Summary Sentence:
Vascular leak is increased diffusely in the lungs in pulmonary fibrosis and is not limited to radiographic areas of disease involvement.
Introduction:
Vascular leak is a cardinal response to tissue injury [1,2]. When dysregulated vascular leak has been shown to contribute to the development of pulmonary fibrosis in the bleomycin mouse model [3]. Specifically targeting vascular endothelial growth factor, initially described as vascular permeability factor [4] and a key mediator regulating capillary permeability, attenuates the development of pulmonary fibrosis in vivo [5]. Gadofosveset (Ablavar®) is a FDA-approved, gadolinium-based, albumin-binding contrast agent. Gadofosveset has been used to detect vascular permeability in mouse models [9] and to perform vascular imaging clinically. We hypothesized that gadofosveset-enhanced lung magnetic resonance imaging (MRI) could detect albumin extravasation in subjects with pulmonary fibrosis and demonstrate the location of ongoing tissue injury.
Methods:
This study was approved though the Partners Institutional Review Board (IRB) and written consent provided. Six subjects with pulmonary fibrosis and four healthy subjects were included. Five subjects had idiopathic pulmonary fibrosis (IPF), and 1 subject had scleroderma-associated interstitial lung disease with a usual interstitial pneumonia pattern. Subjects were excluded for congestive heart failure, pneumonia within 6 weeks of study entry, cigarette smoking within 6 months of study entry, or contraindications to undergoing MRI or receiving gadolinium.
MRI was performed using a commercial 3T MRI scanner (SKYRA, Siemens Healthcare AG) with an 18-channel body array and 12 channel spine array. Images were obtained during free breathing and acquired using pointwise encoding time reduction with radial acquisition (PETRA) with a flip angle of 25°, TE = 0.050 ms, TR = 2.24 ms, 40K radials, 3D field of view of 400mm, and pixel resolution of 1.56 × 1.56 × 1.56mm. Baseline imaging was performed followed by intravenous injection of gadofosveset (Ablavar®, Lantheus Medical Imaging) at single dose of 0.03 mmol per kilogram, and imaging was repeated for up to 32.5 minutes post injection.
Images were analyzed using the freeware Dicom reader OsiriX. For each subject, regions of interest (ROIs) were drawn in the ascending and descending aorta at five different standardized locations, two of which were in coronal planes and three of which were in axial planes. ROIs were drawn by hand to outline subpleural regions of each lung, excluding the most medial portions. The lung ROIs were specifically chosen to avoid central blood vessels so as not to confound changes in lung parenchyma signal with potential differences in vasculature between IPF and healthy subjects. The mean signal intensity (SI) of each ROI was measured pre-gadofosveset injection and 5–7.5 minutes, 10–12.5 minutes, and 15–17.5 minutes after injection of gadofosveset. We calculated our primary outcome measure, the albumin extravasation index (AEI), as a surrogate measure of pulmonary vascular permeability. The AEI is defined as the change in signal intensity in the parenchyma after gadofosveset injection divided by the change in signal intensity in the aorta after gadofosveset injection.
We averaged the AEIs from the right and left lung at each location and then across the three time points to use for subsequent calculations. We calculated total, coronal, and axial AEIs to assess the degree of vascular permeability from measured lung regions and AEIs for each anatomic section to assess the anatomic distribution of alveolar-capillary permeability. Statistical analyses were performed using Prism 6.0 using Mann-Whitney U and one-way ANOVA as appropriate with p values less than 0.05 considered statistically significant. Data are reported as median plus range. To visualize the location of albumin extravasation, subtraction images with color overlay were constructed (Figure 1).
Figure 1. Coronal Subtraction Images with Color Overlay.
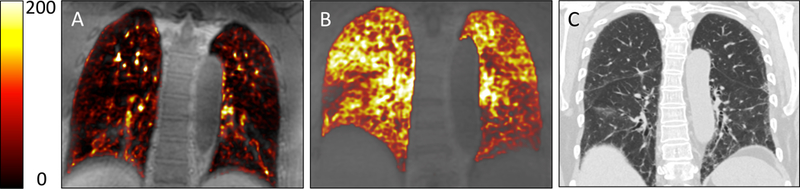
Subtraction of baseline pre-gadofosveset image from 15 minute post-gadofosveset image (color) super-imposed on baseline image. (A) Healthy Control. (B) Subject with IPF. (C) CT scan of the same subject with IPF. In panel A, signal enhancement is isolated to areas of known blood vessels with the majority of the lung unenhanced. In panel B, regions of signal enhancement extend well beyond major vessels and into the lung parenchyma consistent with increased albumin extravasation. When comparing panel B to panel C, regions of increased signal intensity occur in areas of radiographically normal lung in addition to areas with known fibrosis.
Results:
Subjects with pulmonary fibrosis had a total AEI of 0.37 (0.32 – 0.46) vs 0.17 (0.12 – 0.28) for controls, p = 0.01. The AEI was increased in subjects with IPF across coronal [0.34 (0.30 – 0.42) vs 0.16 (0.13 – 0.26), p = 0.01] and axial [0.43 (0.32 – 0.56) vs 0.20 (0.06 – 0.30), p = 0.01] measurements. The AEI was also increased across both anterior [0.39 (0.26 – 0.45) vs 0.15 (0.07 – 0.22), p = 0.01] and posterior [0.32 (0.26 – 0.39) vs 0.18 (0.16 – 0.30), p = 0.02] coronal locations as well as upper [0.45 (0.40 – 0.51) vs 0.21 (0.10 – 0.32), p = 0.01], middle [0.40 (0.31 – 0.57) vs 0.21 (0.05 – 0.30), p = 0.01], and lower [0.44 (0.33 – 0.56) vs 0.20 (0.08 – 0.30), p = 0.01] axial locations in IPF subjects. The AEI was increased similarly across axial locations for UIP subjects. Significance for all AEIs persisted even when excluding the scleroderma-UIP values.
Subtraction images demonstrate a marked increase in signal intensity in IPF patients compared to healthy controls, e.g. Figure 1. For the healthy control (A), the majority of the lung is non-enhanced and high signal enhancement areas are limited to areas of the vascular tree consistent with the expected distribution of a blood pool MR contrast agent. For the subject with IPF (B), however, regions of enhancement extend well beyond major vessels, were located diffusely throughout the lung fields, and did not display a basilar nor a subpleural preference. When compared to CT Chest imaging (C), increased signal intensity occurred in radiographically normal lung as well as areas with fibrotic changes.
Discussion:
We present the novel application of gadofosveset-enhanced MRI to detect pulmonary vascular leak by directly imaging albumin extravasation for the first time. We found that vascular leak was increased in the lungs of individuals with pulmonary fibrosis compared to healthy controls. These findings build on previous observations indicating disruption of the alveolar-capillary membrane in IPF [6–8]. By specifically imaging albumin extravasation from the vascular compartment, our results provide potentially important information about the spatial distribution of ongoing lung injury in pulmonary fibrosis.
Gadofosveset is a small molecule gadolinium-based contrast agent that binds reversibly to serum albumin. After administration, about 80 – 90% of the contrast agent is bound to albumin with a Kd of 90 μM [10]. We hypothesized that in vascular leak, the extravascular, extracellular albumin concentration will increase due to extravasation of albumin, and the unbound gadofosveset would extravasate rapidly from the blood into the lung interstitial space and bind to albumin present there. In the absence of gadofosveset extravasation, the AEI would equal the blood volume fraction in the region of lung tissue analyzed. Higher AEI values would indicate vascular leak. While we did not validate our albumin extravasation index with albumin extravasation histologically, gadofosveset has been used to assess vascular permeability and response to treatment in the setting of mouse models of accelerated atherosclerosis with correlation of vessel wall MRI signal changes with Evans blue dye [9].
Albumin extravasation was increased in subjects with pulmonary fibrosis similarly across all regions of the lung. Our results suggest that lung injury occurring in pulmonary fibrosis is more diffuse than what is traditionally thought, and this finding questions why basilar fibrosis predominates in conditions like IPF. Other factors, such as tractional stress or collapse induration as have been hypothesized [11–13], may create regional differences in wound healing responses that can promote fibrosis rather than normal repair. Insight into the mechanobiologic drivers of fibrosis and signaling pathways upregulated by stretch, including transforming growth factor beta (TGF-beta) [14], may elucidate this further. Albumin extravasation was not limited to fibrotic regions and occurred in areas of radiographically normal lung. Whether some component of these findings could be explained by very early fibrotic changes or neovascularization is not known. Lung regions with increased vascular permeability may represent areas at risk for developing radiographically apparent fibrosis. Additional research is needed to assess for associations between the amount of vascular leak and disease progression. Our results represent an important step towards the use of molecular imaging to assess known processes directly involved in the development of fibrosis.
Acknowledgements:
We would like to gratefully acknowledge the important contributions from Mary O’Hara and Larry White of the Athinoula A. Martinos Center for Biomedical Imaging.
Sources of Support: This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health centers, or the National Institutes of Health. This work was also supported in part by grants from the National Institute of Health (F32HL129789 to SBM, K08HL105656 to BSS, and R01HL131907 to PC and AMT) and the Parker B. Francis Foundation (SBM).
References:
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Tumors: wounds that do not heal: similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315(26):1650–9. [DOI] [PubMed] [Google Scholar]
- 3.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol 2010;43:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983;219:983–985. [DOI] [PubMed] [Google Scholar]
- 5.Iyer AKV, Ramesh V, Castro CA, Kaushik V, Kulkarni YM, Wright CA, Venkatadri R, Rojanasakul Y, Azad N. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J Cell Biochem 2015;116:2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells AU, Hansell DM, Harrison NK, Lawrence R, Black CM, Bois du RM. Clearance of inhaled 99mTc-DTPA predicts the clinical course of fibrosing alveolitis. Eur Respir J 1993;6:797–802. [PubMed] [Google Scholar]
- 7.Mogulkoc N, Brutsche MH, Bishop PW, Murby B, Greaves MS, Horrocks AW, Wilson M, McCullough C, Prescott M, Egan JJ, Greater Manchester Pulmonary Fibrosis Consortium. Pulmonary (99m)Tc-DTPA aerosol clearance and survival in usual interstitial pneumonia (UIP). Thorax 2001;56:916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J 2009;33:77–84. [DOI] [PubMed] [Google Scholar]
- 9.Phinikaridou A, Andia ME, Protti A, Indermuehle A, Shah A, Smith A, Warley A, Botnar RM. Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation 2012;126:707–719. [DOI] [PubMed] [Google Scholar]
- 10.Eldredge HB, Spiller M, Chasse JM, Greenwood MT, Caravan P. Species dependence on plasma protein binding and relaxivity of the gadolinium-based MRI contrast agent MS-325. Invest Radiol 2006;41:229–43. [DOI] [PubMed] [Google Scholar]
- 11.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med 2012;136:591–600. [DOI] [PubMed] [Google Scholar]
- 12.Lutz D, Gazghar A, Lopez-Rodriquez E, Ruppert C, Mahavadi P, Günther A, Klepetko W, Bates J, Smith B, Geiser T, Ochs M, Knudsen L. Alveolar Derecruitment and Collapse Induration as Crucial Mechanisms in Lung Injury and Fibrosis. Am J Resp Cell Mol Biol 2015;52(2):232–43. [DOI] [PubMed] [Google Scholar]
- 13.Todd NW, Atamas SP, Luzina IG, Galvin J. Permanent Alveolar Collapse is the Predominant Mechanism in Idiopathic Pulmonary Fibrosis. Expert Rev Respir Med 2015; 9(4):411–418. [DOI] [PubMed] [Google Scholar]
- 14.Froese AR, Shimbori C, Bellaye P-S, Inman M, Obex S, Fatima S, Jenkins G, Gauldie J, Ask K, Kolb M. Stretch-induced Activation of Transforming Growth Factor-β 1in Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;194:84–96. [DOI] [PubMed] [Google Scholar]


