Background
T cells for cancer immunotherapy
CD8+ T cells play a critical role in adaptive immunity by virtue of their ability to initiate killing following receptor-mediated engagement by antigens expressed on the surface of tumor cells.1 CD8+ T cell-mediated cytotoxicity requires direct contact with target cells, thereby limiting damage to bystander cells. The attractiveness of target-specific approaches lies in avoidance of the serious side effects of other conventional treatments such as chemotherapy and radiation that have relatively non-specific mechanisms of action. A unique feature of the immune response, unlike conventional cancer therapies, is that it can elicit long-term protection from recurring disease (immunological memory).2 Another significant advantage of T cell-based immunotherapies is that T cells can search out and traffic to widely disseminated heterogeneous tumor cell targets by using chemokine-chemokine receptor interaction and generalized Lévy walks.3,4
Current status of adoptive T cell therapy
Significant advances have been made in the development of adoptive cell therapy (ACT) aiming to boost the immune response directed against chronic viral infections and various cancers.5–14 ACT using autologous tumor-infiltrating lymphocytes (TILs) has been used clinically for several decades, and was found to mediate objective tumor regression in 50–70% of patients with IL-2 refractory metastatic melanoma in combination with lymphodepleting chemotherapy and systemic high-dose IL-2 administration.14,15 Additionally, ACT using genetic modification of peripheral blood lymphocytes with a T cell receptor (TCR) or a chimeric antigen receptor (CAR) specific for tumor-specific antigen can mediate regression in multiple cancer histologies.5–12 Both technologies can augment T cell function by altering receptor specificity and signaling functions that control proliferative capacity and other cellular functions.5–12 In the former approach, T cells with enhanced affinity or novel specificity are created by expression of TCR α/β heterodimers in peripheral blood T cells.10–13 The endogenous repertoire for TCRs is generally of low affinity when targeting shared tumor-associated antigen because of the impact of central tolerance; however, TCRs targeting neoantigens, where mutations in the cancer genome create neo-epitopes, have high affinity.16,17 Furthermore, the affinity and functional avidity of tumor-antigen specific TCRs can be enhanced by high-throughput genetic approaches.18–20 NY-ESO-1 is expressed in a variety of cancers, but not in normal adult tissues, except for germ cells of the testis, making it an ideal target for immunotherapy.21,22 NY-ESO-1-specific TCR-engineered T cells have generated clinical responses in patients with advanced multiple myeloma, melanoma, and synovial cell sarcoma.11–13 TCR-based targeting approaches are; however, often susceptible to the common tumor escape mechanisms of major histocompatibility complex (MHC) down-modulation and altered peptide processing.23
The concept of CAR technology dates to the 1980’s when Eshhar and colleagues engineered and expressed chimeric T-cell receptor genes comprising antigen-binding domains fused to T-cell signaling domains.24 Because the target-binding moiety is derived from antibodies with higher affinity than TCRα/β, CARs enable highly specific targeting of antigen in an MHC-independent fashion.5–9 Adoptive transfer of CD19-directed CAR-T cells has generated complete and durable remissions in patients with refractory and relapsed B cell malignancies such as acute lymphoblastic leukemia, chronic lymphocytic leukemia, and non-Hodgkin’ lymphoma.6–9 The growing optimism in the field as we develop a better understanding of these technologies continues to unveil the potential present in adoptive T cell therapy.
Limitations to adoptive T cell therapy for solid malignancies
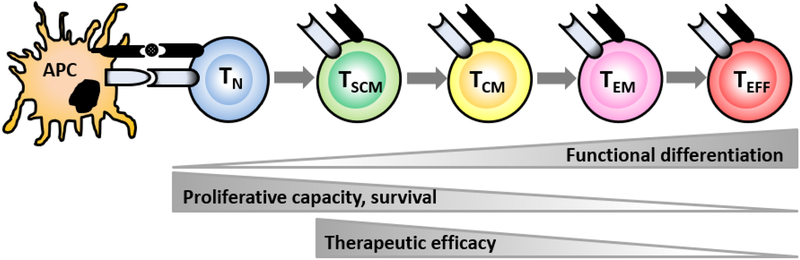
While adoptive T cell therapy demonstrated impressive clinical response with long-term remission for hematological malignancies, this success has not yet been concluded in solid malignancies.25 Despite encouraging results in preclinical models and in patients, poor survival of infused T cells and the existence of immune suppressive pathways appear to restrict the full potential of ACT for solid tumors. Current clinical ACT protocols require extensive ex vivo manipulation of autologous T cells in order to obtain large numbers, resulting in the generation of fully differentiated effector T cells. While these differentiated T cells are equipped with full effector function, they are severely impaired in their proliferative capacity (Figure 1).26–28 Trafficking of infused T cells to the tumor is a critical step for successful immunotherapy that correlates with clinical responses in patients.29 However, the tumor microenvironment (TME) characterized by abnormal tumor vessels and interstitium limits lymphocyte adhesion, extravasation, and infiltration.30 As a result, only a fraction of ex vivo expanded T cells can infiltrate into the tumor tissue.29
Figure 1. Differentiation status of adoptively transferred T cells inversely correlates with therapeutic efficacy.
APC: antigen-presenting cell, TN: naïve T cells, TSCM: stem cell memory T cells, TCM: central memory T cells, TEM: effector memory T cells, TEFF: effector T cells.
Data from Sallusto F, Lanzavecchia A. Memory in disguise. Nat Med 2011; 17(10): 1182–1183.
Cancer cells reprogram their metabolism to meet the rapid energy requirements for proliferation, survival and metastasis.31 Glycolytic metabolism of glucose results in lactic acid, which can acidify the TME.32 Acidosis and hypoxia are considered as biochemical hallmarks of the TME33,34 that not only modulate cancer cell metabolism but also influence T-cell proliferation and effector function.35 Hypoxia induces FoxP3, a key transcriptional regulator for regulatory T cells (Tregs),36 and polarizes CD4+ T cells towards a Th2 phenotype,37 allowing the resultant IL-4 and IL-13 to induce macrophage M2 polarization.38
Tumor associated macrophages (TAMs) are the major immunoregulatory cells in tumors, considered to have an M2 phenotype and secrete an array of cytokines, chemokines and enzymes that can suppress T-cell effector function.39 TAMs secrete chemokines, CCL5, CCL20, CCL22 that recruit natural Treg cells (nTreg) and Arginase I that inhibit TCR ζ chain re-expression in activated T cells by the depletion of L-arginine.40 IL-10 and TGFβ produced by TAMs can induce regulatory functions by the upregulation of the Foxp3 and cytotoxic T lymphocyte antigen 4 (CTLA-4) in CD4+ T cells, and the expression of the programmed death-ligand 1 (PDL1) in monocytes – a co-inhibitory molecule that can inhibit CD8+ T cell functions.40–44 Through HIF-1 α signaling, myeloid-derived suppressor cells (MDSCs) and TAMs in the hypoxic TME upregulate PD-L1 on macrophages.45 Continuous exposure to chronically expressed tumor antigens drives T cells into senescence and exhaustion, characterized by expression of co-inhibitory molecules such as T cell immunoglobulin, mucin domain-containing protein 3 (TIM-3), lymphocyte activation gene 3 protein (LAG-3), programmed cell death protein 1 (PD-1) and CTLA-4 with impaired effector functions and proliferative capacity.27,46–48
Ideal T-cell subsets for adoptive T cell therapy
These limitations signify the necessity of identifying T-cell subsets that maintain the ability to proliferate, effectively traffic to the TME, exhibit robust effector function, and mediate regression of tumors for ACT. Accumulating evidence from preclinical and clinical studies has shown that less-differentiated “younger” T cells with longer telomere persist longer and exhibit more potent anti-tumor efficacy than differentiated T cells after adoptive transfer.26,27,49–54 Using murine B16 melanoma model with Pmel-1 T-cell receptor (TCR) transgenic mice specific for the gp100 antigen expressed on B16 tumors, adoptive transfer of central memory T cells (TCM: CD62Lhi CD44hi) exhibited superior expansion, persistence, and anti-tumor efficacy in vivo compared with effector memory T cells (TEM: CD62Llo CD44hi KLRG-1lo) or terminally differentiated effector T cells (TEFF: CD62Llo CD44hi KLRG-1hi).26,27,49 Even more resounding was the result that stem cell memory T cells (TSCM: CD62Lhi CD44lo Stem cell antigen-1hi CD122hi) were even more potent than TCM on a per-cell basis.52–55 Preclinical and clinical studies found a significant correlation between T cell differentiation status and anti-tumor efficacy, indicating the superiority of TSCM cells over other memory CD8+ T-cell subsets.52–54 Finally, in addition to evaluating memory and effector subsets individually, the ability of natural Ag-specific TEFF derived from different CD8+ T- cell subsets, specifically naïve T cells (TN) and TCM, has also been assessed in both mice and human.56,57 Compared to TEFF derived from TCM, naive-derived TEFF retained the ability to release IL-2 while withholding the acquisition of the senesce marker, KLRG-1.56,57 When adoptively transferred into tumor-bearing mice, TN -derived TEFF demonstrated superior in vivo expansion, persistence, and anti-tumor efficacy relative to TCM -derived TEFF57. In humans, these cells also maintained significantly higher CD27 and longer telomere lengths after ex vivo expansion, suggesting greater proliferative potential.56 These results suggest that the ability of T cells to mediate tumor regression decreases with differentiation. Overall, the increased potential to self-proliferate and differentiate into memory and effector T cell subsets allows less differentiated forms, such as TSCM and TN, to regulate and sustain effective tumor regression and foster superior anti-tumor efficacy relative to differentiated effector cells.
An array of possible approaches has been proposed to enhance the efficacy of ACT. Initial antigen signal strength,58 quality of costimulation,59 and the presence of cytokines, such as IL-2, IL-7 and IL-15,49,60 may influence the relative ratio of TCM to TEM and TEFF generated in response to antigen. Therefore, modulating immunomodulatory cytokines used in ACT along with adapting the duration and nature of T cell ex vivo culture conditions can enhance the in vivo function of tumor-specific CD8+ T cells by selecting and generating optimal memory T cells in cancer patients. Another strategy involves altering metabolism within T cells, primarily inhibiting glycolytic pathways noted to be drivers of terminal effector differentiation. This may promote long-lived CD8+ T cell immunity and enhanced tumor destruction.61 In vitro culturing of T cells in the presence of small molecules provide cell products with superior engraftment, expansion and anti-tumor immunity after adoptive transfer. Inhibition of GSK3, a vital component of the oncogenic WNT signaling pathway, maintains stemness in mature memory CD8+ T cells providing self-renewal capability and multipotency superior to central memory T cells.54 Collectively, less-differentiated tumor antigen-specific T cells are ideal T-cell subsets for ACT; however, generating large numbers of these “younger” T cells is problematic.
Classification of stem cells based on differentiation potential
Stem cells are defined by dual hallmark features of self-renewal and differentiation potential.62–64 These cells are classified into several types according to their capacity to differentiate into specialized cells. A totipotent cell such as zygote (a fertilized egg) and blastomeres during early cleavage of the embryo can give rise to a new organism given appropriate maternal support. They can also differentiate into embryonic and extra-embryonic cell types such as the fetal membranes and placenta.65
Pluripotent stem cells (PSCs) can self-renew and have the ability to form all three embryonic germ layers (i.e., ectoderm, endoderm and mesoderm). Embryonic stem cells (ESCs) epitomize quintessential PSCs that can be isolated from the inner cell mass of blastocysts and cultured as immortal cell lines.66,67 Multipotent stem cells can self-renew, but differentiate into all cell types within one particular lineage.64 These include neural stem cells that are derived from neural tissues and can give rise to all cell types (neurons, astrocytes, and oligodendrocytes) of the nervous system.68 Mesenchymal stem cells are also multipotent stromal cells that can be isolated from the bone marrow.69 They are nonhematopoietic, multipotent stem cells with the capacity to differentiate into mesodermal lineage such as bone cells, cartilage cells, muscle cells, and fat cells.
The rise of induced pluripotency
The understanding of induced pluripotency has developed over the last six decades with the aid of advancing discoveries and technologies. The first PSCs cultured in vitro were derived from a type of germ line tumor called teratocarcinoma.70 The breakthrough in the field came when researchers showed that PSCs can be isolated from mouse blastocysts and propagated in vitro as immortalized, non-transformed cell lines.66,67 Later, Thompson et al. showed PSCs can be derived from human embryos.71 However, ethical concerns using human zygotes and immune rejection of grafted stem cells limit the use of human ESCs.
In 2006 Takahashi and Yamanaka demonstrated that the transient expression of only four transcription factors (Oct4, Sox2, Klf4, and c‐Myc) was sufficient to convert murine fibroblasts into induced pluripotent stem cells (iPSCs), which are ESC‐like cells that demonstrate the same pluripotency and self-renewal properties.72 Only a year later, the successful derivation of human iPSCs from fibroblast was reported.73,74 Human iPSCs circumvent the ethical controversies and rejection problem associated with using autologous stem cells; they provide a valuable source of patient-specific cells for the study and potential treatment of human diseases. Remarkable progress made in reprogramming technology over the past decade has also facilitated the generation of human iPSCs with a minimally invasive approach from a number of human cell types such as keratinocytes, dental stem cells, oral gingival, oral mucosa fibroblasts, and cord blood cells.75–80
In 2010, three groups reported the generation of human iPSCs from peripheral blood T cells.81–83 The use of peripheral blood cells as a source for iPSCs is a less invasive procedure compared to having patients undergo skin biopsy for obtaining fibroblasts. Although all three groups used the same four transcription factors (Oct4, Sox2, Klf4, and c‐Myc) to generate T-cell derived human iPSCs (T-iPSCs), reprogramming efficiency was different. Seki et al. introduced the four factors with Sendai virus vectors and found that only 1 ml of whole blood was sufficient to generate human iPSCs.81 In addition to higher induction efficiency, Sendai virus vectors have some advantages for the generation of human iPSCs. Unlike integrating viral (e.g. retroviral or lentiviral) vectors, Sendai virus vectors only replicate in the cytoplasm of infected cells and do not integrate into the host genome.84 Moreover, temperature-sensitive mutations in the viral genome allow for rapid removal of residual viral genomic RNA from reprogrammed cells.85 Generation of transgene-free iPSCs by non-integrating Sendai virus vectors minimizes the risk of tumor formation associated with random oncogene activation or tumor supressor inactivation.
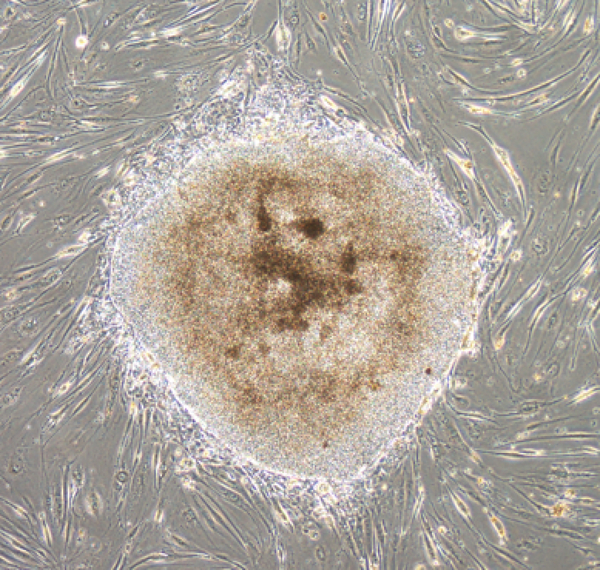
Of note, during normal αβ T cell development, TCRA and TCRB genes are rearranged in the thymus. Detection of TCR gene arrangement in iPSCs is indicative of derivation from cells of the T lineage.81–83 Using Sendai virus vectors, we have also found efficient generation of human iPSCs from peripheral blood T cells (Figure 2). Furthermore, we have shown successful derivation of human iPSCs from melanoma TILs expressing high levels of PD-1.86 A wide variety of TCR gene rearrangement patterns in TIL-derived iPSCs confirmed the heterogeneity of T cells infiltrating melanomas.86 These findings also suggest the feasibility of rejuvenating fully differentiated and exhausted antigen-specific T cells by reprogramming and redifferentiation techniques for adoptive T cell therapy.
Figure 2. Human iPSC derived from peripheral blood T cells under on-feeder condition.
Peripheral blood T cells were reprogramed by viral transduction of a Sendai-virus vector carrying a cassette of the OCT3/4, SOX2, KLF4, and c-MYC. One day after reprogramming, cells were replated on to feeder cells. A human iPSC on feeder cells on day 19 is shown. Scale bar: 500 μm.
Potential of iPSCs to generate T cells for adoptive cell therapy
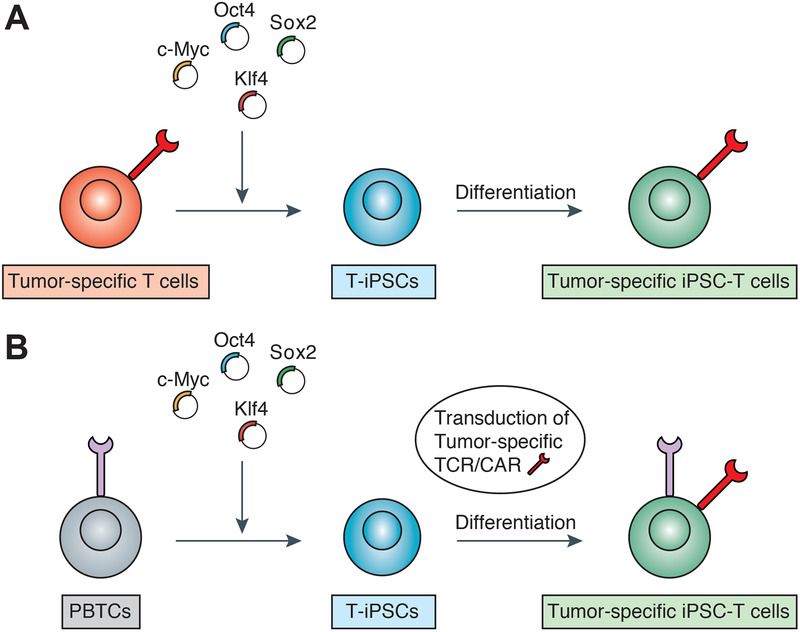
Subsequently, a series of studies have provided insights into the function of rejuvenated antigen-specific T-iPSC-derived T cells. The tumor specificity of T-iPSC-derived T cells can be conferred via two approaches. One is to reprogram tumor-antigen specific T cells and redifferentiate T-iPSCs for the generation of T cells carrying the TCR recognizing the same tumor antigen (Figure 3A). Vizcardo et al. established T-iPSCs from CD8+ T-cell clone specific for the melanoma antigen MART-1 using the Sendai virus reprogramming system.87 All regenerated CD8+ T cells from T-iPSCs were found to express TCR specific for MART-1 antigen and produce IFN-γ in vitro.87 Nishimura et al. rejuvenated HIV-1 specific CD8+ T cell clone and demonstrated that regenerated iPSC-derived T cells have high proliferative capacity, antigen-specific killing activity and elongated telomere.88 Wakao et al. generated T-iPSCs from human cord blood mucosal-associated invariant T (MAIT) cells, innate-like T-cells that recognize derivatives of precursors of bacterial riboflavin presented by the MHC class I-related molecule MR1.89 Regenerated MAIT cells possessed the ability to produce a wide variety of cytokines and chemokines in the presence of bacteria-fed monocytes.89
Figure 3. Two approaches of generating tumor-specific T cells using autologous induced pluripotent stem cells (iPSCs) and reprogramming technology.
(A) Reprogramming of tumor-specific T cells to generate T-cell derived human iPSCs (T-iPSCs) followed by redifferentiation to naïve tumor-specific iPSC-derived T cells. (B) Reprogramming of peripheral blood T cells followed by transduction of T-cell receptor (TCR) or chimeric antigen receptor (CAR) recognizing tumor antigen to T-iPSCs. Genetically engineered T-iPSCs differentiated to naïve tumor-specific TCR/CAR-transduced iPSC-derived T cells.
While these studies utilize a strategy of reprogramming T cells with known antigen specificity and redifferentiating T-iPSCs for the generation of rejuvenated antigen-specific T cells, another approach is to genetically transfer a receptor with known specificity for an antigen into established iPSCs (Figure 3B). Themeli et al. have shown that T-iPSCs transduced with CAR specific for CD19 antigen can generate T cells that display anti-tumor immunity in a xenograft model of lymphoma.90 These studies suggest that iPSCs with CAR genetic modification have the potential to generate functional and expandable T cells specialized for tumor eradication.
In vivo anti-tumor efficacy of iPSC-derived T cells against solid malignancies
Although these studies suggest in vivo anti-tumor efficacy of T-iPSC-derived T cells, it remains uncertain whether iPSC-derived T cells escape immune rejection (immunogenicity) and mediate effective regression of established tumor following adoptive transfer in immunocompetent host. Some studies have shown that certain iPSC-derived cells such as smooth muscle cells and cardiomyocytes are immunogenic while other cell types such as retinal pigment epithelial, hepatocytes, and neuronal cells exhibit little to no immunogenicity.91–94
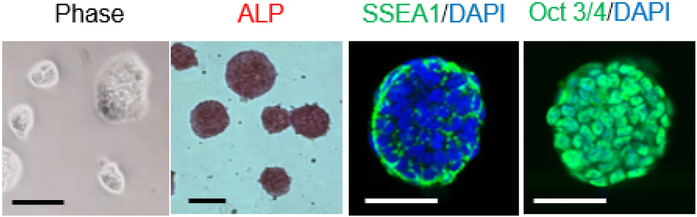
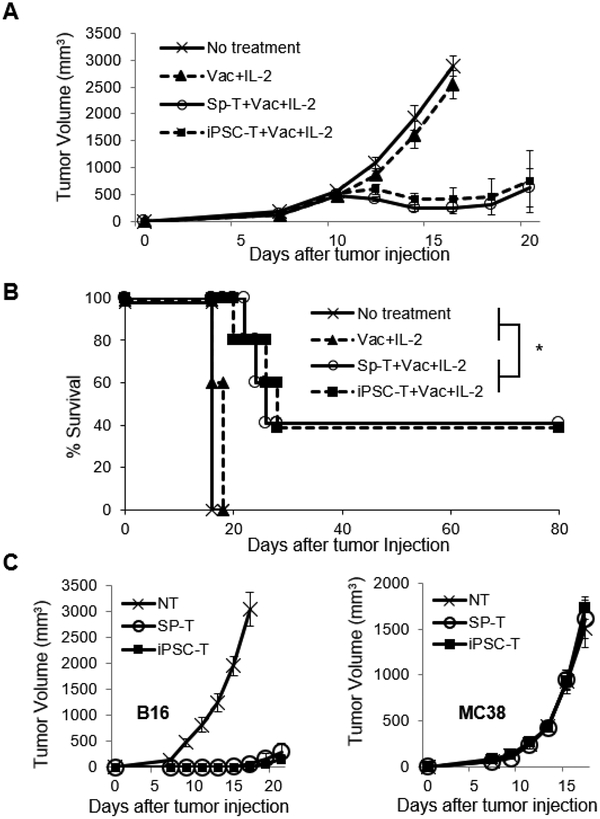
To this end, we have recently established a preclinical murine model in which Pmel-1 TCR transgenic CD8+ T cells able to recognize gp100 antigen were rejuvenated to iPSC-derived T cells utilizing the Sendai virus reprogramming system (Figure 4).95 This novel preclinical model allows us to discover a variety of new findings that have unveiled insights of not only the reprogramming process of iPSC technology, but also its therapeutic potential through in vitro and in vivo analysis in an immunocompetent mouse model. We demonstrated for the first time that murine T cells, like human T cells, can be reprogrammed into iPSCs with the Sendai virus reprogramming system without the use of gene knockout mice or drug-inducible gene expression systems.96,97 Of equal importance was our finding that dual inhibition (2i) of both prodifferentiation MEK and GSK-3 pathways that was shown to support the establishment of mouse iPSCs from partially reprogrammed cells98 was required for reprogramming of Pmel-1 T cells. Rejuvenated iPSC-derived T cells were less-differentiated phenotypes that expressed memory T cell markers and acquired effector functions producing IFN-γ and TNF-α after stimulation with the cognate antigen, gp100.95 Furthermore, adoptive transfer of iPSC-derived regenerated T cells significantly delayed B16 tumor growth and improved overall survival in a lethal murine model of melanoma (Figure 5A and B).95 Importantly, an establishment of antigen-specific immunological memory provides insight into immunogenicity of iPSC-derived T cells, and reveals the feasibility of generating long-lived tumor-specific T cells via reprogramming to pluripotency and redifferentiation (Figure 5C).95
Figure 4. Generation of iPSCs from Pmel-1 TCR transgenic CD8+ T cells.
Morphology, alkaline phosphatase (ALP) activity and expression of pluripotency and surface markers (SSEA1 and Oct3/4) in Pmel-1 iPSCs. Scale bar: 200 μm.
From Saito H, Okita K, Chang AE, et al. Adoptive Transfer of CD8+ T Cells Generated from Induced Pluripotent Stem Cells Triggers Regressions of Large Tumors Along with Immunological Memory. Cancer Res 2016;76(12):3473–3483; with permission.
Figure 5. Adoptively transferred iPSC-derived CD8+ T cells mediate effective regression of large tumors and establishes immunological memory.
(A and B) Tumor growth curves (A) and survival curves (B) in C57BL/6 mice bearing B16 melanomas established for 11 days in different treatment groups. Vac: vaccination with the gp100 antigen, anti-CD40 mAb, poly (I:C), and imiquimod cream. Tumor volume results are the mean of measurements from 5 mice per group. (*=P < 0.0001 using log-rank (Mantel-Cox) test.) (C) Surviving mice (n=4) after adoptive transfer of Pmel-1 iPSC-derived or splenic T cells, vaccination and IL-2 were rechallenged with B16 cells into the contralateral flank and MC38 cells on back on day 80. Tumor growth curves are depicted in which T=0 corresponds to the time of injection of secondary tumors. As a control, tumor growth was monitored following inoculation of the same tumor cell dose into non-tumor (NT) experienced naive C57BL/6 mice (n=5).
From Saito H, Okita K, Chang AE, et al. Adoptive Transfer of CD8+ T Cells Generated from Induced Pluripotent Stem Cells Triggers Regressions of Large Tumors Along with Immunological Memory. Cancer Res 2016;76(12):3473–3483; with permission.
Challenges / Future Directions
ESCs and iPSCs are tumorigenic cells that can give rise to teratoma upon transplantation.99 For clinical translation of iPSC-derived T-cell therapies, the tumorigenic potential of contaminated iPSCs and the malignant transformation of differentiated iPSCs (tumorigenicity) are major safety concerns.100,101 While the tumorigenic risks of iPSC-derived products can be reduced by several methods,102–105 they may not be satisfactory because tumorigenic risk arises not only from contamination with undifferentiated iPSCs, but also from intermediate products having altered proliferation potential and/or with tumorigenic transformed cells.100
Current method of in vitro differentiation of T lymphocytes from human iPSCs uses co-culture with murine OP9 bone marrow stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1).106 To translate this strategy into routine clinical practice, it will be essential to find a way to differentiate iPSCs under xeno-free conditions. Furthermore, regenerated human iPSC-derived T cells express CD3, TCRαβ, and CD8α, but not CD8β. Therefore, these regenerated iPSC-derived T cells possess CD8αα homodimers, not CD8αβ heterodimers.107 CD8αα homodimer has been found only on a small portion of developing thymocytes, gut intraepithelial lymphocytes (IEL) and a subset of NK cells and dendritic cells.108 Although both forms of the CD8 molecule bind to MHC class I with similar affinity, studies have shown that the CD8αα homodimer is a functionally weaker co-receptor than CD8αβ for TCR-based activation.109,110 Moreover, Themeli et al. have shown that CAR-iPSC-derived T cells possess an innate γδ T cell-like profile.90 In contrast, we have found that regenerated murine iPSC-derived T cells express both CD8α and CD8β (CD8αβ heterodimer), which might be because of the use of the sorting procedure performed before activation with the cognate antigen.95 In line with our study, Maeda et al. have recently shown that isolating CD4+CD8+ double positive (DP) T cells before activation with anti-CD3 antibody (Ab) can generate human CD8αβ iPSC-derived T cells.107
Nevertheless, development of feeder-free and xeno-free culture procedures for the generation of CD8αβ T cells will be ideal for clinical use of iPSC-derived T cells. Of note, Vizcardo et al. recently developed a 3D thymic culture system in preclinical model and showed successful generation of murine CD8αβ iPSC-derived T cells without the use of OP9-DL1 feeder cells.111
Lastly, TCRα gene rearrangement takes place when T cells are at the CD4+CD8+ DP stage in thymus.112,113 Additional rearrangement of TCR α chain may occur when iPSC-derived T cells become CD4+CD8+ DP T cells. This may produce T cells with unpredictable antigen specificity, and adoptive transfer of these T cells may cause unpredictable autoimmune reactions because they do not go through thymic positive and negative selection. A possible solution would be to downregulate the expression of the recombination activating genes 1 and 2 (RAG-1 and RAG-2) to stop further endogenous TCRα gene rearrangement by CRISPR/Cas9 genome editing technique.114–116
Conclusions
Adoptive cell therapy with antigen-specific T cells is a promising approach for treating patients with a variety of malignancies. Despite remarkable success seen in the treatment of hematological malignancies, difficulty with generating sufficient numbers of tumor-specific T cells harboring characteristics necessary for in vivo effectiveness remains a major roadblock to ACT for solid malignancies. Use of iPSCs to provide an unlimited number of autologous less-differentiated antigen-specific T cells can theoretically overcome these limitations, and hold great promise for adoptive T cell therapy. While autologous iPSC-derived T cells provide a bright future for personalized cancer treatment, many challenges still remain before these cells can be utilized clinically in patients. Safety and therapeutic efficacy of iPSC-derived T cells need to be further evaluated in preclinical models before they are translated into clinic.
Synopsis:
Current approaches to adoptive T cell therapy for solid malignancies are limited by the difficulty of obtaining sufficient numbers of less-differentiated tumor-specific T cells with superior in vivo expansion, persistence, and anti-tumor efficacy relative to differentiated effector T cells. The use of induced pluripotent stem cells (iPSCs) that self-renew and provide an unlimited number of autologous less-differentiated antigen-specific T cells can theoretically overcome these limitations. Accumulating evidence suggests T cell-derived iPSCs can generate less-differentiated antigen-specific T cells that harbor long telomeres and increased proliferative capacity, and exhibit potent anti-tumor efficacy in vitro and in vivo. While this strategy holds great promise for adoptive T cell therapy, development of clinically applicable protocol for the generation of human iPSC-derived T cells is required prior to the translation of iPSC technology into the clinical setting.
Key Points.
Despite full effector function, differentiated T cells currently available for adoptive cell therapy (ACT) exhibit less expansion, persistence, and anti-tumor efficacy in vivo against solid malignancies compared with less-differentiated T cells.
Induced pluripotent stem cells (iPSCs) can self-renew and provide unlimited number of autologous less-differentiated antigen-specific T cells that can mediate effective regression of established tumor and establish antigen-specific immunological memory in vivo.
Development of highly reproducible and robust differentiation protocols for clinically applicable large scale production of tumor-specific iPSC-derived T cells is needed.
Acknowledgements
We would like to acknowledge funding support from the Roswell Park Alliance Foundation, the Melanoma Research Alliance, the Sarcoma Foundation of America, and the National Cancer Institute (NCI) grant, K08CA197966 (F. Ito)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
The authors have nothing to disclose.
Contributor Information
Sunny Patel, Medical College of Georgia, 1120 Fifteen Street, Augusta, GA 30912-3600.
Takayoshi Yamauchi, Department of Molecular Enzymology, Faculty of Life Sciences, Kumamoto University, Kumamoto, 860-8556, Japan.; Center for Metabolic Regulation of Healthy Aging, Faculty of Life Sciences, Kumamoto University, Kumamoto, 860-8556, Japan.
Fumito Ito, Center for Immunotherapy, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Elm and Carlton Streets, CCC-539, Buffalo, NY 14263.
References
- 1.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. [DOI] [PubMed] [Google Scholar]
- 3.Harris TH, Banigan EJ, Christian DA, et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486(7404):545–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21(5):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Sherry RM, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–1680. [DOI] [PubMed] [Google Scholar]
- 16.Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375(23):2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran E, Ahmadzadeh M, Lu YC, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350(6266):1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chervin AS, Aggen DH, Raseman JM, Kranz DM. Engineering higher affinity T cell receptors using a T cell display system. J Immunol Methods. 2008;339(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23(3):349–354. [DOI] [PubMed] [Google Scholar]
- 20.Kuball J, Hauptrock B, Malina V, et al. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. The Journal of experimental medicine. 2009;206(2):463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94(5):1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer research. 2003;63(18):6076–6083. [PubMed] [Google Scholar]
- 23.Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol. 1999;162(4):2073–2080. [PubMed] [Google Scholar]
- 24.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86(24):10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff CA, Rosenberg SA, Restifo NP. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat Med. 2016;22(1):26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Lanzavecchia A. Memory in disguise. Nature Medicine. 2011;17:1182. [DOI] [PubMed] [Google Scholar]
- 29.Pockaj BA, Sherry RM, Wei JP, et al. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer. 1994;73(6):1731–1737. [DOI] [PubMed] [Google Scholar]
- 30.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10(7):505–514. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 32.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. [DOI] [PubMed] [Google Scholar]
- 33.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8(1):56–61. [DOI] [PubMed] [Google Scholar]
- 34.Brahimi-Horn MC, Bellot G, Pouyssegur J. Hypoxia and energetic tumour metabolism. Current opinion in genetics & development. 2011;21(1):67–72. [DOI] [PubMed] [Google Scholar]
- 35.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8(1):74–80. [DOI] [PubMed] [Google Scholar]
- 36.Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109(41):E2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Ma C, Liu S, et al. Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin. Immunology. 2009;128(1 Suppl):e237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Frontiers in immunology. 2013;4:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daurkin I, Eruslanov E, Stoffs T, et al. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer research. 2011;71(20):6400–6409. [DOI] [PubMed] [Google Scholar]
- 42.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Frontiers in immunology. 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol. 2013;191(8):3973–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PDL1. The Journal of experimental medicine. 2009;206(6):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. [DOI] [PubMed] [Google Scholar]
- 49.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16(24):6122–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175(10):7046–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12(10):671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell–like properties. Nature Medicine. 2011;17(10):1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gattinoni L, Zhong X-S, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lugli E, Dominguez MH, Gattinoni L, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123(2):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinrichs CS, Borman ZA, Gattinoni L, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117(3):808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinrichs CS, Borman ZA, Cassard L, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences. 2009;106(41):17469–17474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174(9):5341–5350. [DOI] [PubMed] [Google Scholar]
- 59.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4(4):355–360. [DOI] [PubMed] [Google Scholar]
- 60.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sukumar M, Liu J, Ji Y, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123(10):4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Los Angeles A, Ferrari F, Xi R, et al. Hallmarks of pluripotency. Nature. 2015;525(7570):469–478. [DOI] [PubMed] [Google Scholar]
- 63.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143(4):508–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daley GQ. Stem cells and the evolving notion of cellular identity. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2015;370(1680):20140376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Condic ML. Totipotency: what it is and what it is not. Stem cells and development. 2014;23(8):796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. [DOI] [PubMed] [Google Scholar]
- 67.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nature reviews Neuroscience. 2010;11(3):176–187. [DOI] [PubMed] [Google Scholar]
- 69.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regenerative medicine. 2010;5(6):933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hogan BL. Changes in the behaviour of teratocarcinoma cells cultivated in vitro. Nature. 1976;263(5573):136–137. [DOI] [PubMed] [Google Scholar]
- 71.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 74.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. [DOI] [PubMed] [Google Scholar]
- 75.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–1284. [DOI] [PubMed] [Google Scholar]
- 76.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem cells and development. 2010;19(4):469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Egusa H, Okita K, Kayashima H, et al. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One. 2010;5(9):e12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyoshi K, Tsuji D, Kudoh K, et al. Generation of human induced pluripotent stem cells from oral mucosa. Journal of bioscience and bioengineering. 2010;110(3):345–350. [DOI] [PubMed] [Google Scholar]
- 79.Haase A, Olmer R, Schwanke K, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5(4):434–441. [DOI] [PubMed] [Google Scholar]
- 80.Giorgetti A, Montserrat N, Aasen T, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5(4):353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7(1):11–14. [DOI] [PubMed] [Google Scholar]
- 82.Loh YH, Hartung O, Li H, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7(1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Staerk J, Dawlaty MM, Gao Q, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7(1):20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li HO, Zhu YF, Asakawa M, et al. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J Virol. 2000;74(14):6564–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saito H, Okita K, Fusaki N, Sabel MS, Chang AE, Ito F. Reprogramming of Melanoma Tumor-Infiltrating Lymphocytes to Induced Pluripotent Stem Cells. Stem Cells International. 2016;2016(8394960):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vizcardo R, Masuda K, Yamada D, et al. Regeneration of Human Tumor Antigen-Specific T Cells from iPSCs Derived from Mature CD8(+) T Cells. Cell Stem Cell. 2013;12(1):31–36. [DOI] [PubMed] [Google Scholar]
- 88.Nishimura T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(1):114–126. [DOI] [PubMed] [Google Scholar]
- 89.Wakao H, Yoshikiyo K, Koshimizu U, et al. Expansion of functional human mucosal-associated invariant T cells via reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(5):546–558. [DOI] [PubMed] [Google Scholar]
- 90.Themeli M, Kloss CC, Ciriello G, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao T, Zhang ZN, Westenskow PD, et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell. 2015;17(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Almeida PE, Meyer EH, Kooreman NG, et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nature communications. 2014;5:3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. [DOI] [PubMed] [Google Scholar]
- 94.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–412. [DOI] [PubMed] [Google Scholar]
- 95.Saito H, Okita K, Chang AE, Ito F. Adoptive Transfer of CD8+ T Cells Generated from Induced Pluripotent Stem Cells Triggers Regressions of Large Tumors Along with Immunological Memory. Cancer research. 2016;76(12):3473–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eminli S, Foudi A, Stadtfeld M, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41(9):968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS biology. 2008;6(10):e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–277. [DOI] [PubMed] [Google Scholar]
- 100.Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19(8):998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nori S, Okada Y, Nishimura S, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem cell reports. 2015;4(3):360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang C, Lee AS, Volkmer JP, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29(9):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben-David U, Gan QF, Golan-Lev T, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12(2):167–179. [DOI] [PubMed] [Google Scholar]
- 104.Lee MO, Moon SH, Jeong HC, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013;110(35):E3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tateno H, Onuma Y, Ito Y, et al. Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem cell reports. 2015;4(5):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5(4):410–417. [DOI] [PubMed] [Google Scholar]
- 107.Maeda T, Nagano S, Ichise H, et al. Regeneration of CD8alphabeta T Cells from T-cell-Derived iPSC Imparts Potent Tumor Antigen-Specific Cytotoxicity. Cancer research. 2016;76(23):6839–6850. [DOI] [PubMed] [Google Scholar]
- 108.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 109.Renard V, Romero P, Vivier E, Malissen B, Luescher IF. CD8 beta increases CD8 coreceptor function and participation in TCR-ligand binding. The Journal of experimental medicine. 1996;184(6):2439–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Witte T, Spoerl R, Chang HC. The CD8beta ectodomain contributes to the augmented coreceptor function of CD8alphabeta heterodimers relative to CD8alphaalpha homodimers. Cell Immunol. 1999;191(2):90–96. [DOI] [PubMed] [Google Scholar]
- 111.Vizcardo R, Klemen ND, Islam SMR, et al. Generation of Tumor Antigen-Specific iPSC-Derived Thymic Emigrants Using a 3D Thymic Culture System. Cell Rep. 2018;22(12):3175–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009;21(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.von Boehmer H Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84:201–238. [DOI] [PubMed] [Google Scholar]
- 114.Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31(3):230–232. [DOI] [PubMed] [Google Scholar]
- 115.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]