Nearly 75% of patients with stage IV melanoma will develop brain metastases as a sequelae of their illness and approximately 50% will succumb to progression of their intracranial disease. Standard therapy includes surgery, but the therapeutic landscape for metastatic melanoma is being transformed by immunotherapy blockade treatment possibilities. Considering the limited data informing the combination of surgery and immunotherapy for metastatic melanoma, the study reported here investigated the timing of neurosurgical intervention and immune checkpoint blockade.
Keywords: Immunotherapy, Metastatic melanoma, Brain metastases
Abstract
Background.
Immune checkpoint blockade has systemic efficacy in patients with metastatic melanoma, including those with brain metastases (MBMs). However, immunotherapy‐induced intracranial tumoral inflammation can lead to neurologic compromise, requiring steroids, which abrogate the systemic efficacy of this approach. We investigated whether upfront neurosurgical resection of MBM is associated with a therapeutic advantage when performed prior to initiation of immunotherapy.
Material and Methods.
An institutional review board‐approved, retrospective study identified 142 patients with MBM treated with immune checkpoint blockade between 2010 and 2016 at Massachusetts General Hospital, of whom 79 received surgery. Patients were classified based on the temporal relationship between immunotherapy, surgery, and development of central nervous system metastases. Overall survival (OS) was calculated from the date of diagnosis of MBM until death from any cause. Multivariate model building included a prognostic Cox model of OS, the effect of immunotherapy and surgical sequencing on OS, and the effect of immunotherapy and radiation sequencing on OS.
Results.
The 2‐year overall survival for patients treated with cytotoxic T‐lymphocyte antigen 4, programmed death 1, or combinatorial blockade was 19%, 54%, and 57%, respectively. Among immunotherapy‐naïve melanoma brain metastases, surgery followed by immunotherapy had a median survival of 22.7 months (95% confidence interval [CI], 12.6–39.2) compared with 10.8 months for patients treated with immunotherapy alone (95% CI, 7.8–16.3) and 9.4 months for patients treated with immunotherapy followed by surgery (95% CI, 4.1 to ∞; p = .12). On multivariate analysis, immunotherapy‐naïve brain metastases treated with immunotherapy alone were associated with increased risk of death (hazard ratio, 1.72; 95% CI, 1.00–2.99) compared with immunotherapy‐naïve brain metastases treated with surgery followed by immunotherapy.
Conclusion.
In treatment‐naïve patients, early surgical resection for local control should be considered prior to commencing immunotherapy. A prospective, randomized trial comparing the sequence of surgery and immunotherapy for treatment‐naïve melanoma brain metastases is warranted.
Implications for Practice.
In this retrospective study of 142 patients with melanoma brain metastases treated with immune checkpoint blockade, the development of melanoma brain metastases following immunotherapy was associated with decreased survival compared with diagnosis of immunotherapy‐naïve brain metastases. The benefit of surgical intervention was seen in immunotherapy‐naïve brain metastases in contrast to brain metastases that developed on immunotherapy. These results suggest that upfront local control with surgery for immunotherapy‐naïve melanoma brain metastasis may provide a bridge toward immunotherapy‐mediated systemic control.
Introduction
Brain metastases are the most frequent cause of intracranial malignancy in adults and continue to occur even when extracranial disease is well controlled [1], [2]. With improved systemic therapies leading to longer survival in patients with metastatic disease, the overall incidence of brain metastases is increasing. Melanoma is the third most common primary tumor to metastasize to the brain [3]. Among patients with stage IV melanoma, nearly 75% will develop brain metastases as sequelae of their illness [3], [4], [5], and approximately 50% will succumb to progression of their intracranial disease [6]. Standard therapy for melanoma brain metastases (MBMs) has historically consisted of surgery and radiosurgery, treatment modalities associated with a median survival of 8 months [5].
Over the past decade, the therapeutic landscape for metastatic melanoma has been transformed. Since 2011, eight drugs and three combinations have been approved by the U.S. Food and Drug Administration, including three immune checkpoint inhibitors, which reverse immune cell exhaustion. Ipilimumab, a monoclonal antibody that targets cytotoxic T‐lymphocyte antigen 4 (CTLA‐4), prolongs survival in approximately 20% of patients [7] and was the first agent to demonstrate an overall survival advantage in patients with advanced melanoma. Nivolumab and pembrolizumab are monoclonal antibodies that inhibit the programmed death 1 (PD‐1) receptor on T cells and have been shown, as single agents, to be associated with outcomes comparable to ipilimumab. Furthermore, the combination of nivolumab and ipilimumab leads to unprecedented complete, durable response rates superior to ipilimumab alone [8], [9], [10], [11].
Despite these encouraging results, patients with brain metastases were excluded from many these initial immunotherapy trials. The documented efficacy of CTLA‐4, PD‐1, and combinatorial blockade in extracranial disease has stimulated attention for the treatment of patients with central nervous system disease. Following an encouraging post hoc analysis of a phase III trial investigating ipilimumab for MBM [12], a phase II single‐agent study using ipilimumab for patients with MBM [13] demonstrated a response rate of 25% in steroid‐naïve patients compared with 10% for patients with symptomatic lesions requiring steroids. Promising results from a 3‐year follow‐up have also been reported from the open‐label, single‐arm, phase II trial evaluating the combination of ipilimumab and fotemustine in advanced melanoma. Among patients with brain metastases, the median overall survival was 12.7 months (95% confidence interval [CI], 2.7–22.7 months) and 3‐year survival was 27.8% [14], [15].
More recently, clinical trials have demonstrated excellent efficacy of single‐agent PD‐1 inhibitors and the combination of ipilimumab and nivolumab. In a nonrandomized, open‐label, phase II trial, patients with treatment‐naïve, asymptomatic MBM achieved a 22% intracranial response rate (4 out of 18 patients) when treated with pembrolizumab [16]. Of note, responses were durable and there was a high concordance between extracranial and intracranial responses. Encouraging results have been reported by the Anti‐PD1 Brain Collaboration in asymptomatic MBM with patients achieving a 46% intracranial response rate and 17% complete response when nivolumab and ipilimumab are given as first‐line therapy. Similarly, single‐agent nivolumab resulted in a 20% intracranial response and 12% complete response rate. Of note, a third cohort consisting of patients with symptomatic MBM or tumors that had failed local therapy were treated with nivolumab and had a 6% intracranial response rate but no complete responders [17]. Lastly, the recent study by Tawbi et al. demonstrated that the combination of nivolumab and ipilimumab led to intracranial clinical benefit (measured by stable disease for at least 6 months, complete response, or partial response) in 57% of patients. At 6 months after treatment, 25% of patient had a complete response, 30% had a partial response, and 2% had stable disease. Additionally, there were concordant response rates of intracranial and extracranial lesions [18].
The traditional role of surgery in the treatment of a single, newly diagnosed brain metastasis has been demonstrated in two, randomized clinical trials comparing surgery and whole brain radiation (WBRT) with WBRT alone [19], [20]. These findings collectively led the American Association of Neurological Surgeons to recommend surgery plus WBRT for patients with good performance status, limited extracranial disease, and a newly diagnosed, surgically amenable, single brain metastasis [21]. Surgery is typically pursued in situations requiring a histologic diagnosis, in patients where neurologic symptoms do not resolve with supportive care, or in patients who have a dominant lesion that is too large for stereotactic radiosurgery [22]. Beyond these cases, the decision to undertake surgery for patients with multiple metastases has required the incorporation of factors including performance status, patient comorbidities, tumor size and location, and proximity to eloquent regions [22]. With advances in immunotherapy for metastatic melanoma and the increasing use of this treatment modality in MBM management, the role of surgery in these cases warrants investigation. Specifically, there is a detriment to immunosuppressive doses of corticosteroids at the onset of immune checkpoint inhibitor therapy, and craniotomy to remove a lesion that is symptomatic could lessen steroid requirements and may allow immunotherapy to be more efficacious. Given the limited data informing the combination of surgery and immunotherapy for MBM, the purpose of our study was to investigate the timing of neurosurgical intervention and immune checkpoint blockade.
Materials and Methods
Medical records for all patients with MBM treated with immunotherapy at Massachusetts General Hospital were reviewed after obtaining approval from the Partners Institutional Review Board. We identified 142 patients with MBM treated with immunotherapy between 2010 and 2016; 79 (55.6%) underwent craniotomy for resection of intracranial metastasis. Immunotherapy regimens included CTLA‐4 inhibition, PD‐1 inhibition, or a combination of these treatments. When documented in the patient's chart, the following data were obtained within 30 days of brain metastases diagnosis: lactate dehydrogenase (LDH), Karnofsky performance status, Diagnosis‐Specific Graded Prognostic Assessment score, and ECOG performance status (ECOG PS).
For patients undergoing craniotomy, the date was recorded and identified as having a gross total resection, subtotal resection, or biopsy. For patients undergoing radiation therapy, the following information was recorded: the date(s) and type of radiation, the individual and total radiation dose, the number of treated targets, and the location of treated targets.
For immunotherapy regimens, treatment data were recorded as receiving CTLA‐4 blockade, PD‐1 blockade, or both agents. Among patients receiving both agents, patients were either started on combined therapy upfront or progressed on CTLA‐4 blockade and were subsequently started on a PD‐1 inhibitor. The number of doses and dates were recorded in addition to central nervous system response and extracranial response. The use of alternative immunotherapy regimens, such as interferon‐γ, granulocyte‐macrophage colony‐stimulating factor, and interleukin‐2, was recorded.
Statistical Analysis
The main objective of the study was to investigate whether upfront neurosurgical resection of MBM is associated with a therapeutic advantage, as measured by overall survival, when performed prior to initiation of immunotherapy. Overall survival (OS) was defined as the time from date of diagnosis of brain metastasis until death from any cause. Follow‐up of patients still alive was censored at the date of last vital status. To explore the relationships between patient demographics, disease, and treatment factors (Table 1, Table 2, supplemental online Table 1, and supplemental online Table 2) and OS, four models were predefined in our analysis plan. The first was a prognostic model. Additional models investigated the relationship between OS and the relative timing of immunotherapy and radiation and the effect upon OS of the relative timing of central nervous system development, immunotherapy, and surgery. A fourth model explored the effect of immunotherapy and surgery upon OS and was based on the subgroup of 79 patients who received surgery.
Table 1. Patient demographics at the time of brain metastasis diagnosis.
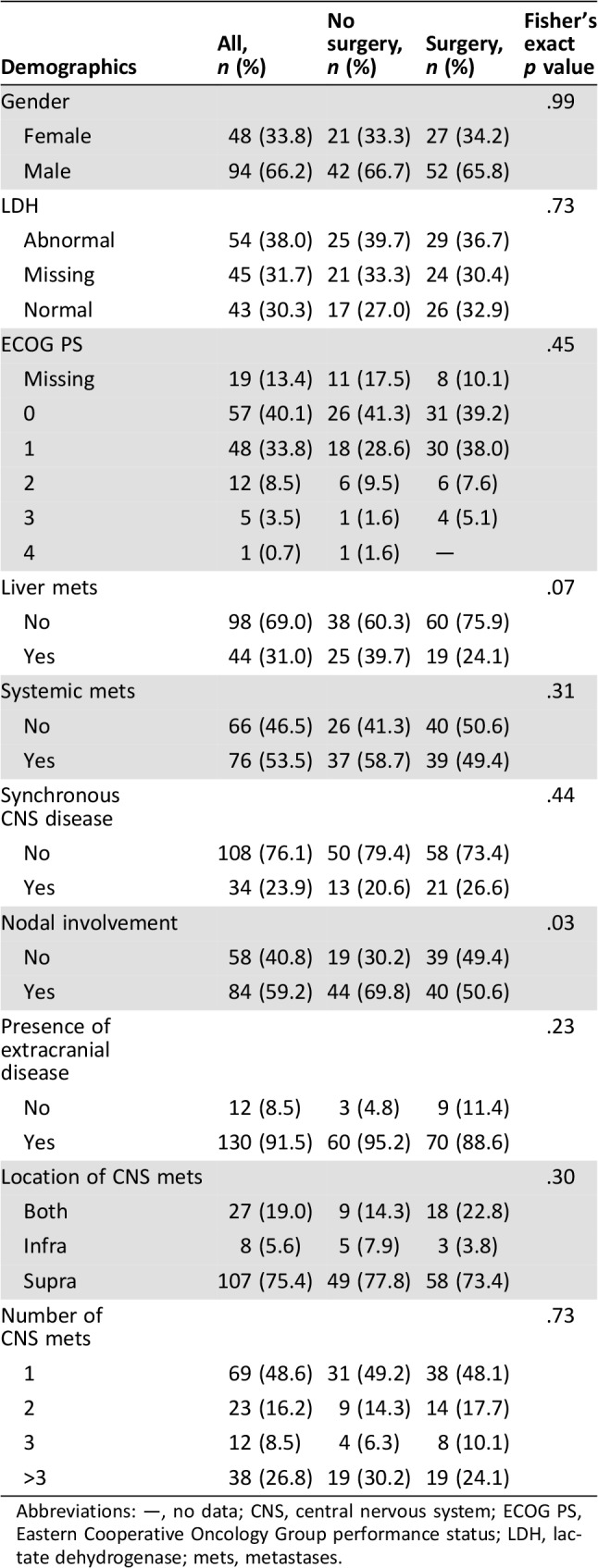
Abbreviations: —, no data; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; mets, metastases.
Table 2. Treatment features following melanoma brain metastasis diagnosis.
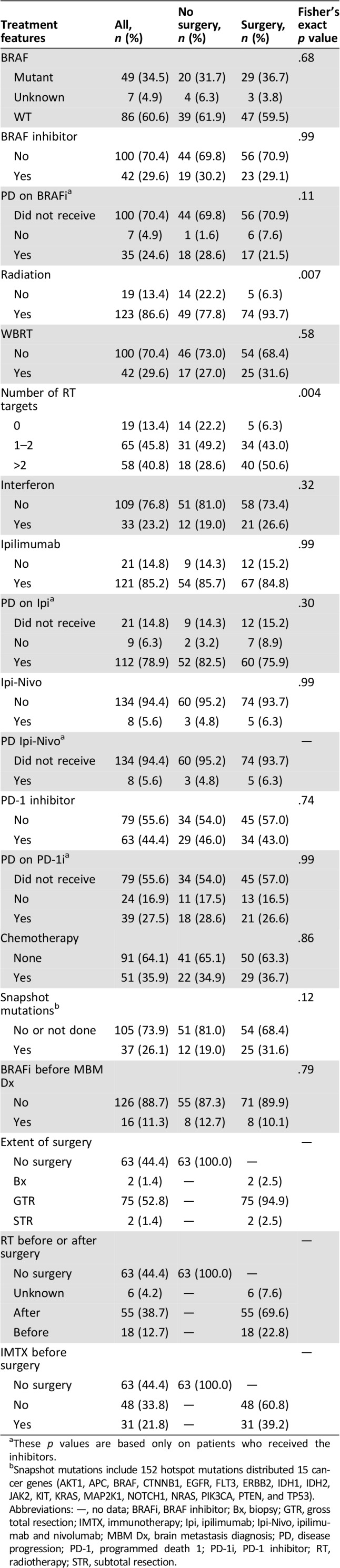
These p values are based only on patients who received the inhibitors.
Snapshot mutations include 152 hotspot mutations distributed 15 cancer genes (AKT1, APC, BRAF, CTNNB1, EGFR, FLT3, ERBB2, IDH1, IDH2, JAK2, KIT, KRAS, MAP2K1, NOTCH1, NRAS, PIK3CA, PTEN, and TP53).
Abbreviations: —, no data; BRAFi, BRAF inhibitor; Bx, biopsy; GTR, gross total resection; IMTX, immunotherapy; Ipi, ipilimumab; Ipi‐Nivo, ipilimumab and nivolumab; MBM Dx, brain metastasis diagnosis; PD, disease progression; PD‐1, programmed death 1; PD‐1i, PD‐1 inhibitor; RT, radiotherapy; STR, subtotal resection.
The immune‐sensitizing potential of radiotherapy (RT) was addressed by creating a new predictor with three categories: No RT (n = 19), RT within 2 months of starting immunotherapy (n = 60), and receipt of RT during other times (n = 63). The temporal relationship between immunotherapy (IMTX), surgery (SURG), and development of central nervous system metastases (CNS) was based on five groups: brain metastasis diagnosis followed by immunotherapy (CNS‐IMTX, n = 48), brain metastasis diagnosis followed by immunotherapy and subsequent surgery (CNS‐IMTX‐SURG, n = 11), brain metastasis diagnosis followed by surgery and subsequent immunotherapy (CNS‐SURG‐IMTX, n = 49), immunotherapy followed by brain metastasis diagnosis (IMTX‐CNS, n = 15), and immunotherapy followed by brain metastasis diagnosis and subsequent surgery (IMTX‐CNS‐SURG, n = 19).
The distribution of OS was summarized using the method of Kaplan‐Meier. Standard model‐building techniques were used for all models. Univariate Cox models were fit to each candidate variable. Variables were recoded if the distributions of OS were similar between categories. Recoded variables included LDH (recoded as missing or normal vs. abnormal), ECOG PS (recoded as 0 or 1 vs. 2, 3, or 4 vs. missing), and number of brain metastases (recoded as 1, 2, or 3 vs. >3). Variables with univariate log‐rank p values <.2 were then carried forward into the multivariable models.
After the initial univariate comparisons, multivariable Cox models were fit using forward, backward, and stepwise techniques to verify consistency. The overall prognostic model was stratified by the treatment factors of immunotherapy and surgery to allow for underlying differences in the baseline hazard. To address the potential for guarantee‐time bias in the models involving surgery, model building was based on the extended Cox model with surgery as a time‐dependent covariate.
Median follow‐up was based on a Kaplan‐Meier estimate with inverted censor. Univariate comparisons of patient characteristics according to categories defined by surgery or the sequencing of central nervous system metastasis, surgery, and immunotherapy were based on Fisher's exact tests for categorical characteristics and Wilcoxon rank‐sum or Kruskal‐Wallis tests for characteristics measured on a continuous scale. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC.) Statistical significance was defined as p < .05. There were no corrections for multiple comparisons.
Results
Patient characteristics are outlined in Table 1, Table 2, supplemental online Table 1, and supplemental online Table 2 and included a median follow‐up from the time of brain metastasis diagnosis of 27 months and 92 (64.8%) deaths. Surgical and nonsurgical cohorts were equivalent across the majority of patient characteristics on multivariate anlaysis. Notable differences include nodal involvement, radiation treatment, and number of radiation targets. There was no difference between surgical and nonsurgical patients regarding disease progression with CTLA‐4, PD‐1, or CTLA‐4 and PD‐1 inhibition. The median time between immunotherapy and surgery in the surgery‐after‐immunotherapy group was 5.9 months (interquartile range [IQR], 3.4–14.3 months) and in the surgery‐before‐immunotherapy group was 1.6 months (IQR, 0.8–6.1 months). Indications for surgery, radiation, and immunotherapy for central nervous system disease were discussed at a weekly multidisciplinary brain metastases tumor board and were consistent between newly diagnosed tumors and patient progressing on immunotherapy. Indications for surgery included obtaining tissue for diagnosis and resection of symptomatic lesions causing mass effect.
Prognostic Model of OS
The prognostic model of OS contained five predictors (Table 3). Significant increases in the hazard of death were noted for patients with abnormal LDH (hazard ratio [HR], 2.2; 95% CI, 1.3–3.5; p = .002), ECOG PS of 2 or higher at the time of central nervous system diagnosis (HR, 2.90; 95% CI, 1.6–5.4; p = .004), and patients who developed brain metastases after prior immunotherapy (HR, 2.05; 95% CI, 1.2–3.6). A lower number of brain metastases (HR, 0.42; 95% CI, 0.3–0.7; p = .001) and absence of extracranial disease at the time of brain metastases diagnosis (HR, 0.32; 95% CI, 0.12–0.80; p = .02) conferred reductions in the hazard of death and improved median overall survival (supplemental online Fig. 1).
Table 3. Prognostic model of overall survival.
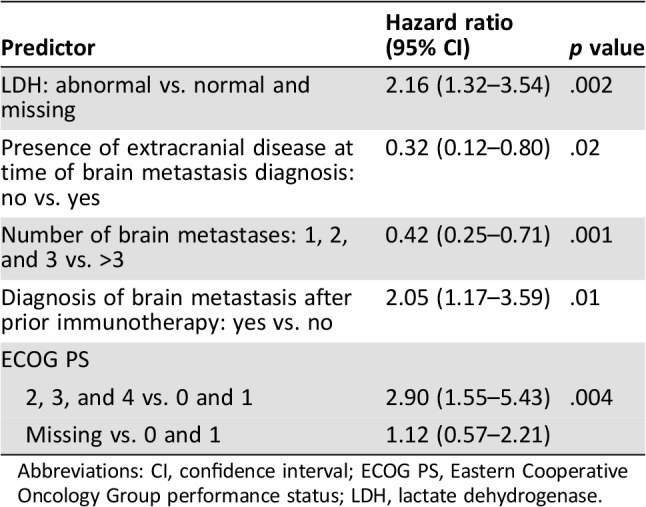
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase.
Predictors of Overall Survival Among Surgical Patients
This analysis was restricted to 79 (55.6%) patients who underwent surgery (75 gross total and 4 subtotal resections) and the model of overall survival contained four predictors (Table 4), which included patients older than the median age of 58 years at the time of primary diagnosis (HR, 2.47; 95% CI, 1.26–4.85; p = .009), patients with brain metastases diagnosis after immunotherapy compared with patients diagnosed with brain metastases prior to initiation of immunotherapy (HR, 2.8; 95% CI, 1.3–6.0; p = .007), and the relative timing of radiation with respect to immunotherapy. Compared with patients who received radiation within 2 months before immunotherapy, patients with no radiation had a sevenfold increase in the hazard of death (HR, 7.4; 95% CI, 1.7–31.3; p = .02); however, there was no difference noted between the groups of patients who received radiation, regardless of timing relative to initiation of immunotherapy. Lastly, there was a significant reduction in the hazard of death for patients who had three or fewer brain metastases (HR, 0.5; 95% CI, 0.2–0.995; p = .049).
Table 4. Predictors of survival among surgical patients.
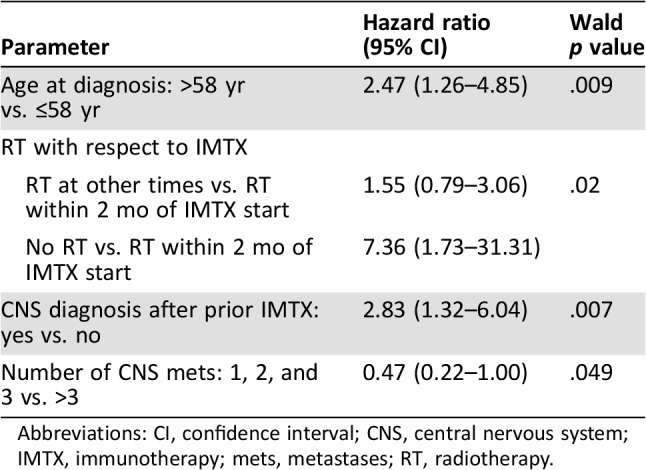
Abbreviations: CI, confidence interval; CNS, central nervous system; IMTX, immunotherapy; mets, metastases; RT, radiotherapy.
Effect of Treatment Sequencing with Development of Central Nervous System Metastasis on Overall Survival
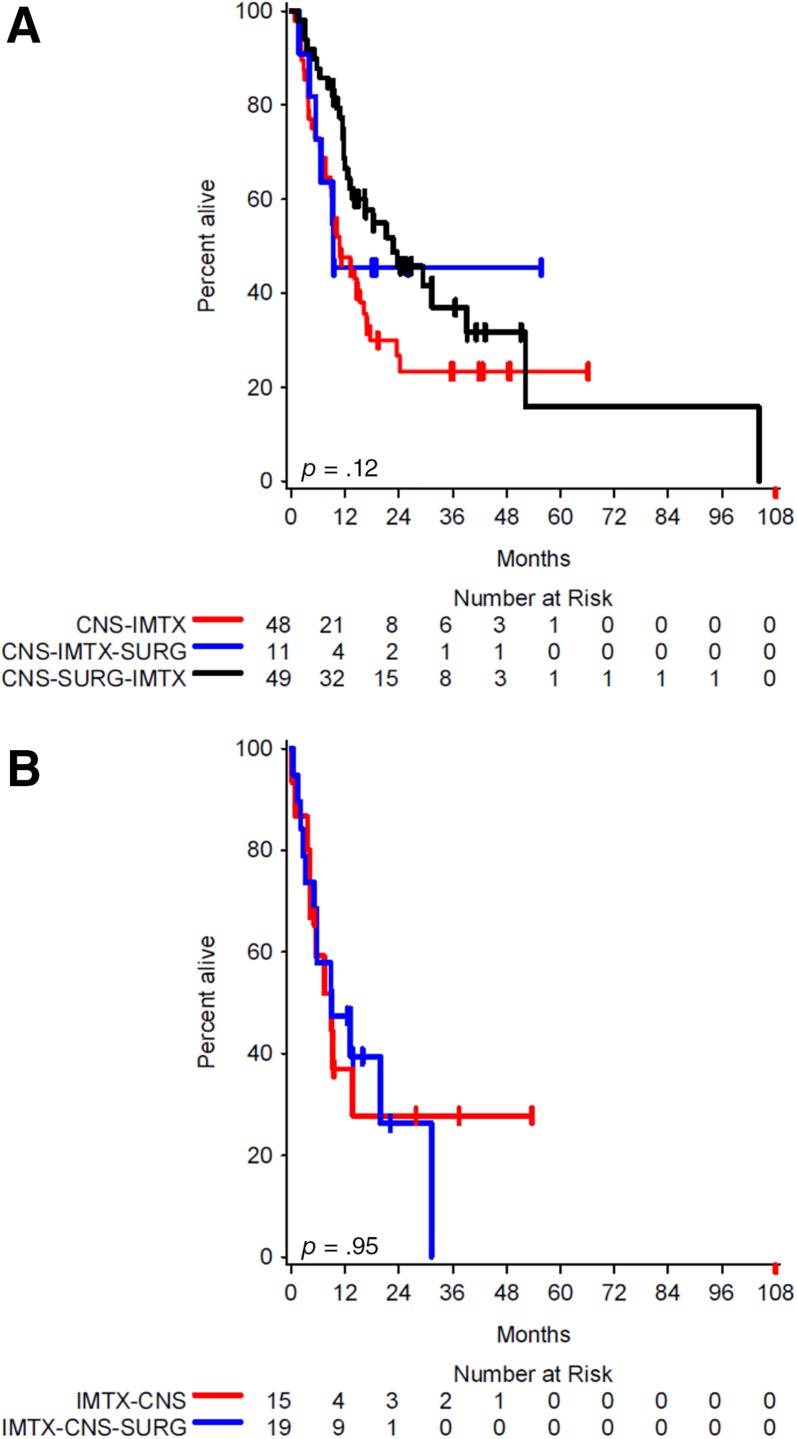
We proceeded to investigate a model of the effect of treatment sequencing for all patients. We first analyzed the median overall survival by treatment sequencing of immunotherapy‐naïve brain metastases (Fig. 1A) and the overall survival by treatment sequencing in patients with prior immunotherapy (Fig. 1B). Among immunotherapy‐naïve brain metastases, the median overall survival was 22.7 months for brain metastases treated with surgery followed by immunotherapy (95% CI, 12.6–39.2), 10.8 months for brain metastases treated with immunotherapy alone (95% CI, 7.8–16.3), and 9.4 months for brain metastases treated with immunotherapy followed by surgery (95% CI, 4.1 to ∞) with an overall p value of .12 (Fig. 1A). These findings suggest that surgery was most beneficial among immunotherapy‐naïve brain metastases treated with upfront surgery followed by immunotherapy.
Figure 1.
Kaplan‐Meier estimates of overall survival by timing of surgery. (A): Median overall survival for CNS‐IMTX (10.8 months; 95% confidence interval [CI], 7.8–16.3), CNS‐IMTX‐SURG (9.4 months; 95% CI, 4.1 to ∞), and CNS‐SURG‐IMTX (22.7 months; 95% CI, 12.6–39.2). (B): Median overall survival for IMTX‐CNS (9.1 months; 95% CI, 3.7 to ∞) and IMTX‐CNS‐SURG (9.0 months; 95% CI, 3.2–31.3).
Abbreviations: CNS‐IMTX, brain metastasis diagnosis treated with immunotherapy alone; CNS‐IMTX‐SURG, brain metastasis diagnosis treated with immunotherapy followed by surgery; CNS‐SURG‐IMTX, brain metastasis diagnosis treated with surgery followed by immunotherapy; IMTX‐CNS, immunotherapy followed by brain metastasis diagnosis; IMTX‐CNS‐SURG, immunotherapy followed by brain metastasis diagnosis and subsequent surgery.
For patients with brain metastases following immunotherapy, the median overall survival for patients treated with upfront immunotherapy followed by brain metastases diagnosis was 9.1 months (95% CI, 3.7 to ∞) compared with 9.0 months for upfront immunotherapy followed by brain metastases diagnosis and subsequent surgery (95% CI, 3.2–31.3) with an overall p value of .95 (Fig. 1B). With the approximately equivalent OS between the IMTX‐CNS and IMTX‐CNS‐SURG groups, this finding suggested that surgery had a minimal impact among these patients. These two groups were subsequently combined into a single group to reflect immunotherapy followed by central nervous system progression regardless of surgery (IMTX‐CNS‐R, 34 patients).
Using the entire cohort, we divided patients into CNS‐IMTX (n = 48), CNS‐IMTX‐SURG (n = 11), CNS‐SURG‐IMTX (n = 49), or IMTX‐CNS‐R (n = 34; supplemental online Table 2). Model building identified five variables significant for overall survival (Table 5). Significant increases in the hazard of death were noted for abnormal LDH (HR, 1.98; 95% CI, 1.20–3.26; p = .008), presence of extracranial disease at time of brain metastasis diagnosis (HR, 4.30; 95% CI, 1.62–11.44; p = .004), no radiation treatment (HR, 2.98; 95% CI, 1.40–6.36; p = .005), and ECOG PS of 2 or higher (HR, 3.02; 95% CI, 1.61–5.69; p = .003). Three or fewer brain metastases conferred a lower risk of death (HR, 0.49; 95% CI, 0.29–0.82; p = .007). Additionally, across all patients, the timing of surgery, immunotherapy, and diagnosis of brain metastasis was marginally significant (p = .06). However, compared with patients who had surgery for treatment‐naïve brain metastasis followed by immunotherapy (CNS‐SURG‐IMTX), a significantly higher risk of death was observed in either patients treated with immunotherapy for treatment‐naïve brain metastasis and no surgery (CNS‐IMTX; HR, 1.72; 95% CI, 1.00–2.99) or patients treated with immunotherapy followed by central nervous system progression (IMTX‐CNS‐R; HR, 1.87; 95% CI, 1.004–3.48).
Table 5. Predictors of overall survival controlling for the time‐dependent covariate of surgery.
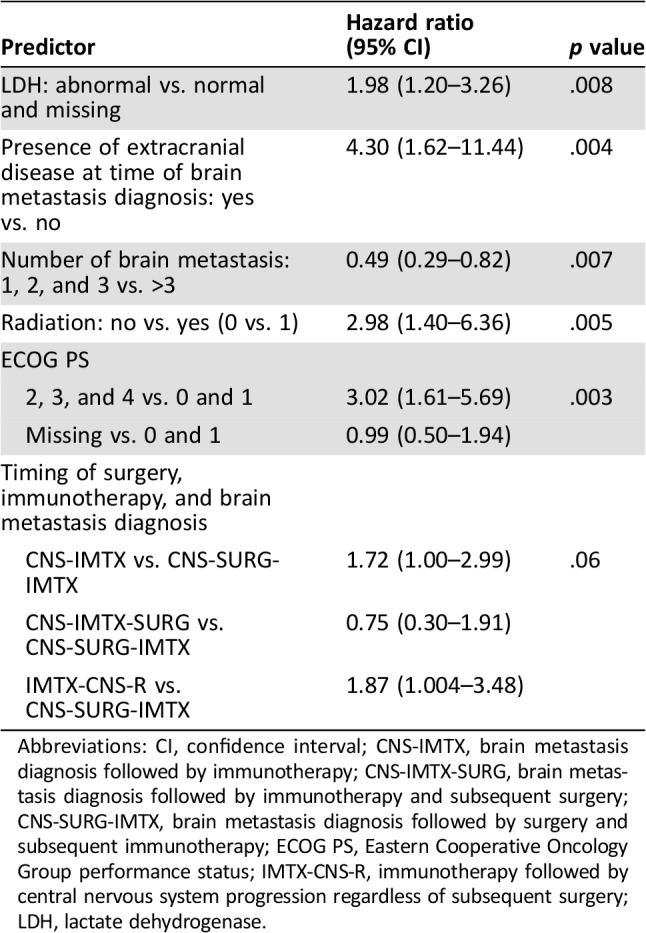
Abbreviations: CI, confidence interval; CNS‐IMTX, brain metastasis diagnosis followed by immunotherapy; CNS‐IMTX‐SURG, brain metastasis diagnosis followed by immunotherapy and subsequent surgery; CNS‐SURG‐IMTX, brain metastasis diagnosis followed by surgery and subsequent immunotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; IMTX‐CNS‐R, immunotherapy followed by central nervous system progression regardless of subsequent surgery; LDH, lactate dehydrogenase.
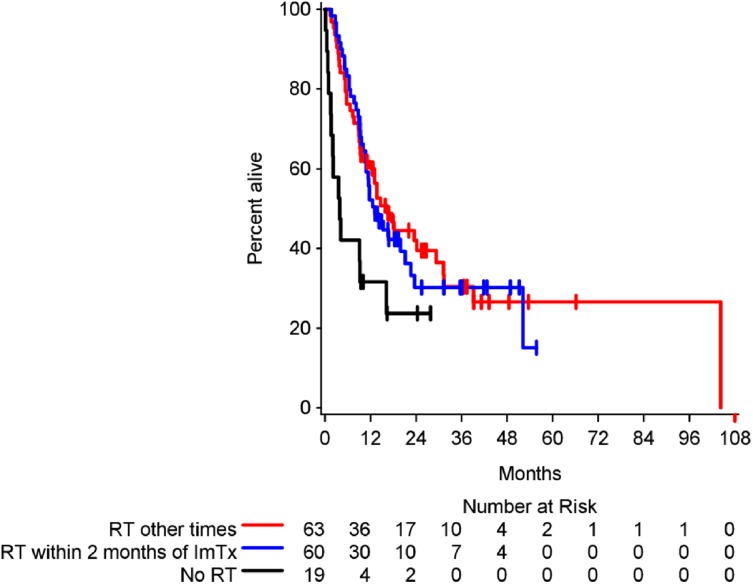
Given the importance of timing between surgery and immunotherapy, we explored if a similar relationship existed between the timing of immunotherapy and radiation across the entire cohort of patients. Recent studies have suggested that radiation may work synergistically with immunotherapy to augment the systemic antitumor response through the abscopal effect [23], [24]. Although exposure to radiation was associated with a benefit in overall survival compared with patients not receiving radiation, this was not dependent on the sequencing of radiation and immunotherapy (Fig. 2).
Figure 2.
Kaplan‐Meier estimates of overall survival by timing of radiation. The cohort was divided into patients who did not receive RT (median overall survival [OS], 4.0 months; 95% confidence interval [CI], 1.5–16.3), those who received RT within 2 months prior to starting ImTx (median OS, 13.2 months; 95% CI, 10.8–21.2), and all others (median OS, 16.5 months; 95% CI, 10.2–29.4). Although radiation was associated with an increase in overall survival, this was not dependent upon the timing of radiation and ImTx (overall p = .009; for the two RT groups, p = .76).
Abbreviations: ImTx, immunotherapy; RT, radiotherapy.
Discussion
With the increasing evidence that patients with brain metastases also benefit from immunotherapy, a closer analysis into the role of metastatic brain tumor resection in the context of CTLA‐4 and PD‐1 blockade is warranted. In this single‐institution, retrospective analysis, patients who underwent surgery of treatment‐naïve MBM followed by immunotherapy had improved survival compared with patients with treatment‐naïve MBM followed by immunotherapy alone. Additionally, patients treated with only immunotherapy for MBM or who developed brain metastases after immunotherapy had significantly increased risk of death on multivariate analysis compared with patients undergoing surgery for treatment‐naïve MBM followed by immunotherapy. These results suggest that immunotherapy‐naïve melanoma brain metastases treated by surgery followed by immunotherapy represent a subset of patients that may benefit from surgery for melanoma brain metastases in the immunotherapy era. As a result, for treatment‐naïve melanoma brain metastases, the approach of upfront local control followed by immunotherapy for systemic disease utilizes surgery as a bridge toward achieving systemic disease control with immunotherapy. This is particularly the case with symptomatic intracranial metastases given their neurologic consequences. An added benefit of surgery prior to immunotherapy is the facilitation of steroid discontinuation shortly after surgery and prior to initiation of immunotherapy, which otherwise might impair the effectiveness of immunotherapy [13], [25], [26]. Further studies will be needed to investigate the time to steroid discontinuation between various upfront treatment approaches including surgery and radiosurgery.
In order to investigate our hypothesis that upfront neurosurgical intervention provides a therapeutic bridge prior to initiation of immunotherapy, we analyzed the sequencing of surgery and immunotherapy within the surgical cohort and across all patients. Within the surgical cohort, factors associated with increased risks of death included age greater than 58, the timing of radiation with respect to immunotherapy, the diagnosis of brain metastases after immunotherapy, and the number of brain metastases. Across the entire cohort of patients, predictors of OS included abnormal LDH, presence of extracranial disease at time of brain metastasis diagnosis, ECOG PS of 2 or higher, no radiation treatment, and more than three brain metastases.
The timing of surgery, immunotherapy and diagnosis of brain metastases included treatment groups that were associated with increased hazards of death and were reflective of differences between immunotherapy‐naïve melanoma brain metastases and patients who developed central nervous system progression on immunotherapy. Consistent with poor outcomes among all patients developing brain metastases following immunotherapy, patients with surgery for treatment‐naïve MBM followed by immunotherapy (CNS‐SURG‐IMTX) had significant reduction in hazard of death compared with patients who developed brain metastases following immunotherapy (IMTX‐CNS‐R). However, patients in the CNS‐SURG‐IMTX group were also associated with a significant reduction in hazard of death compared with immunotherapy‐naïve brain metastases treated only with immunotherapy (CNS‐IMTX).
We also investigated with multivariate analysis whether the timing of radiation and its potential role as a radiosensitizer to immunotherapy was predictive of patient outcomes. Although radiation was clearly associated with improved patient outcomes and reduced hazard of death, the timing of radiation and immunotherapy was not associated with a difference in OS. These findings suggest that the timing between upfront surgery and immunotherapy is a critical clinical decision that is unique compared with the initiation of radiation and immunotherapy, although this needs to be validated in larger studies. Further investigation is warranted to study which local brain therapy should be administered prior to initiation of immunotherapy.
Our findings build upon initial reports demonstrating an objective response to immunotherapy among patients with surgically resected brain metastases [27], [28] and have several therapeutic implications. Surgery has been a critical component for the treatment of intracranial metastases in patients with a favorable performance status, a single brain lesion, controlled extracranial disease, or uncertain histology. However, in an era where immunotherapy is providing patients with significant hope for a durable response, it requires a reassessment of surgery's role in this patient population. In particular, our findings suggest that surgery may serve a critical role in patients with treatment‐naïve MBM who are scheduled to receive subsequent immunotherapy. Multiple clinical trials have demonstrated that blockades of CTLA‐4 and the PD‐1 and PD‐L1 pathway have the potential of controlling extracranial disease, and these treatment modalities are increasingly becoming the standard of care. Our findings are consistent with this notion but also advance our understanding of their role for intracranial disease when combined with neurosurgical intervention.
With the recent improvement in the treatment for metastatic melanoma, immunotherapy for brain metastases is an increasingly pressing challenge. However, the literature is currently lacking evidence to guide decision making for surgery in the setting of immunotherapy and intracranial disease. Our findings demonstrate that surgery for treatment‐naïve intracranial disease followed by immunotherapy is associated with increased OS compared with patients who were solely treated with immunotherapy after a brain metastases diagnosis or who developed brain metastases following immunotherapy.
Given the retrospective nature of this study, there are a number of intrinsic limitations including the small cohort size, disparate immunotherapy regimens, baseline patient characteristics between surgical and nonsurgical groups, variations in steroid utilization, and the timing of salvage treatments and corresponding response rates. Further studies are warranted, particularly among treatment‐naïve MBM, to investigate the clinical utility of timing for surgery and the initiation of immunotherapy among a larger, matched cohort that is ideally carried out in a prospective, randomized controlled setting. Features to consider include the number, size, and location of upfront brain metastases, the number of lesions or tumor volume that should optimally be resected, the role and timing of radiation with surgery, and the optimal timing of steroid discontinuation after surgery. Additionally, our cohort consisted of a variety of immunotherapy treatment regimens, and many patients were treated prior to the use of PD‐1 inhibitors. Future work is needed to extend our findings among patients undergoing a standardized immunotherapy treatment regimen.
Conclusion
These findings suggest that surgery should be considered for patients with intracranial melanoma metastases prior to the initiation of immunotherapy, particularly for those patients on corticosteroids for symptomatic disease. This aggressive surgical approach provides an opportunity to achieve unprecedented clinical benefit of emerging immunotherapies in patients previously though to have end‐stage disease.
See http://www.TheOncologist.com for supplemental material available online.
Contributed equally
Footnotes
For Further Reading: James J. Harding, Federica Catalanotti, Rodrigo R. Munhoz et al. A Retrospective Evaluation of Vemurafenib as Treatment for BRAF‐Mutant Melanoma Brain Metastases. The Oncologist 2015;20:789–797.
Implications for Practice: Vemurafenib is active for BRAF‐mutant intracranial melanoma metastases in an unselected patient population typical of routine oncologic practice. Patients with poor performance status appear to have poor outcomes despite vemurafenib therapy. Preliminary data indicate that co‐occurring or secondary alterations in the phosphatidylinositol 3‐kinase‐AKT (PI3K‐AKT) pathway are involved in resistance to RAF inhibition, thus providing a rationale for dual MAPK and PI3K‐AKT pathway inhibition in this patient population.
Author Contributions
Conception/design: Christopher Alvarez‐Breckenridge, Daniel P. Cahill, Ryan Sullivan, Priscilla K. Brastianos
Provision of study material or patients: Corey M. Gill, Mia Bertalan
Collection and/or assembly of data: Christopher Alvarez‐Breckenridge, Corey M. Gill, Mia Bertalan, Jackson Stocking, Alexander Kaplan, Naema Nayyar
Data analysis and interpretation: Christopher Alvarez‐Breckenridge, Anita Giobbie‐Hurder, Corey M. Gill, Donald P. Lawrence, Daniel P. Cahill, Ryan Sullivan, Priscilla K. Brastianos
Manuscript writing: Christopher Alvarez‐Breckenridge, Anita Giobbie‐Hurder, Corey M. Gill, Mia Bertalan, Jackson Stocking, Donald P. Lawrence, Keith T. Flaherty, Helen A. Shih, Kevin Oh, Tracy T. Batchelor, Daniel P. Cahill, Ryan Sullivan, Priscilla K. Brastianos
Final approval of manuscript: Christopher Alvarez‐Breckenridge, Anita Giobbie‐Hurder, Daniel P. Cahill, Ryan Sullivan, Priscilla K. Brastianos
Disclosures
Keith T. Flaherty: Novartis (RF), Novartis, Roche, Array BioPharma, Merck, Bristol‐Myers Squibb, Amgen (SAB); Kevin Oh: Merck, Elekta (RF); Tracy T. Batchelor: Merck, Champions Biotechnology (C/A), NXDC, Genomicare (SAB); Daniel P. Cahill: Merck (H), Eli Lilly & Co. (SAB); Ryan Sullivan: Merck (RF), Merck, Novartis (SAB); Priscilla K. Brastianos: Angiochem, Eli Lilly & Co., Tesaro (C/A), Merck (RF), Merck, Genentech (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Mehta MP, Tsao MN, Whelan TJ et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence‐based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 2005;63:37–46. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev 2003;29:533–540. [DOI] [PubMed] [Google Scholar]
- 3.Sampson JH, Carter JH Jr, Friedman AH et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 1998;88:11–20. [DOI] [PubMed] [Google Scholar]
- 4.Nieder C, Adam M, Astner ST. Disease presentation and outcome in young patients (<40 years) with brain metastases from malignant melanoma. Anticancer Res 2008;28:1325–1327. [PubMed] [Google Scholar]
- 5.Fife KM, Colman MH, Stevens GN et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004;22:1293–1300. [DOI] [PubMed] [Google Scholar]
- 6.Brastianos HC, Cahill DP, Brastianos PK. Systemic therapy of brain metastases. Curr Neurol Neurosci Rep 2015;15:518. [DOI] [PubMed] [Google Scholar]
- 7.Schadendorf D, Hodi FS, Robert C et al. Pooled analysis of long‐term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, Chiarion‐Sileni V, Gonzalez R et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4‐year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–1492. [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Chiarion‐Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan MK, Kluger H, Postow MA et al. Nivolumab plus ipilimumab in patients with advanced melanoma: Updated survival, response, and safety data in a phase I dose‐escalation study. J Clin Oncol 2017:JCO2017722850. [DOI] [PMC free article] [PubMed]
- 12.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 19;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolin K, Ernstoff MS, Hamid O et al. Ipilimumab in patients with melanoma and brain metastases: an open‐label, phase 2 trial. Lancet Oncol 2012;13:459–465. [DOI] [PubMed] [Google Scholar]
- 14.Di Giacomo AM, Ascierto PA, Pilla L et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT‐M1): An open‐label, single‐arm phase 2 trial. Lancet Oncol 2012;13:879–886. [DOI] [PubMed] [Google Scholar]
- 15.Di Giacomo AM, Ascierto PA, Queirolo P et al. Three‐year follow‐up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)‐M1 phase II study. Ann Oncol 2015;26:798–803. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SB, Gettinger SN, Mahajan A et al. Pembrolizumab for patients with melanoma or non‐small‐cell lung cancer and untreated brain metastases: Early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol 2016;17:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long GV, Atkinson V, Lo S et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol 2018;19:672–681. [DOI] [PubMed] [Google Scholar]
- 18.Tawbi HA, Forsyth PA, Algazi A et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018;379:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patchell RA, Tibbs PA, Walsh JW et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494–500. [DOI] [PubMed] [Google Scholar]
- 20.Vecht CJ, Haaxma‐Reiche H, Noordijk EM et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583–590. [DOI] [PubMed] [Google Scholar]
- 21.Kalkanis SN, Kondziolka D, Gaspar LE et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence‐based clinical practice guideline. J Neurooncol 2010;96:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal S, Silk AW, Tian S et al. Clinical management of multiple melanoma brain metastases: A systematic review. JAMA Oncol 2015;1:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Callahan MK, Barker CA et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamell EF, Wolchok JD, Gnjatic S et al. The abscopal effect associated with a systemic anti‐melanoma immune response. Int J Radiat Oncol Biol Phys 2013;85:293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbour KC, Mezquita L, Long N et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 26.Chasset F, Pages C, Biard L et al. Single‐center study under a French Temporary Authorization for Use (TAU) protocol for ipilimumab in metastatic melanoma: Negative impact of baseline corticosteroids. Eur J Dermatol 2015;25:36–44. [DOI] [PubMed] [Google Scholar]
- 27.Lonser RR, Song DK, Klapper J et al. Surgical management of melanoma brain metastases in patients treated with immunotherapy. J Neurosurg 2011;115:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PS, Cahill DP, Brastianos PK et al. Ipilimumab and craniotomy in patients with melanoma and brain metastases: A case series. Neurosurg Focus 2015;38:E5. [DOI] [PubMed] [Google Scholar]