This article reports the final analysis of a single‐center prospective study that investigated the effect of circulating tumor cells on survival in patients with high‐grade non‐muscle invasive bladder cancer.
Keywords: Cancer‐specific survival, Circulating tumor cells, Non‐muscle‐invasive bladder cancer, Overall survival, Risk variables
Abstract
Background.
Clinical behavior of non‐muscle‐invasive bladder cancer (NMIBC) is largely unpredictable, and even patients treated according to European Association of Urology recommendations have a heterogeneous prognosis. High‐grade T1 (HGT1) bladder cancer is the highest‐risk subtype of NMIBC, with an almost 40% rate of recurrence and 20% of progression at 5 years. Nomograms predicting risk of recurrence, progression, and cancer‐specific survival (CSS) are not available specifically within HGT1 bladder cancer, and the identification of robust prognostic biomarkers to better guide therapeutic strategies in this subgroup of patients is of paramount importance. Strategies to identify putative biomarkers in liquid biopsies from blood and urine collected from patients with bladder cancer have been intensively studied in the last few years.
Subjects, Materials, and Methods.
We here report the final analysis of a single‐center prospective study aimed to investigate the impact of circulating tumor cells (CTCs) on CSS and overall survival (OS) in 102 patients with HGT1 bladder cancer, in a median follow‐up of 63 months.
Results.
We here demonstrate that the presence of even a single CTC is predictive of shorter CSS and OS, as compared with the standard predictive variables. Points of attention in this multivariable analysis are the long‐term follow‐up and the adequate number of outcome events.
Conclusion.
The accurate risk stratification provided by CTCs might be essential for determining the best surveillance strategy for patients after diagnosis. A closer follow‐up, an early radical surgery, or even a systemic treatment might be recommended in patients with super‐high‐risk non‐muscle‐invasive bladder cancer.
Implications for Practice.
Circulating tumor cells identify patients with super‐high‐risk non‐muscle‐invasive bladder cancer who require closer monitoring for local recurrence and/or progression of disease. This super‐high‐risk subgroup of patients might also require more aggressive treatment interventions, which should be evaluated in large prospective cohorts.
Introduction
The vast majority of bladder cancers are classified as non‐muscle‐invasive bladder cancer (NMIBC) at diagnosis. NMIBC are heterogeneous cancers, at the biological and clinical level, with a large degree of variation in clinical presentation, course, and outcomes. T1 tumors (lesions that extend to the lamina propria layer of the bladder) account for approximately 20% of all NMIBC and have the highest rate of recurrence, progression, and cancer‐specific mortality. High‐grade T1 (HGT1) bladder cancer is the highest‐risk subtype of NMIBC, with an almost 40% rate of recurrence and 20% of progression at 5 years, despite primary intravesical therapy with Bacillus Calmette‐Guérin (BCG) [1]. The pathophysiology behind the aggressive nature of HGT1 bladder cancer has not yet been fully elucidated. It has been hypothesized that the poor clinical outcome of some patients is either due to the understaging of T2 lesions or to an inherent biological aggressiveness of the tumor [2]. Clinical and pathological features have been shown to be major determinants of long‐term outcomes. In the last decade, two prognostic models, derived from two large European research consortia, the European Organisation for Research and Treatment of Cancer and Spanish Urological Club for Oncological Treatment, have been proposed to estimate the risk of recurrence and progression to muscle‐invasive disease. Both models have been proposed for use in the broader NMIBC population and calculate the risk profile on the basis of tumor grade and stage, prior recurrences, tumor size, multifocality, and the presence of carcinoma in situ (CIS) [3]. Nomograms predicting risk of recurrence, progression, and cancer‐specific survival (CSS) are not available specifically within HGT1 bladder cancer, and current models have shown limited predictive value in this high‐risk subtype of bladder cancer [4]. An accurate prediction of progression is critically important in the management of NMIBC. Indeed, owing to limitations in risk stratification, patients with T1 bladder cancer are at risk of being either undertreated, with persistent use of BCG despite recurrence, or overtreated with avoidable early cystectomy. The identification of robust prognostic biomarkers could better guide therapeutic strategies for patients with high‐risk non‐muscle‐invasive bladder cancer, which remains a very challenging disease. Over the past decades, the knowledge of the molecular background of bladder cancer has dramatically improved and deregulation of tumor suppressor genes, oncogenes, cell cycle regulators, growth factors and receptors, and cell adhesion molecules has been recognized. So far, molecular markers are rarely discriminatory enough for clinical use as individual markers, although the combination with clinical data may improve their prognostic power [5]. There is general agreement that by adding biomarkers that reflect the biologic behavior of HGT1 bladder cancer to established parameters in nomograms, significant improvements in risk stratification could be achieved. Strategies to identify putative biomarkers in liquid biopsies from blood and urine collected from patients with bladder cancer are being developed to monitor subclinical systemic disease and to identify candidate targets for therapeutic intervention [6]. Circulating tumor cells (CTCs), in particular, are regarded as surrogate for early dissemination of disease and have been qualified as accurate prognostic biomarkers in different cancer types, including bladder cancer [7]. We have previously demonstrated that, in a cohort of 102 patients with HRT1 bladder cancer, the presence of CTCs (detectable in 20% of patients) predicted both decreased time to first recurrence and time to progression (median follow‐up 24.3 months), thus identifying a subgroup of patients with super‐high‐risk CTC‐positive non‐muscle‐invasive bladder cancer [8]. We here report the updated outcome analysis and mature results for cancer‐specific survival and overall survival (OS) in a median follow‐up of 63 months.
Subjects, Materials, and Methods
We conducted a prospective single‐center analysis in 102 patients with pathologically confirmed high‐risk T1G3 transitional cell tumor, treated at our institution between September 2010 and January 2013, according to standard international guidelines: transurethral resection of bladder (TURB) followed by re‐TURB and BCG induction plus maintenance 1–3 years. Patients follow‐up was planned with a cystoscopy and urinary cytology every 3 months and a contrast‐enhanced computed tomography scan every 12 months. CTCs were isolated before the first TURB from 7.5 mL of blood collected into evacuated blood draw tubes through CellSearch system (Menarini Silicon Biosystems, Castel Maggiore, Bologna, Italy), according to manufacturer's instructions [9]. We had previously demonstrated that the presence of even a single CTC is significantly associated with shorter time to first recurrence (TFR) and time to progression (TTP) in a median follow‐up of 24 months, attesting for the first time the powerful prediction of disease recurrence and progression provided by CTCs in patients with high‐grade T1 bladder cancer [8]. Updated outcome analysis and mature CSS and OS results are reported here in a median follow‐up of 63 months. Descriptive statistics were used to summarize pertinent study information. The association between variables was tested by the Pearson chi‐square test or the Fisher's exact test. Survival was calculated by the Kaplan‐Meier product‐limit method. The log‐rank test was used to assess differences between subgroups. Univariate Cox proportional hazards models were built using gender, age, presence of CTC, CIS, lymph vascular invasion, multifocal, tumor size as covariates for TFR, metastasis‐free survival (MFS), TTP, time to second recurrence (TSR), OS, and CSS. Significance was defined at the p ≤ .05 level. A multivariate Cox proportional hazards model was also developed using stepwise regression (forward selection) by selecting those predictive variables that were significant upon univariate analysis; enter limit and remove limit were p = .05 and p = .10, respectively. Statistical analysis was performed with SPSS software (SPSS version 21.0; SPSS Inc., Chicago, IL).
Results
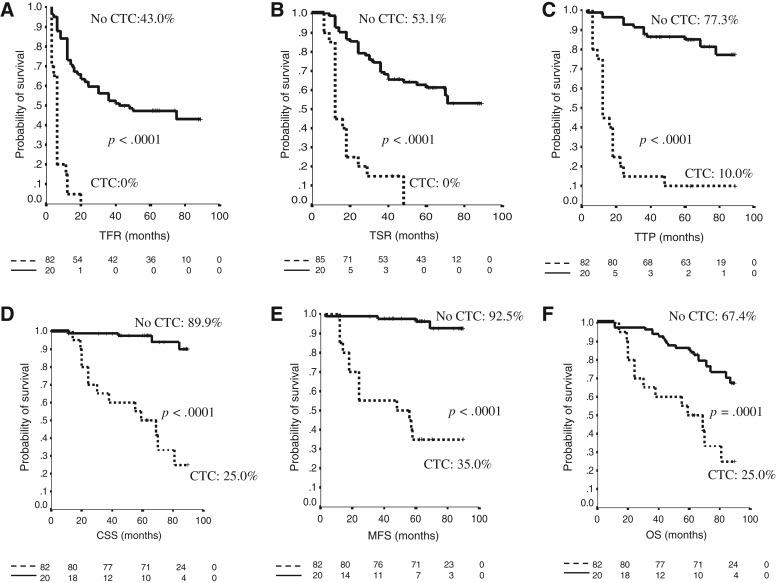
In the study population, CTCs were found in 20/102 (20%) patients. Median CTCs number was 1 (range 1–50). Median follow‐up of this final analysis was 63 months (range 11–90). Patients that were still alive at the end of the study were followed for at least 61 months. General characteristics of the study population are described in Table 1. Kaplan‐Meier analysis revealed a correlation between CTCs and long‐term survival. The final analysis substantiated results obtained at 24 months of median follow‐up showing a statistically significant p value for TFR (p < .0001), TSR (p < .0001), and TTP (p < .0001). The presence of at least one CTC was also found to be clearly associated with shorter MFS (p < .0001), CSS (p < .0001), and OS (p = .0001; Fig. 1). Multivariable analysis confirmed that CTCs, as compared with the standard predictive variables (multifocality, CIS, lymph vascular invasion), have the strongest negative prognostic impact for all the outcomes analyzed (TFR hazard ratio [HR] 4.26 [2.13–8.52] p < .0001; TSR HR 3.53 [1.81–6.87] p < .0001; TTP 7.32 [3.29–16.27] p < .0001; MFS HR 18.75 [6.01–58.47] p < .0001; CSS HR 13.68 [4.27–43.8] p < .0001; OS HR 3.68 [1.81–7.51] p < .0001). Points of attention in this multivariable analysis are the long‐term follow‐up and the adequate number of outcome events [10]. Multivariable analysis is detailed in Table 2. Number of events per variable in CTC‐positive versus CTC‐negative subgroups are shown in Table 3.
Table 1. Main baseline characteristics of the study population (Number: 102).
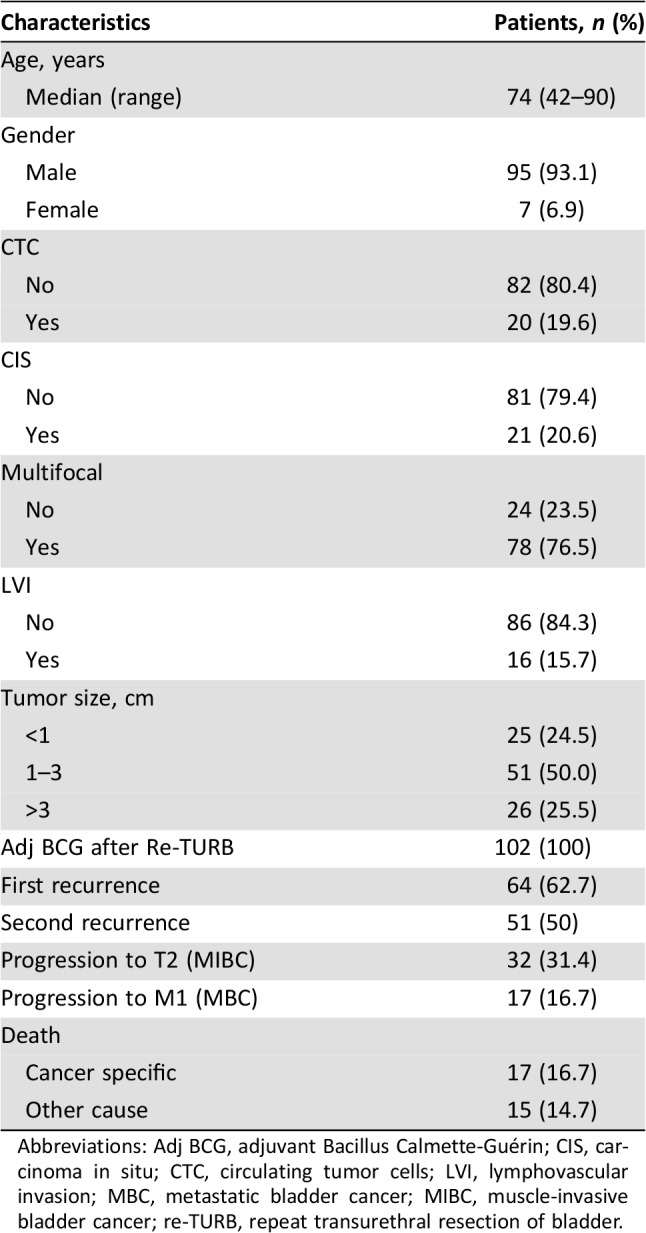
Abbreviations: Adj BCG, adjuvant Bacillus Calmette‐Guérin; CIS, carcinoma in situ; CTC, circulating tumor cells; LVI, lymphovascular invasion; MBC, metastatic bladder cancer; MIBC, muscle‐invasive bladder cancer; re‐TURB, repeat transurethral resection of bladder.
Figure 1.
Kaplan‐Meier curves. (A): TFR. (B): TSR. (C): TTP. (D): CSS. (E): MFS. (F): OS.
Abbreviations: CTC, circulating tumor cells; CSS, cancer‐specific survival; MFS, metastasis‐free survival; OS, overall survival; TFR, time to first recurrence; TSR, time to second recurrence; TTP, time to progression.
Table 2. Multivariate Cox proportional hazards model.
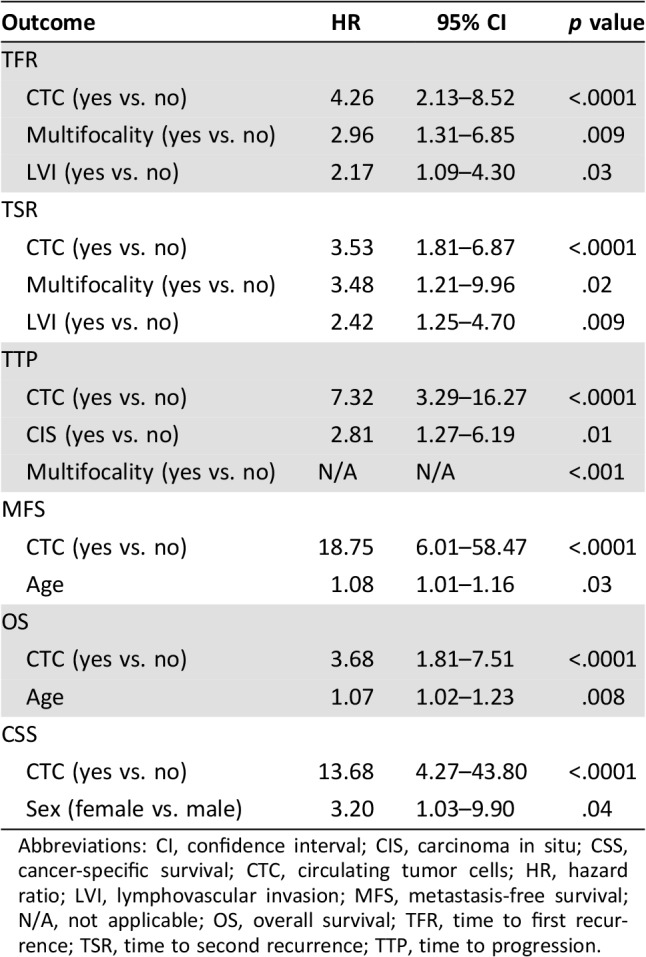
Abbreviations: CI, confidence interval; CIS, carcinoma in situ; CSS, cancer‐specific survival; CTC, circulating tumor cells; HR, hazard ratio; LVI, lymphovascular invasion; MFS, metastasis‐free survival; N/A, not applicable; OS, overall survival; TFR, time to first recurrence; TSR, time to second recurrence; TTP, time to progression.
Table 3. Number of events per variable in CTC‐positive versus CTC‐negative subgroups.
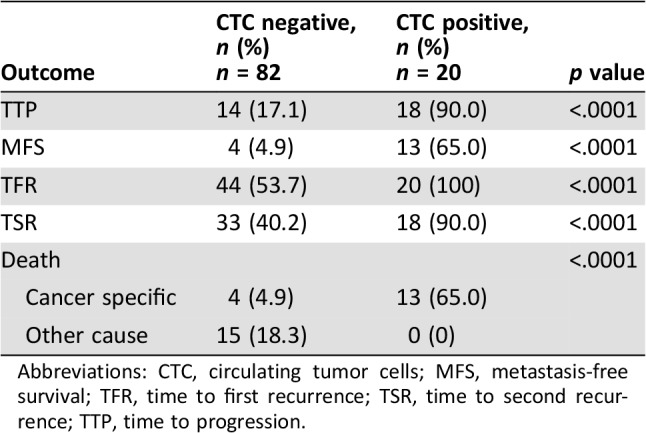
Abbreviations: CTC, circulating tumor cells; MFS, metastasis‐free survival; TFR, time to first recurrence; TSR, time to second recurrence; TTP, time to progression.
Discussion
Since the introduction of intravesical BCG therapy almost 40 years ago, the medical management of NMIBC has remained static. Multiple novel agents to be used in combination with BCG, as an alternative to BCG or after BCG failure, are being explored and show great promise [11]. Current models fail to accurately identify patients with NMIBC who are at the highest risk of progression to muscle‐invasive and/or metastatic disease, despite adjuvant treatment with BCG immunotherapy. Furthermore, we still lack biomarkers for the assessment of the biologic potential and clinical behavior of this complex disease, which could allow for a decisive improvement of patients’ outcomes [12]. The results from this study suggest that in a subgroup of patients with HRT1 bladder cancer, CTCs might indicate a subclinical systemic disease. The accurate risk stratification provided by CTCs analysis might be used, in the near future, for determining the best surveillance strategy for patients after diagnosis. A closer follow‐up, an early radical surgery, or even a systemic treatment might be recommended in this super‐high‐risk CTC‐positive non‐muscle‐invasive bladder cancer subgroup. The term “liquid biopsy” refers to a noninvasive test that can identify markers of various types of cancer in fluid samples such as blood or urine. Among the different approaches for liquid biopsy, the analysis of CTCs is certainly the oldest technique. The CellSearch platform for the isolation of circulating tumor cells from peripheral blood has been on the market for 12 years; it was originally cleared by the U.S. Food and Drug Administration in 2004 for in vitro diagnostic use in patients with metastatic breast cancer and subsequently showed clinical relevance in many other cancer types [7]. The study reported here was started early in 2010, when the first patients with HRT1 bladder cancer was enrolled. We here report the final analysis of results after a median follow‐up of 63 months. This reveals that the presence of even a single CTC in peripheral blood of HGT1 patients is clearly associated with shorter overall and cancer‐specific survival. We are aware that, since this study was conceived and then conducted, the knowledge of the molecular background of NMIBC has significantly evolved and that since then a wide variety of isolation and detection methods have been developed to enrich and enumerate CTCs. Besides, in the last few years, it became clear that specific molecular differences exist between high‐ and low‐grade NMIBC and that the genetic alterations found in high‐grade NMIBC more closely resemble those present in MIBC [13]. Recent studies have demonstrated that cancer‐associated mutations and other DNA alterations can be detected in body fluids, thus providing impetus to the technological advances for circulating tumor DNA analysis in blood and urine from patients with bladder cancer [14]. Given the increasingly complex mutational landscape and intratumor heterogeneity of the disease, it is unlikely that a single biomarker will able to fill the gap in the discrimination of patients at the highest risk of progression to muscle‐invasive disease and/or metastatic dissemination. Current risk stratification models do not include any molecular markers or any analyte from body fluids. It will be important to determine whether the molecular knowledge of HRT1 bladder cancer can improve the management of patients, particularly those at very high risk of progression. Combining new prognostic factors into risk‐prediction nomograms with validation in prospective cohorts would be of undeniable value.
Conclusion
The presence of even a single CTC in peripheral blood of patients with HRT1 bladder cancer identifies patients with super‐high‐risk non‐muscle‐invasive bladder cancer at a very high risk of local recurrence and/or progression of disease. This updated outcome analysis reports mature results for cancer‐specific survival and overall survival in a median follow‐up of 63 months, demonstrating that patients with CTC‐positive HRT1 bladder cancer have shorter metastasis‐free survival, cancer‐specific survival, and overall survival, thus connoting CTCs as a reliable tool to improve the accuracy of current risk stratification models.
See http://www.TheOncologist.com for supplemental material available online.
Contributed equally
Contributed equally
Author Contributions
Conception/design: Paola Gazzaniga, Cristina Raimondi
Provision of study material or patients: Gian Maria Busetto, Enrico Cortesi, Ettore de Berardinis
Collection and/or assembly of data: Chiara Nicolazzo, Isabella Sperduti, Francesco del Giudice, Flavia Loreni
Data analysis and interpretation: Chiara Nicolazzo, Angela Gradilone, Isabella Sperduti, Francesco del Giudice
Manuscript writing: Cristina Raimondi
Final approval of manuscript: Chiara Nicolazzo, Gian Maria Busetto, Angela Gradilone, Isabella Sperduti, Francesco del Giudice, Flavia Loreni, Enrico Cortesi, Ettore de Berardinis, Paola Gazzaniga, Cristina Raimondi
Disclosures
The authors indicated no financial relationships.
References
- 1.Reisz PA, Laviana AA, Chang SS. Management of high‐grade T1 urothelial carcinoma. Curr Urol Rep 2018;19:103. [DOI] [PubMed] [Google Scholar]
- 2.Jordan B, Meeks JJ. T1 bladder cancer: Current considerations for diagnosis and management. Nat Rev Urol 2019;16:23–34. [DOI] [PubMed] [Google Scholar]
- 3.Martin‐Doyle W, Leow JJ, Orsola A et al. Improving selection criteria for early cystectomy in high‐grade T1 bladder cancer: A meta‐analysis of 15,215 patients. J Clin Oncol 2015;33:643–650. [DOI] [PubMed] [Google Scholar]
- 4.Gershman B, Boorjian SA, Hautmann RE. Management of T1 urothelial carcinoma of the bladder: What do we know and what do we need to know? Bladder Cancer 2015;2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Kessel KEM, van der Keur KA, Dyrskjøt L et al. Molecular markers increase precision of the European Association of Urology non‐muscle‐invasive bladder cancer progression risk groups. Clin Cancer Res 2018;24:1586–1593. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Fan W, Deng Q et al. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta‐analysis of 30 published studies. Oncotarget 2017;8:59527–59538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riethdorf S, O'Flaherty L, Hille C et al. Clinical applications of the CellSearch platform in cancer patients. Adv Drug Deliv Rev 2018;125:102–121. [DOI] [PubMed] [Google Scholar]
- 8.Gazzaniga P, de Berardinis E, Raimondi C et al. Circulating tumor cells detection has independent prognostic impact in high‐risk non‐muscle invasive bladder cancer. Int J Cancer 2014;135:1978–1982. [DOI] [PubMed] [Google Scholar]
- 9.Coumans F, Terstappen L. Detection and characterization of circulating tumor cells by the CellSearch approach. Methods Mol Biol 2015;1347:263–278. [DOI] [PubMed] [Google Scholar]
- 10.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710–718. [DOI] [PubMed] [Google Scholar]
- 11.Nykopp TK, Batista da Costa J, Mannas M et al. Current clinical trials in non‐muscle invasive bladder cancer. Curr Urol Rep 2018;19:101. [DOI] [PubMed] [Google Scholar]
- 12.Soria F, Krabbe LM, Todenhöfer T et al. Molecular markers in bladder cancer. World J Urol 2019;37:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butterfield A, Gupta S. Next‐generation sequencing in non‐muscle‐invasive bladder cancer‐A step towards personalized medicine for a superficial bladder tumor. Transl Androl Urol 2017;6:1198–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandekerkhove G, Todenhöfer T, Annala M et al. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin Cancer Res 2017;23:6487–6497. [DOI] [PubMed] [Google Scholar]