Abstract
Efforts from diverse disciplines, including evolutionary studies and biomechanical experiments, have yielded new insights into the genetic, signaling, and mechanical control of tooth formation and functions. Evidence from fossils and non-model organisms has revealed that a common set of genes underlie tooth-forming potential of epithelia, and changes in signaling environments subsequently result in specialized dentitions, maintenance of dental stem cells, and other phenotypic adaptations. In addition to chemical signaling, tissue forces generated through epithelial contraction, differential growth, and skeletal constraints act in parallel to shape the tooth throughout development. Here we review recent advances in understanding dental development from these studies and discuss important gaps that can be filled through continued application of evolutionary and biomechanical approaches.
Keywords: stem cells, progenitor cells, evolution, mechanical force, tooth, morphogenesis, non-model organisms
Graphical Abstract
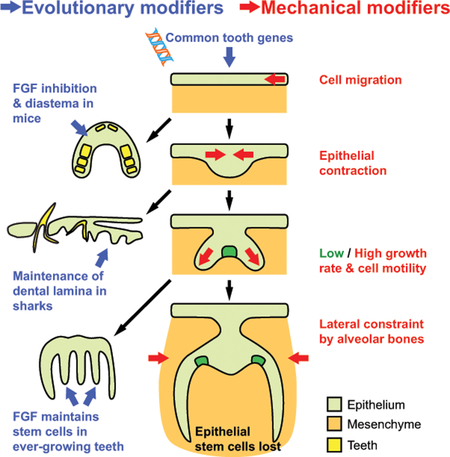
1. Introduction
A fundamental question in developmental and stem cell biology is how organs acquire specific shapes and maintain structures to support normal function throughout an animal’s life. The tooth is an excellent model system to study this topic because of its simple structure, ease of manipulation, and clinical relevance. Research over the past few decades using the mouse molar, which is similar to human rooted teeth and easily cultured ex vivo, has yielded key insights into the signaling pathways and genetic changes required for correct dental development.[1–5] More recently, the continuously growing rodent incisor has emerged as a model to study adult stem cells and their role in homeostasis and repair of a fully-grown organ.e.g.[6–8] Results from these studies have provided the basic framework to develop stem-cell-based strategies for regenerative medicine, as human teeth cannot regenerate enamel, the outermost protective layer of the tooth, due to the loss of epithelial stem cells after tooth eruption.[6] Despite these efforts, major challenges remain for derivation of dental stem cells, precise control of cell proliferation and differentiation in engineered tissues, and guidance of specific populations of cells to produce a distinct tooth shape. These questions are at the heart of recent studies using approaches ranging from phylogenetic and comparative studies to cutting-edge imaging and biomechanical techniques.[8–12] Results from these experiments have provided clues to the evolutionary, genetic, and biomechanical mechanisms that sculpt developing teeth and their adult morphology through time and space. In this review, we focus on evidence from these different fields and examine how their continued integration will improve understanding of tooth developmental and stem cell biology.
2. Epithelial and mesenchymal interactions drive tooth formation
Teeth are composed of crowns, roots, and supporting structures. The crown of the tooth is in direct contact with food and the opposing tooth and therefore must resist abrasion during mastication. For this purpose, the crown is covered by the hardest substance in the body, enamel.[13,14] Enamel is produced by ameloblasts, a group of specialized cells derived from dental epithelium during development. Prior to enamel production, odontoblasts of neural-crest-derived mesenchymal origin deposit a softer inner layer of calcified tissue known as dentin.[14,15] Dentin surrounds other mesenchymal tissues in the dental pulp, including nerves and vasculature.[14] In tooth roots, cementum deposited outside the dentin helps anchor the tooth to the adjacent alveolar bone via the periodontal ligament.[14]
2.1. Mouse molar development is initiated by a group of Fgf8-expressing dental precursor cells
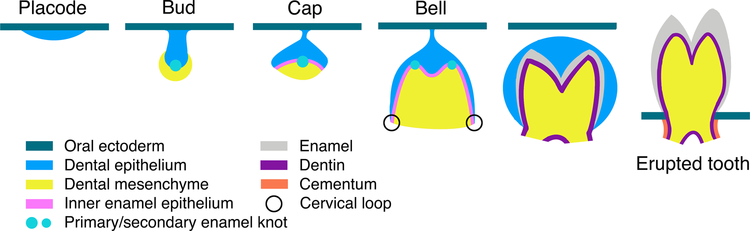
The formation of distinct tooth morphologies in different species and different positions in the jaw, which evolved in part as adaptations to different dietary needs,[2] is determined during development and involves complex reciprocal interactions between dental epithelium and mesenchyme.[15,16] Mice have only incisors and molars, and do not develop canines and premolars; in mouse molars, the dental epithelium originates from a group of Fgf8-expressing oral epithelial cells arranged as a large rosette-like structure (100–200 μm in diameter) in the proximal mandible at embryonic day 11.5 (E11.5).[11] Once released from the rosette, dental epithelial progenitors move anteriorly toward a Shh-expressing signaling center, where they contribute to the thickening epithelial tissue known as the molar placode at E11.5 (Fig. 1).[1,4] Local thickening of the epithelium results from cell divisions at the placodal field and the ensuing generation of stratified suprabasal cells.[17] This process is, in part, regulated by FGF signaling; chemical inhibition of the FGF pathway impedes cell proliferation and subsequent stratification of the molar tooth germ. Signaling crosstalk between the dental epithelium and mesenchyme results in further invagination of the epithelium as the tooth progresses through stages known as the bud, cap, and bell, based on the shape of the developing structure in cross-section (Fig. 1).[1]
Fig. 1. Developmental stages of rooted teeth.
FGF signaling regulates the local thickening of dental epithelium to form the tooth placode, which begins to invaginate; mechanical forces during these early stages of tooth development play an especially important role in buckling the epithelium towards the mesenchyme (see section 4). Signaling crosstalk takes place between the epithelium and mesenchyme, resulting in mesenchymal condensation and epithelial invagination. During the bud stage, the primary enamel knot forms and triggers further cell proliferation in the neighboring epithelium, which extends around the condensing mesenchyme to form a cap shape. During the ensuing bell stage, as cells from the upper inner enamel epithelium begin to differentiate into ameloblasts, cervical loops at the growing end of the invaginating epithelium provide a niche to maintain progenitor cell proliferation and epithelial elongation. Adjacent to the inner enamel epithelium, mesenchymal cells differentiate into odontoblasts, which produce dentin. Cervical loops are lost during root formation and some mesenchymal cells migrate to the surface of developing roots to form cementum, the material to which periodontal ligaments attach to anchor the tooth to the jaw.
Different from molars, the mouse incisor is derived from a group of Isl1-expressing epithelial cells at the distal mandibular process at E9, where ISL1 and BMP4 form a positive autoregulatory loop to specify incisor formation.[18] Whether incisor epithelial thickening is similarly driven by suprabasal stratification has not been tested; subsequently, the mouse incisor then develops through equivalent morphologically-defined stages as molars.
2.2. Signaling-controlled proliferation and differentiation regulate dental epithelial elongation and stem cell maintenance
During the bud to cap transition, the signaling center known as the primary enamel knot (EK) forms at the basal-most point of the epithelium.[19] The EK consists of a group of non-proliferative cells that express cell cycle inhibitor p21 and an array of signaling molecules, including SHH, BMPs, and FGFs.[19,20] These molecules may promote continuing proliferation in the adjacent epithelium, resulting in epithelial elongation as the tooth enters the cap stage.[21,22] Secondary EKs form during the bell stage mainly from undifferentiated epithelial cells, with the buccal secondary EK receiving contributions from cells of the primary EK.[23,24] The secondary EKs are also non-proliferative and help determine both future cusp positions and the eventual crown shape.[2] During the bell stage, cells in the inner enamel epithelium neighboring the secondary EK begin to differentiate into ameloblasts, and the underlying mesenchyme gives rise to odontoblasts.[1] Meanwhile, dental epithelia distal to the EK continue to invaginate.[1] A structure known as the cervical loop forms at the end of the epithelial extension, where stem cells are maintained to sustain epithelial growth and produce additional ameloblasts.[25,26] In rooted teeth (e.g., all human teeth and mouse molars), as the period of crown formation ends, the progenitor cells residing in the cervical loop are lost as a result of the cessation of Fgf10 expression in the mesenchyme, and the epithelium thins.[27–29] The inner and outer enamel epithelial cells form Hertwig’s epithelial root sheath, eventually fenestrating to allow mesenchymal cells to migrate to the external surface of the tooth and form cementoblasts, which make cementum for periodontal ligament attachment.[3,30–32] As a result, rooted teeth lose the potential to regenerate enamel, and the remaining mesenchymal tissues have limited capacity to regenerate dentin, cementum, and pulp. Certain specialized teeth, such as rodent incisors, retain dental epithelial and mesenchymal stem cells throughout the animal’s life,[25,32–34] resulting in continuous crown growth that compensates for tooth wear from chewing and gnawing on food and other materials. Consequently, these teeth never undergo the transition from crown to root formation, and they provide a system for comparative and mechanical studies to understand tooth morphogenesis and dental stem cell proliferation, differentiation, and maintenance responsible for the great diversity of teeth across different species.
3. Dental stem cell origins, development, and maintenance are revealed through evolution and the fossil record
Teeth are among the most important structures for understanding vertebrate evolution, because the hard, mineralized tissues of teeth are easily preserved during fossilization.[1] Although teeth can provide a wealth of information about the diet,[35,36] ecology,[37,38] and evolutionary relationships of vertebrates,[39–41] aspects of their evolution with direct relevance to understanding tooth development and stem cell biology are unresolved, including questions about the embryonic tissues that first contribute epithelia to teeth, the maintenance or loss of tooth replacement, and the origins of hypselodont, or ever-growing, teeth. Some of these questions are difficult to address in fossils, because dental stem cell niches are not preserved along with the teeth;[34] however, examining odontogenic stem cells across extant species and the ways they contribute to different tooth phenotypes and applying the findings to the fossil record will be a powerful tool for understanding how teeth are formed and develop.
3.1. Did dental epithelium evolve from endoderm or ectoderm, and does it matter?
To understand how teeth arrived at their position at the margins of the jaws and the regulatory mechanisms that induce tooth formation at these specific locations, researchers have investigated whether endodermal or ectodermal epithelia contribute to the earliest stages of tooth development. There are two main hypotheses for the origin of oral teeth: “outside to inside,” in which odontodes (i.e., dermal scales or denticles) with ectodermal origins migrated into the oral cavity to form marginal teeth; or “inside to outside,” in which endodermally-derived pharyngeal denticles migrate outward to the jaw margin to form teeth.[42–44] Studies across multiple classes of jawed vertebrates suggest teeth can be generated from epithelia with endodermal, ectodermal, or mixed endo- and ectodermal origins.[45–49] Mammalian dental epithelium appears to arise entirely from the ectoderm during development;[48] however, incisors and molars express different gene profiles, with molars taking on a more endodermal-like signature.[46] The ability of both endodermal and ectodermal epithelia to form teeth has led to the hypothesis that, regardless of the source of epithelium, odontodes will form anywhere a competent epithelium expressing a specific set of genes is juxtaposed with the correct cranial neural crest-derived mesenchyme.[44] This hypothesis focuses on the signaling and contributions from mesenchyme that pattern tooth-forming epithelium, rather than the embryonic source of the epithelium.[44,50,51] Indeed, comparing teeth to other odontodes reveals nearly identical sets of genes involved in their development,[50,52,53] suggesting the gene expression profile, rather than epithelial origin, is what matters. One major difference between teeth and other odontodes appears to be expression of Sox2 in teeth, which establishes epithelial progenitor cells with regenerative capabilities that are unique to teeth.[52] The importance of Sox2 in stem cell maintenance, especially in dental epithelial stem cells, has been broadly demonstrated in teeth of mammals, reptiles, and fish,[52–56] providing evidence for the deep homology of tooth patterning mechanisms regardless of the origin of epithelial tissue.
No less important for tooth evolution are the contributions of mesenchymal cells to tooth development, although the developmental origin of these tissues is generally less controversial. Dental mesenchymal cells are derived from cranial neural crest.[57–60] Signaling interactions between mesenchyme and epithelium through a number of pathways, including FGF, BMP, WNT, EDA/EDAR, and SHH, designate where teeth form, the number of teeth formed, how they are replaced, and even their shapes.[1,2,61,62] These interactions thus produce phylogenetically informative dental characters like the rodent diastema, a toothless region occupied by premolars and canines in many other mammals.[63] In this context, the diastema forms when FGF signaling is inhibited by the Sprouty family proteins (encoded by Spry2 and 4 in epithelium and mesenchyme, respectively) and WNT signaling is repressed by WISE, suppressing tooth formation.[63,64] The formation of replacement teeth can also be controlled in part by the dental mesenchyme: when WNT/β-catenin signaling is hyperactivated in mesenchymal tissues, replacement teeth fail to develop, possibly due to disruption of delicately balanced feedback between WNT and its inhibitors in both epithelium and mesenchyme.[65,66] Conversely, hyperactivation of WNT in epithelial tissues can result in the formation of extra teeth.[67–70] Disruptions of this balance between epithelial and mesenchymal signaling during tooth development may provide molecular explanations for evolution of tooth number and replacement frequency. Such mechanisms can help us understand how dental variation within and between fossil and extant species arose.
Comparative analyses of signaling molecule expression in dental tissues of extant species have also provided evidence for the role mesenchyme plays in tooth morphogenesis. Experiments using mouse and vole molars showed that FGF10 operates in the mesenchyme in combination with NOTCH to pattern the shape of the tooth, in part by supporting the formation and maintenance of the stem cell niche, and the cessation of Fgf10 expression has been tied to root formation as well.[2,26,27,71] Investigating the role of FGF10 in stem cell maintenance will be important for understanding the complete life cycle of stem cells in regenerating organs. More recently, the development of new bioinformatics and sequencing techniques has further contributed to a greater understanding of mesenchymal tissues in teeth; for example, gene co-expression analyses have revealed that the mesenchyme of the periodontium and the dental pulp are maintained by two distinct populations of stem cells.[8] Resolving the evolutionary implications of which stem cells contribute to the maintenance of different parts of teeth, especially within tissues previously thought to have a single origin, may help explain interspecific variation in tooth morphology.
3.2. The dental lamina is a source of odontogenic stem cells for tooth replacement in polyphyodonts
Research focused on the evolution of tooth replacement strategies, either through multiple generations of teeth or the origination of single teeth that continuously produce crown, has contributed to the understanding of how tooth stem cells are maintained.[72,73] The timing and manner of tooth replacement varies considerably across vertebrate species, but can be grouped into two main strategies: by initially forming a large number of replacement teeth for each functional tooth that move into position as needed; or by forming a single replacement tooth for each functional tooth successively, a process in which the new replacement tooth forms only after the previous replacement tooth becomes the functional tooth.[74] In most cases, the first-generation teeth form in the early-developing dental lamina (also known as the odontogenic band), which subsequently expands deeper into the mesenchyme to form the successional lamina that is responsible for the generation of replacement teeth.[53,73] As with other structures that are replenished or replaced by epithelial stem cells, tooth replacement draws on a deeply conserved set of genes across vertebrate species, including Lef1 and Bmp4, which appear to confer odontogenic competence on epithelia for not just the initial tooth but its replacements as well.[50,53,73–75] Regardless of the mode of tooth replacement found in the dentitions of extant jawed vertebrates, molecular data reveal the broad importance of these dental epithelia for housing odontogenic stem cells for multiple generations of teeth, although heterochronic shifts in the timing of stem cell activation may have resulted in different epithelial layers (i.e., the entire odontogenic band of the oral epithelium, the early-forming dental lamina, or the successional lamina) appearing to confer tooth-forming competence in different clades.[73] Assessing differences (or lack thereof) between the mechanisms that activate odontogenic stem cells for replacement teeth in these taxa regardless of the epithelial layer the cells originate from will provide major insight into how tooth competence can be activated for human tooth regeneration.
3.3. Truncating development led to the loss of polyphyodont tooth replacement
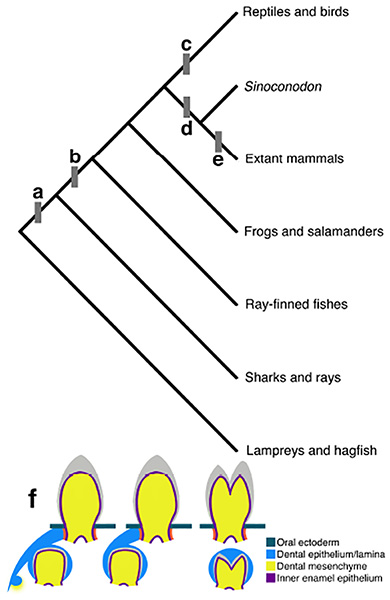
Teeth are an invaluable window into mammalian evolutionary history; because selective pressures of dietary and behavioral needs influence tooth morphology, the cusps of fossil and extant species are a record of changing diets and environments through time.[1,33] Most crown mammals (the clade of all mammals, fossil or living, that descended from the most recent common ancestor of all extant mammals) are diphyodont, meaning they have two generations of teeth: deciduous teeth, usually lost during juvenile development, and permanent teeth, which must last through adult life.[74] The earliest mammals were likely polyphyodont, however, meaning they replaced their teeth multiple times as they were lost throughout life, as are most non-mammalian toothed vertebrates (Fig. 2).[74] The early mammal Sinoconodon, for example, had multiple replacements of its incisors and canines.[76,77] The loss of polyphyodonty in mammals is potentially linked to changes in the growth patterns of the skull. Initially rapid skull growth that slows or stops in adulthood is a hallmark of placental mammal development.[76–78] Teeth generally cannot change size after eruption, and thus multiple tooth replacements in species with skulls that grow continuously throughout life serve the purpose of allowing larger teeth to fill the dentary.[78] Rapid early skull growth that does not continue into adulthood truncates the period during which an intermediate jaw requires intermediate sized teeth, thus reducing the pressure to form multiple generations of intermediately-sized teeth.[77,78] Consequently, reducing the number of generations of teeth may also be related to changes in tooth attachment. Early mammals had teeth that fully ankylosed to the bones of the jaw, whereas the teeth of crown mammals are attached to the bone by the periodontal ligament, called a gomphosis.[79–81] Preserved evidence for periodontal ligaments in early mammal species with ankylosing teeth have been interpreted as evidence that gomphoses represent an early stage in tooth development that ends in ankylosis.[81] Perhaps the shortened period of cranial bone growth thought to be responsible for reducing the number of tooth generations was, more broadly, a shortening of the developmental period of multiple structures in the cranium, including teeth. The continued discovery of early mammal fossil materiale.g.,[82,83] can provide additional specimens to further investigate the link between skull growth rate and tooth replacement, furnishing important historical context for the morphological setting in which the modification of the dental stem cells occurred.
Fig. 2. Evolutionary transitions in vertebrate tooth replacement.
a) Sharks and rays have multiple replacement teeth at the same time for each functional tooth in a “many-for-one” tooth replacement system. Multiple generations of replacement teeth form in the soft tissues of the dental lamina on the lingual side of the functional tooth and migrate toward the oral ectoderm as functional teeth are lost, finally attaching to the cartilage of the jaws as they erupt.[74] b) Bony fish (Osteichthyes) have “one-for-one” or “many-for-one” tooth replacement, while amphibians and mammals tend to exhibit “one-for-one” replacement. In this system, a single replacement tooth forms in a bony cavity beneath the functional tooth. Fish and amphibians replace teeth in this manner throughout life; in amphibians the functional teeth mostly detach from the dental lamina, but maintain some level of connection through replacement tooth generations.[74,135] c) Reptiles with teeth (extant bird, turtles, and tortoises are edentulous), much like fish and amphibians, have replacement teeth connected to the oral surface by the dental lamina; however they predominantly exhibit many-for-one polyphyodonty.[2,74] d) Early mammals like Sinoconodon retained polyphyodont replacement in some teeth while reducing generations toward diphyodonty (two generations of replacement teeth).[76,77] e) Most extant mammals have diphyodont tooth replacement, while some have evolved monophyodont dentitions (no replacement) or edentulism. A small number of mammals have achieved polyphyodont replacement by the continuous addition of molars at the end of the tooth row, which move forward as the anterior-most molar wears down and is lost.[2] f) From left to right, the many-for-one model of tooth replacement in which teeth remain connected to the dental lamina, one-for-one replacement with connected replacement teeth, and one-for-one replacement in which the dental lamina regresses (found in mammals).
3.4. Ever-growing teeth are linked to morphological and environmental changes
The evolution of ever-growing, or hypselodont, teeth may have been a response to the loss of successive generations of teeth; teeth that continuously erupt crown material (requiring lifelong maintenance of the adult stem cells that reside in the cervical loops of the tooth) provide a constant chewing surface without the need for successive generations of new teeth with finite crown growth.[26,34] Hypselodonty has evolved multiple times across Mammalia and is by no means restricted to single originations within any of the clades in which it appears. There are 9 extant mammalian orders in which all or some teeth have become hypselodont, from Glires (the clade of rodents and their relatives, the lagomorphs—rabbits, hares, and pikas), elephants, and walruses with hypselodont incisors to sloths with ever-growth homodont dentitions.[33,84] Extinct clades with ever-growing teeth include the mysterious notoungulates of South America, some of which have superficially similar dentitions to those of rodents, featuring incisors and molars separated by a large diastema.[85] Comparative analyses of high-crowned and ever-growing teeth in notoungulates and rodents suggested that the diastema and mesial drift of molars may be tied to morphological changes needed to accommodate these teeth.[86] If hypselodonty is linked to the maintenance of stem cells in some teeth and the suppression of the tooth germ and agenesis of diastema teeth, then it is possible the molecular patterning of hypselodont teeth (e.g., the Sprouty genes already discussed)[63,64] is also connected to broad morphological changes beyond stem cell maintenance and should be investigated in greater detail for correlations with cranial and mandibular variation in hypselodont clades.
Although studying hypselodonty promises insights into the lifelong maintenance of tooth stem cells, it is also studied in the context of ecological changes and as a possible indicator of plant communities and aridity of ancient environments. The increased prevalence of high-crowned and ever-growing cheek teeth starting approximately 40 million years ago has long been linked to an increase in abrasion in diets, either through the incorporation of silica-rich plants as grasslands spread or through ingestion of dust or grit in dry environments.[10,37,87,88] The evolution of hypselodonty as a response to greater tooth abrasion is logical; however, recent studies favor aridity and open habitats over abrasive grasses as drivers of crown height evolution, especially when the scale of faunal diversity under study is matched to regional rather than global climate data.[89] In rodents, however, molar crown heights appear to have trended toward hypselodonty through time regardless of environmental variables, suggesting that lifelong maintenance of stem cells in these teeth may simply result from gradual, continuous change toward higher crowns.[34] Further investigations of the environmental pressures correlated with the evolution of lifelong maintenance of tooth stem cells, especially focused on potential convergent evolution of signaling mechanisms to preserve these stem cells, will elucidate connections between the external and oral environment that promote continued stem cell replication.
3.5. Tooth shapes are correlated with bite forces
Teeth must resist not only abrasion, but also the stress generated by bite force; the bones, teeth, and muscles of the jaw form a unit that produces forces, which in turn can influence the evolution of jaw and tooth morphology. Studying how the mechanical demands of producing bite force have shaped jaws and teeth has been a powerful tool for understanding tooth morphology and reconstructing the feeding ecology of extinct animals.[90] Variation in diet and bite force are correlated with the morphology of the skull and teeth across species.[91–93] Tooth morphology is also correlated with ability to withstand bite force; the height of cusps relative to the width of the tooth, the distance of the cusp from the side of the tooth, the slope angle of the cusp sides, and enamel thickness are all predictive of a tooth’s ability to resist force without cracking.[94,95] Polyphyodont tooth replacement can also assist in the response of teeth to the way bite force requirements change with different food sources animals exploit throughout development. As alligator jaws grow, their replacement teeth become relatively rounder and better able to resist the force required to crush bone, matching the increased access to bony prey conferred by their larger body size.[96] These morphological changes between successive generations of teeth must involve modifications of dental stem cell regulation, which is an interesting topic for future investigation. Continued research on the ability of bite force and feeding performance to influence tooth evolution will be aided by efforts, discussed in the next section, focusing on the way forces at scales both large and small affect tooth development and adult dental stem cells.
4. Micro- and macro-forces regulate stem cell proliferation and tooth shape
While the importance of chemical signaling in tooth morphogenesis and maintenance, as revealed through both evolutionary approaches and conventional model systems, cannot be overstated, the mechanical forces involved in these processes are increasingly being recognized for their role in proper tooth formation through contributions to shaping the tooth germ and stem cell regulation.[97] Forces affecting teeth and their stem cells can be divided into two categories: local forces produced as a result of actomyosin tension and cell-cell/cell-matrix interactions and the large-scale forces generated through changes in the bone surrounding the tooth or mastication.[97,98] Improved technologies for modeling and measuring these forces at different scales, as well as live imaging techniques that enable observation of cellular changes related to tissue forces, have greatly contributed to understanding the mechanical stresses shaping dental tissues during development and stem cells in adult animals.
4.1. How do micro-forces contribute to dental epithelium invagination?
Molar development begins when dental epithelial progenitor cells migrate away from a rosette-like structure in the oral epithelium, as discussed above. Various developing tissues from different organisms,[99] including epithelia in Drosophila,[100] kidneys in Xenopus,[101] and neural tubes in vertebrates[102,103] also form from rosettes. In particular, large rosettes, such as those formed in neural cell culture, may have larger mechanical constraints, leading to enhanced radial migration.[104] How this guides tooth morphogenesis mechanically and functionally in vivo remains to be tested, but it may explain the more active and directed migration of dental epithelial cells within the rosette-like structure than neighboring non-rosette cells.[11]
Shortly after placode formation, dental epithelium begins to invaginate, a process central to the development of many ectodermally derived organs.[17] Experiments done in other tissues have shown that several different cellular behaviors can promote epithelial invagination, including apical constriction, basal relaxation, apical cable-driven buckling, and vertical telescoping.[105] However, these processes are usually associated with epithelial monolayers, while the developing tooth germ is stratified, and stratification alone is not sufficient to drive the downward bending of the tissue into the mesenchyme.[17]
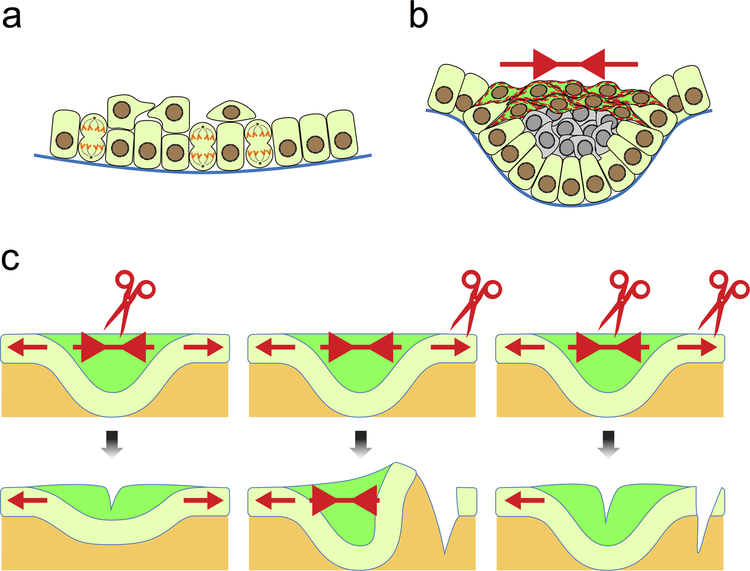
One potential mechanism for epithelial invagination is planar contraction of the suprabasal layer, creating lateral forces necessary for bending the epithelium (Fig. 3a and b). Indeed, recent findings showed increased actin bundles and phosphomyosin staining in horizontally elongated suprabasal cells of the developing molar, indicative of tensile forces distributed planarly within the suprabasal layer.[12] This was further demonstrated through experimentally cutting tissues to produce local relaxation; the direction, as well as the relative magnitude, of forces can be detected by observing how tissues restore force equilibrium through recoil. In the developing molar tooth germ, an incision within the suprabasal layer resulted in a bidirectional recoil pulling the epithelium away from the cut (Fig. 3c),[12] suggesting the suprabasal layer exerts a contraction force related to the downward bending of the tissue. Attachment of dental epithelium to the flanking non-dental epithelium would resist this contraction, as a lateral incision in this region caused further bending of the tooth germ. When the tensile force within the dental epithelium was first relieved through a suprabasal cut, the subsequent lateral incision was unable to induce tissue recoil and bending. Using live imaging, these authors showed that cell intercalation generates the observed contraction force. Some of the peripheral basal cells near the edge of the placode, intercalate with suprabasal cells and draw towards the center of the placode while still anchored to the basal lamina, effectively pulling on the basal layer, which bends in response to the contraction (Fig. 3b). This process also seals the top of the tooth germ, allowing cell proliferation below the constriction to further propel epithelial buckling toward the mesenchyme.
Fig. 3. Mechanical input for dental epithelial invagination.
a) Dental placode is formed as a result of vertical cell division, which generates suprabasal cells and thus the initial thickening of the epithelium. b) Basal cells (light green) at the edges of the placode intercalate with suprabasal cells, which also intercalate within themselves at the more apical portion of the placode (dark green). This creates a contractile tension (red arrows) and leads to the bending of the epithelium. The observation of thick actin bundles and strong phosphomyosin staining (shown as red dashed lines in dark green cells) reflects such tension in the epithelium. c) The tensional forces can also be detected by cutting the epithelium at different points. Cutting within the suprabasal layer relieves the contraction, resulting in a more relaxed and shallow tooth germ. Cutting through the adjacent oral epithelium causes the tooth germ to bend further as the contraction is no longer resisted. Cutting in both the suprabasal layer and the neighboring epithelium abrogates these effects, as the net force is again equivalent between the two regions.
From the signaling perspective, SHH is likely responsible for regulating the tissue contraction and epithelial bending, as chemical inhibition of Hh signaling hinders invagination, resulting in a shallower and wider tooth germ.[17]. Future experiments are required to examine how SHH controls this process and the intermediate steps leading to changes in cell shapes and force generation. It will also be interesting to measure more precisely the magnitude of the contractile force that narrows the apical tooth bud during molar invagination using recently developed techniques such as vinculin or oil microdroplet force sensors.[106,107] Finally, it should be noted that the incisor tooth germ does not undergo apical narrowing as in molars; whether incisor invagination is regulated by a similar process must be examined further.
4.2. Differential tissue growth generates force for tooth morphogenesis
The tooth begins to take its shape at the cap stage, deviating from the round bud structure while the non-EK epithelium elongates. This process is in part regulated by differential growth rates within the tissue.[24] Higher growth rates in the epithelium surrounding the EK than in the EK itself have been detected using live imaging of molar slice explants. The rapid growth around the EK could lead to higher pressure within the EK. In combination with the mechanical constraint of the underlying mesenchyme,[108] this differential growth rate likely resulted in the higher anisotropic deformation and buccal-lingual stretching observed in the EK.[24] As the tooth germ transitions from the cap to the bell stage, high anisotropy in the elongating epithelium, associated with increased actin-dependent cell motility and oriented cell division, propels the continued downward epithelial growth and extension. The mechanical influence of these structures on tooth morphogenesis remains unclear, and experiments focused on the interplay between mechanical forces and cell behavior during this process are needed. Indeed, theoretical models can only predict accurate tooth shapes after implementing the mechanical properties of the cells and tissues.[109,110]
Different from molars, in the single cuspid mouse incisor only a primary EK forms, through a de novo process from cells located in the posterior lower half of the incisor bud.[23,62] Incisor epithelium rotates posteriorly at the cap stage, instead of downwards as in molars. Molecularly, the formation of the incisor EK is dependent on alpha-catenin-mediated inhibition of YAP activity,[111] such that restriction of YAP in the cytoplasm allows cells to cease proliferation and become specialized in signal secretion. The regulation of YAP and tooth morphogenesis is also separable from the cell adhesion function of alpha-catenin. Given the roles of alpha-catenin and YAP in mechano-sensing,[112,113] it is plausible that these molecules may be involved in responding to changes in tissue pressure and compression due to differential growth at the bud to cap transition to control EK formation.
4.3. Do mechanical properties of dental mesenchyme direct cell differentiation and epithelial morphogenesis?
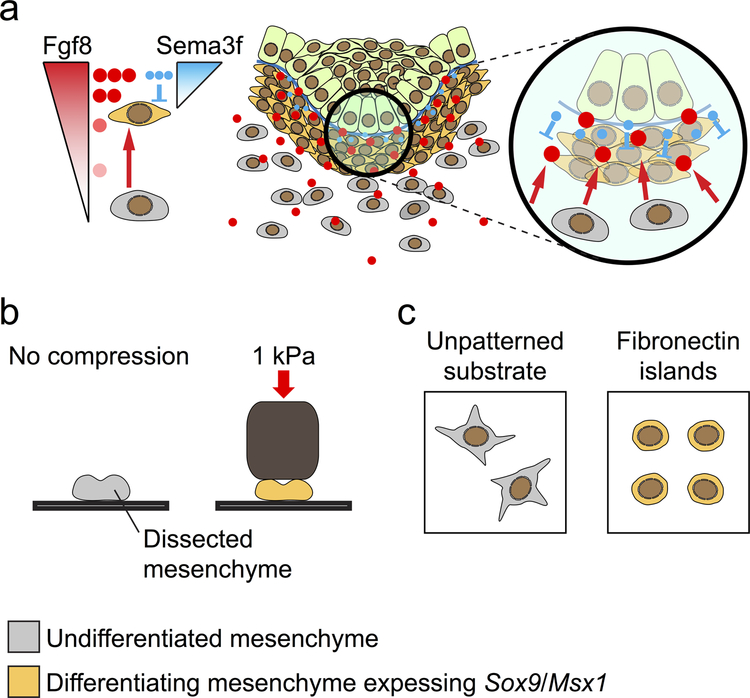
The reciprocal interaction between the epithelium and the underlying mesenchyme is critical for the regulation of tooth morphogenesis and cell proliferation and differentiation. One example during odontogenesis is the epithelial induction of mesenchymal differentiation at E12.5 in mice, when mesenchymal cells begin to condense around the developing tooth bud and express odontogenic markers Pax9 and Msx1.[114–116] Mesenchyme condenses similarly prior to the differentiation of cartilage, kidney, tendon, and feathers.[117] In these latter examples, condensation can be triggered by signaling molecules, such as BMPs,[118] and cell compaction may result from the new genetic identity imparted onto the differentiating cells. In developing molars, FGF8 secreted from the dental epithelium acts as a long-range chemo-attractant to draw mesenchymal cells towards the invaginating tooth bud.[119] The action of SEMA3F, a short-range repulsive signal, enhances cell aggregation at the epithelial-mesenchymal boundary, further crowding mesenchymal cells around the epithelium (Fig. 4a). These condensed cells have reduced RhoA activity and are in fact smaller than cells in non-condensed regions. When such physical constraint on cells is mimicked in culture, either by plating cells on micro-patterned adhesive islands or by directly compressing freshly dissected mandibular mesenchyme, cells begin to express the differentiation markers Pax9 and Msx1, even in the absence of FGF signal from the epithelium. Condensation thus provides a mechanical signal that is sufficient to induce mesenchymal differentiation (Fig. 4b and c). To sustain cell compaction and differentiation, mesenchymal condensation also induces the expression of collagen VI, which is stabilized by lysyl oxidase (LOX) and forms part of the extracellular matrix (ECM) in the mesenchyme. In the absence of a stabilized ECM scaffold, as in the case of LOX inhibition by b-aminopropionitrile (BAPN), mesenchymal condensation is reduced and Pax9 expression decreases.[120] Consequently, just as mesenchymal cell fate switching can be regulated by mechanical signals,[121] dental mesenchyme can also respond to the physical environment to initiate differentiation and odontogenesis.[9,119] Finally, in addition to being embedded in an ECM matrix that contains numerous collagen and laminin molecules, mesenchymal cells immediately adjacent to the epithelium are also in contact with the basement membrane made of matrix proteins, including fibronectin and tenascin, which may also contribute to mesenchymal differentiation.[122] Investigating the mechanical roles of different ECM components and how those signals are mediated to induce changes in cellular behavior and gene expression will be important for future research in this field.
Fig. 4. Mesenchymal differentiation is regulated by mechanical compression.
a) Dental epithelium secretes FGF8 and SEMA3F to induce mesenchymal condensation. FGF8 acts as a long-range chemoattractant (red arrows) to trigger directional movement of undifferentiated mesenchymal cells towards the epithelium. SEMA3F is a short-range repellant (blue blunted lines) and promotes further cell compaction around the invaginating dental epithelium. As a result, condensing mesenchymal cells are mechanically compressed and begin to express odontogenic markers, such as Sox9 and Msx1. b) Dissected mandibular mesenchyme can be induced to express odontogenic markers through direct mechanical compression. c) Restricting cell size by plating mesenchymal cells on micro-patterned fibronectin islands mimics mechanical compression and also results in the induction of Sox9/Msx1 expression.
Could changes in the mechanical property of the mesenchyme in turn modulate the folding of the overlying dental epithelium? The initial invagination of the dental epithelium appears to be tissue-autonomous and may not require much mechanical guidance from the mesenchyme.[12] However, it is possible that the dental mesenchyme provides mechanical cues for subsequent epithelial buckling, turning, and invagination. During the morphogenesis of feathers[123] and mouse gut villi,[124,125] as well as in engineered tissues,[126] mesenchyme plays an important role in driving the formation of local curvatures. A recent theoretical model posited that, during tooth morphogenesis, the mesenchyme serves as a mechanical constraint to aid the bud-to-cap deformation of the epithelium.[108] Consistent with this idea, abundant F-actin and phospho-myosin are present in the dental mesenchyme, indicative of its capability to impart mechanical inputs for the morphogenesis of the overlying dental epithelium. Further experiments are required to test these ideas and may provide invaluable information for developing strategies towards tooth bioengineering.
4.4. Macro-forces shape teeth, but contributions to stem cell replication remain unclear
In addition to local tissue forces, larger scale mechanical forces imparted by surrounding tissues or mastication are also involved in the positioning and morphology of teeth. Teeth cultured in vitro lose some of their species-specific morphology, such as offset cusps.[98] This effect was initially overlooked because commonly cultured mouse teeth have a subtle cusp offset; only attempts to culture vole teeth, with their strongly offset cusps, revealed this morphological change. Using computational models of different forces on developing teeth in conjunction with artificial mechanical constraints on teeth developing in culture, researchers showed that lateral compression on the tooth germ tissues, similar to the forces imposed by the bones that surround a developing tooth in vivo, was sufficient to form offset cusps, and even caused cusp offsets in mouse teeth.[98] In essence, although progenitor cells in developing teeth undergo morphogenesis on their own, external forces are nevertheless required to generate correct tooth shape; thus, the physical constraints imposed by human alveolar bone must also be considered in efforts toward therapeutic stem-cell driven tooth replacement.
Mechanical loading on teeth through mastication or orthodontics may also have implications for stem cell maintenance. Studies have shown that strain applied to extracellular matrix through orthodontic treatments results in increased inflammatory, osteogenic, and angiogenic responses, activating bone progenitor cells that reshape the bone surrounding the tooth and the periodontal ligament.[127–129] Given the role force places in stimulating changes in these tissues, it is reasonable to conclude they may also affect other aspects of tooth biology, such as tooth eruption. This notion is consistent with recent findings that SHH-secreting neurovascular bundles maintain dental mesenchymal homeostasis,[7] but these bundles may also provide mechanical loading through musculature to regulate stem cells. The observation that a subset of dental mesenchymal stem cells increase their rate of proliferation (and thus the rate of tooth eruption) after rodent incisors are cut prompted hypotheses that changes in the mechanical forces experienced by the clipped teeth through loss of occlusion were responsible.[130] However, reducing mechanical force from occlusion by clipping only one incisor produced no observed difference in growth rates, suggesting that loss of occlusion force was not sufficient to alter growth rates in mesenchymal cells.[130] Nonetheless, previous research has shown that molecular mechanisms of tooth eruption in ever-growing teeth may differ from those of teeth with finite growth.[131–133] Thus, whether occlusal dynamics and mechanical force influence stem cell proliferation in other tooth and cell types, such as epithelial stem cells of adult incisors, requires further investigation. Such a relationship would be consistent with the observation that proliferation of these stem cells is regulated through an integrin-YAP signaling axis capable of mechanotransduction.[134] Further research will clarify these questions and may aid the development of new strategies integrating chemical and mechanical signaling to control dental stem cell proliferation and differentiation for clinical applications.
5. Conclusions and outlook
Understanding the regulation of progenitor and stem cells during tooth development and renewal and the mechanisms governing the acquisition of correct tooth shapes and compositions is paramount to developing stem-cell-based therapies for human tooth regeneration. A great deal of work has gone into researching the signaling and genetic control of dental tissues, especially in model species like mice and rats; however, there are important insights to gain from studying the effects of evolution and mechanical forces in tooth morphogenesis and maintenance. Moving forward, advances in modeling and measuring macro- and micro-forces, live imaging of tissues with the aid of more sophisticated computational and genetic tools, comparative analyses of gene expression and genomics in non-model organisms, and the continued application of fossil evidence to understand the past and present of tooth development and stem cell regulation will be needed. Such studies will help address outstanding questions in dental biology, including how teeth acquire their shapes, how stem cells can be derived and maintained, how tooth replacement is regulated, and how root formation is controlled. These future explorations have the potential not only to reach a deeper understanding of organogenesis and stem cells, but also to lead to a better design of dental regenerative medicine.
Acknowledgments
We thank Rebecca Kim and the reviewers for helpful suggestions. Work in the Klein laboratory was funded by NIH R35-DE026602 to O.D.K. and K99-DE025874 to J.K.-H.H. The authors have no conflicts of interest to declare.
References
- [1].Tucker A, Sharpe P, Nat. Rev. Genet 2004, 5, 499. [DOI] [PubMed] [Google Scholar]
- [2].Jernvall J, Thesleff I, Development 2012, 139, 3487. [DOI] [PubMed] [Google Scholar]
- [3].Hu JK-H, Mushegyan V, Klein OD, Genesis 2014, 52, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim R, Green JBA, Klein OD, Dev. Dyn 2017, 246, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li J, Parada C, Chai Y, Development 2017, 144, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mitsiadis TA, Harada H, Regen. Med 2015, 10, 5. [DOI] [PubMed] [Google Scholar]
- [7].Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, Chai Y, Cell Stem Cell 2014, 14, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seidel K, Marangoni P, Tang C, Houshmand B, Du W, Maas RL, Murray S, Oldham MC, Klein OD, Elife 2017, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mu C, Lv T, Wang Z, Ma S, Ma J, Liu J, Yu J, Mu J, Biomed Res. Int 2014, 2014, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dunn RE, Strömberg CAE, Madden RH, Kohn MJ, Carlini AA, Science (80-. ) 2015, 347, 258. [DOI] [PubMed] [Google Scholar]
- [11].Prochazka J, Prochazkova M, Du W, Spoutil F, Tureckova J, Hoch R, Shimogori T, Sedlacek R, Rubenstein JL, Wittmann T, Klein OD, Dev. Cell 2015, 35, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Panousopoulou E, Green JBA, PLoS Biol 2016, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park S, Wang DH, Zhang D, Romberg E, Arola D, J. Mater. Sci. Mater. Med 2007, 19, 2317. [DOI] [PubMed] [Google Scholar]
- [14].Nanci A, Ten Cate’s Oral Histology, Elsevier, St. Louis, 2018. [Google Scholar]
- [15].Thesleff I, Hurmerinta K, Differentiation 1981, 18, 75. [DOI] [PubMed] [Google Scholar]
- [16].Mina M, Kollar EJ, Arch. Oral Biol 1987, 32, 123. [DOI] [PubMed] [Google Scholar]
- [17].Li J, Chatzeli L, Panousopoulou E, Tucker AS, Green JBA, Development 2016, 143, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mitsiadis TA, Development 2003, 130, 4451. [DOI] [PubMed] [Google Scholar]
- [19].Jernvall J, Åberg T, Kettunen P, Keränen S, Thesleff I, Development 1998, 125, 161. [DOI] [PubMed] [Google Scholar]
- [20].Butler PM, J. Dent. Res 1962, 41, 1261. [Google Scholar]
- [21].Kettunen P, Karavanova I, Thesleff I, Dev. Genet 1998, 22, 374. [DOI] [PubMed] [Google Scholar]
- [22].Thesleff I, Keranen S, Jernvall J, Adv. Dent. Res 2001, 15, 14. [DOI] [PubMed] [Google Scholar]
- [23].Du W, Hu JK-H, Du W, Klein OD, J. Biol. Chem 2017, 292, 15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morita R, Kihira M, Nakatsu Y, Nomoto Y, Ogawa M, Ohashi K, Mizuno K, Tachikawa T, Ishimoto Y, Morishita Y, Tsuji T, PLoS One 2016, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harada H, Kettunen P, Jung H-S, Mustonen T, Wang YA, Thesleff I, J. Cell Biol 1999, 147, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tummers M, Thesleff I, Development 2003, 130, 1049. [DOI] [PubMed] [Google Scholar]
- [27].Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H, Development 2002, 129, 1533. [DOI] [PubMed] [Google Scholar]
- [28].Yamashiro T, Tummers M, Thesleff I, J. Dent. Res 2003, 82, 172. [DOI] [PubMed] [Google Scholar]
- [29].Kumakami-Sakano M, Otsu K, Fujiwara N, Harada H, Exp. Cell Res 2014, 325, 78. [DOI] [PubMed] [Google Scholar]
- [30].Thomas HF, Kollar EJ, Arch. Oral Biol 1989, 34, 27. [DOI] [PubMed] [Google Scholar]
- [31].Ten Cate AR, Oral Dis 1996, 2, 55. [DOI] [PubMed] [Google Scholar]
- [32].Tummers M, Thesleff I, Evol. Dev 2008, 10, 187. [DOI] [PubMed] [Google Scholar]
- [33].Renvoisé E, Michon F, Front. Physiol 2014, 5 August, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tapaltsyan V, Eronen JT, Lawing AM, Sharir A, Janis C, Jernvall J, Klein OD, Cell Rep 2015, 11, 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].DeMiguel D, Fortelius M, BMC Evol. … 2008, 8, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Famoso, Feranec RS, Davis EB, Palaeogeogr. Palaeoclimatol. Palaeoecol 2013, 387, 211. [Google Scholar]
- [37].Selkin PA, Strömberg CAE, Dunn R, Kohn MJ, Carlini AA, Siân Davies-Vollum K, Madden RH, Geology 2015, 43, 567. [Google Scholar]
- [38].Janis CM, Ecol. Brows. grazing 2008. [Google Scholar]
- [39].Anquetin J, Antoine P-O, Tassy P, Zool. J. Linn. Soc 2007, 151, 577. [Google Scholar]
- [40].Robovský Y, Řičánková V, Zrzavý J, Zool. Scr 2008, 37, 571. [Google Scholar]
- [41].Gatesy J, Arctander P, Syst. Biol 2000, 49, 515. [DOI] [PubMed] [Google Scholar]
- [42].Huysseune A, Sire JY, Witten PE, J. Appl. Ichthyol 2010, 26, 152. [Google Scholar]
- [43].Witten PE, Sire JY, Huysseune A, J. Appl. Ichthyol 2014, 30, 636. [Google Scholar]
- [44].Smith MM, Johanson Z, in Gt. Transform. Vertebr. Evol (Eds.: Dial KP, Shubin N, Brainerd EL), The University Of Chicago Press, Chicago and London, 2015, pp. 9–29. [Google Scholar]
- [45].Soukup V, Epperlein HH, Horácek I, Cerny R, Nature 2008, 455, 795. [DOI] [PubMed] [Google Scholar]
- [46].Ohazama A, Haworth KE, Ota MS, Khonsari RH, Sharpe PT, Genesis 2010, 48, 382. [DOI] [PubMed] [Google Scholar]
- [47].Atukorala ADS, Inohaya K, Baba O, Tabata MJ, Ratnayake RAR, Abduweli D, Kasugai S, Mitani H, Takano Y, Arch. Histol. Cytol 2011, 73, 139. [DOI] [PubMed] [Google Scholar]
- [48].Rothova M, Thompson H, Lickert H, Tucker AS, Dev. Dyn 2012, 241, 1183. [DOI] [PubMed] [Google Scholar]
- [49].Donoghue PCJ, Rücklin M, Evol. Dev 2016, 18, 19. [DOI] [PubMed] [Google Scholar]
- [50].Fraser GJ, Hulsey CD, Bloomquist RF, Uyesugi K, Manley NR, Streelman JT, PLoS Biol 2009, 7, 0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fraser GJ, Cerny R, Soukup V, Bronner-Fraser M, Streelman JT, BioEssays 2010, 32, 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Martin KJ, Rasch LJ, Cooper RL, Metscher BD, Johanson Z, Fraser GJ, Proc. Natl. Acad. Sci 2016, 113, 14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rasch LJ, Martin KJ, Cooper RL, Metscher BD, Underwood CJ, Fraser GJ, Dev. Biol 2016, 415, 347. [DOI] [PubMed] [Google Scholar]
- [54].Sun Z, Yu W, Sanz Navarro M, Sweat M, Eliason S, Sharp T, Liu H, Seidel K, Zhang L, Moreno M, Lynch T, Holton NE, Rogers L, Neff T, Goodheart MJ, Michon F, Klein OD, Chai Y, Dupuy A, Engelhardt JF, Chen Z, Amendt BA, Development 2016, 143, 4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Juuri E, Jussila M, Seidel K, Holmes S, Wu P, Richman J, Heikinheimo K, Chuong C-M, Arnold K, Hochedlinger K, Klein O, Michon F, Thesleff I, Development 2013, 140, 1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li J, Feng J, Liu Y, Ho T-V, Grimes W, Ho HA, Park S, Wang S, Chai Y, Dev. Cell 2015, 33, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lumsden AGS, Development 1988, 103 Supple, 155. [DOI] [PubMed] [Google Scholar]
- [58].Osumi-Yamashita N, Ninomiya Y, Doi H, Eto K, Dev. Biol 1994, 164, 409. [DOI] [PubMed] [Google Scholar]
- [59].Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM, Development 2000, 127, 1671. [DOI] [PubMed] [Google Scholar]
- [60].Sharpe PT, Development 2016, 143, 2273. [DOI] [PubMed] [Google Scholar]
- [61].Jernvall J, Thesleff I, Mech. Dev 2000, 92, 19. [DOI] [PubMed] [Google Scholar]
- [62].Ahtiainen L, Uski I, Thesleff I, Mikkola ML, J. Cell Biol 2016, 214, DOI 10.1083/jcb.201512074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR, Dev. Cell 2006, 11, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ahn Y, Sanderson BW, Klein OD, Krumlauf R, Development 2010, 137, 3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Järvinen E, Shimomura-Kuroki J, Balic A, Jussila M, Thesleff I, Development 2018, dev. 158048. [DOI] [PubMed] [Google Scholar]
- [66].Chen J, Lan Y, Baek JA, Gao Y, Jiang R, Dev. Biol 2009, 334, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Andl T, Reddy ST, Gaddapara T, Millar SE, Dev. Cell 2002, 2, 643. [DOI] [PubMed] [Google Scholar]
- [68].Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I, Proc. Natl. Acad. Sci 2006, 103, 18627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE, Dev. Biol 2008, 313, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang X-P, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, Kucherlapati R, Maas RL, Development 2009, 136, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yokohama-Tamaki T, Ohshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, Ohuchi H, Harada H, Development 2006, 133, 1359. [DOI] [PubMed] [Google Scholar]
- [72].Huysseune A, Thesleff I, BioEssays 2004, 26, 665. [DOI] [PubMed] [Google Scholar]
- [73].Smith MM, Fraser GJ, Mitsiadis TA, J. Exp. Zool. Mol. Dev. Evol 2009, 312B, 260. [DOI] [PubMed] [Google Scholar]
- [74].Tucker AS, Fraser GJ, Semin. Cell Dev. Biol 2014, 25–26, 71. [DOI] [PubMed] [Google Scholar]
- [75].Seppala M, Fraser G, Birjandi A, Xavier G, Cobourne M, J. Dev. Biol 2017, 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang F, Crompton AW, Luo Z-X, Schaff CR, Vertebr. Pal Asiat 1998, 36, 197—217. [Google Scholar]
- [77].Luo Z-X, Kielan-Jaworowska Z, Cifelli RL, Bull. Carnegie Museum Nat. Hist 2004, 36, 159. [Google Scholar]
- [78].O’Meara RN, Asher RJ, Paleobiology 2016, 42, 439. [Google Scholar]
- [79].Edmund AG, Life Sci. Div. R. Ontario Museum 1960, 52, 190. [Google Scholar]
- [80].Peyer B, Comparative Odontology, University Of Chicago Press, Chicago, 1968. [Google Scholar]
- [81].LeBlanc ARH, Reisz RR, Brink KS, Abdala F, J. Clin. Periodontol 2016, 43, 323. [DOI] [PubMed] [Google Scholar]
- [82].Meng QJ, Grossnickle DM, Liu D, Zhang YG, Neander AI, Ji Q, Luo ZX, Nature 2017, 548, 291. [DOI] [PubMed] [Google Scholar]
- [83].Panciroli E, Benson RBJ, Walsh S, Pap. Palaeontol 2017, 3, 373. [Google Scholar]
- [84].Hautier L, Gomes Rodrigues H, Billet G, Asher RJ, Sci. Rep 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gomes Rodrigues H, Herrel A, Billet G, Proc. Natl. Acad. Sci 2017, 114, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Gomes Rodrigues H, Lefebvre R, Fernández-Monescillo M, Quispe BM, Billet G, Soc R. Open Sci 2017, 4, DOI 10.1098/rsos.170494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Stebbins GL, Ann. Missouri Bot. Gard 1981, 68, 75. [Google Scholar]
- [88].Kohn MJ, Strömberg CAE, Madden RH, Dunn RE, Evans S, Palacios A, Carlini AA, Palaeogeogr. Palaeoclimatol. Palaeoecol 2015, 435, 24. [Google Scholar]
- [89].Strömberg CAE, Annu. Rev. Earth Planet. Sci 2011, 39, 517. [Google Scholar]
- [90].Tseng ZJ, Flynn JJ, PLoS One 2015, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Maestri R, Patterson BD, Fornel R, Monteiro LR, de Freitas TRO, J. Evol. Biol 2016, 29, 2191. [DOI] [PubMed] [Google Scholar]
- [92].Dollion AY, Measey GJ, Cornette R, Carne L, Tolley KA, da Silva JM, Boistel R, Fabre AC, Herrel A, Funct. Ecol 2017, 31, 671. [Google Scholar]
- [93].Tseng ZJ, Flynn JJ, Sci. Adv 2018, 4, eaao5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Constantino PJ, Bush MB, Barani A, Lawn BR, Soc JR. Interface 2016, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chai H, Acta Biomater 2018, 75, 279. [DOI] [PubMed] [Google Scholar]
- [96].Gignac PM, Erickson GM, J. Zool 2015, 295, 132. [Google Scholar]
- [97].Li D, Zhou J, Chowdhury F, Cheng J, Wang N, Wang F, Regen. Med 2011, 6, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Renvoisé E, Kavanagh KD, Lazzari V, Häkkinen TJ, Rice R, Pantalacci S, Salazar-Ciudad I, Jernvall J, Proc. Natl. Acad. Sci 2017, 114, 9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Harding MJ, McGraw HF, Nechiporuk A, Development 2014, 141, 2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Blankenship JT, Backovic ST, Sanny JSP, Weitz O, Zallen JA, Dev. Cell 2006, 11, 459. [DOI] [PubMed] [Google Scholar]
- [101].Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G, Nat. Genet 2012, 44, 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Afonso C, Henrique D, J. Cell Sci 2006, 119, 4293. [DOI] [PubMed] [Google Scholar]
- [103].Haigo SL, Hildebrand JD, Harland RM, Wallingford JB, Curr. Biol 2003, 13, 2125. [DOI] [PubMed] [Google Scholar]
- [104].Ziv O, Zaritsky A, Yaffe Y, Mutukula N, Edri R, Elkabetz Y, PLoS Comput. Biol 2015, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Pearl EJ, Li J, Green JBA, Philos. Trans. R. Soc. B Biol. Sci 2017, 372, DOI 10.1098/rstb.2015.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Campàs O, Mammoto T, Hasso S, Sperling RA, Connell O, Bischof AG, Maas R, Weitz DA, Nat. Methods 2014, 11, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA, Nature 2010, 466, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Takigawa-Imamura H, Morita R, Iwaki T, Tsuji T, Yoshikawa K, J. Theor. Biol 2015, 382, 284. [DOI] [PubMed] [Google Scholar]
- [109].Salazar-Ciudad I, Curr. Top. Dev. Biol 2008, 81, 341. [DOI] [PubMed] [Google Scholar]
- [110].Salazar-Ciudad I, Jernvall J, Nature 2010, 464, 583. [DOI] [PubMed] [Google Scholar]
- [111].Li CY, Hu J, Lu H, Lan J, Du W, Galicia N, Klein OD, Nat. Commun 2016, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S, Nature 2011, 474, 179. [DOI] [PubMed] [Google Scholar]
- [113].Lecuit T, Nat. Cell Biol 2010, 12, 522. [DOI] [PubMed] [Google Scholar]
- [114].Peters H, Neubüser A, Kratochwil K, Balling R, Genes Dev 1998, 12, 2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Satokata I, Maas R, Nat. Genet 1994, 6, 348. [DOI] [PubMed] [Google Scholar]
- [116].Vainio S, Karavanova I, Jowett A, Thesleff I, Cell 1993, 75, 45. [PubMed] [Google Scholar]
- [117].Hall BK, Miyake T, BioEssays 2000, 22, 138. [DOI] [PubMed] [Google Scholar]
- [118].Lim J, Tu X, Choi K, Akiyama H, Mishina Y, Long F, Dev. Biol 2015, 400, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mammoto T, Mammoto A, Torisawa Y, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D, Ingber DE, Dev. Cell 2011, 21, 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mammoto T, Mammoto A, Jiang A, Jiang E, Hashmi B, Ingber DE, Dev. Dyn 2015, 244, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Engler AJ, Sen S, Sweeney HL, Discher DE, Cell 2006, 126, 677. [DOI] [PubMed] [Google Scholar]
- [122].Thesleff I, Vainio S, Jalkanen M, Int. J. Dev. Biol 1989, 33, 91. [PubMed] [Google Scholar]
- [123].Shyer AE, Rodrigues AR, Schroeder GG, Kassianidou E, Kumar S, Harland RM, Science (80-. ) 2017, 357, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Walton KD, Kolterud Å, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL, Proc. Natl. Acad. Sci 2012, 109, 15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Walton KD, Whidden M, Kolterud Å, Shoffner SK, Czerwinski MJ, Kushwaha J, Parmar N, Chandhrasekhar D, Freddo AM, Schnell S, Gumucio DL, Development 2016, 143, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hughes AJ, Miyazaki H, Coyle MC, Zhang J, Laurie MT, Chu D, Vavrušová Z, Schneider RA, Klein OD, Gartner ZJ, Dev. Cell 2018, 44, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Howard P, Kucich U, Taliwal R, Korostoff J, J Periodontal Res 1998, November; 33, 500. [DOI] [PubMed] [Google Scholar]
- [128].Miyagawa A, Chiba M, Hayashi H, Igarashi K, J. Dent. Res 2009, 88, 752. [DOI] [PubMed] [Google Scholar]
- [129].Feller L, Khammissa RAG, Schechter I, Thomadakis G, Fourie J, Lemmer J, Sci. World J 2015, Article ID, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].An Z, Sabalic M, Bloomquist RF, Fowler TE, Streelman T, Sharpe PT, Nat. Commun 2018, 9, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cielinski MJ, Jolie M, Wise GE, Marks SC, Connect. Tissue Res 1995, 32, 165. [DOI] [PubMed] [Google Scholar]
- [132].Wise GE, Grier IV RL, Lumpkin SJ, Zhang Q, Clin. Anat 2001, 14, 204. [DOI] [PubMed] [Google Scholar]
- [133].Wise GE, King GJ, J. Dent. Res 2008, 87, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hu JKH, Du W, Shelton SJ, Oldham MC, DiPersio CM, Klein OD, Cell Stem Cell 2017, 21, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gillette R, Am. J. Anat 1955, 96, 1. [DOI] [PubMed] [Google Scholar]