Abstract
Drugs that target monoaminergic transmission represent a first-line treatment for major depression. Though a full understanding of the mechanisms that underlie antidepressant efficacy is lacking, evidence supports a role for enhanced excitatory transmission. This can occur through two non-mutually exclusive mechanisms. The first involves increased function of excitatory neurons through relatively direct mechanisms such as enhanced dendritic arborization. Another mechanism involves reduced inhibitory function, which occurs with the rapid antidepressant ketamine. Consistent with this, GABAergic interneuron-mediated cortical inhibition is linked to reduced gamma oscillatory power, a rhythm also diminished in depression. Remission of depressive symptoms correlates with restoration of gamma power.
Due to strong excitatory input, reliable GABA release and fast firing, PV-expressing neurons (PV neurons) represent critical pacemakers for synchronous oscillations. PV neurons also represent the predominant GABAergic population enveloped by perineuronal nets (PNNs), lattice-like structures that localize glutamatergic input. Disruption of PNNs reduces PV excitability and enhances gamma activity.
Studies suggest that monoamine reuptake inhibitors reduce integrity of the PNN. Mechanisms by which these inhibitors reduce PNN integrity, however, remain largely unexplored. A better understanding of these issues might encourage development of therapeutics that best upregulate PNN modulating proteases.
We observe that the serotonin/norepinephrine reuptake inhibitor venlafaxine increases hippocampal MMP-9 levels as determined by ELISA and concomitantly reduces PNN integrity in murine hippocampus as determined by analysis of sections following their staining with a fluorescent PNN-binding lectin. Moreover, venlafaxine treated mice (30 mg/kg/day) show an increase in carbachol-induced gamma power in ex vivo hippocampal slices as determined by local field potential recording and Matlab analyses. Studies with mice deficient in matrix metalloproteinase-9 (MMP-9), a protease linked to PNN disruption in other settings, suggest that MMP-9 contributes to venlafaxine-enhanced gamma power. In conclusion, our results support the possibility that MMP-9 activity contributes to antidepressant efficacy through effects on the PNN that may in turn enhance neuronal population dynamics involved in mood and/or memory.
Graphical Abstract
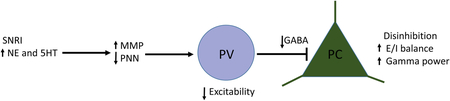
VI. Summary schematic
The serotonin/norepinephrine reuptake inhibitor venlafaxine can increase brain levels of serotonin (5-hydroxytryptamine or 5HT) and norepinephrine (NE) to increase the expression of perineuronal net (PNN) degrading matrix metalloproteinases (MMPs). Consistent with this hypothesis, results shown herein demonstrate that venlafaxine increases hippocampal levels of MMP-9. Attenuation of the PNN may reduce the excitability of parvalbumin (PV) expressing, gamma-aminobutyric acid (GABA) releasing, interneurons to affect an overall increase in pyramidal cell (PC) activity. Increased PC activity may in turn increase excitatory/inhibitory (E/I) balance and gamma power. Results shown herein also support this hypothesis in that venlafaxine reduces PNN integrity and increases carbachol-induced gamma power in ex vivo hippocampal slices. Since increased gamma power correlates with remission from a depressive phenotype, these findings imply that MMP activity could contribute to antidepressant efficacy. These findings also suggest it may be worthwhile to develop and test novel therapeutics that can target the PNN in a circuit specific or adaptive manner.
Introduction
Major depressive disorder (MDD) is a debilitating condition that affects ~12–17% of individuals in the U.S.at some point during their lifetime. This places a substantial burden on those affected and on family and friends. Moreover, untreated depression may increase one’s risk for substance abuse and Alzheimer’s disease (Ownby et al. 2006, Quello et al. 2005).
While a full understanding of molecular underpinnings is lacking, studies suggest that depression is linked to reductions in the strength of glutamatergic synaptic subsets in regions including hippocampus and prefrontal cortex (PFC). Animal models have shown changes including reduced GluA1 expression in layer V pyramidal cells, and impaired hippocampal long-term potentiation (LTP) [reviewed in (Thompson et al. 2015)].
In terms of effective treatment, one mechanism by which antidepressants may enhance glutamatergic transmission involves generation of new glutamatergic synapses. Animal models support this as successful treatment has been linked to an increase in the number of dendritic spines, which represent post-synaptic processes for the majority of excitatory synapses in the central nervous system (Chen et al. 2010). Enhanced dendritic spine number could potentially follow from antidepressant-mediated increases in neurotrophins.
A non-mutually exclusive mechanism by which primary or adjunct therapeutics could improve glutamatergic signaling is through reduced inhibition of glutamatergic neurons. This possibility is supported by the rapid antidepressant activity of ketamine, which can stimulate disinhibition by acting as a preferential antagonist for GluNs localized to inhibitory interneurons (Thompson et al. 2015). Importantly, new research has shown that negative allosteric modulation of the α5 GABA-A receptor subunit increases electroencephalographic gamma power (Zanos et al. 2017), a rhythm that is reduced in human depression and an animal model of depression, and also normalized with remission (Khalid et al. 2016, Fitzgerald & Watson 2018). The same allosteric modulator concomitantly improves performance in the forced swim test (Zanos et al. 2017).
An alternative means to affect cortical disinhibition is through manipulation of the perineuronal net (PNN), a lattice like extracellular matrix that is predominantly localized to parvalbumin (PV) expressing interneurons (Lensjo et al. 2017a, Sorg et al. 2016). The PNN facilitates properly localized glutamatergic input to PV cells (Frischknecht et al. 2009), thus enhancing PV mediated inhibition. Disruption of the PNN leads to increased lateral diffusion of glutamate receptor subunits on PV cells (Frischknecht et al. 2009), and it may also result in increased diffusion of glutamate. Consistent with this, enzymatic degradation of the PNN reduces the frequency of excitatory post synaptic currents in hippocampal fast spiking interneurons (Hayani et al. 2018). Recent work also suggests that PNN disruption can enhance cortical excitation and gamma activity (Lensjo et al. 2017b).
In terms of antidepressant therapy and the PNN, it is of interest that chronic treatment with fluoxetine decreases PNN staining in murine medial PFC and hippocampus (Ohira et al. 2013). Recently published work has also shown that increased PNN staining in a chronic stress-induced rat model of depression (Riga et al. 2017).
A relatively unexplored question is whether PNN reduction makes a substantial contribution to antidepressant efficacy and, if so, whether therapeutics or combinations beyond fluoxetine may best achieve this. The significance of this possibility is underlined by the widespread distribution of PNN enwrapped PV interneurons. As opposed to GABA-A α5 subunits, which have a depression-relevant but relatively restricted expression in forebrain, PNN enveloped PV neurons are found in multiple brain areas important to mood and memory.
Studies that have examined PNN disruption for its potential to mediate cortical disinhibition have typically employed hyaluronidase, which targets the PNN backbone, or chondroitinase, which targets chrondroitin sulfate proteoglycan side chains. Physiologically relevant modulators of PNN integrity are, however, expressed in the central nervous system. Important among these modulators are proteases belonging to the metalloproteinase (MMP) family.
MMP-9, MMP-13, and select transmembrane MMPs have been well linked to PNN component cleavage in vivo (Lorenzo Bozzelli et al. 2018). For example, light reintroduction after dark exposure triggers MMP-9 dependent PNN degradation (Murase et al. 2017). In addition, seizure activity has been shown to increase MMP-9 levels and PNN breakdown while pretreatment with a broad-spectrum MMP inhibitor abrogates these effects (Pollock et al. 2014). Further, in a murine model of Fragile X syndrome, selective genetic reduction of MMP-9 also reduces PNN remodeling and restores PNN formation surrounding PV cells in the auditory cortex (Wen et al. 2017).
Of interest, PNN degrading MMPs may be upregulated by monoamines or reuptake inhibitors (Bijata et al. 2017). Consistent with this possibility is work demonstrating increased MMP-9 expression in venlafaxine treated rats (Tamasi et al. 2014). While effects of specific monoamines on MMP release from brain-derived cells have not been extensively studied, in non-neural cells, norepinephrine has been shown to enhance MMP expression as well as MMP dependent endpoints (Yang et al. 2006).
In the present study, we focus on the question of whether one of the most commonly prescribed antidepressants, venlafaxine, can stimulate PNN proteolysis and disinhibition as measured by its ability to upregulate carbachol-induced gamma power. Our studies focus on the hippocampus, a region increasingly implicated in depression (MacQueen & Frodl 2011). We also explore a role for MMP-9 in venlafaxine-stimulated disinhibition
Materials and Methods
Chemicals and reagents
Venlafaxine and carbamoylcholine chloride (carbachol) were purchased from Sigma Chemical (St. Louis, MO; catalogue numbers PHR1736 and C4382). Carbachol was maintained in frozen 50 mM aliquots and reconstituted in ACSF to a final concentration of 40 μM just prior to slice treatment.
Subjects and drug administration
Both male and female C57BL/6J (Jackson Laboratory, RRID:IMSR_JAX:000664) or MMP-9 homozygous null mice (Jackson Laboratory, RRID:IMSR_JAX:007084) that had been backcrossed to C57BL/6J mice for at least five generations were used for experiments. Experimental groups were matched in terms of the male to female ratio for each comparison and animals arbitrarily assigned to groups. Both mouse strains were bred in-house. Food and water were provided throughout life ad libitum. At approximately 4 weeks of age, a time at which PNN and/or PNN component deposition is complete in varied brain regions including hippocampus (Noguchi et al. 2017, Lensjo et al. 2017a) mice were treated for 2 weeks with saline or saline containing 30 mg/kg venlafaxine. While lower doses act predominantly on serotonin reuptake, this is a dose that has been shown to reduce uptake of both serotonin and norepinephrine (David et al. 2003). Treatments were delivered in a total volume of 200 μl and administered daily by intraperitoneal injection. To minimize animal stress and suffering, and to provide expert and consistent administration across subjects, we hired an experienced veterinary technician to deliver saline or venlafaxine. Animals were quickly scruffed, their heads tilted downwards so that organs would move forwards, and then injected in the lower left or right quadrant via a small needle (26 and 3/8 gauge). The injection site altered between right and left sides with each day. Since the injection was brief (1–2 seconds), no pain medication was administered during the pretreatment or treatment period. Cages were equipped with balconies and nesting material for enrichment and no animals were single housed. Experiments were performed in accordance with National Institutes of Health guidelines and an institutionally approved protocol (Number 2016–1117). For euthanasia, animals from which brains were harvested for immunostaining or protein lysates were rendered unconscious via CO2 inhalation in a veterinary medicine established and approved chamber/delivery rate that was available to us in the surgical suite of the Department of Comparative Medicine (DCM). Following apparent loss of consciousness and an inability to respond to strong paw pinching, animals were rapidly decapitated. For euthanasia in advance of slice physiology experiments, animals were rendered unconscious/insensate in the laboratory using a DCM approved isoflurane chamber and protocol. When unconscious and unresponsive to pain animals were rapidly decapitated.
Preparation of brain lysates and ELISA
Following saline or venlafaxine treatment and euthanasia, hemi brains were dissected and hippocampi were extracted and lysed in RIPA buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 0.1% SDS, 1% IGEPAL, and 1X protease and phosphatase cocktail (Thermo Scientific 1861281)]. Lysates were sonicated for 10 seconds, placed on ice for 20 minutes, and centrifuged for 15 minutes at 14,000 rpm in 4 °C. Lysate supernatants were saved for analysis. Pro-MMP9 protein concentration in hippocampal lysates were measured by ELISA, performed according to the manufacturer’s protocol (Mouse Pro-MMP-9, R&D systems, catalogue number MMP900B). Of note, ELISA of lysates from MMP-9 null animals, in which the same amount of total protein was loaded as had been for wild types, showed no detectable signal (data not shown).
PNN staining and microscopy
Following saline or venlafaxine treatment and euthanasia, hemi brains were fixed overnight in 4% PFA/sucrose at 4° C. Brains were then paraffin-embedded and sectioned at 15 microns. Sections were washed 2–3 times with 1X-PBS, permeabilized with 1X-PBS containing .1% Triton X-100, blocked with 10% normal goat serum, and incubated with anti-parvalbumin (1:500, Sigma, P3088) overnight at 4° C. Following subsequent washes with 1x phosphate buffered saline (PBS) and incubation with a fluorescent secondary antibody for PV immunostaining and fluorescein-labeled Wisteria floribunda lectin (WFA) (1:1000, Vector Laboratories, FL-1351) for 2 hours at room temperature, sections were washed three times with 1X-PBS, counterstained with DAPI and mounted with Hydromount (National Diagnostics, HS-106) and allowed to dry several days at 4° C prior to confocal imaging.
PV and PNN cell quantification
Images were acquired using a Leica SP8 laser scanning confocal microscope with an oil immersion, 20X objective with .40 numerical aperture. Laser intensity, gain, and pinhole settings were kept constant for all samples. Images were taken through a z-plane (8.5 μm) within the center of the tissue section, containing 20 stacks (0.4 μm/stack) from the dorsal hippocampus. Quantification of PV numbers with and without an associated PNN were counted for each image that was acquired from regions of interest (ROI) using 5–8 mice per group, and 1–3 slides per mouse (total of 13–15 images/group). Due to regional differences in PNN intensity, comparable regions were blindly analyzed for each animal. K.C. labeled the slides in a coded manner and S.A. performed analyses before being un-blinded. For PNN intensity, a semi-automated analysis, “PIPSQUEAK” macro plugin, was used [described in (Slaker et al. 2016)]. The macro uses a region of interest (ROI) protocol for double-labeled cell based quantification. After background subtraction within an appropriate radius, WFA or PV cells were identified based on threshold limit requirements. ROIs were identified around WFA labeled PV expressing cells and the mean intensity was measured. 7–8 mice were used per group and data points represent averages for ROIs from 1–2 images from each of these animals.
Slice preparation
Following treatment with vehicle (saline) or venlafaxine mice were anaesthetized with deep isoflourane inhalation and then rapidly decapitated. The whole brain was subsequently removed and chilled in cold (0° C) sucrose-based cutting artificial cerebrospinal fluid (sACSF) containing (in mM) 252 sucrose; 3 KCL; 2 CaCl2; 2 MgSO4; 1.25 NaH2PO4; 26 NaHCO3; 10 dextrose and bubbled by 95% O2, 5% CO2. Hippocampal slices (480 um thick unless otherwise indicated) were cut in horizontal sections from dorsal to ventral brain with a vibratome (Leica, VT1000S). Dorsal and ventral most slices were excluded due to potential PNN density differences along this axis. ACSF contained (in mM) NaCl, 132; KCl, 3; CaCl2, 2; MgSO4, 2; NaH2PO4, 1.25; NaHCO3, 26; dextrose 10; and saturated with 95% O2, 5% CO2 at 26° C. Slices were incubated for at least 120 minutes before being moved to the recording chamber.
Local field potential (LFP) recordings
Low resistance glass microelectrodes (approximately 150K tip resistance) were used for LFP recordings of gamma frequency oscillations. Electrodes were filled with 1M NaCl in 1% agar, which prevents leakage of the electrode solution that could potentially alter conditions at the recording site. The recordings were done in a submerged chamber, and slices were perfused on both sides at a high flow rate (20 ml /min). Recordings were performed in CA1 stratum oriens proximal to CA2. For exposure of slices to carbachol, infusion was switched from ACSF to ACSF containing carbachol at 40 μM. After 150 seconds, the next 315 seconds were used for analysis (see next section).
Local Field Potential (LFP) Analysis
The LFP signal was analyzed in 315s blocks, beginning 150s after the onset of carbachol perfusion. Analysis was performed with a custom MATLAB algorithm available on request from Adam Caccavano or Katherine Conant (ac1625@georgetown.edu; kec84@georgetown.edu). A Gaussian FIR band-pass filter with corrected phase delay was applied between 1–1000Hz, after which additional band-pass filters were applied for theta (4–12Hz), low gamma (25–55Hz), and high gamma (65–85Hz) ranges. The power was computed by integrating the entire 315s band-pass filtered signals.
Statistical analysis
Sample size was not predetermined but based on experience indicating that 4–8 animals per group are typically necessary to observe physiologically relevant changes in MMP levels. Data was entered into a Graph Pad Prism 7.0 program and statistical analysis performed using Student’s two tailed unpaired t-test for 2 group comparisons or ANOVA for comparisons of more than 2 groups. Significance was set at p </= 0.05. Shapiro-Wilk normality testing was performed to confirm a normal distribution for tests that were based on the assumption of a normal distribution and ROUT testing was performed to identify outliers. We include “n” as the number of separate animals and “N” as the number of total slides or slices analyzed per animal per group. No animals were excluded from analysis.
Results
I. MMP-9 protein levels are increased, and PNN immunoreactivity reduced, in venlafaxine treated mice
Previous studies have linked increased expression of molecules including brain derived neurotrophic factor (BDNF) to monoamine reuptake inhibitor effects (Nibuya et al. 1996). This work does not, however, preclude a significant role for additional molecular effectors of antidepressant efficacy. Previously published data has shown that venlafaxine is linked to increased mRNA expression of MMP-9 in the rat brain (Tamasi et al. 2014). With respect to the potential to disrupt PV to pyramidal cell input, which would likely have a profound influence on widespread population activity, MMP-9 has also been shown to diminish PNN integrity (Murase et al. 2017, Wen et al. 2017). Since MMP-9 has been linked to reductions in PNN levels in the setting of both FXS (Wen et al. 2017) and light reintroduction following dark exposure (Murase et al. 2017), we examined PNN levels in control and venlafaxine treated mice.
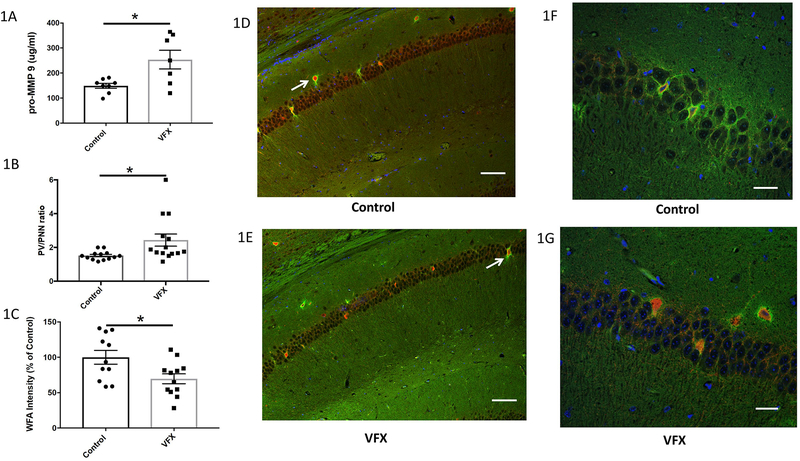
Hippocampal lysates were analyzed by ELISA for levels of MMP-9. As shown in figure 1A, venlafaxine treatment significantly increased hippocampal concentrations of this enzyme (n=7–8; p=0.0129; Student’s t-test). For results shown in figure 1B, hippocampal sections were stained for both PV and PNN immunoreactivity and the ratio of PV positive to PNN positive cells quantified. Though not numerous in terms of overall cell number in the hippocampus, it is important to note that each PV expressing neuron can make inhibitory contact with hundreds of pyramidal cells (Anderson et al. 2006). In venlafaxine treated animals, the PV/PNN ratio was significantly increased (n=8; N= 13–14 p=0.023; Student’s t-test). PV numbers were not increased (control 8.15 +/− 0.87; venlafaxine 7.93 +/− 0.85; p= 0.84), suggesting that PNN positivity was selectively reduced. In figure 1C we show results for WFA fluorescence intensity using a published protocol (Slaker et al. 2016) in control and venlafaxine treated mouse hippocampi. This technique also demonstrated a significant difference between groups (p=0.0187). Hippocampal PV and PNN staining for slice subsections are shown in figures 1D and E. The scale bar represents 75 μm and representative PNN enwrapped PV cells are indicated by arrows. High power images are shown in 1F and 1G. The scale bar in these images represents 25 μm.
I. MMP-9 levels and PNN immunoreactivity in hippocampi from venlafaxine treated mice.
1A) MMP-9 concentrations in brain lysates from 8 control and 7 venlafaxine (VFX) treated mice. Venlafaxine treatment significantly increased hippocampal concentrations of this enzyme (Control 149.4 +/− 9.9; VFX 253.8 +/− 37.3; p=0.0129; Student’s t-test). IB) A quantitative analysis of PV/PNN ratios from control and venlafaxine treated animals (n=8 animals and N= 13–14 slides for each group) revealed a statistically significant increase in the venlafaxine group (p=0.023; Student’s t-test). PV numbers were not increased (control 8.15 +/− 0.87; venlafaxine 7.93 +/− 0.85; p= 0.84; not shown graphically), suggesting that PNN positivity was selectively reduced. 1C) WFA fluorescence intensity in control and venlafaxine treated mouse hippocampi shows a reduction in the venlafaxine treated group (*p= 0.0187, n= 7–8 mice; N data points each representing the average ROI values for 1–2 images per mouse). 1D-E) PV (red) and PNN (green) immunoreactivity in murine hippocampi in slices from a control and venlafaxine treated animal as indicated. The scale bar represents 75 μm and representative PNN enwrapped PV cells are indicated by arrows. IF-G) High power images of PNN staining in murine hippocampi. In these images the scale bar represents 25 μm.
II. Venlafaxine increases the power of carbachol-induced gamma activity in ex vivo hippocampal slices
The hippocampus receives cholinergic input from the medial septum-diagonal band of Broca and this input is thought to be an important effector of physiological gamma (Fisahn et al. 1998). In isolated hippocampal slices, the cholinergic agonist carbachol is widely used to induce gamma (Fisahn et al. 1998). Because chondroitinase mediated PNN degradation has been linked to increased gamma power in the visual cortex, and because alterations in gamma power may be of relevance to the depressive phenotype (Fitzgerald & Watson 2018), we examined the possibility that venlafaxine treatment could impact this rhythm. In figure 2A we show representative local field potential (LFP) recordings between treatments, as well as filtered signals in the theta (4–12Hz) and low and high gamma ranges (25–55 and 65–85Hz respectively). In figure 2B we show there is a statistically significant increase in low and high gamma power for the venlafaxine treated animals (p = 0.008 and 0.05 respectively; Student’s t-test; n= 4–6 animals and 1–2 slices per animal for each group). Of note, the fold change in low gamma is more appreciable than that for high gamma, suggesting that low gamma power is particularly well increased following venlafaxine. In figure 2C, we show the ratio of post- to pre- carbachol gamma power for each slice. This approach may partially address potential confounds introduced by differences in slice viability or electrode placement. Again we detect a significant difference between slices from wild type saline and venlafaxine treated animals in low gamma (p=0.0246) and a tendency towards significance in high gamma (p= 0.084). There was no statistically significant difference in theta power (4–12 Hz) in slices from venlafaxine as compared to treated mice (data not shown).
II. Venlafaxine increases the power of carbachol-induced gamma activity in ex vivo hippocampal slices.
2A) Representative local field potential (LFP) recordings from slices obtained from a saline or venlafaxine treated animal. The LFP was filtered in the theta (4–8Hz), low and high gamma (25–55Hz and 65–85Hz respectively) ranges. 2B) The average low and high gamma power of a 315s window beginning 150s after onset of carbachol perfusion was compared between treatment. For n= 4–6 animals, 1–2 slices per animal for each group, there is a statistically significant increase in low and high gamma power for the venlafaxine treated animals (p = 0.008 and 0.05 respectively; Student’s t test). 2C), we show the ratio of post- to pre- carbachol gamma power for each slice. Again we detect a significant difference between slices from wild type saline and venlafaxine treated animals in low gamma (p=0.0246) and a tendency towards significance in high gamma (p= 0.084).
III. Venlafaxine stimulated changes in PNN integrity are reduced in MMP-9 null mice
MMP-9 has been shown to reduce PNN integrity in mice that experience light reintroduction after deprivation, and also in a mouse model of FXS (Murase et al. 2017, Wen et al. 2017). MMP-9 may directly target PNN components or activate other proteases that do the same. To examine the possibility that this MMP-9 activity contributes to venlafaxine associated changes in the PNN, we treated MMP-9 null mice with saline or venlafaxine beginning at the same age and with the same dose and duration that had been utilized for wild type animals. Of note, previously published work suggests that basal PNN levels are comparable between wild type and MMP-9 null animals (Murase et al. 2017).
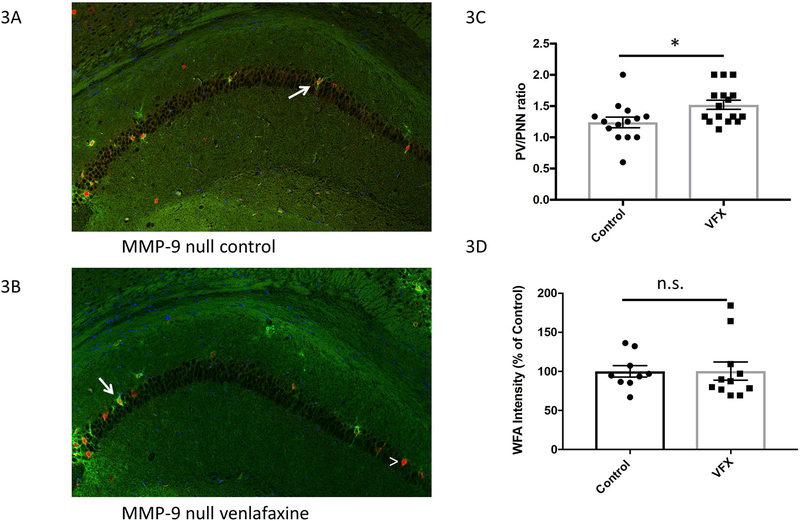
Hippocampal PV and PNN staining for slice subsections are shown in figures 2A and B. PNN enwrapped PV cells are indicated by arrows, while a PV cell without an appreciable PNN is noted by the arrowhead. In 3C we show the PV/PNN ratio for both groups. Though reduced in magnitude as compared to wild type animals (1.6 fold increase in WT; 1.2 fold increase in MMP-9 null), the PV/PNN ratio was nonetheless increased in the venlafaxine group (*Student’s t test p= 0.017). In 3D we show WFA intensity in control and venlafaxine treated MMP-9 null mice. Though the PV/PNN ratio was increased by venlafaxine in the MMP-9 nulls, as opposed to what was observed in wild type animals venlafaxine did not reduce the intensity of WFA fluorescence in the knockout animals. We also performed a 2-way ANOVA for PV/PNN and WFA intensity, using data shown in figures 1B and2C as well as 1C and 3D respectively. The PV/PNN analysis showed no effect of genotype but a significant effect of treatment, with a significant difference observed only between the wild type saline group and the wild type venlafaxine group (= 0.0079; Tukey’s post hoc analysis). There was no significant difference between the MMP-9 null groups. 2-way ANOVA of WFA intensity values showed no significant effect of either genotype or treatment, but post hoc analysis with Dunnett’s multiple comparison test showed that the difference between wild type with and without venlafaxine was close to significance (p = 0.056), while that between MMP-9 null animals with and without venlafaxine was not near significance (p > 0.999).
III. Venlafaxine stimulated changes in PNN integrity are reduced in MMP-9 null mice.
3A-B) PNN and PV staining in slice subsections from treated and untreated MMP-9 null mice. Representative PNN enwrapped PV cells are indicated by arrows, while a PV cell without an appreciable PNN is noted by the arrowhead. Scale bar represents 75μM. 3C) PV/PNN ratio for both groups, with data from wild type mice shown for comparison. For this analysis, n= 4–6 animals per group with 1–3 slides analyzed per animal. As in wild type animals, the number of PV positive cells was not affected by venlafaxine (control 6.87 +/− 0.79, N=14; venlafaxine 5.75 +/− 0.59, N=16; p= 0.26). Though reduced in magnitude as compared to wild type animals, the PV/PNN ratio was increased in the venlafaxine group (*Student’s t test p= 0.017). 3D) WFA intensity in control and venlafaxine treated MMP-9 null mice. Though the PV/PNN ratio was affected by venlafaxine in the MMP-9 nulls, in contrast to what was observed with wild type animals, the intensity of WFA flourescence was not reduced.
IV. Venlafaxine does not significantly increase the power of carbachol-induced gamma activity in ex vivo hippocampal slices from MMP-9 knockout mice
Given that venlafaxine stimulated a lesser fold reduction of PNN integrity in MMP-9 nulls as compared to wild type, we also examined carbachol-stimulated gamma in saline and venlafaxine animals on the MMP-9 null background. Previous studies have examined baseline neurotransmission in these animals and observed that this was not impaired (Nagy et al. 2006). Similarly MMP-9 inhibitors have no effects on baseline properties of neurotransmission and spine size or morphology [reviewed in (Ferrer-Ferrer & Dityatev 2018)].
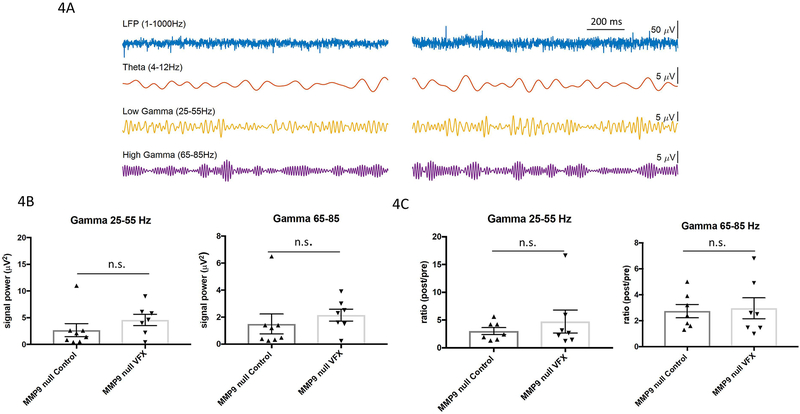
In figure 3A we show representative LFP recordings and filtered signals in the theta, low and high gamma ranges from slices obtained from saline or venlafaxine-treated MMP-9 knockout animals. In 4B we show that in MMP9 knockout mice, we observed no significant increase in low or high gamma power with venlafaxine treatment (Student’s t test). In figure 3C we show post to pre carbachol ratio data in slices from saline and venlafaxine treated mice. The post to pre carbachol ratio in low or high gamma is not significantly different between control and treatment groups in MMP-9 null animals. We again performed a 2-way ANOVA for low and high gamma power, separately analyzing low and high gamma values shown in figures 2B–C and 3B–C. Low gamma power showed no effect of genotype but a significant effect of treatment, with a significant difference observed between the wild type saline group and the wild type venlafaxine group (= 0.0093; Tukey multiple comparisons). There was no significant difference between the MMP-9 null groups. 2-way ANOVA of high gamma values showed no significant effect of either genotype or treatment.
IV. Venlafaxine does not significantly increase the power of carbachol-induced gamma activity in ex vivo hippocampal slices from MMP-9 knockout mice.
4A) Representative LFP recordings and filtered data from slices obtained from saline or venlafaxine treated MMP-9 null animals. 4B) Analyses of average low and high gamma power revealed no statistically significant increase due to venlafaxine treatment in MMP-9 null animals (n= 4–6 animals per group and N represents the average value across epochs from 1–2 slices from each n; p > 0.05, Student’s t test). 4C) Post- to pre- carbachol ratio in slices from saline and venlafaxine treated mice. The post to pre carbachol ratio in low or high gamma is not significantly different between control and treatment in MMP-9 null animals.
V. Schematic overview
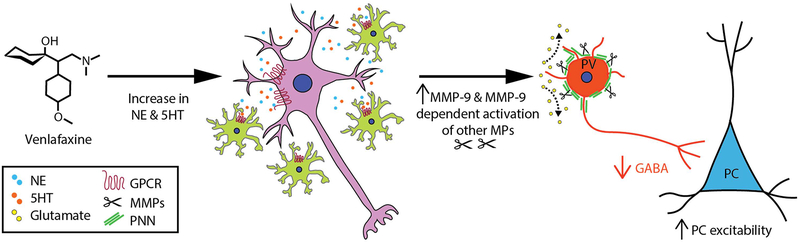
In figure 4 we show a hypothetical overview by which venlafaxine can influence PNN integrity and gamma power. First, hippocampal levels of serotonin and norepinephrine are increased in the background of venlafaxine treatment. These monoamines interact with G protein coupled receptors expressed on neurons and glia. Gαs protein coupled monoamine receptors in particular, including 5HT7 and the β1 adrenergic receptor (ADRB1), have been linked to increased MMP-9 expression [(Bijata et al. 2017), Alaiyed S. et al., unpublished observations]. Increased levels of MMP-9 can in turn target PNN components and/or activate additional proteases that do the same. Reduced PNN integrity, likely localized to regions where monoaminergic transmission typically occurs, can in turn disrupt PV mediated inhibition of pyramidal cell activity with consequent effects on gamma power.
V. Hypothetical Overview.
Hippocampal levels of serotonin and norepinephrine are increased in response to venlafaxine treatment (far left). These monoamines interact with G-protein coupled receptors expressed on neurons (purple) and non-neuronal cells such as microglia (green) to increase expression of MMP-9. Increased levels of MMP-9 can in turn target PNN components and/or activate additional metalloproteases (MPs) that also target PNN substrates. Reduced PNN integrity can in turn disrupt PV-mediated inhibition of pyramidal cell activity with consequent effects on gamma power.
Discussion
In the present study, we find that chronic treatment of mice with the serotonin/norepinephrine reuptake inhibitor venlafaxine concomitantly increases PNN proteolysis and hippocampal gamma power. We further observe that venlafaxine enhanced gamma power is significantly reduced in mice that are deficient in MMP-9, a family member that is expressed in response to neuronal activity (Dziembowska et al. 2012) and monoamine exposure (Bijata et al. 2017).
Prior studies have observed regional alterations in gamma power in association with major depressive illness and bipolar disorder (Liu et al. 2012), as well as increases in gamma with spontaneous remission or selective serotonin reuptake inhibitor (SSRI) treatment in humans and rodent models (Arikan et al. 2018, Khalid et al. 2016). However, the role of PNN-degrading MMPs has not previously been explored as a mediator of enhanced gamma or antidepressant efficacy. Of interest, the SSRI fluoxetine has been linked to reductions in PNN integrity (Ohira et al. 2013), and conversely, a recent study observed increased hippocampal PNN deposition in a social defeat induced persistent stress rat model of depression (Riga et al. 2017). Moreover, exogenous chondroitinase, which degrades PNN components, improved stress-associated cognitive deficits in this model (Riga et al. 2017). In contrast, in mice that are deficient in the PNN protein neurocan, mania-like symptomatology with increased sucrose preference and reduced immobility in the forced swim test are observed (Miro et al. 2012). Also of relevance are human studies that have examined the PNN in the background of depression and observed alterations including PNN sulfation patterns that render the PNN more resistant to proteolysis (Pantazopoulos et al. 2015).
Changes in PNN integrity have the potential to influence oscillatory activity in varied brain regions. This specialized extracellular matrix (ECM) is predominantly localized to PV expressing fast spiking GABA releasing interneurons, which are in turn critical to rhythmic oscillations in the gamma frequency range. At the single cell level, PNNs have been shown to decrease membrane capacitance allowing fast spiking interneurons to fire at high frequency (Tewari et al. 2018). In addition, disruption of the PNN has been linked to reduced glutamateric input to PV expressing interneurons (Hayani et al. 2018) through mechanisms that may include increased lateral diffusion of GluAs and presynaptically released glutamate (Frischknecht et al. 2009). Similarly, genetic deletion of the PNN component brevican has been associated with altered intrinsic properties and reduced synaptic input to PV interneurons through changes in potassium channel localization and levels of GluAs (Favuzzi et al. 2017). Of interest, antidepressants including venlafaxine have been linked to an increased risk of seizures and disinhibition could contribute to the same.
In terms of effects on population events to which PV activity contributes, gamma magnitude has been shown to correlate with sharp wave magnitude (Sullivan et al. 2011) and the latter would be expected to increase with pyramidal cell disinhibition. Consistent with this, PNN disruption has been shown to increase gamma power in the visual cortex (Lensjo et al. 2017b). Indeed, chondroitinase removal of PNNs was shown to enhance both spontaneous gamma power in the 30–40 Hz range and monocular deprivation-linked gamma activity (Lensjo et al. 2017b). These effects may seem at odds with the critical role that PV interneurons play in gamma and ripple generation, and with data linking knock down of GluA1 expression on PV cells to reduced gamma power (Fuchs et al. 2007). PNN attenuation, however, as opposed to a complete loss of this structure, could enhance gamma power through PC disinhibition in the background of PV cells that retain their ability to respond to the particularly strong rhythmic excitatory post synaptic potentials arising from pyramidal cells during oscillatory activity (Mann et al. 2005). Consistent with this are results linking a partial block of GluAs on interneurons to gamma generation (Traub et al. 1996). In addition, ketamine, which preferentially inhibits GluNs at fast spiking interneurons (Homayoun & Moghaddam 2007), has been associated with increases in gamma power (Hakami et al. 2009, Ye et al. 2018). Similarly, intermittent theta burst transcranial magnetic stimulation reduces PV expression and increases EEG gamma power (Benali et al. 2011). Furthermore, reducing NMDA mediated drive of cortical interneurons increases pyramidal cell firing rate (Homayoun & Moghaddam 2007) and modeling studies are also consistent with a causative role for PV deficits in terms of oscillatory power (Sauer et al. 2015).
Despite potential similarities between effects of PNN disruption and models of reduced GABA mediated inhibition, we acknowledge that effects of chondroitinase and monoamine uptake inhibitors could be relatively complex. With respect to the latter, given the non-mutually exclusive potential for MMPs to enhance excitatory neurotransmission through relatively direct effects on PCs (Nagy et al. 2007), future studies are warranted to parse out the relative contribution of PNN dependent and independent mechanisms to MMP associated changes in gamma power. One possibility would be to use PNN sulfation mutants that are relatively more resistant to proteolysis (Foscarin et al. 2017). It would also be of interest to evaluate gamma in PNN link protein mutants, which display juvenile plasticity and attenuated PNNs (Carulli et al. 2010). It might also be of value to examine the relationship between PNN levels and carbachol induced gamma power in murine models of schizophrenia, in that increased gamma band activity has been reported in affected patients as have been regional reductions in PNN integrity (Mauney et al. 2013, McNally & McCarley 2016).
In terms of the potential for increased gamma to contribute to antidepressant efficacy, we acknowledge that future behavioral studies will be needed to address this issue. Gamma changes might simply represent a correlate of antidepressant efficacy. Given that increased gamma is observed not only with spontaneous remission but with improvements on the Hamilton Depression scale (Arikan et al. 2018), however, the association is strong. Moreover, in a mouse model, deficits in gamma are inversely correlated with behavioral despair (Sauer et al. 2015). In addition, gamma activity is thought to improve cognitive features that are impaired in major depressive disorder including short-term memory and attention (MacQueen & Frodl 2011). Of additional interest, depressed patients have poor dream recall as compared to controls (Zanasi et al. 2008), and both venlafaxine and increased gamma activity have independently been linked to lucid dreaming or enhanced dream recall (Voss et al. 2014, Tribl et al. 2013). An unanswered question, however, relates to whether antidepressant associated changes in gamma power would be wholly beneficial. This is of relevance given that changes in the PNN integrity and gamma power may not be beneficial in the background of schizophrenia (Mauney et al. 2013, McNally & McCarley 2016).
In experiments herein, we focused on carbachol-induced gamma in the hippocampus, a region that has been increasing implicated in MDD (reviewed in (MacQueen & Frodl 2011)). For example, depression can be induced by stress in varied rodent models and the hippocampus is sensitive to stress induced atrophy (MacQueen & Frodl 2011). Moreover, hippocampal dependent functions including explicit memory are impaired with depression (MacQueen & Frodl 2011). The hippocampus is also part of a functional network, which includes regions such as prefrontal cortex, that is dysregulated in MDD (MacQueen & Frodl 2011). Of interest, the hippocampus is also the focus of a study examining kainate-induced gamma in ex vivo rat slices following acute or chronic administration of fluoxetine or imipramine (Mendez et al. 2012). Differences in species and animal ages, which influence PNN levels (Murase et al. 2017, Wen et al. 2017), may confound direct comparisons with the present study, as may the use of alternate antidepressants and a different gamma-inducing stimulus. Interestingly, however, while gamma power in chronically treated rats was not increased when examined over a broad range, the power trace for fluoxetine at frequencies below 40 Hz was elevated relative to control and imipramine (Mendez et al. 2012).
Herein we also focused on evaluation of gamma in ex vivo slices after chronic antidepressant administration. Monoamine reuptake inhibitors typically require several weeks to show a clinical benefit. We posit that changes in PNN integrity could take days to weeks to develop but future studies will be necessary to address the time course by which venlafaxine influences PNN integrity. Importantly, however, we note that acute treatment of ex vivo hippocampal slices with monoamines or serotonin reuptake inhibitors can reduce gamma power suggesting that results described in the present study are likely due to changes that follow chronic as opposed to acute antidepressant exposure (Wojtowicz et al. 2009, Mendez et al. 2012, Hajos et al. 2003).
While our study also demonstrated increased MMP-9 in the CNS of venlafaxine treated animals as well as an MMP-9 dependent reduction in PNN intensity, we acknowledge that other MMPs may contribute to venlafaxine-associated changes in the PNN. This is supported by the observation that the PV/PNN ratio is also increased in the venlafaxine treated knockouts. The observation that ratio differences are more robust in the wild type mice and that PNN intensity diminution is significant only in the wild type mice, however, is not unexpected. As compared to other family members, neurons express high levels of MMP-9 when stimulated with glutamate or monoamines (Bijata et al. 2017, Dziembowska et al. 2012). MMP-9 is also rapidly transcribed with neuronal activity and LTP (Dziembowska et al. 2012, Nagy et al. 2006), and the latter may be enhanced by monoamines (Bijata et al. 2017). It is thus possible that localized increases in monoamine levels can increase neuronal expression of MMP-9 in a relatively selective manner. Light reintroduction and FXS related changes in fragile X mental retardation protein may do the same (Janusz et al. 2013), and MMP-9 has been implicated in the PNN remodeling that occurs in both settings (Murase et al. 2017), (Wen et al. 2017). Once upregulated, MMP-9 could also activate additional metalloproteinases that can in turn process PNN components.
In summary, we have shown that chronic treatment with the monoamine reuptake inhibitor venlafaxine increases PNN proteolysis and gamma power in murine hippocampus. We suggest that future studies are warranted to determine whether PNN proteolysis contributes to antidepressant efficacy. While we acknowledge that not all patients respond to monoamine reuptake inhibitors, these medications are effective in a substantial percentage. The identification of new molecular targets could thus lead to the design of drugs or combination therapies. Our results raise the possibility that preferential targeting of GPCRs which best activate signaling molecules linked to increased MMP expression might be pursued.
Acknowledgements
We would like to acknowledge outstanding veterinary support from the Department of Comparative Medicine and we would also like to apologize to investigators whose excellent work was not directly cited.
Funding and Disclosure Katherine Conant is a handling editor for the Journal of Neurochemistry. Katherine Conant received funds for support and supplies from Deborah Wilson and Anthony Herman through the Georgetown University Partners in Research program. Seham Alaiyed received support from the Saudi Arabian government, Qassim University scholarship program. P. Lorenzo Bozzelli was supported with funding from T32 NS041218 and Adam Caccavano was supported by the NIH/NCATS, TL1TR001431. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. There are no competing financial interests for any of the authors in relation to the work described. An earlier version of this manuscript was uploaded to biorxiv: http://www.biorxiv.org/content/early/2018/10/01/432419
Abbreviations:
- PNN
perineuronal net
- NMDA
N-methyl-D-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- PV
parvalbumin
- MMP
matrix metalloproteinase
- GABA
Gamma-Aminobutyric Acid
- GluA
AMPA receptor
- GluN
NMDA receptor
- MDD
major depressive disorder
References
- Andersen P, Morris R, Amaral D, Bliss T, and O’Keefe J eds. (2006) The Hippocampus Book New York: Oxford University Press, 2006; Oxford Scholarship Online, 2009. doi: 10.1093/acprof:oso/9780195100273.001.0001. [DOI] [Google Scholar]
- Arikan MK, Metin B and Tarhan N (2018) EEG gamma synchronization is associated with response to paroxetine treatment. J Affect Disord, 235, 114–116. [DOI] [PubMed] [Google Scholar]
- Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R and Funke K (2011) Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci, 31, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijata M, Labus J, Guseva D et al. (2017) Synaptic Remodeling Depends on Signaling between Serotonin Receptors and the Extracellular Matrix. Cell Rep, 19, 1767–1782. [DOI] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC et al. (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain, 133, 2331–2347. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G and Nyengaard JR (2010) Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus, 20, 1376–1384. [DOI] [PubMed] [Google Scholar]
- David DJ, Bourin M, Jego G, Przybylski C, Jolliet P and Gardier AM (2003) Effects of acute treatment with paroxetine, citalopram and venlafaxine in vivo on noradrenaline and serotonin outflow: a microdialysis study in Swiss mice. Br J Pharmacol, 140, 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E, Gorkiewicz T, Tiron A, Bramham CR and Kaczmarek L (2012) Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci, 32, 14538–14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R et al. (2017) Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron. [DOI] [PubMed] [Google Scholar]
- Ferrer-Ferrer M and Dityatev A (2018) Shaping Synapses by the Neural Extracellular Matrix. Front Neuroanat, 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH and Paulsen O (1998) Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature, 394, 186–189. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ and Watson BO (2018) Gamma oscillations as a biomarker for major depression: an emerging topic. Transl Psychiatry, 8, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foscarin S, Raha-Chowdhury R, Fawcett JW and Kwok JCF (2017) Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging (Albany NY), 9, 1607–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D and Gundelfinger ED (2009) Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci, 12, 897–904. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO et al. (2007) Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron, 53, 591–604. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Robinson DD, Yu JH and Hajos-Korcsok E (2003) Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo-hippocampal system. Neuropsychopharmacology, 28, 857–864. [DOI] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O’Brien TJ and Pinault D (2009) NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One, 4, e6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayani H, Song I and Dityatev A (2018) Increased Excitability and Reduced Excitatory Synaptic Input Into Fast-Spiking CA2 Interneurons After Enzymatic Attenuation of Extracellular Matrix. Front Cell Neurosci, 12, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H and Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci, 27, 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz A, Milek J, Perycz M, Pacini L, Bagni C, Kaczmarek L and Dziembowska M (2013) The Fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci, 33, 18234–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A, Kim BS, Seo BA, Lee ST, Jung KH, Chu K, Lee SK and Jeon D (2016) Gamma oscillation in functional brain networks is involved in the spontaneous remission of depressive behavior induced by chronic restraint stress in mice. BMC Neurosci, 17, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo KK, Christensen AC, Tennoe S, Fyhn M and Hafting T (2017a) Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo KK, Lepperod ME, Dick G, Hafting T and Fyhn M (2017b) Removal of Perineuronal Nets Unlocks Juvenile Plasticity Through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. J Neurosci, 37, 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Hsieh JC, Chen YS, Tu PC, Su TP and Chen LF (2012) Different patterns of abnormal gamma oscillatory activity in unipolar and bipolar disorder patients during an implicit emotion task. Neuropsychologia, 50, 1514–1520. [DOI] [PubMed] [Google Scholar]
- Lorenzo Bozzelli P, Alaiyed S, Kim E, Villapol S and Conant K (2018) Proteolytic Remodeling of Perineuronal Nets: Effects on Synaptic Plasticity and Neuronal Population Dynamics. Neural Plast, 2018, 5735789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G and Frodl T (2011) The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry, 16, 252–264. [DOI] [PubMed] [Google Scholar]
- Mann EO, Radcliffe CA and Paulsen O (2005) Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol, 562, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S and Woo TU (2013) Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry, 74, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JM and McCarley RW (2016) Gamma band oscillations: a key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr Opin Psychiatry, 29, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Pazienti A, Szabo G and Bacci A (2012) Direct alteration of a specific inhibitory circuit of the hippocampus by antidepressants. J Neurosci, 32, 16616–16628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro X, Meier S, Dreisow ML et al. (2012) Studies in humans and mice implicate neurocan in the etiology of mania. Am J Psychiatry, 169, 982–990. [DOI] [PubMed] [Google Scholar]
- Murase S, Lantz CL and Quinlan EM (2017) Light reintroduction after dark exposure reactivates plasticity in adults via perisynaptic activation of MMP-9. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O and Huntley GW (2007) The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn Mem, 14, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A et al. (2006) Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci, 26, 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ and Duman RS (1996) Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci, 16, 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi A, Matsumoto N, Morikawa S, Tamura H and Ikegaya Y (2017) Juvenile Hippocampal CA2 Region Expresses Aggrecan. Front Neuroanat, 11, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Takeuchi R, Iwanaga T and Miyakawa T (2013) Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Mol Brain, 6, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V and Loewenstein D (2006) Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry, 63, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Markota M, Jaquet F, Ghosh D, Wallin A, Santos A, Caterson B and Berretta S (2015) Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Transl Psychiatry, 5, e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock E, Everest M, Brown A and Poulter MO (2014) Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis, 70, 21–31. [DOI] [PubMed] [Google Scholar]
- Quello SB, Brady KT and Sonne SC (2005) Mood disorders and substance use disorder: a complex comorbidity. Sci Pract Perspect, 3, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga D, Kramvis I, Koskinen MK et al. (2017) Hippocampal extracellular matrix alterations contribute to cognitive impairment associated with a chronic depressive-like state in rats. Sci Transl Med, 9. [DOI] [PubMed] [Google Scholar]
- Sauer JF, Struber M and Bartos M (2015) Impaired fast-spiking interneuron function in a genetic mouse model of depression. Elife, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker ML, Harkness JH and Sorg BA (2016) A standardized and automated method of perineuronal net analysis using Wisteria floribunda agglutinin staining intensity. IBRO Rep, 1, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC and Miquel M (2016) Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci, 36, 11459–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K and Buzsaki G (2011) Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J Neurosci, 31, 8605–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamasi V, Petschner P, Adori C, Kirilly E, Ando RD, Tothfalusi L, Juhasz G and Bagdy G (2014) Transcriptional evidence for the role of chronic venlafaxine treatment in neurotrophic signaling and neuroplasticity including also Glutamatergic [corrected] - and insulin-mediated neuronal processes. PLoS One, 9, e113662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari BP, Chaunsali L, Campbell SL, Patel DC, Goode AE and Sontheimer H (2018) Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat Commun, 9, 4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA and Cai X (2015) An excitatory synapse hypothesis of depression. Trends Neurosci, 38, 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G and Jefferys JG (1996) Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol, 493 (Pt 2), 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribl GG, Wetter TC and Schredl M (2013) Dreaming under antidepressants: a systematic review on evidence in depressive patients and healthy volunteers. Sleep Med Rev, 17, 133–142. [DOI] [PubMed] [Google Scholar]
- Voss U, Holzmann R, Hobson A, Paulus W, Koppehele-Gossel J, Klimke A and Nitsche MA (2014) Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci, 17, 810–812. [DOI] [PubMed] [Google Scholar]
- Wen TH, Afroz S, Reinhard SM, Palacios AR, Tapia K, Binder DK, Razak KA and Ethell IM (2017) Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Interneurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb Cortex, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz AM, van den Boom L, Chakrabarty A, Maggio N, Haq RU, Behrens CJ and Heinemann U (2009) Monoamines block kainate- and carbachol-induced gamma-oscillations but augment stimulus-induced gamma-oscillations in rat hippocampus in vitro. Hippocampus, 19, 273–288. [DOI] [PubMed] [Google Scholar]
- Yang EV, Sood AK, Chen M et al. (2006) Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res, 66, 10357–10364. [DOI] [PubMed] [Google Scholar]
- Ye T, Bartlett MJ, Schmit MB, Sherman SJ, Falk T and Cowen SL (2018) Ten-Hour Exposure to Low-Dose Ketamine Enhances Corticostriatal Cross-Frequency Coupling and Hippocampal Broad-Band Gamma Oscillations. Front Neural Circuits, 12, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanasi M, Pecorella M, Chiaramonte C, Niolu C and Siracusano A (2008) Dreams by persons with mood disorders. Psychol Rep, 103, 381–394. [DOI] [PubMed] [Google Scholar]
- Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD and Thompson SM (2017) A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-Like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice. eNeuro, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]