 2018 that the interventionist is a health care professional. |
Heckel 2015.
| Trial name or title | Acceptability and utility of a telephone outcall program for carers of persons diagnosed with cancer |
| Methods | Randomised trial |
| Participants | Caregivers of persons with cancer recruited from four Australian health services. One hundred and eight carer/person with cancer dyads were randomised to the intervention group and 108 to the control group. Participants who completed the study: 54% were female with the majority (81%) caring for their spouse/partner; mean age of carers was 58 years. All caregivers were 18 or over (confirmed via email by the author). |
| Interventions | Title of intervention: Telephone outcall program Aim: To evaluate acceptability and utility of a telephone outcall program to reduce burden and depression among carers of persons newly diagnosed with cancer Interventionist(s): Cancer Council Helpline nurse Mode of delivery: Telephone Duration: 3 months (carers received three telephone outcalls (mean call duration: 3 min) at three time points (7‐10 days after recruitment, 1 and 3 months later). Content: Carers were screened for distress using the Distress Thermometer (range: 0‐10) and given tailored information and support. Carers with a distress score of > 4 were referred to their GP for follow‐up. Standardisation: no details provided Comparison group: two comparison groups, sham outcalls and a usual support group. 1. Participants in the sham group received three sham outcalls (mean call duration: 22 min) at the same time points as the intervention group and were provided with the Cancer Council Helpline number to contact as needed. 2. Participants in the control arm who chose to contact the Cancer Council Helpline received usual support provided by Helpline nurses (not the outcall program). |
| Outcomes | Participants completed a utility survey one month post‐intervention. |
| Starting date | No details provided |
| Contact information | Leila Heckel, email: leila@deakin.edu.au |
| Notes | Abstract only. Author advised via email that data collection and analysis have been finalised and they are in the process of preparing a manuscript reporting on the main outcomes of the RCT for publication. |
Mavandadi 2017.
| Trial name or title | A randomised pilot trial of a telephone‐based collaborative care management program for caregivers of individuals with dementia |
| Methods | Randomised trial |
| Participants | Caregivers of older veterans with dementia |
| Interventions | Title of the intervention: Modified Telehealth Education Program (TEP) and the Behavioural Health Laboratory (BHL) Aim: To provide caregiver education and psychosocial support Interventionist(s): Care manager (nurse) Mode of delivery: Telephone Duration: Three months (minimum of 3 contacts) Content: Two components: An individualised dementia care manager (CM) provided regular and extended contact between the caregiver (CG), care manager, and when appropriate, the veteran’s primary care provider (PCP). The care manager monitored veterans’ symptoms via CG report and provided support to CGs. TEP was modified for use with individual CGs and was formatted so that CGs could select from a menu of up to seven modules in workbook format covering various content areas evaluated during the course of the CM assessments (e.g. communication skills, behavioural management techniques, stress management and coping skills, long‐term planning). They also received all material made available in the usual care arm. During the first contact, the care manager provided a general overview of the format, content, and goals of the TEP. In addition, the care manager reviewed the recommended subject areas and the CG and care manager collaboratively finalised the list of TEP modules to be covered throughout the course of the individualised program. CGs were permitted to choose as many or as few of the modules as they felt necessary. All CGs, however, were encouraged to participate in a minimum of two introductory sessions. These two sessions explained how symptoms of dementia differ from normal aging and how symptoms change over the course of the illness and introduced problem‐solving techniques. Sessions were scheduled depending upon the availability and preference of the CG. Even if the CG declined all modules, the care manager still contacted the CG for the individualised care management as described above. Standardisation: No detail provided Comparison group: Usual care (were mailed general material providing information about VA and community resources) |
| Outcomes | 1.Burden: Zarit Burden Interview 12‐item (range 0–48) 2. Bother or upset: Revised Memory and Behavior Problems Checklist RMBPC caregiver reaction subscale (range 0–96) 3.Distress Neuropsychiatric Inventory Questionnaire caregiver distress subscale (range 0–50) 4.Coping: Management of Meaning — Reduction of Expectations subscale of the Pearlin Caregiving and Stress Process Scale 3 items, range 3–12) 5. Mastery: Caregiving Mastery subscale of the Lawton Caregiving Appraisal (6‐item scale ranging from 6–30) Data were collected at baseline and at 3‐ and 6‐month follow‐ups. |
| Starting date | Study is complete. |
| Contact information | Shahrzad Mavandadi email: shahrzad.mavandadi@va.gov |
| Notes | Full paper published in 2017 following completion and submission of the review; to be evaluated for inclusion in the next update. |
Nasiriani 2017.
| Trial name or title | The effects of telephone counselling and education on breast cancer screening in family caregivers of breast cancer patients |
| Methods | Randomised trial |
| Participants | Caregivers of people with breast cancer |
| Interventions | Title of the intervention: Counselling and education intervention Aim: not stated Interventionist(s): MSc Nurse Mode of delivery: Telephone Duration: Not stated Content: Counselling and education according to the protocol about breast cancer screening in three phone calls of 60‐90 minutes for each call Standardisation: not stated Comparison group: control group (no intervention but received the counselling and education intervention after the study) |
| Outcomes | Structured questionnaire (demographics, breast cancer screening knowledge, breast cancer risk perception |
| Starting date | Start date not stated but data were collected between May and October 2011 |
| Contact information | far.farnia@yahoo.com |
| Notes | Study complete (results to be included in the review update) |
NCT00646074.
| Trial name or title | Self‐Care Talk Study ‐ promoting Alzheimer's Disease (AD) spousal caregiver health |
| Methods | A randomised controlled trial |
| Participants | Caregivers of persons with dementia |
| Interventions | Title of the intervention: Self‐Care TALK Aim: No detail provided Interventionist(s): Advanced practice nurses Mode of delivery: Telephone Duration: Six weeks Content: The intervention includes creating a health‐promoting, self‐care education and support partnership between caregivers and nurses through the use of weekly telephone conversations. Each conversation focuses on a health‐related topic. The conversations follow a basic format, but are also individualised. Standardisation: No detail provided Comparison group: No intervention; they received written materials related to self‐care and health promotion post‐time 3 (week 24). |
| Outcomes | 1. Physical health: SF‐36 v2, PCS (perceived physical health), SF‐36 v2, MCS (perceived mental health) 2. Burden: M‐CSI; modified (caregiver strain) 3. Self‐efficacy: SRAHP (self‐efficacy for health) 4. Depression: CES‐D Data were collected at baseline, 8 weeks (time 2), and 24 weeks (time 3) after baseline. |
| Starting date | July 2006 |
| Contact information | Cynthia Teel, PhD, RN |
| Notes | Trial registration website indicated that recruitment was complete. |
NCT02505737.
| Trial name or title | Telephone‐based counselling for depression in Parkinson's disease (TH‐CBT) |
| Methods | Parallel randomised trial |
| Participants | Caregiver: A family member or friend (care‐partner) of a person with Parkinson's Disease (PD). Age ranged from 35 to 85 years (confirmed by author via email). Care‐recipients: 35 to 85 years (adult, senior), all sexes eligible for the study, confirmed diagnosis of Parkinson's disease, clinically significant depressive symptoms (e.g. symptoms are pervasive, distressing, and make life harder), the presence of a formal depressive disorder will be determined by study staff based on standardised criteria, stable medication regimen ≥ 6 weeks, no change in mental health treatment in the past 2 months, family member or friend willing to participate, access to a telephone, live in the United States of America (USA). |
| Interventions | Title of the intervention: Telephone‐guided cognitive behavioural self‐help program (TH‐CBT) plus enhanced usual care Aim: To evaluate a 10‐session telephone‐guided cognitive behavioural self‐help program (TH‐CBT) for depression in PD (dPD). Interventionist(s): no details provided. Author confirmed they were licensed Clinical Psychologists and Masters level therapists. Mode of delivery: Telephone Duration: 3‐4 separate educational sessions (30‐60 minutes each), evenly dispersed throughout the 10‐week TH‐CBT treatment period. Content: TH‐CBT will be delivered to the participant with PD and works by teaching people with PD (PWP) the coping skills needed to manage their emotional reactions to the numerous challenges posed by the disease (specifically, the treatment targets maladaptive thought patterns (e.g. I have no control; I am helpless) and behaviours (e.g. social isolation, lack of exercise, poor sleep habits, excessive worry)), and, critically, provides caregivers with the tools needed to encourage the PWPs' practice of their newly acquired coping skills.The study treatment provided to the care‐partner will teach the care‐partner how to best support the participant with PD as he/she tries to incorporate the information learned during the study treatment, in day‐to‐day life. The care‐partner will be asked to participate in separate sessions. Standardisation: no details provided Comparison group: enhanced usual care (routine medical treatment with the provision of written educational materials for effective coping with PD, the close clinical monitoring of depressive symptoms by study staff, and the provision of counselling resources in the local community). Participants assigned to the control group with have the opportunity to receive the experimental intervention (TH‐CBT) after the data collection period (e.g. after the 6‐month follow‐up evaluation). |
| Outcomes | Caregiver distress inventory (confirmed by author via email) |
| Starting date | July 2015 |
| Contact information | Roseanne D Dobkin, PhD email: dobkinro@rwjms.rutgers.edu |
| Notes | Roseanne Dobkin contacted and confirmed that no data are available to share for this review. |
NCT02806583.
| Trial name or title | Talking Time: telephone support groups for informal caregivers of people with dementia |
| Methods | Cluster‐adjusted randomised controlled trial |
| Participants | Caregivers are eligible if they are 18 or older, have cared for the PwD for at least four hours on four days per week in the last six months, have access to a telephone connection for participation in the intervention and study evaluation. Exclusion criteria are lack of knowledge of the German language of informal caregiver, risk of suicide in the informal caregiver, actual psychiatric diagnosis of mental illness of the caregiver and ICD‐10 diagnosis of 'dementia in other diseases classified elsewhere', except dementia in primary Parkinson disease and Lewy Body disease. |
| Interventions | Aim: The Talking Time project aims to close the supply gap (i.e. caregivers ability to attend on‐site support groups) by providing structured telephone‐based support groups in Germany for the first time. Interventionist(s): no details provided Mode of delivery: Telephone Duration: Three months Title of intervention: Telephone‐based structured support groups Content:Telephone‐based Support Groups, information booklet, and telephone‐based preparatory meeting prior to the telephone‐based support groups Standardisation: no details provided Comparison group: Usual care (intervention as experimental group after T1 data collection (end of intervention, after 3 months). |
| Outcomes | 1. Well‐being: Subjective well‐being using the Mental Component Summary of the General Health Questionaires Short Form 12 (SF‐12), psychological quality of life of the caregivers 2. Physical Component Summary of the SF‐12, physical quality of life of the caregivers 3. Social support: Perceived Social Support Caregiving Scale, perceived social support of the caregivers 4. Burden: Caregiver Reaction Scale, caregivers burden Data were collected at baseline and T1 (end of intervention, the 3‐month time point). |
| Starting date | November 2015 |
| Contact information | Martin Berwig, Dr. University of Leipzig email: martin.berwig@medizin.uni‐leipzig.de |
| Notes | Dr. Berwig has confirmed that the trial is at the data analysis stage. |
Soellner 2015.
| Trial name or title | The Tele.TAnDem intervention: study protocol for a psychotherapeutic intervention for family caregivers of people with dementia |
| Methods | Non‐blinded two‐armed parallel RCT |
| Participants | Caregiving partners, children and children‐in‐law who have key responsibility for the relative with at least a low grade dementia diagnosis. Caregivers are excluded if they are in receipt of ongoing psychotherapeutic treatment, have a severe physical illness/medically diagnosed psychiatric disorder or the person with dementia is institutionalised or institutionalisation is planned for the next 6 months. |
| Interventions | Aim: The primary objective was to evaluate whether telephone‐based cognitive‐behavioural therapy (TEL) improves depressiveness, burden of care, health complaints, and problem‐solving ability compared to usual care. Interventionist(s): Psychotherapists Mode of delivery: Telephone Duration: 6 months (weekly for 4 weeks, two‐weekly for 6 further sessions and monthly for the last two sessions) Title of intervention: Cognitive‐behavioural telephone‐based intervention for family caregivers of people with dementia. Content: The intervention is based on the principles and methods used in cognitive behavioural therapy. Therapy strategies were adapted for caregivers of people with dementia. The intervention which is standardised and manualised consists of 10 different therapy modules individualised by the therapist to the needs of each participant. Standardisation: according to the manual Comparison group: usual care |
| Outcomes | 1. Quality of life using the WHOQoL‐BREF, a standardised and normed questionnaire with 26 items measuring subjective physical and mental well‐being as well as satisfaction with social relations and the environment 2. Burden: A self‐developed thermometer scale (0‐100, vertical) 3. Depression: A self‐developed thermometer scale (0‐100, vertical) and the Allgemeine Depressionsskala (ADS). ADS consists of 20 item covering emotional, motivational, cognitive, and somatic aspects. 4. Physical health: complaints assessed on four domains (fatigue, stomach problems, heart problems, and joint pain) by using the Gießener Beschwerdebogen instrument. 5. Problem‐solving using the Goal Attainment Scaling, a non‐standardised manual‐based instrument providing process‐orientated information on how far participants are from reaching individual therapy goals 6. Anxiety: The anxiety subscale of the Hospital Anxiety and Depression Scale 7. Cost: Cost‐effectiveness analysis will be conducted from the perspective of statutory health insurance with a time horizon of 6 months. This consisted of the costs of the intervention and of the health care utilisation of the caregiving relatives. The latter were assessed by the FIMA questionnaire. Time spent on informal care was measured by a modified version of the Resource Utilisation in Dementia (RUD) questionnaire. Effectiveness was measured using the subjectively rated health status of caregiving relatives and quality of life, measured through the EQ‐5D. Data were collected at T1, end of intervention (6‐month time point) and T2, 12 months (the 6‐month follow‐up time point). |
| Starting date | Not stated |
| Contact information | Renate Soellner email:soellner@uni‐hildesheim.de |
| Notes | Findings for this study were not published at the time of our search. |
Wilz 2018.
| Trial name or title | The Tele.TAnDem Intervention: telephone‐based CBT for family caregivers of people with dementia |
| Methods | Randomised trial |
| Participants | Family caregivers of people with dementia |
| Interventions | Title of the intervention: CBT‐based telephone intervention Aim: To improve caregiver depressiveness, burden of care, health complaints, and problem‐solving Interventionist(s): Psychotherapists Mode of delivery: Telephone Duration: Six months (12 50‐minute therapy sessions, weekly first 4 sessions, biweekly for 6 sessions, monthly for the two last sessions) Content: Cognitive behaviour therapy consisting of 10 different therapy modules which could be used and combined by the therapist according to the individual needs of the participant Standardisation: Standardised and manual‐based Comparison group: Usual care group. Received written information on dementia and caregiving |
| Outcomes | 1. Burden: A self‐developed thermometer scale (0‐100, vertical) 2. Depression: The German Version of the Center for Epidemiologic Studies Depression Scale 3. Emotional well‐being: Visual analogue scale (0‐100) 4. Physical health: The four domains (fatigue, stomach problems, heart problems, and joint pain) by using the Gießener Beschwerdebogen instrument 5. Coping: Coping with burden of care (single item rating scale 0‐4); coping with challenging behaviour (single item from the German Version of BEHAVE‐AD rating scale 0‐4). Data were collected at T1, end of intervention (6‐month time point) and T2, 12 months (6‐month follow‐up). |
| Starting date | Not stated |
| Contact information | Gabriele Wilz email: gabriele.wilz@uni‐jena.de |
| Notes | Draws on the work of Soellner 2015; the design has been changed. Full paper published in 2018 following completion and submission of the review; to be evaluated for inclusion in the next update of this review. |
AD: Alzheimer's disease ADS: Allgemeine Depressionsskala AMD: Age‐related macular degeneration BEHAVE‐AD: Behavioral pathology in alzheimer’s disease rating scale BHL: Behavioural health laboratory CBS: Caregiver burden scale CES‐D: Center for epidemiological depression scale CM: care manager dPD: Depression in Parkinson's disease EQ‐5D: EuroQol health related quality of life ‐ 5 dimensions EQ‐5D‐5L: EuroQol health related quality of life ‐ 5 dimensions ‐5 levels EUROQoL: EuroQol health related quality of life FIMA: Questionnaire for the use of medical and non‐medical services in old age GP: General practitioner ICD‐10: International statistical classification of disease ‐ 10 M‐CBT: Mail‐delivered cognitive behavioural therapy MCS: Mental component summary M‐CSI: Modified caregiver strain index MSc: Master of science PCP: Primary care provider PD: Parkinson's disease PwD: People with dementia PWP: Persons with Parkinson's RMBPC: Revised memory and behavior problems checklist RUD: Resource utilisation in dementia SF‐12: Short Form ‐ 12 SF‐36 v2: Short Form ‐36 version 2 SRAHP: Self‐rated abilities for health practices scale TALK: Definition unable to be found, may not be an acronym TAP‐VA: Tailored activity program – veterans administration TEL: Telephone‐based cognitive‐behavioural therapy Tele.TAnDem: Telephone‐based short‐term intervention for family caregivers of people with dementia TEP: Telehealth education program TH‐CBT: Telephone‐based cognitive behavioural therapy T1: Time 1 T2: Time 2 VA: Veteran Affairs WHOQoL‐BREF: World Health Organisation Quality of Life Abbreviated Version
Differences between protocol and review
The title has been amended slightly to read "Telephone interventions, delivered by healthcare professionals, for providing education and psychosocial support for informal caregivers of adults with diagnosed illnesses". Our approach to data analysis and the absence of planned subgroup analysis due to insufficient data differs from our published protocol. The outcome 'social activity' has been re‐categorised from the taxonomy 'Skill Acquisition' to 'Health status and well‐being'. Furthermore, while we specified in our protocol that we would consider blinding separately for different outcomes, where appropriate (for example, blinding may have the potential to affect differently subjective versus objective outcome measures), as all our included outcomes were assessed using a variety of scales that could be considered subjective measures (i.e. caregiver‐reported), we assessed all outcomes equally for blinding of outcome assessment. Greg Sheaf, librarian in the Library of Trinity College Dublin, Dublin, Ireland joined the review team.
Contributions of authors
Margarita Corry (MC) co‐ordinated the review, wrote the first draft, contributed to subsequent drafts and prepared the final draft for submission. All other authors ‐ Valerie Smith (VS), Kathleen Neenan (KN) and Sally Brabyn (SB) ‐ contributed to the drafting of the review and reviewed it for intellectual content. Margarita Corry is the review's guarantor.
VS provided overall guidance and support to MC and acted as adjudicator on aspects of the review where there was disagreement or uncertainty amongst the other authors. She independently checked the outcome data entered into RevMan against the data extraction sheets and helped with data analysis and write‐up of the review.
KN and SB assisted with study selection, data extraction, and drafting of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland.
Awarded a Cochrane fellowship to MC for the conduct of this systematic review
Declarations of interest
Margarita Corry: I have completed a pilot feasibility study of a telephone support intervention for caregivers of people with multiple sclerosis using the RCT design. I was not involved in assessing the study for inclusion, extracting, or analysing data from that study.
Valerie Smith: none known
Kathleen Neenan: none known
Sally Brabyn: none known
New
References
References to studies included in this review
Bishop 2014 {published data only (unpublished sought but not used)}
- Bishop D, Miller I, Weiner D, Guilmette T, Mukand J, Feldmann E, et al. Family Intervention: Telephone Tracking (FITT): a pilot stroke outcome study. Topics in Stroke Rehabilitation 2014;21(Suppl 1):S63‐74. [DOI] [PubMed] [Google Scholar]
- Miller IW, Bishop DS, Epstein‐Lubow G. Reduction in post‐stroke depressive symptoms among patients and caregivers: the FITT study. American Journal of Geriatric Psychiatry 2010;18(3 SUPPL.):S61‐S62.. [Google Scholar]
Connell 2009 {published and unpublished data}
- Connell CM, Janevic MR. Effects of a telephone‐based exercise intervention for dementia caregiving wives: a randomized controlled trial. Journal of Applied Gerontology 2009;28(2):171‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janevic MR, Connell CM. Effects of a Telephone‐Based Exercise Intervention among Female Spouse Caregivers of People with Dementia [PhD thesis]. Vol. 3057974, Michigan: Ann Arbor University of Michigan, 2002. [Google Scholar]
- Janevic MR, Connell CM. Exploring self‐care among dementia caregivers: the role of perceived support in accomplishing exercise goals. Journal of Women & Aging 2004;16(1‐2):71‐86. [DOI] [PubMed] [Google Scholar]
Corry 2015 {published and unpublished data}
- Corry, M. An Exploratory Randomised Trial of a Nurse‐Led Telephone Support Intervention for People who support People with Multiple Sclerosis (The MNTISS trial) [PhD thesis]. Vol. 1 and 2, Dublin, Ireland: University of Dublin, Trinity College, 2015. [Google Scholar]
- NCT01302431. Nurse‐led manualized telephone support intervention. ClinicalTrials.gov/show/NCT01302431 (first received 24 February 2011). [NCT01302431]
Davis 2011 {published and unpublished data}
- Davis JD, Tremont G, Bishop DS, Fortinsky RH. A telephone‐delivered psychosocial intervention improves dementia caregiver adjustment following nursing home placement. International Journal of Geriatric Psychiatry 2011;26(4):380‐7. [DOI] [PubMed] [Google Scholar]
Gallagher‐Thompson 2007 {published and unpublished data}
- Gallagher‐Thompson D, Gray HL, Tang PCY, Pu C‐Y, Leung Laurie YL, Wang P‐C, et al. Impact of in‐home behavioral management versus telephone support to reduce depressive symptoms and perceived stress in Chinese caregivers: results of a pilot study. American Journal of Geriatric Psychiatry 2007;15(5):425‐34. [DOI] [PubMed] [Google Scholar]
Glueckauf 2012 {published and unpublished data}
- Glueckauf RL, Davis W, Shuford W, Floyd SD, Gustafson DJ, Hayes J, et al. Telephone‐based, cognitive‐behavioral therapy for African American dementia caregivers with depression: initial findings. Rehabilitation Psychology 2012;57(2):124‐39. [DOI] [PubMed] [Google Scholar]
- NCT00769769. Comparing delivery methods of cognitive behavioral therapy for depressed African‐American dementia caregivers. ClinicalTrials.gov/show/NCT00769769 (first received 9 October 2008). [NCT00769769]
Kwok 2013 {published data only}
- Kwok T, Wong B, Ip I, Chui K, Young D, Ho F. Telephone‐delivered psychoeducational intervention for Hong Kong Chinese dementia caregivers: a single‐blinded randomized controlled trial. Clinical Interventions in Aging 2013;8:1191‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martindale‐Adams 2013 {published and unpublished data}
- Martindale‐Adams J, Nichols LO, Burns R, Graney MJ, Zuber J. A trial of dementia caregiver telephone support. Canadian Journal of Nursing Research 2013;45(4):30‐48. [DOI] [PubMed] [Google Scholar]
- NCT00119561. Testing the effectiveness of telephone support for dementia caregivers (CONNECT). ClinicalTrials.gov/show/NCT00119561 (first received 13 July 2005). [NCT00119561]
- Nichols LO, Martindale‐Adams JL, Burns R, Graney MJ. The use of telephone support groups to increase free time for dementia caregivers. HSRD National Meeting, 2008. www.hsrd.research.va.gov/meetings/2008/display_abstract.cfm?RecordID=336.
NCT00646217 {published data only}
- NCT00646217. Self care TALK study ‐ promoting stroke caregiver health. ClinicalTrials.gov/show/NCT00646217 (first received 28 March 2008). [NCT00646217]
Pfeiffer 2014 {published and unpublished data}
- Pfeiffer K, Beische D, Hautzinger M, Berry JW, Wengert J, Hoffrichter R, et al. Telephone‐based problem‐solving intervention for family caregivers of stroke survivors: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2014;82(4):628‐43. [DOI] [PubMed] [Google Scholar]
Piamjariyakul 2015 {published and unpublished data}
- Piamjariyakul U, Werkowitch M, Wick J, Russell C, Vacek JL, Smith C. Caregiver coaching program effect: reducing heart failure patient rehospitalizations and improving caregiver outcomes among African Americans. Heart & Lung 2015;44(6):466‐73. [DOI] [PubMed] [Google Scholar]
Powell 2014 {published and unpublished data}
- NCT00692575. Telephone intervention for caregivers of persons with traumatic brain injury. ClinicalTrials.gov//show/NCT00692575 (first received 6 June 2008). [NCT00692575]
- Powell JM, Fraser R, Ann J, Brockway A, Temkin N, Bell K. A telehealth approach to improving outcomes for caregivers of adults with traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2014;95(10):e61. [Google Scholar]
- Powell JM, Fraser R, Brockway JA, Temkin N, Bell KR. A telehealth approach to caregiver self‐management following traumatic brain injury: a randomized controlled trial. Journal of Head Trauma Rehabilitation 2016;31(3):180‐90. [DOI] [PubMed] [Google Scholar]
- Powell JM, Wise EK, Brockway JA, Fraser R, Temkin N, Bell KR. Characteristics and concerns of caregivers of adults with traumatic brain injury. Journal of Head Trauma Rehabilitation 2017;32(1):E33‐41. [DOI] [PubMed] [Google Scholar]
Shaw 2016 {published and unpublished data}
- Shaw JM, Young JM, Butow PN, Badgery‐Parker T, Durcinoska I, Harrison JD, et al. Improving psychosocial outcomes for caregivers of people with poor prognosis gastrointestinal cancers: a randomized controlled trial (Family Connect). Supportive Care in Cancer 2016;24(2):585‐95. [DOI] [PubMed] [Google Scholar]
Shum 2014 {published and unpublished data}
- NCT01519895. Effectiveness of telephone intervention for colorectal cancer caregivers. ClinicalTrials.gov/show/NCT01519895 (first received 27 January 2012). [NCT01519895]
- Shum NF, Lui YL, Law WL, Fong YTD. A nurse‐led psycho‐education programme for Chinese carers of patients with colorectal cancer. Cancer Nursing Practice 2014;13(5):31‐9. [Google Scholar]
Smith and Toseland 2006 {published data only}
- Smith TL, Toseland RW. The effectiveness of a telephone support program for caregivers of frail older adults. Gerontologist 2006;46(5):620‐9. [DOI] [PubMed] [Google Scholar]
Toye 2016 {published and unpublished data}
- Toye C, Parsons R, Slatyer S, Aoun SM, Moorin R, Osseiran‐Moisson R, et al. Outcomes for family carers of a nurse‐delivered hospital discharge intervention for older people (the Further Enabling Care at Home Program): single blind randomised controlled trial. International Journal of Nursing Studies 2016;64:32‐41. [DOI] [PubMed] [Google Scholar]
Tremont 2008a {published and unpublished data}
- Tremont G, Davis JD. Bishop DS, Fortinsky RH. Telephone‐delivered psychosocial intervention reduces burden in dementia caregivers. Dementia 2008;7(4):503‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vazquez 2016 {published and unpublished data}
- NCT02292394. Brief telephone psychological intervention for depressive symptoms in caregivers (RCDS). ClinicalTrials.gov Identifier/show/NCT02292394 (first received 17 November 2014).
- Vázquez FL, Torres Á, Otero P, Blanco V, Díaz O, Estévez LE. Analysis of the components of a cognitive‐behavioral intervention administered via conference call for preventing depression among non‐professional caregivers: a pilot study. Aging & Mental Health 2016;21(9):938‐46. [DOI] [PubMed] [Google Scholar]
Wilz 2016a {published and unpublished data}
- Schinköthe D, Wilz G. The assessment of treatment integrity in a cognitive behavioral telephone intervention study with dementia caregivers. Clinical Gerontologist 2014;37(3):211‐34. [Google Scholar]
- Wilz G, Meichsner F, Soellner R. Are psychotherapeutic effects on family caregivers of people with dementia sustainable? Two‐year long‐term effects of a telephone‐based cognitive behavioral intervention. Aging & Mental Health 2017;21:774‐81. [DOI] [PubMed] [Google Scholar]
- Wilz G, Soellner R. Evaluation of a short‐term telephone‐based cognitive behavioral intervention for dementia family caregivers. Clinical Gerontologist 2016;39(1):25‐47. [DOI: 10.1080/07317115.2015.1101631] [DOI] [Google Scholar]
Winter 2006 {published data only}
- Winter L, Gitlin LN. Evaluation of a telephone‐based support group intervention for female caregivers of community‐dwelling individuals with dementia. American Journal of Alzheimer's Disease and Other Dementias 2006;21(6):391‐7. [DOI] [PubMed] [Google Scholar]
Wray 2010 {published data only}
- NCT00105638. Telehealth education program for caregivers of veterans with dementia. ClinicalTrials.gov/show/NCT00105638 (first submitted 17 March 2005). [NCT00105638]
- Wray LO, Shulan MD, Toseland RW, Freeman KE, Vasquez BE, Gao J. The effect of telephone support groups on costs of care for veterans with dementia. Gerontologist 2010; Vol. 50, issue 5:623‐31. [NCT00105683] [DOI] [PubMed]
References to studies excluded from this review
Achie 2015 {published data only}
- Achey MA, Beck CA, Beran DB, Boyd CM, Schmidt PN, Willis AW, et al. A randomized controlled trial of telemedicine for Parkinson's disease (Connect.Parkinson) in the United States: interim assessment of investigator and participant experiences. Movement Disorders 2015;30:S64. [Google Scholar]
ACTRN12616000467437 {published data only}
- ACTRN12616000467437. Further enabling care at home for people living with dementia. www.anzctr.org.au/actrn12616000467437.aspx (first received 18 March 2016). [ACTRN12616000467437]
Aguirrezabal 2013 {published data only}
- Aguirrezabal A, Duarte E, Rueda N, Cervantes C, Marco E, Escalada F. Effects of information and training provision in satisfaction of patients and carers in stroke rehabilitation. Neuro Rehabiliation 2013;33(4):639‐47. [DOI] [PubMed] [Google Scholar]
Badger 2007 {published data only}
- Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nursing Research 2007;56(1):44‐53. [DOI] [PubMed] [Google Scholar]
Badr 2015 {published data only}
- Badr H, Smith CB, Goldstein NE, Gomez JE, Redd WH. Dyadic psychosocial intervention for advanced lung cancer patients and their family caregivers: results of a randomized pilot trial. Cancer 2015;121(1):150‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 1997 {published data only}
- Bailey DA, Mion LC. Improving care givers' satisfaction with information received during hospitalization. Journal of Nursing Administration 1997;27(1):21‐7. [DOI] [PubMed] [Google Scholar]
Bakas 2009a {published and unpublished data}
- Bakas T, Austin JK, Buelow JM, Habermann B, Li Y, McLennon SM, et al. Preliminary efficacy of a stroke caregiver intervention program for reducing depressive symptoms. Stroke 2010;41(4):e357. [Google Scholar]
- Bakas T, Farran CJ, Austin JK, Given BA, Johnson EA, Williams LS. Stroke caregiver outcomes from the Telephone Assessment and Skill‐building Kit (TASK). Topics in Stroke Rehabilitation 2009;16(2):105‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, Li Y, Habermann B, McLennon S, Weaver MT. Developing a cost template for a nurse‐led stroke caregiver intervention program. Clinical Nurse Specialist 2011;25(1):41‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00264745. Telephone assessment and skill‐building kit (TASK): a new program for family caregivers of stroke survivors. Clinicaltrials.gov/show/NCT00264745 (first received 13 December 2005). [00264745]]
Bakas 2015 {published and unpublished data}
- Bakas T, Austin JK, Habermann B, Jessup NM, McLennon SM, Mitchell PH, et al. Telephone assessment and skill‐building kit for stroke caregivers: a randomized controlled clinical trial. Stroke 2015;46(12):3478‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, Austin JK, Habermann B, Jessup NM, McLennon SM, Mitchell PH, et al. Telephone assessment and skill‐building kit for stroke caregivers: a randomized controlled clinical trial. Stroke 2015; Vol. 46, issue 12:3478‐87. [DOI] [PMC free article] [PubMed]
- Bakas T, Farran CJ, Austin JK, Given BA, Johnson EA, Williams LS. Content validity and satisfaction with a stroke caregiver intervention program. Journal of Nursing Scholarship 2009;41(4):368‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, Jessup NM, McLennon SM, Habermann B, Weaver MT, Morrison G. Tracking patterns of needs during a telephone follow‐up programme for family caregivers of persons with stroke. Disability and Rehabilitation 2016;27(18):1780‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakas T, McLennon SM, Miller ET. Satisfaction with the stroke caregiver telephone assessment and skill‐building kit. Stroke. American Heart Association/American Stroke Association; 2017. 2017; Vol. 48:A22‐A22.
- NCT01275495. Telephone assessment and skill‐building intervention for stroke caregivers (TASKII). ClinicalTrials.gov Identifier/show/NCT01275495 (first received 12 January 2011). [01275495]
Barclay 2016 {published data only}
- Barclay TR, Forsberg AC, Thomas AJ, Crow JM, Haley‐Franklin H, Hanson LR. Outcomes of a dementia resource and education program imbedded in a health care system. Alzheimer's and Dementia 2016;12 Suppl 7:605. [Google Scholar]
Bauman 2015 {published data only}
- Bauman JR, Schleicher S, Nipp RD, El‐Jawahri A, Pirl WF, Greer JA, et al. Feasibility of a pilot study of an intervention to enhance communication during hospice care with oncology (ECHO). Journal of Clinical Oncology 2015;33(29 SUPPL. 1):51. [Google Scholar]
Bauman 2018 {published data only}
- Bauman JR, Schleicher SM, Nipp R, El‐Jawahri A, Pirl WF, Greer JA, et al. Enhancing communication between oncology care providers and patient caregivers during hospice. Journal of Community and Supportive Oncology 2018;16:e72‐e80. [Google Scholar]
Bell 2005 {published data only}
- Bell KR, Temkin NR, Esselman PC, Doctor JN, Bombardier CH, Fraser RT, et al. The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Archives of Physical Medicine and Rehabilitation 2005;86(5):851‐6. [DOI] [PubMed] [Google Scholar]
Belle 2006 {published data only}
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher‐Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Annals of Internal Medicine 2006;145(10):727‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AF, Burgio LD, Decoster J. Enhancing caregiver health: findings from the resources for enhancing Alzheimer's caregiver health II intervention. Journal of the American Geriatrics Society 2010;58(1):30‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LO, Chang C, Lummus A, Burns R, Martindale‐Adams J, Graney MJ, et al. The cost‐effectiveness of a behavior intervention with caregivers of patients with Alzheimer's disease. Journal of the American Geriatrics Society 2008;56(3):413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berwig 2017 {published data only}
- Berwig M, Heinrich S, Spahlholz J, Hallensleben N, Brähler E, Gertz HJ. Individualized support for informal caregivers of people with dementia – effectiveness of the German adaptation of REACH II. BMC Geriatrics 2017;17(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenthal 2009 {published data only}
- Blumenthal JA, Keefe FJ, Babyak MA, Fenwick C, Virginia J, Julie M, et al. Caregiver‐assisted coping skills training for patients with COPD: background, design, and methodological issues for the INSPIRE‐II study. Clinical Trials 2009;6(2):172‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00736268. Coping skills for patients with chronic obstructive pulmonary disease (COPD) and their caregivers. ClinicalTrials.gov/show/NCT00736268 (first received 15 August 2008). [NCT00736268]
Brown 1999 {published data only}
- Brown R, Pain K, Berwald C, Hirschi P, Delehanty R, Miller H. Distance education and caregiver support groups: comparison of traditional and telephone groups. Journal of Head Trauma Rehabilitation 1999;14(3):257‐68. [DOI] [PubMed] [Google Scholar]
Callahan 2006 {published data only}
- Callanan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA 2006;295(18):2148‐57. [DOI] [PubMed] [Google Scholar]
Chambers 2014 {published data only}
- Chambers S, Girgis A, Occhipinti S, Hutchison S, Turner J, Carter R, et al. Improving the psychosocial health of people with cancer and their carers: a community‐based approach. Psycho‐Oncology 2010;19(Suppl 2):S193‐S194.. [Google Scholar]
- Chambers SK, Girgis A, Occhipinti S, Hutchison S, Turner J, Carter R, et al. Beating the blues after cancer: randomised controlled trial of a tele‐based psychological intervention for high distress patients and carers. BMC Cancer 2009;9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SK, Girgis A, Occhipinti S, Hutchison S, Turner J, McDowell M, et al. A randomized trial comparing two low‐intensity psychological interventions for distressed patients with cancer and their caregivers. Oncology Nursing Forum 2014;41(4):E256‐66. [DOI] [PubMed] [Google Scholar]
- Chambers SK, Girgis A, Occhipinti S, Hutchison S, Turner J, McDowell M, et al. Randomized controlled trial of psychological intervention for high distress cancer patients and carers. Psycho‐Oncology 2014;23:47‐8. [Google Scholar]
- Chambers SK, Girgis A, Occhipinti S, Turner J, Carter R, Dunn J. Matching treatment intensity to need: preliminary findings from a community based randomized trial of tele‐based psychological intervention for high distress patient and carers. Asia‐Pacific Journal of Clinical Oncology 2012;8:185. [Google Scholar]
Chang 2004 {published data only}
- Chang BL, Nitta S, Carter PA, Markham YK. Perceived helpfulness of telephone calls ‐ providing support for caregivers of family members with dementia. Journal of Gerontological Nursing 2004;30(9):14‐21. [DOI] [PubMed] [Google Scholar]
Chodosh 2015 {published data only}
- Chodosh J, Colaiaco BA, Connor KI, Cope DW, Liu H, Ganz DA, et al. Dementia care management in an underserved community: the comparative effectiveness of two different approaches. Journal of Aging and Health 2015;27(5):864‐93. [DOI] [PubMed] [Google Scholar]
Cox 2012 {published data only}
- Cox CE, Porter LS, Hough CL, White DB, Kahn JM, Carson SS, et al. Development and preliminary evaluation of a telephone‐based coping skills training intervention for survivors of acute lung injury and their informal caregivers. Intensive Care Medicine 2012;38(8):1289‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czaja 2013 {published data only}
- Czaja SJ, Loewenstein D, Schulz R, Nair SN, Perdomo DA. Videophone psychosocial intervention for dementia caregivers. American Journal of Geriatric Psychiatry 2013;21(11):1071‐81. [DOI] [PubMed] [Google Scholar]
Dellasega 2002 {published data only}
- Dellasega C, Zerbe TM. Caregivers of frail rural older adults. Effects of an advanced practice nursing intervention. Journal of Gerontological Nursing 2002;28(10):40‐9. [DOI] [PubMed] [Google Scholar]
Demiris 2011 {published data only}
- Demiris G, Oliver DP, Wittenberg‐Lyles E, Washington K. Use of videophones to deliver a cognitive‐behavioural therapy to hospice caregivers. Journal of Telemedicine and Telecare 2011;17(3):142‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demiris 2012 {published data only}
- Demiris G, Parker OD, Wittenberg‐Lyles E, Washington K, Doorenbos A, Rue T, et al. A non‐inferiority trial of a problem‐solving intervention for hospice caregivers: in person versus videophone. Journal of Palliative Medicine 2012;15(6):653‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 2017 {published data only}
- Duncan PW, Bushnell CD, Rosamond WD, Berkeley SB, Gesell SB, D’Agostino RB, et al. The COMprehensive post‐acute stroke services (COMPASS) study: design and methods for a cluster‐randomized pragmatic trial. BMC Neurology 2017;17(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 2009 {published data only}
- Elliott TR, Berry JW, Grant JS. Problem‐solving training for family caregivers of women with disabilities: a randomized clinical trial. Behaviour Research and Therapy 2009;47(7):548‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erten‐Lyons 2017 {published data only}
- Erten‐Lyons D, Lindauer A, Mincks K, Silbert L, Lyons B, Dodge H, et al. Alzheimer’s Care via Telemedicine for OregoN (ACT‐ON), phase II: a pilot study of to‐the‐home dementia care via telemedicine. Neurology 2017;88(16):5‐081. [Google Scholar]
Finkel 2007a {published data only}
- Finkel S, Czaja S, Sara J, Schulz R, Martinovich Z. E‐Care: a telecommunications technology intervention for family caregivers of dementia patients. American Journal of Geriatric Psychiatry 2007;15(5):443‐8. [DOI] [PubMed] [Google Scholar]
Gant 2007a {published data only}
- Gant JR, Steffen AM, Lauderdale SA. Comparative outcomes of two distance‐based interventions for male caregivers of family members with dementia. American Journal of Alzheimer's Disease and other Dementias 2007;22(2):120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garand 2002 {published data only}
- Garand L, Buckwalter KC, Lubaroff D, Tripp‐Reimer T, Frantz RA, Ansley TNA. A pilot study of immune and mood outcomes of a community‐based intervention for dementia caregivers: the PLST intervention. Archives of Psychiatric Nursing 2002;16(4):156‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaugler 2008 {published data only}
- Gaugler JE, Roth DL, Haley WE, Mittelman MS. Can counselling and support reduce burden and depressive symptoms in caregivers of people with Alzheimer's disease during the transition to institutionalization? Results from the New York University caregiver intervention study. Journal of the American Geriatrics Society 2008;56(3):421‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilliss 1992 {published data only}
- Gilliss CL, Belza BL. A framework for understanding family caregivers' recovery work after cardiac surgery. Family & Community Health 1992;15(2):41‐8. [Google Scholar]
Gitlin 2003 {published data only}
- Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill‐building program on the caregiver‐care recipient dyad: 6‐month outcomes from the Philadelphia REACH Initiative. Gerontologist 2003;43(4):532‐46. [DOI] [PubMed] [Google Scholar]
Gitlin 2010 {published data only}
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. Journal of the American Geriatrics Society 2010;58(8):1465‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2010a {published data only}
- Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WWA. Biobehavioral home‐based intervention and the well‐being of patients with dementia and their caregivers: the COPE randomized trial. JAMA 2010;304(9):983‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gitlin 2016 {published data only}
- Gitlin LN, Piersol CV, Hodgson N, Marx K, Roth DL, Johnston D, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: design and methods of a randomized clinical trial. Contemporary Clinical Trials 2016;49:92‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonyea 2016 {published data only}
- Gonyea JG, Lopez LM, Velasquez EH. The effectiveness of a culturally sensitive cognitive behavioral group intervention for Latino Alzheimer's caregivers. Gerontologist 2016;56(2):292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham‐Philips 2016 {published data only}
- Graham‐Phillips A, Roth DL, Huang J, Dilworth‐Anderson P, Gitlin LN. Racial and ethnic differences in the delivery of the resources for enhancing Alzheimer's caregiver health II intervention. Journal of the American Geriatrics Society 2016;64.(8):1662‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 1999 {published data only}
- Grant JS. Social problem‐solving partnerships with family caregivers. Rehabilitation Nursing 1999;24(6):254‐60. [DOI] [PubMed] [Google Scholar]
Grant 2002 {published data only}
- Grant JS, Elliott TR, Weaver M, Bartolucci AA, Giger JN. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke 2002;33(8):2060‐5. [DOI] [PubMed] [Google Scholar]
Greaves 2016 {published data only}
- Greaves CJ, Wingham J, Deighan C, Doherty P, Elliott J, Armitage W. Optimising self‐care support for people with heart failure and their caregivers: development of the Rehabilitation Enablement in Chronic Heart Failure (REACH‐HF) intervention using intervention mapping. Pilot and Feasibility Studies 2016;2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasan 2015 {published data only}
- Hasan AA, Callaghan P, Lymn JS. Evaluation of the impact of a psycho‐educational intervention for people diagnosed with schizophrenia and their primary caregivers in Jordan: a randomized controlled trial. BMC Psychiatry 2015;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hicken 2017 {published data only}
- Hicken BL, Daniel C, Luptak M, Grant M, Kilian S, Rupper RW. Supporting caregivers of rural veterans electronically (SCORE). Journal of Rural Health 2017;33(3):305‐13. [DOI] [PubMed] [Google Scholar]
Hirsch 2014 {published data only}
- Hirsch O, Leyh J, Karch C, Ferlings R, Schafer D. Impact of a training program for caregivers of neurological patients on depression, prostration, and subjective burden. Journal of Neuroscience Nursing 2014;46(2):97‐105. [DOI] [PubMed] [Google Scholar]
Hori 2009 {published data only}
- Hori M, Kubota M, Ando K, Kihara T, Takahashi R, Kinoshita A. The effect of videophone communication (with skype and webcam) for elderly patients with dementia and their caregivers. Cancer & Chemotherapy 2009;36(Suppl 1):36‐8. [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang H‐L, Kuo L‐M, Chen Y‐S, Liang J, Huang H‐L, Chiu Y‐C, et al. A home‐based training program improves caregivers' skills and dementia patients' aggressive behaviors: a randomized controlled trial. American Journal of Geriatric Psychiatry 2013;21(11):1060‐70. [DOI] [PubMed] [Google Scholar]
Hudson 2015 {published data only}
- Hudson P, Trauer T, Kelly B, O'Connor M, Thomas K, Zordan R, et al. Reducing the psychological distress of family caregivers of home based palliative care patients: longer term effects from a randomised controlled trial. Psycho‐Oncology 2015;24(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2018 {published data only}
- Johnson AM, Jones SB, Duncan PW, Bushnell CD, Coleman SW, Mettam LH, et al. Hospital recruitment for a pragmatic cluster‐randomized clinical trial: lessons learned from the COMPASS study. Trials 2018;19(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kozachik 2001 {published data only}
- Kozachik SL, Given CW, Given BA, Pierce SJ, Azzouz F, Rawl SM, et al. Improving depressive symptoms among caregivers of patients with cancer: results of a randomized clinical trial. Oncology Nursing Forum 2001;28(7):1149‐57. [PubMed] [Google Scholar]
Kuo 2017 {published data only}
- Kuo L‐M, Huang H‐L, Liang J, Kwok Y‐T, Hsu W‐C, Su P‐L, et al. A randomized controlled trial of a home‐based training programme to decrease depression in family caregivers of persons with dementia. Journal of Advanced Nursing 2017;73(3):585‐98. [DOI] [PubMed] [Google Scholar]
Kwok 2012 {published data only}
- Kwok T, Lam L, Chung J. Case management to improve quality of life of older people with early dementia and to reduce caregiver burden. Hong Kong Medical Journal 2012;18 Suppl 6:4‐6. [PubMed] [Google Scholar]
Lindauer 2016 {published data only}
- Lindauer A, Seelye A, Lyons B, Mincks K, Mattek N, Gregor M, et al. Alzheimer's care via telemedicine for Oregon (ACT‐ON): phase i ‐ establishing the feasibility and reliability of standard measures used with telemedicine. Alzheimer's and Dementia 2016;12(7 (Suppl)):149‐150. [Google Scholar]
Linton 2018 {published data only}
- Linton KF, Kim BJ. A pilot study of Trabajadora de Salud, a lay health worker intervention for Latinas/os with traumatic brain injuries and their caregivers. Disability and Health Journal 2018;11(1):161‐4. [DOI] [PubMed] [Google Scholar]
Livingston 2013 {published data only}
- Heckel L, Fennell KM, Orellana L, Boltong A, Byrnes M, Livingston PM. A telephone outcall program to support caregivers of people diagnosed with cancer: utility, changes in levels of distress, and unmet needs. Supportive Care in Cancer 2018;15(1):1. [DOI] [PubMed] [Google Scholar]
- Heckel L, Fennell KM, Reynolds J, Boltong A, Botti M, Osborne RH, et al. Efficacy of a telephone outcall program to reduce caregiver burden among caregivers of cancer patients [PROTECT]: a randomised controlled trial. BMC Cancer 2018;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston P, Osborne R, Botti M, Chirgwin J, Mihalopoulos C, Mcguigan S, et al. Efficacy and cost‐effectiveness of a telephone outcall program to reduce caregiver burden and depression among carers of cancer patients [PROTECT]. Rationale and design of a multi‐centre randomised controlled trial. Cancer care coming of age. Clinical Oncology Society of Australia's 40th Annual Scientific Meeting 2013. Australia: John Wiley & Sons, 2013:159.
- Livingston PM, Osborne RH, Botti M, Mihalopoulos C, McGuigan S, Heckel L, et al. Efficacy and cost‐effectiveness of an outcall program to reduce carer burden and depression among carers of cancer patients [PROTECT]: rationale and design of a randomized controlled trial. BMC Health Services Research 2014;14(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston T, Osborne R, Botti M, Chirgwin J, Mihalopoulos C, McGuigan S, et al. Efficacy and cost‐effectiveness of a telephone outcall program to reduce carer burden and depression among carers of cancer patients [PROTECT]. Rationale and design of a multi‐state, multi‐centre randomised controlled trial. Asia‐Pacific Journal of Clinical Oncology 2013;9(Meeting Abstracts):159. [Google Scholar]
Martín‐Carrasco 2009 {published data only}
- Martín‐Carrasco M, Martín MF, Valero CP, Millán PR, García CI, Montalbán SR, et al. Effectiveness of a psychoeducational intervention program in the reduction of caregiver burden in Alzheimer's disease patients' caregivers. International Journal of Geriatric Psychiatry 2009;24(5):489‐99. [DOI] [PubMed] [Google Scholar]
- NCT00119561. Testing the effectiveness of telephone support for dementia caregivers. ClinicalTrials.gov/show/NCT00119561 (first received 13 July 2005). [NCT00119561]
Mazanec 2017 {published data only}
- Mazanec SR, Miano S, Baer L, Campagnaro EL, Sattar A, Daly BJ. A family‐centered intervention for the transition to living with multiple myeloma as a chronic illness: a pilot study. Applied Nursing Research 2017;35:86‐9. [DOI] [PubMed] [Google Scholar]
McCann 2015 {published data only}
- McCann TV, Songprakun W, Stephenson J. Effectiveness of guided self‐help in decreasing expressed emotion in family caregivers of people diagnosed with depression in Thailand: a randomised controlled trial. BMC Psychiatry 2015;15:258. [DOI: 10.1186/s12888-015-0654-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCann 2015a {published data only}
- McCann TV, Songprakun W, Stephenson J. A randomized controlled trial of guided self‐help for improving the experience of caring for carers of clients with depression. Journal of Advanced Nursing 2015;7(17):1600‐10. [DOI] [PubMed] [Google Scholar]
McLennon 2016 {published data only}
- McLennon SM, Hancock RD, Redelman K, Scarton LJ, Riley E, Sweeney B, et al. Comparing treatment fidelity between study arms of a randomized controlled clinical trial for stroke family caregivers. Clinical Rehabilitation 2016;30(5):495‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendyk 2018 {published data only}
- Mendyk AM, Duhamel A, Bejot Y, Leys D, Derex L, Dereeper O, et al. Controlled Education Of Patients after Stroke (CEOPS) ‐ nurse‐led multimodal and long‐term interventional program involving a patient’s caregiver to optimize secondary prevention of stroke: study protocol for a randomized controlled trial. Trials 2018;19(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2015 {published data only}
- Morgan RO, Bass DM, Judge KS, Liu CF, Wilson N, Snow AL, et al. A break‐even analysis for dementia care collaboration: partners in dementia care. Journal of General Internal Medicine 2015;30(6):804‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosher 2018 {published data only}
- Mosher CE, Secinti E, Johns SA, O’Neil BH, Helft PR, Shahda S, et al. Examining the effect of peer helping in a coping skills intervention: a randomized controlled trial for advanced gastrointestinal cancer patients and their family caregivers. Quality of Life Research 2018;27(2):515‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00052104 {published data only}
- NCT00052104. Telephone‐based support for caregivers of patients With dementia. ClinicalTrials.gov/show/NCT00052104 (first received 23 January 2003). [NCT00052104]
- Tremont G, Davisa JD, Papandonatosc GD, Ottd BR, Fortinsky RH, Gozalog P, et al. Psychosocial telephone intervention for dementia caregivers: a randomized, controlled trial. Alzheimer’s & Dementia 2014;11(5):5411‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00067171 {published data only}
- NCT00067171. Telephone enhancement procedure for continuing care. ClinicalTrials.gov/show/NCT00067171 (first received 13 August 2005). [NCT00067171]
NCT00131092 {published data only}
- NCT00131092. The CHAT (Community Health Advice by Telephone) study. ClinicalTrials.gov/show/NCT00131092 (first received 17 August 2005). [NCT00131092]
NCT00247000 {published data only}
- NCT00247000. A strategy of home telehealth for management of congestive heart failure(STARTEL). ClinicalTrials.gov/show/NCT00247000 (first received 1 November 2005). [NCT00247000]
NCT00271739 {published data only}
- NCT00271739. Randomized trial of telemedicine for diabetes care (IDEATel). ClinicalTrials.gov Identifier/show/NCT00271739 (first received 4 January 2006). [NCT00271739]
NCT00288132 {published data only}
- NCT00288132. The diabetes TeleCare study. ClinicalTrials.gov/show/NCT00288132 (first received 7 February 2006). [NCT00288132]
NCT00483522 {published data only}
- NCT00483522. Department of Education telephone intervention after traumatic brain injury. ClinicalTrials.gov/show/NCT00483522 (first received 7 June 2007). [NCT00483522]
NCT00693563 {published data only}
- NCT00693563. Scheduled telephone intervention for individuals with spinal cord injury and their families. ClinicalTrials.gov/show/NCT00693563 (first received 9 June 2008). [NCT00693563]
NCT00721383 {published data only}
- NCT00721383. Telephone resources and assistance for caregivers (TRAC). ClinicalTrials.gov/show/NCT00721383 (first received 24 July 2008). [NCT00721383]
NCT00822510 {published data only}
- NCT00822510. Telephone counseling: men with prostate cancer & partners. ClinicalTrials.gov/show/NCT00822510 (first received 14 January 2009). [NCT00822510]
NCT00829361 {published data only}
- NCT00829361. Stroke telemedicine for Arizona rural residents trial. clinicaltrials.gov/show/NCT00829361 (first received 27 January 2009). [NCT00829361]
NCT01993550 {published data only}
- Mosher CE, Winger JG, Hanna N, Jalal SI, Einhorn LH, Birdas TJ, et al. Randomized pilot trial of a telephone symptom management intervention for symptomatic lung cancer patients and their family caregivers. Journal of Pain and Symptom Management 2016;52(4):469‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01993550. Telephone support program for lung cancer patients and their family caregivers. clinicaltrials.gov/ct2/show/record/NCT01993550 (first received 11 November 2013). [NCT01993550]
NCT02036294 {published data only}
- NCT02036294. Improving patient‐centered care delivery among patients with chronic obstructive pulmonary disease. Clinicaltrials.gov/show/NCTt02036294 (first received 15 January 2014).
NCT02094846 {published data only}
- NCT02094846. Evaluation of an intervention based on cell phones. ClinicalTrials.gov/show/NCT02094846 (first received 24 February 2013). [NCT02094846]
NCT02347202 {published data only}
- NCT02347202. Tools for distance delivery of an evidence‐based AD family caregiver intervention. Clinicaltrials.gov/show/NCT02347202 (first received 27 January 2015).
NCT02364505 {published data only}
- NCT02364505. A telemedicine mindfulness‐based intervention for people with multiple sclerosis and their caregivers. clinicaltrials.gov/show/NCT02364505 (first received 18 February 2015). [NCT02364505]
NCT02475954 {published data only}
- NCT02475954. Telehealth cognitive behavioral therapy for depression in Parkinson's Disease (PD) (TH‐CBT). ClinicalTrials.gov/show/NCT02475954 (first received 19 June 2015). [NCT02475954]
NCT02483494 {published data only}
- NCT02483494. Effectiveness of advanced practice nurse‐led telehealth on readmissions. ClinicalTrials.gov/show/NCT02483494 (first received 29 June 2015). [NCT02483494]
NCT02703532 {published data only}
- NCT02703532. Evaluation of a care partner‐integrated telehealth rehabilitation program for persons with stroke. ClinicalTrials.gov/show/NCT02703532 (first received 9 March 2016). [NCT02703532]
NCT03127930 {published data only}
- NCT03127930. Improving caregiver mediated medication management ‐ the 3M study. Clinicaltrials.gov/show/NCTt03127930: (first received 25 April 2017).
NCT03142841 {published data only}
- NCT03142841. Spanish intervention for caregivers of veterans with stroke (RESCUE Espanol). ClinicalTrials.gov Identifier/show/NCT03142841 (first received 5 May 2017). [NCT03142841]
NCT03164239 {published data only}
- NCT03164239. Aktiv i Sør: a randomized controlled trial. ClinicalTrials.gov/show/NCT03164239 (first received 23 May 2017). [NCT03164239]
NCT03177447 {published data only}
- Bakitas M, Dionne‐Odom JN, Pamboukian SV, Tallaj J, Kvale E, Swetz KM, et al. Engaging patients and families to create a feasible clinical trial integrating palliative and heart failure care: results of the ENABLE CHF‐PC pilot clinical trial. BMC Palliative Care 2017;16(1):45. [DOI: 10.1186/s12904-017-0226-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03177447. ENABLE: CHF‐PC (Comprehensive Heartcare For Patients and Caregivers). ClinicalTrials.gov Identifier: NCT03177447 (first received 6th June 2017).
NCT03378050 {published data only}
- NCT03378050. A trial of a multi‐component individualized telephone‐based support intervention for adult‐child caregivers caring for parents with dementia. ClinicalTrials.gov IdentifierNCT03378050 (first received 19 December 2017).
NCT03506945 {published data only}
- NCT03506945. Mobile web‐based behavioral intervention for improving caregiver well‐being. Clinicaltrials.gov/show/NCT03506945 (first received 24 April 2018).
NCT03635151 {published data only}
- NCT03635151. Caregiver self‐management needs through skill‐building. Clinicaltrials.gov/show/NCT03635151 (first received 17 August 2018).
Nichols 2011 {published data only}
- Nichols LO, Martindale‐Adams J, Burns R, Graney MJ, Zuber J. Translation of a dementia caregiver support program in a health care system ‐ REACH VA. Archives of Internal Medicine 2011;171(4):353‐9. [DOI] [PubMed] [Google Scholar]
Nobili 2004 {published data only}
- Nobili A, Riva E, Tettamanti M, Lucca U, Liscio M, Petrucci B, et al. The effect of a structured intervention on caregivers of patients with dementia and problem behaviors: a randomized controlled pilot study. Alzheimer's Disease and Associated Disorders 2004;18(2):75‐82. [DOI] [PubMed] [Google Scholar]
Penner 2016 {published data only}
- Penner JL, Sabiston CM, Ducharme F, Cohen SR. Home‐based physical activity for family caregivers of people with advanced cancer: a pilot randomized controlled trial. Journal of Pain and Symptom Management 2016;52(6):e89‐e90. [Google Scholar]
Piamjariyakul 2012 {published data only}
- Piamjariyakul U, Smith CE, Russell C. Translation and evaluation of evidence‐based telephone coaching program to improve heart failure (HF) self‐management. Clinical and Translational Science 2012;5(2):173. [Google Scholar]
Piamjariyakul 2013 {published data only}
- Piamjariyakul U, Smith CE, Russell C, Werkowitch M, Elyachar A. The feasibility of a telephone coaching program on heart failure home management for family caregivers. Heart & Lung 2013;42(1):32‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piette 2015 {published data only}
- Piette JD, Striplin D, Marinec N, Chen J, Aikens JEA. Randomized trial of mobile health support for heart failure patients and their informal caregivers. Medical Care 2015;53(8):692‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirrraglia 2005 {published data only}
- Pirraglia PA, Bishop D, Herman DS, Trisvan E, Lopez RA, Torgersen CS, et al. Caregiver burden and depression among informal caregivers of HIV‐infected individuals. Journal of General Internal Medicine 2005;1(20):510‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 2011 {published data only}
- Porter LS, Keefe FJ, Garst J, Baucom DH, McBride CM, McKee DC, et al. Caregiver‐assisted coping skills training for lung cancer: results of a randomized clinical trial. Journal of Pain and Symptom Management 2011;41(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prick 2015 {published data only}
- Prick A‐E, Lange J, Twisk J, Pot AM. The effects of a multi‐component dyadic intervention on the psychological distress of family caregivers providing care to people with dementia: a randomized controlled trial. International Psychogeriatrics 2015;27(12):2031‐44. [DOI] [PubMed] [Google Scholar]
Radziewicz 2009 {published data only}
- Radziewicz RM, Rose JH, Bowman KF, Berila RA, O'Toole EE, Given B. Establishing treatment fidelity in a coping and communication support telephone intervention for aging patients with advanced cancer and their family caregivers. Cancer Nursing 2009;32(3):193‐202. [DOI] [PubMed] [Google Scholar]
Reeves 2018 {published data only}
- Reeves M. A randomized trial of a social worker led home‐based case management to improve outcomes for caregivers of acute stroke patients during the transition period. Stroke 2018;3(1 (Supplement 1)):125. [Google Scholar]
- Reeves MJ, Hughes AK, Woodward AT, Freddolino PP, Coursaris CK, Swierenga SJ, et al. Improving transitions in acute stroke patients discharged to home: the Michigan stroke transitions trial (MISTT) protocol. BMC Neurology 2017;17(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2007 {published data only}
- Richardson A, Plant H, Moore S, Medina J, Cornwall A, Ream E. Developing supportive care for family members of people with lung cancer: a feasibility study. Supportive Care in Cancer 2007;15(11):1259‐69. [DOI] [PubMed] [Google Scholar]
Rivera 2008 {published data only}
- Rivera PA, Elliott TR, Berry JW, Grant JS. Problem‐solving training for family caregivers of persons with traumatic brain injuries: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2008;89(5):931‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samus 2014 {published data only}
- Samus QM, Johnston D, Black BS, Hess E, Lyman, C, Vavilikolanu A, et al. A multidimensional home‐based care coordination intervention for elders with memory disorders: the maximizing independence at home (MIND) pilot randomized trial. American Journal of Geriatric Psychiatry 2014;22(4):398‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schinköthe 2014 {published data only}
- Schinköthe D, Wilz G. The assessment of treatment integrity in a cognitive behavioral telephone intervention study with dementia caregivers. Clinical Gerontologist 2014;37(3):211‐34. [Google Scholar]
Schure 2006 {published data only}
- Schure LM, Heuvel ETP, Stewart RE, Sanderman R, Witte LP, Meyboom‐de Jong B. Beyond stroke: description and evaluation of an effective intervention to support family caregivers of stroke patients. Patient Education and Counseling 2006;62(1):46‐55. [DOI] [PubMed] [Google Scholar]
Schwarz 2008 {published data only}
- Schwarz KA, Mion LC, Hudock D, Litman G. Telemonitoring of heart failure patients and their caregivers: a pilot randomized controlled trial. Progress in Cardiovascular Nursing 2008;23(1):18‐26. [DOI] [PubMed] [Google Scholar]
Shanley 2008 {published data only}
- Shanley C. Supporting family carers through telephone‐mediated group programs: opportunities for gerontological social workers. Journal of Gerontological Social Work 2008;51(3/4):199‐209. [PubMed] [Google Scholar]
Sherrod 2013 {published data only}
- Sherrod AM, Wells NL, Dietrich MS, Murphy BA. Examining caregiver perceptions and distress related to patient pain. American Society of Clinical Oncology 2013;31(15 Suppl 1):e20555. [Google Scholar]
Sherwood 2012 {published data only}
- Sherwood PR, Given BA, Given CW, Sikorskii A, You M, Prince J. The impact of a problem‐solving intervention on increasing caregiver assistance and improving caregiver health. Supportive Care in Cancer 2012;20(9):1937‐47. [DOI] [PubMed] [Google Scholar]
Silveira 2016 {published data only}
- Silveira M. Cancer care partners: can family caregivers help cancer patients to manage their symptoms?. Journal of Pain and Symptom Management 2016;51(2):330‐1. [Google Scholar]
Sneed 1997 {published data only}
- Sneed NV, Finch NJ, Michel Y. The effect of psychosocial nursing intervention on the mood state of patients with implantable cardioverter defibrillators and their caregivers. Progress in Cardiovascular Nursing 1997;12(2):4‐14. [PubMed] [Google Scholar]
Stewart 2001 {published data only}
- Stewart MJ, Hart G, Mann K, Jackson S, Langille L, Reidy M. Telephone support group intervention for persons with hemophilia and HIV/AIDS and family caregivers. International Journal of Nursing Studies 2001;38(2):209‐25. [DOI] [PubMed] [Google Scholar]
Teel 2005 {published data only}
- Teel CS, Leenerts MH. Developing and testing a self‐care intervention for older adults in caregiving roles. Nursing Research 2005;54(3):193‐201. [DOI] [PubMed] [Google Scholar]
Tompkins 2009 {published data only}
- Tompkins SA, Bell PA. Examination of a psychoeducational intervention and a respite grant in relieving psychosocial stressors associated with being an Alzheimer's caregiver. Journal of Gerontological Social Work 2009;52(2):89‐104. [DOI] [PubMed] [Google Scholar]
Tremont 2014 {published data only}
- Tremont G, Davis J, Bryant K, Ott BR, Papandonatos G, Fortinsky R, et al. Effect of a telephone‐based dementia caregiver intervention on use of community support services and health care resources. Alzheimer's & Dementia 2014;10:226‐7. [Google Scholar]
- Tremont G, Davis J, Grover C, Bryant K, Ott B, Papandonatos G, et al. Randomized controlled trial of a telephone‐delivered intervention (FITT‐Caregiver) for dementia caregivers. Alzheimer's and Dementia 2013;9(4 Suppl 1):324‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tremont 2015 {published data only}
- Tremont G, Davis JD, Papandonatos GD, Ott BR, Fortinsky RH, Gozalo P, et al. Psychosocial telephone intervention for dementia caregivers: a randomized, controlled trial. Alzheimer's & Dementia 2015;11(5):541‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tremont 2017 {published data only}
- NCT00735800. Telephone support for dementia caregivers. ClinicalTrials.gov Identifier/show/NCT00735800 (first received 15 August 2008). [00735800]
- Tremont G, Davis J, Papandonatos GD, Grover C, Ott BR, Fortinsky RH, et al. A telephone intervention for dementia caregivers: background, design, and baseline characteristics. Contemporary Clinical Trials 2013;36(2):338‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremont G, Davis JD, Ott BR, Galioto R, Crook C, Papandonatos GD, et al. Randomized trial of the family intervention: telephone tracking‐caregiver for dementia caregivers: use of community and healthcare resources. Journal of the American Geriatrics Society 2017;65(5):924‐30. [DOI: 10.1111/jgs.14684] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2005 {published data only}
- Tsai C‐Y, Li IC, Hou S‐Y. A project to raise the participation rate in education groups by psychiatric patient family members. Journal of Nursing 2005;52(6):30‐9. [PubMed] [Google Scholar]
Uphold 2014 {published data only}
- Uphold CR. Utilizing the RESCUE stroke caregiver website to enhance discharge planning. VA Health Services Research & Development 2014.
Uphold 2015 {published data only}
- Uphold CR, Dang S, Jordan M, Freytes IM, Rutter T, Ruiz D, et al. Pilot test for a low‐cost, telephone and online intervention for caregivers of veterans post‐stroke. VA Health Services Research & Development Annual Conference; 2015; Philadelphia, PA. 2015.
Valeberg 2013 {published data only}
- Valeberg BT, Kolstad E, Smastuen MC, Miaskowski C, Rustoen T. The PRO‐SELF pain control program improves family caregivers' knowledge of cancer pain management. Cancer Nursing 2013;36(6):429‐35. [DOI] [PubMed] [Google Scholar]
Van Knippenberg 2016 {published data only}
- Knippenberg RJM, Vugt ME, Ponds RW, Myin‐Germeys I, Verhey FRJ. Dealing with daily challenges in dementia (DEAL‐ID study): effectiveness of the experience sampling method intervention 'Partner in Sight' for spousal caregivers of people with dementia: design of a randomized controlled trial. BMC Psychiatry 2016;16:136. [DOI: 10.1186/s12888-016-0834-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Mierlo 2012a {published data only}
- Mierlo LD, Meiland FJM, Droes R‐M. Dementia coach: effect of telephone coaching on carers of community‐dwelling people with dementia. International Psychogeriatrics 2012;24(2):212‐22. [DOI] [PubMed] [Google Scholar]
Wilder ongoing {published data only}
- Wilder VS, Roudebush RL. Telephone assessment and skill‐building intervention for informal caregivers. www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141702708 (accessed 18 November 2018).
Williams 2010 {published data only}
- Williams VP, Bishop‐Fitzpatrick L, Lane JD, Gwyther LP, Ballard EL, Vendittelli AP, et al. Video‐based coping skills to reduce health risk and improve psychological and physical well‐being in Alzheimer's disease family caregivers. Psychosomatic Medicine 2010;72(9):897‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamada 2011 {published data only}
- Yamada H, Aoki K, Nishida M, Takechi H. The effects of psychosocial intervention to family caregivers of patients with memory impairment: a randomized controlled trial. Alzheimer's and Dementia 2011;7(4 Suppl 1):S436. [Google Scholar]
Yan 2016 {published data only}
- Yan LL, Chen S, Zhou BO, Zhang J, Xie B, Luo R, et al. A randomized controlled trial on rehabilitation through caregiver‐delivered nurse‐organized service programs for disabled stroke patients in rural china (the RECOVER trial): design and rationale. International Journal of Stroke 2016;11(7):823‐30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Au 2014 {published data only}
- Au A, Wong MK, Leung LM, Leung P, Wong A. Telephone‐assisted pleasant‐event scheduling to enhance well‐being of caregivers of people with dementia: a randomised controlled trial. Hong Kong Medical Journal 2014;20(3 Suppl 3):30‐3. [PubMed] [Google Scholar]
Bass 2017a {published data only}
- Bass DM, Benjamin Rose Institute. Care consultation telephone‐based empowerment intervention. www.rosalynncarter.org/care_consultation_telephone‐based_empowerment_intervention/ (accessed 10 December 2018).
Chodosh 2015a {published data only}
- Chodosh J, Connor K, Vassar S, Pearson M, Lee M, Mittman B, et al. Implementing dementia care management in a medicare managed care plan: a randomized controlled trial. Journal of the American Geriatrics Society 2015;63:S1. [Google Scholar]
Chwalisz 2017 {published data only}
- Chwalisz K, Dollinger CS. Southern Illinois rural caregiver telehealth project. www.rosalynncarter.org/caregiver_intervention_database/rural_caregiving/southern_illinois_rural_caregiver_telehealth_project/ (accessed 10 December 2018).
Gitlin 2018 {published data only}
- Gitlin LN, Arthur P, Piersol C, Hessels V, Wu SS, Dai Y, et al. Targeting behavioral symptoms and functional decline in dementia: a randomized clinical trial. Journal of the American Geriatrics Society 2018;66(2):339‐45. [DOI] [PubMed] [Google Scholar]
Mavandadi {published data only}
- Mavandadi S, Crescenz MJ. Comparative effectiveness of delivery methods for caregiver support and education. www.hsrd.research.va.gov/research/abstracts.cfm?Project_ID=2141704171 (accessed 10 November 2018).
NCT00031265 {published and unpublished data}
- NCT00031265. Efficacy of a family telephone intervention for stroke. ClinicalTrials.gov/show/NCT00031265 (first received 1 March 2002). [NCT00031265]
NCT00183781 {published data only}
- NCT00183781. Effectiveness of a telephone intervention program in improving depression, coping, and family functioning in HIV‐infected individuals and caregivers. ClinicalTrials.gov Identifier/show/NCT00183781 (first received 16 September 2005). [NCT00183781]
NCT00416078 {published data only}
- NCT00416078. Online caregiver psychoeducation and support for Alzheimer's. Clinicaltrials.gov/show/NCT00416078 (first received 27 December 2006). [NCT00416078]
NCT00869739 {published data only}
- NCT00869739. Helping African American prostate cancer survivors and their partners cope with challenges after surgery for prostate cancer. Clinical trails.gov/show/NCT00869739 (first received 26 March 2009).
NCT02152033 {published data only}
- NCT02152033. Home‐based continuing care for young adults leaving residential substance abuse treatment. Clinicaltrials.gov/show/NCT02152033 (first received 2 June 2014). [NCT02152033]
NCT02215187 {published data only}
- NCT02215187. Telehealth‐education‐based program for military caregivers of injured service members with head injuries. ClinicalTrials.gov/show/NCT02215187 (first received 13 August 2014). [NCT02215187;]
NCT02505425 {published data only}
- NCT02505425. ENABLE CHF‐PC (Comprehensive heartcare for patients and caregivers). ClinicalTrials.gov Identifier: NCT02505425 (first received 22 July 2015).
- Wells R, Stockdill ML, Dionne‐Odom JN, Ejem D, Burgio KL, Durant RW, et al. Educate, nurture, advise, before life ends comprehensive heartcare for patients and caregivers (ENABLE CHF‐PC): study protocol for a randomized controlled trial. Trials 2018;19(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03260608 {published data only}
- NCT03260608. Effectiveness of a psychoeducation and support protocol by phone in the aid of caregivers of patients with dementia. ClinicalTrials.gov Identifier: NCT03260608 (first received 24 August 2017).
References to ongoing studies
Gitlin 2013 {published data only}
- Gitlin LN, Mann WC, Vogel WB, Arthur PB. A non‐pharmacologic approach to address challenging behaviors of veterans with dementia: description of the tailored activity program ‐ VA randomized trial. BMC Geriatrics 2013;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01357564. Tailored Activity Program ‐ VA [Telehealth education program for caregivers of veterans with dementia]. ClinicalTrials.gov/show/NCT01357564 (first received 20 May 2011).
Gopinah 2017 {published and unpublished data}
- ACTRN12616001461482. Caring for the carer’: implementing a comprehensive support service model for family caregivers looking after persons with age‐related macular degeneration [.]. www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12616001461482 (first received 19 October 2016).
- Gopinath B, Craig A, Kifley A, Liew G, Bloffwitch J, Vu K, et al. Implementing a multi‐modal support service model for the family caregivers of persons with age‐related macular degeneration: a study protocol for a randomised controlled trial. BMJ Open 2017;7(8):e018204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heckel 2015 {published data only}
- Heckel L, Fennell KM, Botti M, Reynolds J, Chirgwin J, Ashley DM, et al. Acceptability and utility of a telephone outcall program for carers of persons diagnosed with cancer. Asia‐Pacific Journal of Clinical Oncology 2015;11:156. [Google Scholar]
Mavandadi 2017 {published data only}
- Mavandadi S, Wright EM, Graydon MM, Oslin DW, Wray LO. A randomised pilot trial of a telephone‐based collaborative care management program for caregivers of individuals with dementia. Psychological Services 2017;14(1):102. [DOI] [PubMed] [Google Scholar]
Nasiriani 2017 {published data only}
- Nasiriani K, Motevasselian M, Farnia F, Shiryazdi SM, Khodayarian M. The effect of telephone counseling and education on breast cancer screening in family caregivers of breast cancer patients. International Journal of Community Based Nursing and Midwifery 2017;5(4):306. [PMC free article] [PubMed] [Google Scholar]
NCT00646074 {published data only}
- NCT00646074. Self ‐ care TALK study ‐ promoting Alzheimer's disease (AD) spousal caregiver health. ClinicalTrials.gov/show/NCT00646074 (first received 28 March 2008). [NCT00646074]
NCT02505737 {published data only}
- NCT02505737. Telephone ‐ based counseling for depression in Parkinson's Disease (TH‐CBT). Clinical trials.gov/show/NCT02505737 (first received 22 July 2015).
NCT02806583 {published data only}
- Berwig M, Dichter MN, Albers B, Wermke K, Trutschel D, Seismann‐Petersen S, et al. Feasibility and effectiveness of a telephone‐based social support intervention for informal caregivers of people with dementia: study protocol of the TALKING TIME project. BMC Health Services Research 2017;17:280. [DOI: 10.1186/s12913-017-2231-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02806583. Diseases talking time: telephone support groups for Informal caregivers of people with dementia. ClinicalTrials.gov/show/NCT02806583 (first received 20 June 2016). [NCT02806583;]
Soellner 2015 {published data only}
- DRKS00006355. Telephone‐based psychotherapy for family‐caregivers of people with dementia ‐ practice transfer of a telephone based psychotherapy by supporting family caregivers. www.drks.de/drks_web/navigate.do;jsessionid=490506598A315F31B945C663084F7932?navigationId=results (first received 29 October 2014).
- Soellner R, Reder M, Machmer A, Holle R, Wilz G. The Tele.TAnDem intervention: study protocol for a psychotherapeutic intervention for family caregivers of people with dementia. BMC Nursing 2015; Vol. 14, issue 11. [DOI: 10.1186/s12912-015-0059-9] [DOI] [PMC free article] [PubMed]
Wilz 2018 {published data only}
- Wilz G, Reder M, Meichsner F, Soellner R. The Tele.TAnDem intervention: telephone‐based CBT for family caregivers of people with dementia. Gerontologist 2018;58:e118‐e129. [DOI] [PubMed] [Google Scholar]
- Wilz G, Weise L, Reiter C, Reder M, Machmer A, Soellner R. Intervention helps family caregivers of people with dementia attain own therapy goals. American Journal of Alzheimer's Disease and Other Dementias 2018;33:301‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Archbold 1990
- Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Research in Nursing & Health 1990;13(6):375‐84. [DOI] [PubMed] [Google Scholar]
Aubin 2012
- Aubin M, Giguère A, Martin M, Verreault R, Fitch MI, Kazanjian A, et al. Interventions to improve continuity of care in the follow‐up of patients with cancer. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD007672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Badr 2016
- Badr H. Psychosocial interventions for patients with advanced cancer and their families. American Journal of Lifestyle Medicine 2016;10(1):53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakas 2009
- Bakas T, Farran CJ, Austin JK, Given BA, Johnson EA, Williams LS. Stroke caregiver outcomes from the telephone assessment and skill‐building kit (TASK). Topics in Stroke Rehabilitation 2009;16(2):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellg 2004
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology 2004;23(5):443. [DOI] [PubMed] [Google Scholar]
Borman 2009
- Bormann J, Warren K, Regalbuto L, Glaser D, Kelly A, Schnack J, et al. A spiritually based caregiver Intervention with telephone delivery for family caregivers of veterans with dementia. Family and Community Health 2009;32(4):345‐53. [DOI] [PubMed] [Google Scholar]
Brereton 2007
- Brereton L, Carroll C, Barnston S. Interventions for adult family carers of people who have had a stroke: a systematic review. Clinical Rehabilitation 2007;21(10):867‐84. [DOI] [PubMed] [Google Scholar]
Buhse 2008
- Buhse M. Assessment of caregiver burden in families of persons with multiple sclerosis. Journal of Neuroscience Nursing 2008;40(1):25‐31. [DOI] [PubMed] [Google Scholar]
Cameron 2013
- Cameron JI, Naglie G, Silver FL, Gignac MA. Stroke family caregivers’ support needs change across the care continuum: a qualitative study using the timing it right framework. Disability and Rehabilitation 2013;35(4):315‐24. [DOI] [PubMed] [Google Scholar]
Cameron 2016
- Cameron JI, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. One‐year outcomes in caregivers of critically ill patients. New England Journal of Medicine . 2016;374(19):1831‐41. [DOI] [PubMed] [Google Scholar]
Candy 2011
- Candy B, Jones L, Drake R, Leurent B, King M. Interventions for supporting informal caregivers of patients in the terminal phase of a disease. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD007617] [DOI] [PubMed] [Google Scholar]
Care Action Network 2013
- Care Action Network. Community caregivers: the backbone for accessible care and support. www.cordaid.org/media/publications/Malawi_CAN_Report_26_July2013.pdf (accessed March 2015).
Care Alliance Ireland 2010
- Care Alliance Ireland. The caring reality of family carers. www.carealliance.ie (accessed March 2015).
Care Alliance Ireland 2015
- Care Alliance Ireland. Family caring in Ireland. www.carealliance.ie (accessed February 2016).
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 1999
- Chang BL. Cognitive‐behavioural interventions for homebound caregivers of persons with dementia. Nursing Research 1999;48:173‐82. [DOI] [PubMed] [Google Scholar]
Charlesworth 2008
- Charlesworth G, Shepstone L, Wilson E, Reynolds S, Mugford M, Price D, et al. Befriending carers of people with dementia: randomised controlled trial. BMJ 2008;336(7636):1295‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2015
- Chi N‐C, Demiris G. A systematic review of telehealth tools and interventions to support family caregivers. Journal of Telemedicine and Telecare 2015;21(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2015
- Coleman EA, Roman SP. Family caregivers' experiences during transitions out of hospital. Journal of Healthcare Quality 2015;37(1):12‐21. [DOI] [PubMed] [Google Scholar]
Consumer Technology Association 2015
- Consumer Technology Association. How mobile phones are changing the developing world. www.cta.tech/News/Blog/Articles/2015/July/How‐Mobile‐Phones‐Are‐Changing‐the‐Developing‐Worl.aspx (accessed August 2016).
Corry 2009
- Corry M, While A. The needs of carers of people with multiple sclerosis: a literature review. Scandinavian Journal of Caring Sciences 2009;23(3):569‐88. [DOI] [PubMed] [Google Scholar]
Corry 2010
- Corry M, Clarke M, While AE, Lalor J. Developing complex interventions for nursing: a critical review of key guidelines. Journal of Clinical Nursing 2010;22(17‐18):2366‐86. [DOI] [PubMed] [Google Scholar]
Corry 2017
- Corry M, Smith V, Neenan K, Brabyn S. Telephone interventions, delivered by healthcare professionals, for educating and psychosocially supporting informal caregivers of adults with diagnosed illnesses. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czerwonka 2015
- Czerwonka AI, Herridge MS, Chan L, Chu LM, Matte A, Cameron JI. Changing support needs of survivors of complex critical illness and their family caregivers across the care continuum: a qualitative pilot study of Towards RECOVER. Journal of Critical Care 2015;30(2):242‐9. [DOI] [PubMed] [Google Scholar]
Dam 2016
- Dam AE, Vugt ME, Klinkenberg IP, Verhey FR, Boxtel MP. A systematic review of social support interventions for caregivers of people with dementia: are they doing what they promise?. Maturitas 2016;85:117‐30. [DOI] [PubMed] [Google Scholar]
Davidson 2012
- Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome – family. Critical Care Medicine 2012;40(2):618‐24. [DOI] [PubMed] [Google Scholar]
Deek 2016
- Deek H, Hamilton S, Brown N, Inglis SC, Digiacomo M, Newton PJ, et al. Family‐centred approaches to healthcare interventions in chronic diseases in adults: a quantitative systematic review. Journal of Advanced Nursing 2016;75(5):968‐79. [DOI: 10.1111/jan.12885] [DOI] [PubMed] [Google Scholar]
Deloitte 2012
- Deloitte. Sub‐Saharan Africa mobile observatory. www.gsma.com/publicpolicy/wp‐content/uploads/2013/01/gsma_ssamo_full_web_11_12‐1.pdf (accessed August 2016).
Deloitte 2014
- Deloitte. [The mHealth opportunity in Sub‐Sahara Africa: the path towards practical application]. www2.deloitte.com/content/dam/Deloitte/nl/Documents/technology‐media‐telecommunications/deloitte‐nl‐mhealth.pdf (accessed August 2016).
Do 2015
- Do YK, Norton EC, Stearns SC, Houtven CH. Informal care and caregivers health. Health Economics 2015;22(2):224‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 2010
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta‐analysis. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD005066.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkel 2007
- Finkel S, Czaja SJ, Martinovich Z, Harris C, Pezzuto D, Schulz R. E‐care: a telecommunications technology intervention for family caregivers of dementia patients. American Journal of Geriatric Psychiatry 2007;15(5):443‐8. [DOI] [PubMed] [Google Scholar]
Forster 2012
- Forster A, Brown L, Smith J, House A, Knapp P, Wright JJ, et al. Information provision for stroke patients and their caregivers. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD001919.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2003
- Foster M, Chaboyer W. Family carers of ICU survivors: a survey of the burden they experience. Scandinavian Journal of Caring Sciences 2003;17(3):205‐14. [DOI] [PubMed] [Google Scholar]
Gant 2007
- Gant JR, Steffen AM, Lauderdale SA. Comparative outcomes of two distance‐based interventions for male caregivers of family members with dementia. American Journal of Alzheimer's Disease and other Dementias 2007;22:120‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gavois 2006
- Gavois H, Paulsson G, Fridlund B. Mental health professional support in families with a member suffering from severe mental illness: a grounded theory model. Scandinavian Journal of Caring Sciences 2006;20(1):102‐9. [DOI] [PubMed] [Google Scholar]
Gearing 2011
- Gearing RE, Bassel N, Ghesquiere A, Baldwin S, Gillies J, Ngeow E. Major ingredients of fidelity: a review and scientific guide to improving quality of intervention research implementation. Clinical Psychology Review 2011;31(1):79‐88. [DOI] [PubMed] [Google Scholar]
Gendron 2013
- Gendron T, Pelco LE, Pryor J, Barsness S, Seward L. A telephone support program for adult day center caregivers: early indications of impact. Journal of Higher Education Outreach and Engagement 2013;17(1):45‐58. [Google Scholar]
Glendinning 2009
- Glendinning C, Tjadens F, Arksey H, Morée M, Moran N, Nies H. Care provision within families and its socio‐economic impact on care providers. europa.eu/epic/docs/eu‐2342‐care‐provision.pdf (accessed April 2015).
Golics 2013
- Golics CJ, Basra MKA, Salek MS, Finlay AY. The impact of patients’ chronic disease on family quality of life: an experience from 26 specialties. International Journal of General Medicine 2013;6:787‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
González‐Fraile 2015
- González‐Fraile E, Solà I, Ballesteros J, Rueda J‐R, Martinez G, Santos B. Information, support and training for informal caregivers of people with dementia. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD006440.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 1990
- Goodman C, Pynoos J. A model telephone information and support program for caregivers of Alzheimer's patients. Gerontologist 1990;30(3):399‐404. [DOI] [PubMed] [Google Scholar]
Greenwood 2010
- Greenwood N, Mackenzie A. Informal caring for stroke survivors: meta‐ethnographic review of qualitative literature. Maturitas 2010;66(3):268‐76. [DOI] [PubMed] [Google Scholar]
Haines 2015
- Haines KJ, Denehy L, Skinner EH, Warrillow S, Berney S. Psychosocial outcomes in informal caregivers of the critically Ill: a systematic review. Critical Care Medicine 2015;43(5):1112‐20. [DOI] [PubMed] [Google Scholar]
Hartke 2003
- Hartke RJ, King RB. Telephone group intervention for older stroke caregivers. Topics in Stroke Rehabilitation 2003;9(4):65‐81. [DOI] [PubMed] [Google Scholar]
Hasson 2010
Heese 2013
- Heese O, Vogeler E, Martens T, Schnell O, Tonn JC, Simon M, et al. End‐of‐life caregivers' perception of medical and psychological support during the final weeks of glioma patients: a questionnaire‐based survey. Neuro‐Oncology 2013;15(9):1251‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbert 2005
- Herbert RD, Bø K. Analysis of quality of interventions in systematic reviews. BMJ 2005;331(7515):507‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 2006
- Herman DS, Bishop D, Anthony JL, Chase W, Trisvan E, Lopez R, et al. Feasibility of a telephone intervention for HIV patients and their Informal caregivers. Journal of Clinical Psychology in Medical Settings 2006;13(1):81‐90. [Google Scholar]
Higgins 2008
- Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials.. Clinical Trials 2008;5(3):225‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hogan 2002
- Hogan BE, Linden W, Najarian B. Social support interventions: do they work?. Clinical Psychology Review 2002;22(3):381‐440. [DOI] [PubMed] [Google Scholar]
International Alliance of Carer Organisations 2016
- International Alliance of Carer Organisations. Caregiving facts. www.internationalcarers.org/carer‐facts/global‐carer‐stats/ (accessed prior to 5 April 2019).
Kaltenbaugh 2015
- Kaltenbaugh DJ, Klem ML, Hu L, Turi E, Haines AJ, Hagerty LJ. Using web‐based interventions to support caregivers of patients with cancer: a systematic review. Oncology Nursing Forum 2015;42(2):156‐64. [DOI] [PubMed] [Google Scholar]
Lavender 2013
- Lavender T, Richens Y, Milan SJ, Smyth R, Dowswell T. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009338.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Legg 2011
- Legg LA, Quinn TJ, Mahmood F, Weir CJ, Tierney J, Stott DJ, et al. Non‐pharmacological interventions for caregivers of stroke survivors. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008179.pub2] [DOI] [PubMed] [Google Scholar]
Lethin 2016
- Lethin C, Leino‐Kilpi H, Roe B, Soto MM, Saks K, Stephan A, et al. Formal support for informal caregivers to older persons with dementia through the course of the disease: an exploratory, cross‐sectional study. BMC Geriatrics 2016;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2010
- Levine C, Halper D, Peist A, Gould DA. Bridging troubled waters: family caregivers, transitions, and long‐term care. Health Affairs 2010;29(1):116‐24. [DOI] [PubMed] [Google Scholar]
Levine 2013
- Levine C, Halper JL, Rutber J, Gould DA. Engaging family caregivers as partners in transitions. TC–QuIC: a quality Improvement collaborative. www.uhfnyc.org/assets/1111 (accessed on 1 April 2016).
Lilly 2012
- Lilly MB, Robinson CA, Holtzman S, Bottorff JL. Can we move beyond burden and burnout to support the health and wellness of family caregivers to persons with dementia? Evidence from British Columbia, Canada. Health & Social Care in the Community 2012;20(1):103‐12. [DOI] [PubMed] [Google Scholar]
Lins 2014
- Lins S, Hayder‐Beichel D, Rücker G, Motschall E, Antes G, Meyer G, et al. Efficacy and experiences of telephone counselling for informal carers of people with dementia. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD009126.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunden 2015
- Lunden I. 6.1B Smartphone users globally by 2020, overtaking basic fixed phone subscriptions. techcrunch.com/2015/06/02/6‐1b‐smartphone‐users‐globally‐by‐2020‐overtaking‐basic‐fixed‐phone‐subscriptions/ (accessed 30 September 2016).
Mahan 1963
- Mahan RA, Bollman SR. Education or information giving?. joe.org/joe/1968summer/1968‐2‐a5.pdf (accessed 3 October 2016).
Mars 2013
- Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor S. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3(11):e003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCabe 2009
- McCabe MP, Firth L, O’Connor E. A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. Journal of Clinical Psychology in Medical Settings 2009;16(4):355‐62. [DOI] [PubMed] [Google Scholar]
Melrose 2015
- Melrose KL, Brown GDA, Wood AM. When is received social support related to perceived support and well‐being? When it is needed. Personality and Individual Differences 2015;77:97‐105. [Google Scholar]
Merckaert 2013
- Merckaert I, Libert Y, Lieutenant F, Moucheux A, Farvacques C, Slachmuylder J‐L, et al. Desire for formal psychological support among caregivers of patients with cancer: prevalence and implications for screening their needs. Psycho‐Oncology 2013;22(2):1389‐95. [DOI] [PubMed] [Google Scholar]
Mittelman 1996
- Mittelman MS, Ferris SH, Shulman E, Steinberg G, Levin B. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA 1996;276(21):1725‐31. [PubMed] [Google Scholar]
Mohler 2009
- Möhler D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosher 2013
- Mosher CE, Champion VL, Hanna N, Jalal SI, Fakiris AJ, Birdas TJ, et al. Support service use and interest in support services among distressed family caregivers of lung cancer. Psycho‐Oncology 2013;22(7):1549‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC 2008
- Medical Research Council. Developing and evaluating complex interventions: new guidance. www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC004871 (accessed 10 March 2016).
Murrow 1996
- Murrow EJ, Oglesby FM. Acute and chronic illness: similarities, differences and challenges. Orthopaedic Nursing 1996;15(5):47‐51. [PubMed] [Google Scholar]
Möhler 2012
- Möhler RG, Bartoszek S, Köpkeand Meyer G. Proposed criteria for reporting the development and evaluation of complex interventions in healthcare (CReDECI): guideline development. International Journal of Nursing Studies 2012;49(1):40‐6. [DOI] [PubMed] [Google Scholar]
Nalder 2012
- Nalder E, Fleming J, Cornwell P, Foster M. Linked lives: the experiences of family caregivers during the transition from hospital to home following traumatic brain injury. Brain Impairment 2012;13(1):108‐22. [Google Scholar]
Navine‐Waliser 2002
- Navaie‐Waliser M, Feldman PH, Gould DA, Levine C, Kuerbis AN, Donelan K. When the caregiver needs care: the plight of vulnerable caregivers. American Journal of Public Health 2002;93(3):409‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
OECD 2011
- Organisation for Economic Co‐operation and Development. Help wanted? Providing and paying for long‐term care. www.oecd.org/els/health‐systems/47884865.pdf (accessed May 2015).
OECD 2013
- Organisation for Economic Co‐operation and Development. Health at a glance 2013: OECD Indicators. dx.doi.org/10.1787/health_glance‐2013‐76‐en (accessed 10 February 2016).
Oguh 2013
- Oguh O, Kwasny M, Carter J, Stell B, Simuni T. Caregiver strain in Parkinson's disease: National Parkinson Foundation quality initiative study. Parkinsonism & Related Disorders 2013;19(11):975‐9. [DOI] [PubMed] [Google Scholar]
Oshio 2015
- Oshio T. How is an informal caregiver’s psychological distress associated with prolonged caregiving? Evidence from a six‐wave panel survey in Japan. Quality of Life Research 2015;24(12):2907‐15. [DOI] [PubMed] [Google Scholar]
Ostwald 2009
- Ostwald S, Bernal MP, Cron SG, Godwin KM. Stress experienced by stroke survivors and spousal caregivers during the first year after discharge from inpatient rehabilitation. Topics in Stroke Rehabilitation 2009;16(2):93‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
O’Shaughnessy 2010
- O’Shaughnessy M, Lee K, Lintern T. Changes in the couple relationship in dementia care: spouse carers’ experiences. Dementia 2010;9(2):237‐58. [Google Scholar]
Penning 2015
Pew Research Centre 2015
- Pew Research Centre. Cell phones in Africa: communication lifeline. www.pewglobal.org/2015/04/15/cell‐phones‐in‐africa‐communication‐lifeline/ (accessed 2 August 2016).
Ravenson 2016
- Ravenson TA, Griva K, Luszczynska A, Morrison V, Panagopoulou E, Vilchinsky N, et al. Caregiving in illness context. UK: Palgrave Macmillan, 2016. [Google Scholar]
Reinhard 2008
- Reinhard SC, Given B, Petlick NH, Bemis A. Supporting family caregivers in providing care. In: Hughes RG editor(s). Patient Safety and Quality: An Evidence‐Based Handbook for Nurses. (US): Agency for Healthcare Research and Quality, 2008:341‐56. [PubMed] [Google Scholar]
Resnick 2005
- Resnick B, Bellg A, Borrelli B, Francesco C, Breger R, Hecht J, et al. Examples of implementation and evaluation of treatment fidelity in the BCC studies: where we are and where we need to go. Annals of Behavioral Medicine 2005;29(1):46‐54. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riess‐Sherwood 2002
- Riess‐Sherwood P, Given BA, Given CW. Who cares for the caregiver: strategies to provide support. Home Health Care Management & Practice 2002;14(2):110‐21. [Google Scholar]
Robinson 2005
- Robinson L, Clare L, Evan K. Making sense of dementia and adjusting to loss: psychological reactions to a diagnosis of dementia in couples. Aging & Mental Health 2005;9(4):337‐47. [DOI] [PubMed] [Google Scholar]
Roick 2007
- Roick C, Heider D, Bebbington PE, Angermeyer MC, Azorin J‐M, Brugha TS, et al. Burden on caregivers of people with schizophrenia: comparison between Germany and Britain. British Journal of Psychiatry 2007;190(4):333‐8. [DOI] [PubMed] [Google Scholar]
Rosland 2008
- Rosland A‐M, Kieffer E, Israel B, Cofield M, Palmisano G, Sinco B, et al. When Is social support important? The association of family support and professional support with specific diabetes self‐management behaviors. Journal of General Internal Medicine 2008;23(12):1992‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosland 2010
- Rosland A‐M, Piette JD. Emerging models for mobilizing family support for chronic disease management: a structured review. Chronic Illness 2010;6(1):7‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Review Group. Study quality guide. cccrg.cochrane.org/author‐resources (accessed 30 September 2016).
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. cccrg.cochrane.org/author‐resources (accessed 29 July 2018).
Ryan 2016a
- Ryan R, Santesso N, Hill S. Preparing summary of findings (SoF) tables. http://cccrg.cochrane.org/author‐resources (accessed 29 July 2018).
Salfi 2005
- Salfi J, Ploeg J, Black ME. Seeking for understand telephone support for dementia caregivers. Western Journal of Nursing Research 2005;27(6):701‐21. [DOI] [PubMed] [Google Scholar]
Santin 2012
- Santin O, Coleman H, Mills M, Cardwell CR, Donnelly M. Psychosocial interventions for informal caregivers of people living with cancer. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherbring 2002
- Scherbring M. Effect of caregiver perception of preparedness on burden in an oncology population. Oncology Nursing Forum 2002;29(6):E70‐6. [DOI] [PubMed] [Google Scholar]
Schwichtenberg 2007
- Schwichtenberg A, Poehlmann J. Applied behaviour analysis: does intervention intensity relate to family stressors and maternal well‐being?. Journal of Intellectual Ability Research 2007;51(8):598‐605. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPD, Vist GE, Glasziou PE, Guyatt GH. Chapter 11: Presenting results and ‘summary of findings' tables. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Selwood 2007
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders 2007;101(1):75‐89. [DOI] [PubMed] [Google Scholar]
Sepulveda 2008
- Sepulveda AR, Lopez C, Macdonald P, Treasure J. Feasibility and acceptability of DVD and telephone coaching‐based skills training for carers of people with an eating disorder. International Journal of Eating Disorders 2008;41(4):318‐25. [DOI] [PubMed] [Google Scholar]
Shozi 2013
- Shozi NA, Pottas D, Mostert‐Phipps N. Perceived benefits of remote data capturing in community home‐based care: the caregivers’ perspective. ci‐journal.net/index.php/ciej/article/view/857 (accessed 18 February 2016).
Smith 2004
- Smith L, Lawrence M, Kerr SM, Langhorne P, Kennedy R. Informal carers’ experience of caring for stroke survivors. Journal of Advanced Nursing 2004;46(3):235‐44. [DOI] [PubMed] [Google Scholar]
Steffen 2000
- Steffan AM. Anger management for dementia caregivers: a preliminary study using video and telephone interventions. Behavioural Therapy 2000;31:281‐99. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting bias. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taylor 2008
- Taylor C. Supporting the carers of individuals affected by colorectal cancer. British Journal of Nursing 2008;17(4):226‐230. [DOI] [PubMed] [Google Scholar]
Thompson 2007
- Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatrics 2007;7(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Topo 2009
- Topo P. Technology studies to meet the needs of people with dementia and their caregivers: a literature review. Journal of Applied Gerontology 2009;28(1):5‐37. [Google Scholar]
Toseland 1989
- Toseland R, Rossiter C, Labrecque MS. The effectiveness of peer‐led and professionally led groups to support family caregivers. Gerontologist 1989;29(4):465‐71. [DOI] [PubMed] [Google Scholar]
Tremont 2008
- Tremont G, Davis JD, Bishop DS, Fortinsky RH. Telephone‐delivered psychosocial intervention reduces burden in dementia caregivers. Dementia 2008;74(4):503‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unson 2016
- Unson C, Flynn D, Haymes E, Sancho D, Glendon MA. Predictors of types of caregiver burden. Social Work in Mental Health 2016;14(1):82‐101. [Google Scholar]
Van Mierlo 2012b
- Mierlo LD, Meiland FJ, Roest HG, Dröes RM. Personalised caregiver support: effectiveness of psychosocial interventions in subgroups of caregivers of people with dementia. International Journal of Geriatric Psychiatry 2012;27(1):1‐14. [DOI] [PubMed] [Google Scholar]
Vermeulen 2015
- Vermeulen B, Lauwers H, Spruytte N, Audenhove C, Magro C, Saunders J, et al. Experiences of family caregivers for persons with severe mental illness: an international exploration. www.caringformentalhealth.org/c4c_reports/c4c_global.pdf (accessed February 2016).
Vernooij‐Dassen 2011
- Vernooij‐Dassen M, Draskovic I, McCleery J, Downs M. Cognitive reframing for carers of people with dementia. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD005318.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2015
- Vos T, Barber RM, Bell B, Bertozzi‐Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the global burden of disease study 2013. Lancet 2015;386(9995):743‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waller 2017
- Waller A, Dilworth S, Mansfield E, Sanson‐Fisher R. Computer and telephone delivered interventions to support caregivers of people with dementia: a systematic review of research output and quality. BMC Geriatrics 2017;17(1):265. [DOI: 10.1186/s12877-017-0654-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2006
- World Health Organisation. Neurological Disorders: Public Health Challenges. Geneva: World Health Organisation Press, 2006. [Google Scholar]
Williams 2015
- Williams R. 7 in 10 of world's population using smartphones by 2020. www.telegraph.co.uk/technology/mobile‐phones/11646593/7‐in‐10‐of‐worlds‐population‐using‐smartphones‐by‐2020.html (accessed 30 September 2016).
Wilz 2016
- Wilz G, Soellner R. Evaluation of a short‐term telephone‐based cognitive behavioral intervention for dementia family caregivers. Clinical Gerontologist 2016;39(1):25‐47. [Google Scholar]
Yoder 2012
- Yoder P, Fey ME, Warren SF. Studying the impact of intensity is important but complicated. International Journal of Speech‐Language Pathology 2012;14(5):410‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwaanswijk 2013
- Zwaanswijk M, Peeters JM, Beek AP, Meerveld JH, Francke AL. Informal caregivers of people with dementia: problems, needs and support in the initial stage and in subsequent stages of dementia: a questionnaire survey. Open Nursing Journal 2013;7:6‐13. [DOI: 10.2174/1874434601307010006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwicker 2010
- Zwicker D. Preparedness for caregiving scale. Movement Disorders 2010;13(1):20‐8. [Google Scholar]


