Abstract
Objectives
Brain sulcation is an indirect marker of neurodevelopmental processes. Studies of the cortical sulcation in bipolar disorder have yielded mixed results, likely due to high variability in clinical phenotype. We investigated whole brain cortical sulcation in a large sample of selected patients with high neurodevelopmental load.
Methods
263 patients with bipolar disorder I and 320 controls were included in a multicentric MRI study. All subjects had a high resolution T1 weighted brain MRI. Images were processed with an automatized pipeline to extract the global sulcal index (g-SI) and the local sulcal indices (l-SI) from twelve a priori determined brain regions covering the whole brain. We compared l-SI and g-SI between patients with or without early-onset bipolar disorder and patients with or without positive history of psychosis adjusting for age, gender and handedness.
Results
Patients with early-onset bipolar disorder had a higher l-SI in the right prefrontal dorsolateral region. Patients with psychotic bipolar disorder had a decreased l-SI in the left superior parietal cortex. No group differences in g-SI or l-SI were found between healthy subjects and the whole patient cohort. We could replicate the early-onset finding in an independent cohort.
Conclusions
Our work suggests that BD is not associated with generalized abnormalities of sulcation, but rather with localized changes of cortical folding restricted to patients with a heavy neurodevelopmental loading. These findings support the hypothesis that BD is heterogeneous but may be disentangled using MRI, and suggest the need for investigations into neurodevelopmental deviations in the disorder.
Keywords: Bipolar disorder, sulcation, psychosis, early-onset, MRI, neurodevelopment
1. Introduction
Early neurodevelopmental deviations are suspected to contribute to the pathophysiology of bipolar disorder (BD), which together with exposure to post-natal environmental factors during childhood and adolescence1,2 lead to onset of the disorder in early adulthood. Epidemiological evidence supports this hypothesis: for instance, prenatal exposure to influenza has been associated with an increased risk for BD in exposed offspring3, influence of the season of birth4,5 and obstetric complications6,7 have been identified as peri-natal risk factors for BD. The second line of evidence comes from genetic studies. Although strongly modulated by environmental factors and familiality8, there is clear evidence for a role for genetic factors in BD with an estimated heritability of about 0.69. In addition, there is some evidence that BD is preceded by prodromal cognitive symptoms during adolescence10 and is associated with subtle neurological signs11 that may reflect a deviation in the developmental trajectory of future bipolar patients.
Not all patients with BD may have impaired neurodevelopmental trajectories, but indirect evidence suggests that more homogeneous clinical subgroups of BD, namely early-onset BD (<18 years) and BD with psychosis, may be particularly associated with impaired neurodevelopmental processes12,13. Indeed, patients with psychotic BD have a younger age at onset14 than non-psychotic BD and early-onset BD share a high degree of intra-familial aggregation15,16 and are associated with a worse prognosis17–19. Moreover, early exposure, including during fetal life, to unfavourable environmental factors may increase the risk of BD with psychosis20 and early-onset BD6. Taken together, these findings suggest that psychotic and early-onset BD may be a clinical BP subgroup with a high load of neurodevelopmental insults21.
Sulcation and gyrification are developmental processes that occur as early as the 10th gestational week extending to the 44th week in the fetal brain22. Cortical folding is a graduated process following a chronological ordering, with a continuum between the primary folds, showing less inter-individual variability, and the tertiary folds, with the most inter-individual variability22. In adults the sulcal index23, which reflects the extent to which the cortex is buried, is considered an indirect retrospective marker of these early developmental processes24. Cortical folding has been quantitatively investigated in neuroimaging studies from two complementary anatomical features of the cortex anatomy, namely by assessing the degree of ‘sulcation’ (i.e. by measuring the normalized surface of the cortex buried in the sulci) or the degree of ‘gyrification’ (i.e. by measuring the normalized gyral surface).
Investigations of the cortical folding in BD patients compared to controls have primarily focused on predetermined prefrontal and limbic regions and have yielded mixed results. Increased25 and decreased26 gyrification of the anterior cingulate region have both been described in adults with BD. More shallow olfactory sulci have also been reported in adults bipolar patients27 as well as reduced prefrontal gyrification28. Other studies have focused on treatment response and have found that non-responders in a mixed sample of unipolar depression patients and BD patients with psychosis had decreased gyrification in the temporal, frontal and insular regions29 and one study has reported a right-sided reduction of the global sulcation index (g-SI) in bipolar patients with treatment-resistant depression30. Concerning adolescent bipolar patients, one study has reported an increased mean sulcal width of the prefrontal cortex and reduced global gyrification in psychotic BD31. To our knowledge only one study has focused on bipolar subgroups by comparing directly early-onset to intermediate-onset BD and found an increased local sulcal index (l-SI) of the prefrontal cortex in early versus intermediate-onset BD patients32. Albeit mixed, these results suggest that specific cortical folding deviations can be detected in patients with BD during adulthood.
An important limitation of these findings is that they were obtained from monocentric studies mostly with sample size ranging from 17 to 141 BD patients thus limiting the statistical power to perform subgroup comparisons and whole-brain analyses. In addition, to our knowledge, no multicenter whole-brain study has tested the hypothesis that cortical sulcation may be specifically altered in clinically homogeneous subgroups of patients with neurodevelopmental deviations. In this context, we performed a whole brain exploratory comparison of global and regional sulcation indices between controls and BD patients in a large international multicentric sample, and searched for specific alterations in patients with putative higher neurodevelopmental deviations. Our sample size allowed us to investigate cortical folding in early-onset BD and BD with psychotic features. We assumed that bipolar patients would exhibit differences in sulcation when compared to controls, but that these differences would be different or present mostly in the early-onset and psychotic bipolar subgroup of patients, reflecting specific neurodevelopmental deviations. To reinforce the validity of our results, we also included another independent sample to perform confirmatory analyses.
2. Materials and methods
2.1 Subjects
Patients with BD and healthy controls were recruited from 6 international participating university-affiliated psychiatry departments: Assistance Publique- Hôpitaux de Paris (Henri Mondor hospitals and Fernand Widal- Lariboisière hospital, Créteil & Paris, France), Grenoble-Alpes University (Grenoble, France), the Western Psychiatric Institute and Clinic, UPMC, (Pittsburgh, USA), the Central Institute for Mental Health (Mannheim, Germany), the Department of Public Health and Community Medicine, Section of Psychiatry and Section of Clinical Psychology, University of Verona (Verona, Italy) and the University Hospital Galway (Galway, Ireland). Controls were recruited from media announcements or registry offices and had no personal or familial history of axis I mood disorder, schizophrenia or schizoaffective disorder and no actual alcohol abuse. Subjects were assessed for diagnosis by trained raters either using the Diagnosis Interview for Genetic Study (at Paris & Créteil), the Structured Clinical Interview for DSM-IV (at Grenoble, German, Irish and US sites) or the Schedules Clinical Assessment Neuropsychiatry (in Italy) and with the Edimburgh questionnaire for handedness in all sites. We defined patients with a positive history of psychotic features if they had at least one episode of depression or mania either with delusions or hallucinations. Early-onset patients were defined as experiencing a first mood episode before 18 years-old as in previous studies33. Subjects were excluded if they had a positive history of neurological disease, head trauma with loss of consciousness or MRI contraindications. Local ethics committee approval was obtained at each center and written informed consent was obtained after complete description of the study to each of the subjects.
2.2 MRI Data collection
High-resolution T1 weighted structural MRI was obtained for each subject using a Siemens Magnetom TrioTim 3T Syngo MR B17 (Siemens Medical Solutions) at Creteil and at German and US sites (echo time = 2.98 ms, repetition time = 2300 ms, 160 slices, 1.0 × 1.0 × 1.0 or 1.1 mm), a 1.5T Siemens Magnetom Symphony at the Irish site (echo time = 4.38 ms, repetition time = 1140 ms, matrix size 256 × 256 interpolated to 512 × 512, voxel size 0.45mm × 0.45mm2), a 1.5 T Siemens Magnetom Symphony (echo time = 3.93 ms, repetition time = 2140 ms, matrix size 512×384 with a slice thickness of 1.25mm) or a 3T Siemens Allegra (echo time = 3.93ms, repetition time = 2300 ms, image size = 256×256, slice thickness 1mm) for the Italian site, a 3T Brucker or Philips Achieva (echo time =9.4ms, repetition time = 2000 ms, matrix size = 279×320 or 320×320 respectively, slice thickness 1mm) at Grenoble. Raw images were assessed visually and quoted for geometrical distortions by two authors (SS and FH) and were included based on a blind polling procedure.
2.3 Data processing
Raw T1 images were processed using the BrainVISA 4.4.2 (http://brainvisa.info) with standard parameters which allows for an automatic delineation of the inner sulci surface and automatic neuroanatomical labelling of cortical sulci in subjects’ native space with minimal manual intervention. This approach was chosen because of the relatively large number of subjects included in our sample and to avoid biases related to the operator and potential shape distortion resulting from group normalization. This procedure has been previously applied to schizophrenia, major depressive disorder and BD23,30,32,34. All processing steps were reviewed visually by SS.
2.3.1- Sulci recognition
An automated pre-processing step skull-stripped T1 MRIs and segmented the brain tissues. No spatial normalization was applied to MRIs to overcome potential bias that may result from the sulcus shape deformations induced by the warping process. The cortical folds were automatically segmented throughout the cortex from the skeleton of the gray matter/cerebrospinal fluid mask, with the cortical folds corresponding to the crevasse bottoms of the “landscape,” the altitude of which is defined by its intensity on the MRIs. This definition provides a stable and robust sulcal surface definition that is not affected by variations in cortical thickness or gray matter/white matter contrast35 due for instance to aging processes or treatment. For each participant, images at each processing step were visually checked. No gross segmentation error (e.g., cortical ribbon thinning, gyrus or sulcus missing) was detected. Image analysis was performed with the Morphologist toolbox using BrainVISA 4.4.2 software and standard parameters (http://brainvisa.info).
The cortical folds were then converted to a graph-based representation of the cortex containing information related to shape (area, depth and length) and spatial organization (position and orientation). Sulci were then automatically recognized using a bayesian probabilistic model which provides the probability of presence of every sulcus at any position of cortex. This recognition step, using a previously validated procedure with a mean accuracy rates of 86%36, resulted in labelled sulci graphs for each hemisphere for each subject not requiring any manual intervention.
2.3.2- Surface area
Brain mask was used to estimate the brain outer surface for each subject following an isotropic closing procedure of the sulcal outer surface. The surface area of the resulting envelope that swaddles the brain surface (referred to as ‘hull’) was collected for the left and right hemisphere of each subject.
2.3.3- Sulcation indexes
Analysis of cortical sulcation were based on 3D mesh-based sulcal indexes, which reflect the degree of cortical burying following the methods by Cachia et al. 23. The global sulcal index (g-SI) for each hemisphere was measured as the ratio between the total sulcal area (i.e. the sum of the areas of all segmented cortical folds) and the total outer cortex area (i.e. hull area). Hence, a g-SI of zero reflects a totally smoothed cortical surface.
G-SI describes the burying of the cortex and is therefore slightly different from the gyrification index (GI), the ratio of the whole gyral contour length to the outer, exposed surface37, which embodies additional information (included in the whole gyral contour length) related to the cortex thickness and the sulcal opening. In addition, the classical GI captures the shape of the cortical folds, which are complex three-dimensional structures from measures on two-dimensional MRI slices37, while the g-SI is based on measures derived from a three-dimensional reconstruction of the sulcal surface. The local sulcal indexes (l-SI) were computed in each hemisphere from twelve a priori determined brain regions covering the whole cortex and including the lateral prefrontal cortex, the medial prefrontal cortex, the precentral region, the central sulcus, the Sylvian fissure, the parietal cortex, the intraparietal fissure, the medial parieto-occipital cortex, the occipital cortex, the external temporal cortex, the basal temporal cortex and the calcarine complex (Figure 1). For each of these regions, the l-SI was obtained as the ratio between the sum of the areas of the cortical folds included in the region of interest and the hull area.
Figure 1. Processing pipeline.
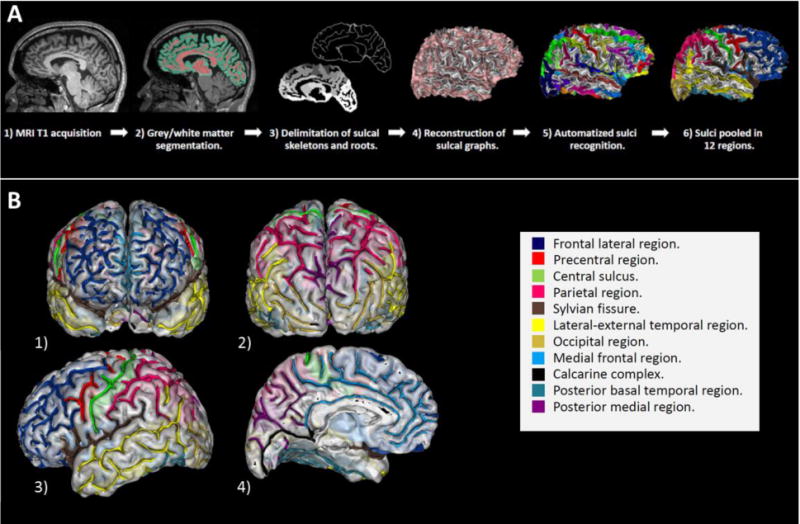
1-A : Processing steps embedded in the BrainVISA morphologist pipeline. 1-B : The twelve predetermined cortical regions investigated with local sulcation index. 1) Anterior view. 2) Posterior view. 3) Lateral view of the left hemisphere. 4) Medial view of the left hemisphere. The 12 cortical regions that have been studied are shown in color. Navy blue: Frontal dorso-lateral region. Red: Precentral region. Green: Central sulcus. Pink: Parietal region. Brown : Sylvian fissure. Yellow: Lateral – external temporal region. Gold: Occipital region. Light blue: Medial frontal region. Dark: Calcarine complex. Green blue: Posterior basal temporal region. Violet: Posterior medial region.
2.4 Statistical Analyses
All statistical analyses were ran after outlier filtering using cook’s distance. Univariate linear mixed modelling was used to 1) compare global and local sulcation indexes in patients and controls and 2) compare subgroups of patients. The linear model included diagnosis, age, sex and handedness as independent variables with a fixed effect and the site as an independent variable with a random effect. As a secondary analysis, we investigated if the age effect was different between the groups considered by adding an age-by-group interaction term. Normality of the linear model residuals was checked using qq-plots and Shapiro-Wilk test. Clinical and demographic continuous variables were compared using the Student’s t-test and categorical variables with the chi-square test when ensured that the appropriate assumptions were met.
No correction for multiple comparisons was applied, our results should therefore be regarded as exploratory. All the statistical analyses were carried out with R 3.3 software (http://www.r-project.org/)38 with ‘lmer’, ‘lmerTest’ and ‘effsize’ packages.
2.5- Replication sample
We ran a post hoc replication analysis on an independent sample of 26 patients with bipolar disorder I. Patients were included at Mondor University Hospital (Créteil, France). All patients underwent a high-resolution T1 MRI (Siemens Prisma Fit 3T, voxel resolution of 1×1×1mm, TR=2300ms and TE=2.98ms). Raw T1 images were processed using the same pipeline than the primary multicenter dataset (BrainVisa). We conducted one linear model with l-SI as the dependent variable and age at onset, age, gender and handedness as independent variables.
3. Results
3.1 Sample characteristics
Among the initial 606 included subjects, 19 patients were excluded due to motion artifacts and geometrical distortions, 3 controls had past alcohol dependence and 1 control had missing data about handedness. The final sample thus comprised 263 patients with BD I and 320 controls. Samples characteristics are detailed in table 1 and data for each site are provided in supplementary materials. Data about psychotic features were missing for 52 patients and data about past history of alcohol dependence was missing in 49 (23.2%) of patients with BD.
Table 1.
Sample characteristics.
| Patients with bipolar disorder I (n=263) |
Healthy subjects (n=320) |
Test value (t or Chi2) | P value | |
|---|---|---|---|---|
| Age (mean (SD)) | 40.46 (11.36) | 36.89 (11.04) | t=−3.83 | <0.001 |
| Women (n (%)) | 153 (58.17%) | 175 (54.7%) | Chi2=0.58 | 0.45 |
| Right- handed (n (%)) | 242 (92.02%) | 285 (89.06)% | Chi2=1.47 | 0.48 |
| Age at onset (mean (SD)) | 25.18 (9.78) | - | ||
| Bipolar patients with psychosis (n=129) | Bipolar patients without psychosis (n=82) | Test value | P value | |
| Age (mean (SD)) | 38.77 (10.32) | 38.74 (11.12) | t=0.02 | 0.98 |
| Women (n (%)) | 68 (52.71%) | 50 (60.97%) | Chi2=1.07 | 0.30 |
| Right-handed (n (%)) | 116 (89.92%) | 80 (97.6%) | Chi2=4.57 | 0.10 |
| Age at onset (mean (SD)) | 23.85 (8.65) | 23.58 (9.44) | t=0.21 | 0.83 |
| Bipolar patients with Early onset (n=58) | Bipolar patients with intermediate/late onset (n=204) | Test value | P value | |
| Age (mean (SD)) | 32.81 (9.38) | 42.60 (10.96) | t=−6.74 | <0.001 |
| Women (n (%)) | 39 (67.24%) | 114 (55.88%) | Chi2=1.95 | 0.16 |
| Right-handed (n (%)) | 56 (96.55%) | 185 (90.68) | Chi2=2.11 | 0.35 |
| Age at onset (mean (SD)) | 14.41 (2.44) | 28.24 (8.88) | t=−19.77 | <0.001 |
3.2 Group- and sub-comparisons
There were no between-group differences on g-SI (F(1,541.07) = 1.76,p = 0.18) or l-SI in BD vs HC (all p > 0.05, table 2).
Table 2. Comparison of Local Sulcal Index (LSI) between patients with bipolar disorder and control subjects for 12 predetermined regions bilaterally.
Abbreviations: n, number; SE, standard error; DoF, degree of freedom.
| Region | Outliers | Bipolar disorder LSI (estimates) | Healthy Subjects LSI (estimates) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | Mean | SE | DoF | F (Group) | Cohen’s d | p | Significant covariates | |
| Left Calcarine | 35 | 0.060 | 0.002 | 0.061 | 0.002 | (1,541.1) | 1.76 | 0.18 | 0.18 | Gender |
| Left Frontal Medial | 28 | 0.179 | 0.009 | 0.178 | 0.009 | (1,542.9) | 0.69 | −0.02 | 0.40 | Gender, Age |
| Left Parietal Occipital Medial | 29 | 0.135 | 0.003 | 0.136 | 0.003 | (1,548.0) | 0.40 | 0.02 | 0.53 | Gender, Age |
| Left Sylvian Fissure | 26 | 0.211 | 0.006 | 0.212 | 0.006 | (1,547.2) | 0.02 | −0.10 | 0.88 | Gender, Age |
| Left Intraparietal Fissure | 29 | 0.195 | 0.003 | 0.194 | 0.003 | (1,539.5) | 0.21 | 0.05 | 0.64 | Gender, Age |
| Left Occipital | 30 | 0.075 | 0.002 | 0.077 | 0.002 | (1,546.8) | 3.01 | −0.06 | 0.08 | Gender, Age, Hand |
| Left Parietal | 25 | 0.045 | 0.001 | 0.047 | 0.001 | (1,552.1) | 2.03 | −0.15 | 0.15 | Age |
| Left Lateral Prefrontal | 29 | 0.312 | 0.009 | 0.314 | 0.009 | (1,542.7) | 0.24 | −0.11 | 0.62 | Gender, Age |
| Left Central Sulcus | 27 | 0.086 | 0.002 | 0.086 | 0.002 | (1,548.5) | 0.04 | 0.01 | 0.85 | None |
| Left Precentral | 32 | 0.088 | 0.003 | 0.088 | 0.003 | (1,544.4) | 0.1 | −0.03 | 0.75 | Age |
| Left Temporal Basal | 24 | 0.143 | 0.006 | 0.145 | 0.006 | (1,547.3) | 2.55 | −0.1 | 0.11 | Age, Gender |
| Left Temporal Lateral | 23 | 0.258 | 0.008 | 0.259 | 0.008 | (1,548.8) | 0.55 | 0.02 | 0.46 | Age, Gender |
| Right Calcarine | 32 | 0.067 | 0.003 | 0.066 | 0.003 | (1,542.5) | 0.75 | 0.18 | 0.39 | None |
| Right Frontal Medial | 27 | 0.180 | 0.008 | 0.180 | 0.008 | (1,543.9) | <0.1 | −0.05 | 0.92 | Age, Gender |
| Right Parietal Occipital Medial | 32 | 0.135 | 0.003 | 0.138 | 0.003 | (1,544.7) | 3.34 | −0.15 | 0.07 | Gender |
| Right Sylvian Fissure | 34 | 0.203 | 0.006 | 0.203 | 0.006 | (1,538.1) | 0.03 | −0.02 | 0.85 | Age, Gender |
| Right Intraparietal Fissure | 35 | 0.195 | 0.003 | 0.197 | 0.003 | (1,539.2) | 0.75 | −0.06 | 0.39 | Age |
| Right Occipital | 24 | 0.090 | 0.002 | 0.088 | 0.002 | (1,552.2) | 1.62 | 0.13 | 0.20 | Age, Gender, Hand |
| Right Parietal | 30 | 0.049 | 0.002 | 0.05 | 0.002 | (1,543.4) | 0.45 | −0.05 | 0.50 | None |
| Right Lateral Prefrontal | 25 | 0.317 | 0.009 | 0.315 | 0.009 | (1,546.8) | 0.89 | 0.03 | 0.34 | Age, Gender |
| Right Central Sulcus | 31 | 0.084 | 0.002 | 0.084 | 0.002 | (1,542.7) | 0.05 | −0.04 | 0.81 | None |
| Right Precentral | 27 | 0.098 | 0.003 | 0.101 | 0.003 | (1,548.7) | 2.03 | −0.14 | 0.15 | Age |
| Right Temporal Basal | 29 | 0.137 | 0.006 | 0.138 | 0.006 | (1,542.3) | 0.13 | 0.002 | 0.72 | Age, Gender |
| Right Temporal Lateral | 21 | 0.275 | 0.009 | 0.272 | 0.009 | (1,550.3) | 2.27 | 0.13 | 0.13 | Age, Gender |
Further analyses revealed specific alterations in l-SI in subgroups of BD patients. Patients with early-onset BD (n=58) were found to have a higher l-SI in the right prefrontal dorsolateral region (F(1,244.4)=4.18,p=0.04), compared to patients with intermediate or late onset BD (n=204) (table 3 and figure 2). In addition, patients with psychotic BD (n=129) had a decreased l-SI in the left superior parietal cortex (F(1,196)=5.91,p=0.02) compared to patients who never experienced psychosis (n=82) (table 4, figure 3). Subgroup analysis did not reveal other differences in g-SI or l-SI (all p >0.05, table 3 and 4).
Table 3. Comparison of Local Sulcal Index (LSI) between patients with and without early-onset BD for 12 predetermined regions bilaterally.
Abbreviations: n, number; SE, standard error; DoF, degree of freedom.
| Region | Outliers | Early-onset Bipolar LSI (estimates) | Intermediate and late-onset Bipolar LSI (estimates) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | Mean | SE | DoF | F (Group) | Cohen’s d | p | Significant covariates | |
| Left Calcarine | 18 | 0.067 | 0.004 | 0.062 | 0.004 | (1,235.5) | 2.71 | 0.25 | 0.10 | Gender |
| Left Frontal Medial | 14 | 0.179 | 0.009 | 0.180 | 0.009 | (1,235.1) | 0.17 | −0.20 | 0.68 | Age |
| Left Parietal Occipital Medial | 12 | 0.136 | 0.005 | 0.137 | 0.004 | (1,210.7) | 0.05 | −0.03 | 0.82 | None |
| Left Sylvian Fissure | 14 | 0.217 | 0.008 | 0.211 | 0.007 | (1,239.3) | 1.35 | 0.09 | 0.25 | None |
| Left Intraparietal Fissure | 11 | 0.194 | 0.005 | 0.199 | 0.004 | (1,238.3) | 2.11 | −0.13 | 0.15 | Age |
| Left Occipital | 9 | 0.077 | 0.004 | 0.075 | 0.003 | (1,235.3) | 0.51 | 0.09 | 0.48 | Gender |
| Left Parietal | 15 | 0.048 | 0.003 | 0.046 | 0.002 | (1,243.0) | 0.77 | 0.27 | 0.38 | Age |
| Left Lateral Prefrontal | 10 | 0.319 | 0.012 | 0.316 | 0.010 | (1,242.5) | 0.14 | 0.10 | 0.71 | Age, Gender |
| Left Central Sulcus | 14 | 0.086 | 0.002 | 0.088 | 0.002 | (1,244.3) | 1.62 | −0.04 | 0.20 | None |
| Left Precentral | 10 | 0.091 | 0.004 | 0.086 | 0.003 | (1,242.8) | 3.39 | 0.35 | 0.07 | None |
| Left Temporal Basal | 11 | 0.146 | 0.007 | 0.146 | 0.006 | (1,237.8) | 0.04 | −0.22 | 0.83 | Age, Gender |
| Left Temporal Lateral | 11 | 0.257 | 0.008 | 0.258 | 0.007 | (1,240.8) | 0.01 | 0.12 | 0.93 | Age |
| Right Calcarine | 11 | 0.065 | 0.004 | 0.063 | 0.003 | (1,246.7) | 0.51 | 0.06 | 0.48 | None |
| Right Frontal Medial | 13 | 0.176 | 0.009 | 0.183 | 0.008 | (1,241.4) | 3.36 | −0.44 | 0.07 | None |
| Right Parietal Occipital Medial | 15 | 0.133 | 0.005 | 0.133 | 0.004 | (1,227.9) | 0.01 | −0.06 | 0.91 | Gender |
| Right Sylvian Fissure | 17 | 0.206 | 0.007 | 0.205 | 0.006 | (1,238.9) | 0.02 | −0.09 | 0.88 | Gender |
| Right Intraparietal Fissure | 19 | 0.199 | 0.005 | 0.196 | 0.004 | (1,220.6) | 0.28 | 0.26 | 0.59 | Age |
| Right Occipital | 12 | 0.090 | 0.005 | 0.091 | 0.004 | (1,242.4) | 0.13 | −0.04 | 0.72 | Age, Gender |
| Right Parietal | 13 | 0.046 | 0.004 | 0.048 | 0.003 | (1,237.5) | 0.43 | −0.13 | 0.51 | None |
| Right Lateral Prefrontal | 11 | 0.330 | 0.011 | 0.318 | 0.010 | (1,244.4) | 4.18 | 0.24 | 0.04 | Age, Gender |
| Right Central Sulcus | 15 | 0.083 | 0.002 | 0.084 | 0.002 | (1,239.8) | 0.32 | −0.06 | 0.57 | None |
| Right Precentral | 13 | 0.102 | 0.005 | 0.099 | 0.004 | (1,238.0) | 1.02 | 0.19 | 0.31 | None |
| Right Temporal Basal | 11 | 0.143 | 0.007 | 0.140 | 0.006 | (1,238.8) | 1.52 | −0.09 | 0.22 | Gender |
| Right Temporal Lateral | 10 | 0.278 | 0.010 | 0.278 | 0.009 | (1,242.8) | 0.02 | −0.05 | 0.88 | Age, Gender |
Figure 2.
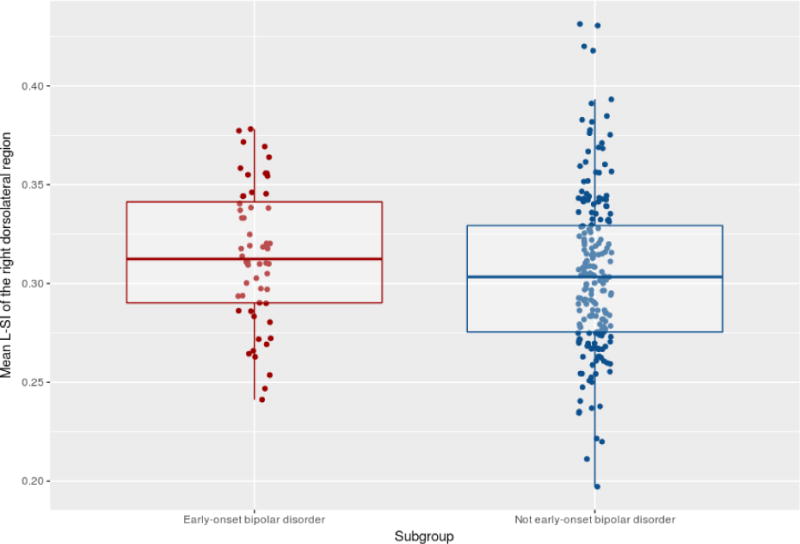
Jitterplot of the Right Lateral Prefrontal Local Sulcal Index (unadjusted) in patients with or without early-onset bipolar disorder.
Table 4. Comparison of Local Sulcal Index (LSI) between patients with and without history of psychosis for 12 predetermined regions bilaterally.
Abbreviations: n, number; SE, standard error; DoF, degree of freedom.
| Region | Outliers | Bipolar with psychosis LSI (estimates) | Bipolar without psychosis LSI (estimates) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | Mean | SE | DoF | F (Group) | Cohen’s d | p | Significant covariates | |
| Left Calcarine | 13 | 0.061 | 0.004 | 0.061 | 0.004 | (1,195.3) | 0.017 | −0.28 | 0.90 | Gender |
| Left Frontal Medial | 12 | 0.169 | 0.004 | 0.171 | 0.004 | (1,54.7) | 0.53 | −0.08 | 0.47 | Age |
| Left Parietal Occipital Medial | 12 | 0.138 | 0.004 | 0.138 | 0.005 | (1,66.9) | 0.09 | 0.09 | 0.77 | None |
| Left Sylvian Fissure | 11 | 0.208 | 0.006 | 0.210 | 0.006 | (1,74.5) | 0.23 | −0.06 | 0.63 | Age |
| Left Intraparietal Fissure | 12 | 0.197 | 0.004 | 0.192 | 0.005 | (1,46.8) | 2.47 | 0.26 | 0.12 | Age |
| Left Occipital | 13 | 0.078 | 0.003 | 0.077 | 0.003 | (1,193.0) | 0.15 | 0.06 | 0.69 | Gender |
| Left Parietal | 12 | 0.045 | 0.003 | 0.05 | 0.004 | (1,196.0) | 5.91 | −0.34 | 0.02 | Age |
| Left Lateral Prefrontal | 10 | 0.308 | 0.008 | 0.309 | 0.009 | (1,181.7) | 0.04 | −0.18 | 0.84 | Age, Gender |
| Left Central Sulcus | 9 | 0.086 | 0.002 | 0.087 | 0.002 | (1,173.9) | 0.44 | −0.27 | 0.51 | Age |
| Left Precentral | 9 | 0.080 | 0.004 | 0.083 | 0.004 | (1,170.8) | 1.46 | −0.28 | 0.23 | Handedness |
| Left Temporal Basal | 10 | 0.146 | 0.006 | 0.142 | 0.006 | (1,195.8) | 1.46 | 0.14 | 0.23 | Gender |
| Left Temporal Lateral | 9 | 0.253 | 0.005 | 0.259 | 0.005 | (1,199.0) | 2.42 | −0.16 | 0.12 | Age, Handedness |
| Right Calcarine | 12 | 0.058 | 0.003 | 0.061 | 0.004 | (1,61.07) | 1.26 | −0.18 | 0.26 | Gender |
| Right Frontal Medial | 9 | 0.168 | 0.004 | 0.172 | 0.005 | (1,125.2) | 1.79 | −0.09 | 0.18 | Age, Gender |
| Right Parietal Occipital Medial | 11 | 0.134 | 0.006 | 0.133 | 0.006 | (1,197.0) | 0.09 | 0.05 | 0.76 | Gender |
| Right Sylvian Fissure | 15 | 0.203 | 0.005 | 0.203 | 0.005 | (1,96.23) | 0.02 | 0.09 | 0.89 | Gender |
| Right Intraparietal Fissure | 13 | 0.198 | 0.005 | 0.197 | 0.005 | (1,40.46) | 0.07 | 0.04 | 0.79 | Age |
| Right Occipital | 8 | 0.092 | 0.003 | 0.094 | 0.004 | (1,198.0) | 0.78 | −0.07 | 0.38 | Age, Gender |
| Right Parietal | 12 | 0.045 | 0.004 | 0.049 | 0.004 | (1,196.0) | 2.43 | −0.21 | 0.12 | None |
| Right Lateral Prefrontal | 8 | 0.307 | 0.009 | 0.312 | 0.010 | (1,192.0) | 0.51 | −0.20 | 0.47 | Age, Gender |
| Right Central Sulcus | 13 | 0.080 | 0.002 | 0.081 | 0.002 | (1,159.5) | 0.12 | −0.24 | 0.73 | None |
| Right Precentral | 10 | 0.091 | 0.004 | 0.094 | 0.004 | (1,198.0) | 1.72 | −0.23 | 0.19 | None |
| Right Temporal Basal | 7 | 0.138 | 0.006 | 0.136 | 0.006 | (1,197.9) | 0.69 | −0.018 | 0.41 | Gender |
| Right Temporal Lateral | 9 | 0.265 | 0.005 | 0.269 | 0.006 | (1,50.19) | 0.73 | −0.04 | 0.39 | Age, Gender |
Figure 3.
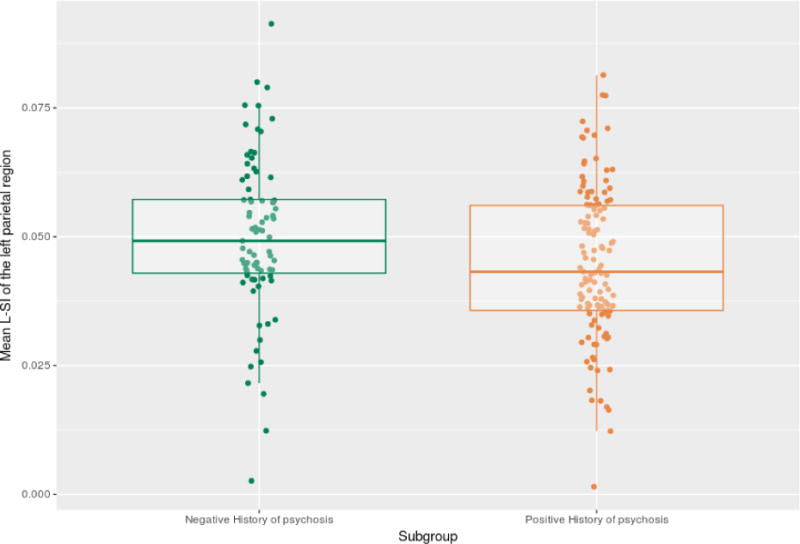
Jitterplot of the Left Parietal Local Sulcal Index (unadjusted) in bipolar patients with or without a positive history of psychotic features.
3.5 Clinical correlations and complementary analyses
We found no difference of hull area between patients depending on their history of psychosis (F(1,129.8)=1.07;p=0.3) or age at onset (F(1,286.5)=0.53,p=0.47). When adding past history of alcohol abuse as a covariate in the model, the effect of age at onset on the prefrontal l-SI remained significant (F(1,164.19)=5.17; p=0.02). However, history of psychotic feature was no longer significant adding the status of past alcohol dependence to the model in patients in which the data was available (F(1,51.99=0.33),p=0.56).
We conducted complementary analyses to investigate whether the difference of age in subgroups based on the age at onset could influence the results. Firstly, we found no effect of duration of illness on the prefrontal l-SI (F(1,242.15)=2.69, p=0.10). Secondly, we found no significant age-by-group interaction on the prefrontal l-SI when considering subgroups of patients based on their age at onset (F(1,242.06)=2.7,p=0.1) or on the parietal l-SI when comparing patients based on their history of psychosis (F(1,195.02)=0.028,p=0.86). Thirdly, we replicated the l-SI difference of the right prefrontal cortex in a sample of 58 early-onset and 58 intermediate/late onset of patients with BD matched for age, gender, handedness and site (t(97.42)=2.02, p=0.046) (Supplementary materials). We also checked that our results were not driven by samples with unbalanced contribution of patients with psychotic BD (Supplementary materials).
Last, we checked that our results were not driven by differences of medication patterns, especially lithium treatment (details on medication data are provided in Supplementary materials). The differences between patients depending on the age at onset (F(1,233.54)=5.57,p=0.019) or on the history of psychotic features (F(1,179.02)=5.87,p=0.016) remained significant when adding the number of current psychotropic medications as a covariate to the model. Moreover, adding medication status to the main models did not substantially change the results (Supplementary materials).
3.6 – Replication analysis
Based on the primary results, we tested whether we could replicate the finding of a higher right prefrontal sulcation associated with an earlier age at onset in 26 patients with bipolar disorder I. Characteristics of the sample are described in Supplementary materials. We found a negative association between age at onset and the right prefrontal l-SI, when controlling for age, gender and handedness (β=−0.003; t=−3.322; p=0.003) (Supplementary materials).
4. Discussion
In this exploratory study on a relatively large international multicenter sample involving more than 580 participants, we found localized sulcation deviations associated with BD but only in subgroups of bipolar patients with putative deviation in neurodevelopmental trajectories, namely early age of onset and positive history of psychosis12,13. To our best knowledge, this multicenter study is the largest sample used suggesting differences of sulcation in subgroups of patients with BD and the first study to suggest differences of sulcation between the psychotic and non-psychotic subgroup of patients with BD.
These findings replicate, using the same methodology but in a completely independent sample, the study of Pentilla and al. 32 reporting increased local sulcation in the prefrontal cortex in early-onset patients. The effect size related to this difference (Cohen’s d = 0.24) was smaller than in the Pentilla et al. study with an estimated Cohen’s d of 1.30. The lower effect size in the current study may come from the statistical analyses which include more covariates to control potential confounding effects. Such observed decreased effect in a large sample is in line with previous neuroimaging studies showing lower effect sizes in larger samples39. We also found, in a smaller but independent sample of patients with bipolar disorder, a linear association between earlier age at onset and higher sulcation of the right prefrontal cortex. Albeit exploratory, our study replicated previous results32 suggesting that an early age of onset is associated to specific impaired sulcation of the right lateral prefrontal cortex, that could be related to specific neurodevelopmental deviations in this subgroup of patients. Of note, a greater inter-subject variation of sulcation pattern has been described in the prefrontal cortex compared to motor and visual areas37. We speculate that the lack of association between illness duration and the sulcation of the lateral prefrontal cortex in the current sample, in line with the investigation by Pentilla et al. 32 may be interpreted in terms of neurodevelopmental deviations and not as a disease-related neurodegenerative process. To confirm such a hypothesis, large cross sectional, as well as longitudinal, studies replicating our findings focusing on the relationship between prefrontal sulcation and cognitive functions in youth at high risk remain particularly needed.
Our results also suggest that the sulcation of the left superior parietal cortex is lower in patients with psychosis, including the superior and transverse sulci as well as the sulcus of the supra marginal gyrus but excluding the intraparietal fissure, contrasting with a recent work reporting higher parietal sulcation in patients with schizophrenia40. Other studies have specifically focused on bipolar disorder with psychosis, i.e by comparing patients with psychotic bipolar disorder to patients with schizophrenia or to controls with heterogeneous results showing higher25, lower31, or mixed41 differences of gyrification indices26. One speculative hypothesis could be that differences of symptoms in the patients recruited in the different studies may explain such discrepancy with our findings. Interestingly, a very recent study reported different parietal gyrification variations in patients with schizophrenia depending on their liability to hallucinate42. Such a symptom-based approach would be of interest to explore this apparent discrepancy, however we were unable to retrieve specific data about auditory hallucinations in our sample. Another hypothesis is the fact that the relationship between gyrification and psychotic symptoms may not related to the disorder per se, but rather could be mediated by different developmental trajectories associated with psychosis. To support this hypothesis, neurological soft signs, considered as clinical markers of neurodevelopmental deviations, are correlated with gyrification in samples of patients with schizophrenia43,44 and in healthy subjects45. Future studies directly comparing sulcation patterns in the psychosis spectrum but exploring their relationship with neurological soft signs would be of importance to understand the relationship between neurodevelopment and sulcation in patients with psychosis.
As sulcation is intimately related to early neurodevelopment46, local and global sulcal indexes are used to retrospectively assess impaired early neurodevelopmental processes occurring in patients. Indeed, the mature morphology of the cortex is considered to result from early processes that shape the cortex anatomy from a smooth lissencephalic structure to a highly convoluted surface47. This complex folded surface has been shown to be an early marker of later functional development48. The precise mechanism underlying the cortex folding is still unknown but several factors likely contribute to prenatal processes that influence the shape of the folded cerebral cortex, including cortical growth, apoptosis, differential expansion of superior and inferior cortical layers, differential growth of the progyral versus the prosulcal regions24 and/or variations of white matter connections49,50. These mechanical constraints lead to a compact layout that optimizes the transmission of neuronal signals between brain regions51 and thus small world characteristics of the brain network. We found no difference of brain hull area between subgroups of patients thus suggesting that the differences of local sulcal index were explained by actual differences of folding rather than indirect effects of global brain volume differences on prefrontal sulcation. Neurotrophic properties of lithium are highly suspected52 and may act as a confounder in this study. However we did not find evidence for our results being driven by medication status, including lithium medication (supplementary materials).
Our results suggest that patients with BD, considered as a single group, had no difference in sulcation compared to healthy controls. One may speculate that, like in schizophrenia23, impaired neurodevelopment might have led to clinically-specific differences in sulcation indexes that may later mediate a different phenotypic presentation. The group-specific changes in local sulcation suggest that different brain areas may be differentially impacted by genetic – environmental interactions during brain growth. Longitudinal studies of high-risk subjects which measure endpoints of sulcation in childhood, adolescence and early adulthood are needed to clarify and confirm our exploratory findings. Finally, our results support the hypothesis that BD is a highly heterogeneous entity21 that can be disentangled using neuroimaging measures.
The results of this study are best understood in the context of a number of methodological issues. Albeit the processing pipeline was the same for all sites, MRI acquisitions were different between sites and we used different structured clinical interviews across sites. This induced diagnostic and measurement heterogeneity despite controlling for site in our statistical analyses. However, this also suggests that our results are independent of these methodological issues. Cross-sectional analyses were used while age and age at onset are highly correlated. A longitudinal design may be more suitable to understand the deviation in sulcation in early-onset BD. We do not know how sulcation landmarks and cytoarchitectonic boarders are related53. It is important to remind that our results have to be considered as exploratory as we did not correct our analyses for multiple comparisons. Albeit large and multicenter studies are considered to better estimate ‘true’ effect sizes in neuroimaging studies39 one must keep in mind that we found relatively small effect sizes. Future confirmatory cross-sectional and longitudinal studies studying sulcation in subgroups of BD would have to include a sufficient number of subjects to detect effect sizes of this magnitude with lower uncertainty.
5. Conclusion
In conclusion, our results suggest subgroup-specific differences in sulcation in patients with BD that may be limited to patients with putative neurodevelopmental impairment (i.e. early-onset and/or psychotic BD). We replicated in 2 samples a previous finding regarding early-onset BD. Sulcal differences do not apply to all patients with BD, but may be subtle and restricted to prefrontal and parietal regions of the cortex. These exploratory findings support the hypothesis that BD is a heterogeneous clinical entity that may be partially disentangled using brain imaging. However, replication studies with larger samples and longitudinal investigations of neurodevelopmental trajectories are needed to confirm these results and to establish that local sulcation index could be used as a neurodevelopmental marker of BD.
Supplementary Material
Acknowledgments
We are grateful to the participating subjects. This work was supported by public funding from the Agence Nationale pour la Recherche (ANR MNP VIP 2008, ANR-11-IDEX-0004 Labex BioPsy and ANR-DFG ANR-14-CE35-0035 FUNDO), the Fondation de l’Avenir (Recherche Médicale Appliquée 2014), the Fondation pour la Recherche Médicale (Appel d’offres analyse bioinformatique pour la recherche en biologie 2014), the Deutsche Forschungsgemeinschaft (SFB636/C6 and We3638/3-1), the NIMH R01 MH076971, the American Psychiatric Institute for Research and Education (APIRE Young Minds in Psychiatry Award), from the Italian Ministry for Education, University and Research (PRIN n. 2005068874), from Veneto StartCup 2007 to Dr Brambilla, and from the Regione Veneto, Italy (159/03, DGRV n. 4087), the Grenoble University Hospital, the French University Institute, the Grenoble Cognition Center, and the Health and Society Research Network of the Pierre Mendes-France University (Grenoble), the Grenoble MRI facility IRMaGE was partly funded by the French program Investissements d’avenir run by the Agence Nationale pour la Recherche; grant Infrastructure d’avenir en Biologie Sante – ANR-11-INBS-0006. S. Sarrazin is supported by a grant from the Labex Bio-Psy & APHP.
Footnotes
DR. JOSSELIN HOUENOU (Orcid ID : 0000-0003-3166-5606)
Financial disclosures
No author has financial disclosure related to this work.
References
- 1.O’Shea KS, McInnis MG. Neurodevelopmental origins of bipolar disorder: iPSC models. Mol Cell Neurosci. 2015 Dec 1; doi: 10.1016/j.mcn.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Bortolato B, Köhler CA, Evangelou E, León-Caballero J, Solmi M, Stubbs B, et al. Systematic assessment of environmental risk factors for bipolar disorder: an umbrella review of systematic reviews and meta-analyses. Bipolar Disord. 2017 Mar;19(2):84–96. doi: 10.1111/bdi.12490. [DOI] [PubMed] [Google Scholar]
- 3.Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013 Jul;70(7):677–85. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- 4.Fuller Torrey E. Epidemiological comparison of schizophrenia and bipolar disorder. Schizophr Res. 1999 Sep;39(2):101–6. doi: 10.1016/s0920-9964(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 5.Disanto G, Morahan JM, Lacey MV, DeLuca GC, Giovannoni G, Ebers GC, et al. Seasonal distribution of psychiatric births in England. PloS One. 2012;7(4):e34866. doi: 10.1371/journal.pone.0034866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chudal R, Sourander A, Polo-Kantola P, Hinkka-Yli-Salomäki S, Lehti V, Sucksdorff D, et al. Perinatal factors and the risk of bipolar disorder in Finland. J Affect Disord. 2014 Feb;155:75–80. doi: 10.1016/j.jad.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guth C, Jones P, Murray R. Familial psychiatric illness and obstetric complications in early-onset affective disorder. A case-control study. Br J Psychiatry J Ment Sci. 1993 Oct;163:492–8. doi: 10.1192/bjp.163.4.492. [DOI] [PubMed] [Google Scholar]
- 8.Kerner B. Toward a Deeper Understanding of the Genetics of Bipolar Disorder. Front Psychiatry. 2015;6:105. doi: 10.3389/fpsyt.2015.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landén M, Lichtenstein P. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015 Mar;17(2):184–93. doi: 10.1111/bdi.12242. [DOI] [PubMed] [Google Scholar]
- 10.Olvet DM, Burdick KE, Cornblatt BA. Assessing the potential to use neurocognition to predict who is at risk for developing bipolar disorder: a review of the literature. Cognit Neuropsychiatry. 2013;18(1–2):129–45. doi: 10.1080/13546805.2012.724193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mrad A, Wassim Krir M, Ajmi I, Gaha L, Mechri A. Neurological soft signs in euthymic bipolar I patients: A comparative study with healthy siblings and controls. Psychiatry Res. 2016 Feb 28;236:173–8. doi: 10.1016/j.psychres.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 12.Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophr Bull. 2014 Mar;40(Suppl 2):S138–46. doi: 10.1093/schbul/sbt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigurdsson E, Fombonne E, Sayal K, Checkley S. Neurodevelopmental antecedents of early-onset bipolar affective disorder. Br J Psychiatry J Ment Sci. 1999 Feb;174:121–7. doi: 10.1192/bjp.174.2.121. [DOI] [PubMed] [Google Scholar]
- 14.Yildiz A, Sachs GS. Age onset of psychotic versus non-psychotic bipolar illness in men and in women. J Affect Disord. 2003 Apr;74(2):197–201. doi: 10.1016/s0165-0327(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 15.Schulze TG, Hedeker D, Zandi P, Rietschel M, McMahon FJ. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006 Dec;63(12):1368–76. doi: 10.1001/archpsyc.63.12.1368. [DOI] [PubMed] [Google Scholar]
- 16.Schürhoff F, Bellivier F, Jouvent R, Mouren-Siméoni MC, Bouvard M, Allilaire JF, et al. Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord. 2000 Jun;58(3):215–21. doi: 10.1016/s0165-0327(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 17.Ozyildirim I, Cakir S, Yazici O. Impact of psychotic features on morbidity and course of illness in patients with bipolar disorder. Eur Psychiatry J Assoc Eur Psychiatr. 2010 Jan;25(1):47–51. doi: 10.1016/j.eurpsy.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Levy B, Medina AM, Weiss RD. Cognitive and psychosocial functioning in bipolar disorder with and without psychosis during early remission from an acute mood episode: a comparative longitudinal study. Compr Psychiatry. 2013 Aug;54(6):618–26. doi: 10.1016/j.comppsych.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geoffroy PA, Etain B, Scott J, Henry C, Jamain S, Leboyer M, et al. Reconsideration of bipolar disorder as a developmental disorder: importance of the time of onset. J Physiol Paris. 2013 Sep;107(4):278–85. doi: 10.1016/j.jphysparis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Canetta SE, Bao Y, Co MDT, Ennis FA, Cruz J, Terajima M, et al. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry. 2014 May;171(5):557–63. doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hozer F, Houenou J. Can neuroimaging disentangle bipolar disorder? J Affect Disord. 2016 May;195:199–214. doi: 10.1016/j.jad.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex N Y N 1991. 2008 Jun;18(6):1444–54. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- 23.Cachia A, Amad A, Brunelin J, Krebs M-O, Plaze M, Thomas P, et al. Deviations in cortex sulcation associated with visual hallucinations in schizophrenia. Mol Psychiatry. 2015 Sep;20(9):1101–7. doi: 10.1038/mp.2014.140. [DOI] [PubMed] [Google Scholar]
- 24.Mangin J-F, Jouvent E, Cachia A. In-vivo measurement of cortical morphology: means and meanings. Curr Opin Neurol. 2010 Aug;23(4):359–67. doi: 10.1097/WCO.0b013e32833a0afc. [DOI] [PubMed] [Google Scholar]
- 25.Nenadic I, Maitra R, Dietzek M, Langbein K, Smesny S, Sauer H, et al. Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia. J Affect Disord. 2015 Oct 1;185:104–7. doi: 10.1016/j.jad.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Nanda P, Tandon N, Mathew IT, Giakoumatos CI, Abhishekh HA, Clementz BA, et al. Local gyrification index in probands with psychotic disorders and their first-degree relatives. Biol Psychiatry. 2014 Sep 15;76(6):447–55. doi: 10.1016/j.biopsych.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T, Malhi GS, Nakamura Y, Suzuki M, Pantelis C. Olfactory sulcus morphology in established bipolar affective disorder. Psychiatry Res. 2014 Apr 30;222(1–2):114–7. doi: 10.1016/j.pscychresns.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh AM, Moorhead TWJ, McKirdy J, Hall J, Sussmann JED, Stanfield AC, et al. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2009 Mar;119(3):192–8. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- 29.Palaniyappan L, Marques TR, Taylor H, Handley R, Mondelli V, Bonaccorso S, et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry. 2013 Oct;70(10):1031–40. doi: 10.1001/jamapsychiatry.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penttilä J, Paillère-Martinot M-L, Martinot J-L, Ringuenet D, Wessa M, Houenou J, et al. Cortical folding in patients with bipolar disorder or unipolar depression. J Psychiatry Neurosci JPN. 2009 Mar;34(2):127–35. [PMC free article] [PubMed] [Google Scholar]
- 31.Janssen J, Alemán-Gómez Y, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, et al. Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr Res. 2014 Sep;158(1–3):91–9. doi: 10.1016/j.schres.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 32.Penttilä J, Cachia A, Martinot J-L, Ringuenet D, Wessa M, Houenou J, et al. Cortical folding difference between patients with early-onset and patients with intermediate-onset bipolar disorder. Bipolar Disord. 2009 Jun;11(4):361–70. doi: 10.1111/j.1399-5618.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 33.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004 May 1;55(9):875–81. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Gay O, Plaze M, Oppenheim C, Mouchet-Mages S, Gaillard R, Olié J-P, et al. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. 2013 Jul;39(4):820–9. doi: 10.1093/schbul/sbs083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangin J-F, Rivière D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, et al. A framework to study the cortical folding patterns. NeuroImage. 2004;23(Suppl 1):S129–38. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Perrot M, Rivière D, Mangin J-F. Cortical sulci recognition and spatial normalization. Med Image Anal. 2011 Aug;15(4):529–50. doi: 10.1016/j.media.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988;179(2):173–9. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- 38.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing [Internet]; Vienna, Austria: 2012. Available from: http://www.R-project.org/ [Google Scholar]
- 39.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 40.Sasabayashi D, Takayanagi Y, Nishiyama S, Takahashi T, Furuichi A, Kido M, et al. Increased Frontal Gyrification Negatively Correlates with Executive Function in Patients with First-Episode Schizophrenia. Cereb Cortex N Y N 1991. 2017 Apr 1;27(4):2686–94. doi: 10.1093/cercor/bhw101. [DOI] [PubMed] [Google Scholar]
- 41.Palaniyappan L, Liddle PF. Diagnostic discontinuity in psychosis: a combined study of cortical gyrification and functional connectivity. Schizophr Bull. 2014 May;40(3):675–84. doi: 10.1093/schbul/sbt050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubera KM, Thomann PA, Hirjak D, Barth A, Sambataro F, Vasic N, et al. Cortical folding abnormalities in patients with schizophrenia who have persistent auditory verbal hallucinations. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2018 Feb;28(2):297–306. doi: 10.1016/j.euroneuro.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Hirjak D, Kubera KM, Wolf RC, Thomann AK, Hell SK, Seidl U, et al. Local brain gyrification as a marker of neurological soft signs in schizophrenia. Behav Brain Res. 2015 Oct 1;292:19–25. doi: 10.1016/j.bbr.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 44.Hirjak D, Wolf RC, Paternoga I, Kubera KM, Thomann AK, Stieltjes B, et al. Neuroanatomical Markers of Neurological Soft Signs in Recent-Onset Schizophrenia and Asperger-Syndrome. Brain Topogr. 2016 May;29(3):382–94. doi: 10.1007/s10548-015-0468-9. [DOI] [PubMed] [Google Scholar]
- 45.Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Thomann PA. Multiparametric mapping of neurological soft signs in healthy adults. Brain Struct Funct. 2016 Apr;221(3):1209–21. doi: 10.1007/s00429-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 46.Welker W. Why Does Cerebral Cortex Fissure and Fold? In: Jones EG, Peters A, editors. Cerebral Cortex: Comparative Structure and Evolution of Cerebral Cortex, Part II [Internet] Boston, MA: Springer US; 1990. pp. 3–136. Available from: [DOI] [Google Scholar]
- 47.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977 Jan;1(1):86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 48.Dubois J, Benders M, Borradori-Tolsa C, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain J Neurol. 2008 Aug;131(Pt 8):2028–41. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bayly PV, Taber LA, Kroenke CD. Mechanical forces in cerebral cortical folding: a review of measurements and models. J Mech Behav Biomed Mater. 2014 Jan;29:568–81. doi: 10.1016/j.jmbbm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White T, Su S, Schmidt M, Kao C-Y, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010 Feb;72(1):36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klyachko VA, Stevens CF. Connectivity optimization and the positioning of cortical areas. Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7937–41. doi: 10.1073/pnas.0932745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rybakowski JK, Suwalska A, Hajek T. Clinical Perspectives of Lithium’s Neuroprotective Effect. Pharmacopsychiatry. 2017 Dec 21; doi: 10.1055/s-0043-124436. [DOI] [PubMed] [Google Scholar]
- 53.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex N Y N 1991. 1995 Aug;5(4):323–37. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


