Anthracycline therapy remains a mainstay for breast cancer (BC) many other cancers. However, anthracyclines incur a >5-fold risk of incident heart failure. Substrate for heart failure is myocardial fibrosis that is preceded by myocardial edema, supported by preclinical studies of AC-induced myocardial damage1. Loss of endothelial progenitor cells (EPCs) has also been viewed as a contributor to myocardial damage2. However, detection of early changes via blood biomarkers for better risk stratification in humans remains challenging3. Recently, cardiac magnetic resonance (CMR) has been successfully applied in the detection of post-treatment anthracycline-induced fibrosis through T1 mapping, paralleling decline in left ventricular ejection fraction (LVEF)4. Given preclinical evidence that myocardial edema precedes fibrosis in AC-induced myocardial damage1, we tested the hypothesis that myocardial edema could be detected using the CMR relaxation parameter T2, comparing its value to EPC assay in human subjects receiving anthracyline therapy for breast cancer.
We prospectively enrolled at Ohio State University’s James Comprehensive Cancer Center consecutive women with non-metastatic BC initiating doxorubicin-containing regimens who provided written informed consent to participate in this Institutional Review Board-approved longitudinal study. Females ≥18 years old treated with doxorubicin for neoadjuvant or adjuvant therapy were included. We excluded those with prior anthracycline exposure, coronary disease, or contraindication to magnetic resonance. Scans were performed pre-treatment (≥2 weeks before anthracycline-initiation), post-cycle 1 of treatment (early), and 2–4 weeks after the last cycle of anthracyclines (post-treatment). Correlative biomarkers, including high-sensitivity troponin-I (TnI), brain natriuretic peptide (BNP), and peripheral blood EPCs were assayed from blood collected at the time of imaging. EPCs were processed according to previously published protocols5. Available CMR data from those presenting for follow-up CMR >1 year post-anthracycline initiation were also assessed.
The primary outcome was change in myocardial T2 following anthracycline initiation. All scans were completed at 1.5 Tesla (Magnetom Avanto, Siemens Healthcare). Acquired data included conventional cine imaging in long and short-axis planes, T2 mapping, and late gadolinium enhancement (LGE) imaging following intravenous administration of 0.2 mmol/kg gadolinium-based contrast agent (gadopentetate dimeglumine). CMR analyses were performed blinded to clinical information and included: i) computation of ventricular volumes from endocardial contour delineation, ii) circumferential systolic strain computation using feature tracking (VVI, Siemens), iii) identification of maximum T2 by manual segmentation, and iv) recording of presence or absence of myocardial damage by full width at half-maximum segmentation of LGE images. Pretreatment characteristics are described with mean ± standard deviation for continuous variables and frequencies for categoric variables. One-way repeated measures analysis of variance (ANOVA) models were fit to model changes in measures over time. Mean differences in measures at timepoints relative to pretreatment (pre-anthracycline exposure) were estimated from the model and presented with standard errors. Statistical significance of CMR measures was set at a Bonferroni-corrected α=0.017 to maintain an overall α=0.05 and account for three tests at time points post-anthracycline initiation (early, post-treatment, and late); a Bonferroni-corrected α=0.025 was used for correlative biomarkers to account for two tests at time points post-anthracycline initiation (early and post-treatment). All tests were two-sided. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Twenty-nine BC patients were studied: age 54±10 years, BMI 31±8 kg/m2, 45% hypertensive, 7% diabetic, 21% on angiotensin-converting-enzyme inhibitor, angiotensin-receptor-blocker, or beta-blocker; 31% on statins; 10% additionally receiving herceptin, baseline LVEF 65.9±6.4%, and baseline T2 of 51.8±3.5 ms; no subject had LGE abnormality at baseline. Median cumulative doxorubicin dose was 237.1±7.6 mg/m2. After the first AC treatment, there was no significant change in LVEF (−0.4±1.2%, p=0.76) or circumferential strain (1.8±0.9%, p=0.05). However, there was a significant increase in myocardial T2 by 3.3±0.8ms (p<0.001); (Figure). After completion of doxorubicin therapy (post-4th cycle), there remained no difference in LVEF (−1.6±1.5%, p=0.30) or circumferential strain (1.7±1.1, p=0.12); however, myocardial T2 had further increased by 5.4±0.8ms (p<0.001). There was no significant change in TnI or BNP across visits. When compared to similar healthy volunteers, BC patients had lower baseline EPC levels (110.7±10.1 vs. 49.6±27.2, p<0.01), but there was no significant difference in EPC levels before and after doxorubicin initiation. There was also no clear correlative pattern between EPC morphology and CMR or cardiac biomarker parameters.
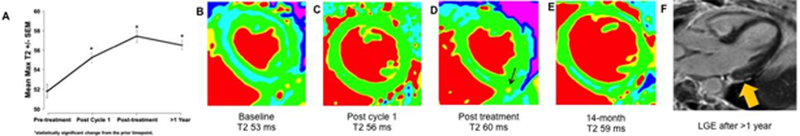
In a cohort of women with breast cancer, myocardial T2 values increased over the course of anthracycline therapy (A). One subject, for instance, had a pre-treatment T2 value of 55 ms that increased to 60ms after the first cycle and 61 ms post-treatment; while T2 then normalized to 56 ms (B-E), late gadolinium enhancement imaging after one year showed evident subepicardial scarring (F, arrow). Of note, her LV ejection fraction remained normal until 14 months when it had fallen to 47%.
Among those with late CMRs [17 BC patients; median 19.8 months (range 13.5–24.6) post-doxorubicin initiation], LVEF decreased by −7.5±1.8% (p<0.001), with 35% (6) seeing >10% decline. Three subjects (18%) developed LGE. However, there was no significant correlation between early change in T2 (Spearman’s r=0.01; p=0.96), circumferential strain (Spearman’s r=−0.22; p=0.39) and late LVEF decline. Limitations include a focus on BC, sample size, and the unavailability of longer-term follow-up.
In summary, patients undergoing anthracycline-therapy are at high-risk for significant reductions in LVEF. The presence of early myocardial inflammation as shown by T2 increase appears to associate with anthracycline administration, even before fibrosis, strain, and decline in LVEF. Additional studies evaluating the prognostic role of CMR-derived early myocardial T2 change as a potential biomarker target for cardioprotective strategies are needed.
Acknowledgements:
The authors acknowledge and thank the patients and their families treated at the Ohio State University Comprehensive Cancer Center. The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: This work was supported by an NIH R21-CA143787–02 award, as well as by R01-CA189947, R01-HL116533, and K12-CA133250 grants.
Disclosures: Dr. Raman receives institutional research support from Siemens. Dr. Wesolowski has received research funding from Acerta Pharma and has provided consulting services to Nepa, Novartis, Agenus, Pfizer and Puma. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Farhad H, Staziaki PV, Addison D, Coelho-Filho OR, Shah RV, Mitchell RN, Szilveszter B, Abbasi SA, Kwong RY, Scherrer-Crosbie M, Hoffmann U, Jerosch-Herold M and Neilan TG. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2016;9:e003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F and Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G, Albrile F, Bobbio M and Merlano M. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148:194–8. [DOI] [PubMed] [Google Scholar]
- 4.Melendez GC, Jordan JH, D’Agostino RB Jr, Vasu S, Hamilton CA and Hundley WG. Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. JACC Cardiovasc Imaging. 2017;10:708–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das H, George JC, Joseph M, Das M, Abdulhameed N, Blitz A, Khan M, Sakthivel R, Mao HQ, Hoit BD, Kuppusamy P and Pompili VJ. Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PloS one. 2009;4:e7325. [DOI] [PMC free article] [PubMed] [Google Scholar]