This article evaluates Oncotype DX ordering practices and chemotherapy use pre‐ and post‐implementation of standardized testing criteria for patients with early‐stage breast cancer.
Keywords: Breast cancer, Quality improvement, Genomic assays
Abstract
Background.
For clinically appropriate early‐stage breast cancer patients, reflex criteria for Oncotype DX ordering (“the intervention”) were implemented at our comprehensive cancer center, which reduced time‐to‐adjuvant chemotherapy initiation. Our objective was to evaluate Oncotype DX ordering practices and chemotherapy use before and after implementation of the intervention.
Materials and Methods.
We examined medical records for 498 patients who had definitive breast cancer surgery at our center. The post‐intervention cohort consisted of 232 consecutive patients who had Oncotype DX testing after reflex criteria implementation. This group was compared to a retrospective cohort of 266 patients who were diagnosed and treated prior to reflex criteria implementation, including patients who did and did not have Oncotype DX ordered. Factors associated with Oncotype DX ordering pre‐ and post‐intervention were examined. We used multivariate logistic regression to evaluate factors associated with chemotherapy receipt among patients with Oncotype DX testing.
Results.
The distribution of Oncotype DX scores, the proportion of those having Oncotype DX testing (28.9% vs. 34.1%) and those receiving chemotherapy (14.3% vs. 19.4%), did not significantly change between pre‐ and post‐intervention groups. Age ≤65 years, stage II, grade 2, 1–3+ nodes, and tumor size >2 cm were associated with higher odds of Oncotype DX testing. Among patients having Oncotype DX testing, node status and Oncotype DX scores were significantly associated with chemotherapy receipt.
Conclusion.
Our criteria for reflex Oncotype DX ordering appropriately targeted patients for whom Oncotype DX would typically be ordered by providers. No significant change in the rate of Oncotype DX ordering or chemotherapy use was observed after reflex testing implementation.
Implications for Practice.
This study demonstrates that implementing multidisciplinary consensus reflex criteria for Oncotype DX ordering maintains a stable Oncotype DX ordering rate and chemotherapy rate, mirroring what was observed in a specific clinical practice, while decreasing treatment delays due to additional testing. These reflex criteria appropriately capture patients who would likely have had Oncotype DX ordered by their providers and for whom the test results are predicted to influence management. This intervention serves as a potential model for other large integrated, multidisciplinary oncology centers to institute processes targeting patient populations most likely to benefit from genomic assay testing, while mitigating treatment delays.
摘要
背景。对于临床上适合的早期乳腺癌患者,我们在综合癌症中心实施了 Oncotype DX 预定的反射标准(简称为“干预”),从而减少了辅助化疗开始的时间。我们的目标是在实施干预之前和之后评估 Oncotype DX 预定的实践和化疗使用。
材料和方法。我们检查了 498 名曾在我们中心接受确定性乳腺癌手术的患者的医疗记录。干预后队列由 232 名在反射标准实施之后接受 Oncotype DX 检测的连续患者组成。我们将此组与回顾性队列进行比较,该回顾性队列由 266 名在反射标准实施之前已接受诊断和治疗的患者组成,其中包括已预定及未预定 Oncotype DX 的患者。我们检查了与干预前后的 Oncotype DX 预定相关的各种因素。在接受 Oncotype DX 检测的患者中,我们利用多变量逻辑回归来评估与接受化疗相关的各种因素。
结果。Oncotype DX 分数的分布、接受 Oncotype DX 检测的患者比例(28.9% vs. 34.1%)以及接受化疗的患者比例(14.3% vs. 19.4%)在干预前组和干预后组之间没有显著变化。年龄 ≤65 岁,II 期,2 级,1–3 个阳性淋巴结以及肿瘤大小 >2 cm,这些因素与 Oncotype DX 检测的较高几率相关。在接受 Oncotype DX 检测的患者中,淋巴结状态和 Oncotype DX 分数与接受化疗明显相关。
结论。我们的 Oncotype DX 预定的反射标准适当地以通常由提供商为其预定 Oncotype DX 的患者为目标。在实施反射试验之后,我们在 Oncotype DX 预定率或化疗使用率中未观察到显著变化。
对临床实践的提示:本研究表明,针对 Oncotype DX 预定实施多学科共识反射标准可以保持稳定的 Oncotype DX 预定率和化疗率,从而反映在特定临床实践中观察到的情况并减少因额外检测而导致的治疗延误。这些反射标准能够适当地捕获可能已经由提供商为其预定 Oncotype DX 且检测结果预计将影响对其的管理之患者。这种干预可作为其他大型综合性多学科肿瘤中心的潜在模型,以便制定各种以最有可能受益于基因组分析试验的患者群体为目标并减少治疗延误的流程。
Introduction
The advent of genomic assays has allowed for a more personalized approach to breast cancer treatment. Oncotype DX (Genomic Health, Inc., Redwood City, CA) is a 21‐gene assay that provides a 10‐year estimate for the risk of distant breast cancer recurrence and the benefit of chemotherapy for patients with early‐stage hormone receptor‐positive (HR+) and human epidermal growth receptor 2‐negative (HER2−) breast cancer [1]. Oncotype DX is widely used by oncologists to assist in adjuvant treatment decisions and is included in all major breast cancer clinical guidelines [2], [3], [4], [5]. It was also recently incorporated in the 8th edition of the American Joint Committee on Cancer (AJCC) Breast Cancer Staging Manual [6]. This is the first time a genomic platform has been included in a staging system, presenting new challenges for providers to obtain and use this information in an efficient and effective manner. Incorporating Oncotype DX testing into clinical practice has been shown to limit unnecessary chemotherapy use, leading to decreased patient morbidity and reduced health care costs [7], [8], [9]. However, additional testing frequently introduces delays into treatment decision‐making and chemotherapy initiation [10], [11], [12], [13], [14].
At the Dana‐Farber/Brigham and Women's Cancer Center (DF/BWCC), reflex Oncotype DX ordering criteria were developed through a multidisciplinary consensus process with input from medical oncologists, breast surgeons, and pathologists with the goal of identifying patients for whom the majority of clinicians would typically consider Oncotype DX testing to inform chemotherapy decisions. The goal of this “intervention” was to reduce treatment delays by having breast surgeons order the test immediately after the postoperative pathology results are available for patients ≤65 years of age with HR+/HER2− tumors who meet certain pathologic criteria: T1cN0 (grade II–III), T2N0 (grade I–II), or T1/T2N1 (grade I–II) disease. In prior work, we found thatthis intervention significantly decreased both the time in receiving Oncotype DX results postoperatively and the timefrom surgery to chemotherapy initiation by over 1 week [15].
Understanding which patients are most likely to benefit from Oncotype DX testing is paramount in balancing the test's benefits of reducing morbidity from unwarranted chemotherapy with the increased costs and delays that additional testing introduces into the breast cancer treatment timeline. However, the impact of reflex ordering criteria on Oncotype DX ordering practices and receipt of chemotherapy has not been widely studied. In this study, we examined whether our Oncotype DX reflex ordering criteria appropriately capture similar patient populations before and after intervention implementation and the impact on chemotherapy use. We evaluated Oncotype DX ordering practices and chemotherapy receipt before and after implementation of reflex criteria in order to explore whether reflex testing contributes to over‐ or under‐testing, to better understand how testing influences treatment decisions, and to inform further refinement of our reflex criteria.
Materials and Methods
Research Setting and Data Sources
DF/BWCC is a National Cancer Institute (NCI)‐designated comprehensive cancer center with over 4,000 unique new breast cancer patients annually. Our center employs a multidisciplinary clinic model in which the vast majority of patients with early‐stage invasive breast cancer are seen at initial consultation by both a medical and surgical oncologist. Patient sociodemographic and clinical characteristics and adjuvant chemotherapy regimens were obtained through manual retrospective chart review and data extraction from clinical information systems. Oncotype DX recurrence scores were obtained from Genomic Health for the period studied. Reflex ordering for Oncotype DX was implemented in January 2016, as described above. Patients >65 years of age were excluded from the reflex criteria for two main reasons: (a) to avoid unnecessary over‐testing given the somewhat different risk‐benefit considerations in older women when considering adjuvant chemotherapy, and (b) given issues with coverage and reimbursement in the immediate (14‐day) postoperative period among patients insured by Medicare. Medical oncologists could also elect to order Oncotype DX testing on any patient with characteristics outside of reflex criteria.
Because this study was a retrospective medical record review, it was determined to be exempt from review by the Dana‐Farber/Harvard Cancer Center Institutional Review Board.
Patient Cohorts
Female patients with HR+ (estrogen receptor‐positive or progesterone receptor‐positive) and HER2− breast cancer were eligible for study inclusion. During the postintervention period (February to November 2016), we identified 242 consecutive patients who had Oncotype DX ordered following definitive breast surgery. Overall, 10 patients were excluded for unilateral or bilateral primary breast cancer of mixed subtypes (HER2+/−), neoadjuvant therapy, medical oncology follow‐up at an outside hospital, if their current diagnosis was a localized recurrence within 3 years of initial diagnosis, or for an erroneous order of Oncotype DX. The final “post‐intervention cohort” included 232 patients. During the post‐intervention period, a total of 680 patients with HR+/HER2− breast cancer underwent surgery, serving as the denominator to calculate the percentage of patients who underwent Oncotype DX testing during this period.
We compared the post‐intervention cohort to a retrospective cohort of patients treated at our center prior to reflex Oncotype DX ordering, which included both patients who had Oncotype DX ordered and those who did not at the discretion of their medical oncology provider. This “preintervention” cohort consisted of 366 consecutive women who underwent definitive breast cancer surgery between October 2014 and March 2015. The faculty at our center was consistent during the period studied. One hundred patients were excluded for stage 0 cancer, receipt of neoadjuvant therapy, medical oncology follow‐up at an outside hospital, a simultaneous second primary cancer, or if their current diagnosis was a localized recurrence within 3 years of initial diagnosis. Thus, 266 patients were included in the pre‐intervention analytic cohort, 77 with Oncotype DX ordered and 189 without. Full inclusion and exclusion criteria for the pre‐ and post‐intervention cohorts are detailed in Figure 1.
Figure 1.
Inclusion and exclusion criteria. *, excluded patients in pre‐intervention cohort: 83 for stage 0 cancer, 8 for neoadjuvant therapy, 3 for a primary recurrence within 3 years, 4 for medical oncology follow‐up at an outside hospital, 2 for simultaneous second primary cancer. +, excluded patients in post‐intervention cohort: 4 for unilateral or bilateral cancer of different subtypes (i.e., one side HER2+ and other HER2−), 2 for neoadjuvant therapy, 2 for medical oncology follow‐up at an outside hospital, 1 for primary recurrence within 3 years, 1 for erroneous Oncotype DX order.
Abbreviations: DF/BWCC, Dana‐Farber/Brigham and Women's Cancer Center; HER2−, human epidermal growth receptor 2‐negative; HR+, hormone receptor‐positive.
Outcomes of Interest
Our primary outcomes of interest were Oncotype DX ordering (yes/no) and chemotherapy receipt (yes/no). Chemotherapy receipt was defined as having any standard of care chemotherapy regimen in the adjuvant setting, ascertained through medical record abstraction. Specific chemotherapy regimens were also captured.
Control Variables of Interest
Covariates of interest were those clinically relevant to treatment decisions and differed for each outcome examined, including age at the time of surgery (<50, 50–65, >65 years), insurance (private vs. public), race (white vs. nonwhite), AJCC 7th edition stage (I, II, III), grade (I, II, III), nodal status (0, 1–3, >3 positive nodes), single versus multifocal tumor, lymphovascular invasion (LVI; none, single focus, multiple), tumor size (≤2 cm vs. >2 cm), surgery type (lumpectomy vs. mastectomy), and Oncotype DX recurrence score (low <18, intermediate 18–30, high >30).
Statistical Analysis
We compared patient demographics and tumor characteristics among all patients with and without Oncotype DX testing using chi‐square analyses. The percentage of patients who received Oncotype DX testing in the pre‐ and post‐intervention cohorts was also compared using chi‐square testing. We then performed a multivariate logistic regression model to examine factors associated with chemotherapy use, examining patients in the pre‐ and post‐intervention cohorts who underwent Oncotype DX testing. Variables included age, insurance type, race, grade, nodal status, multifocal tumors, LVI, tumor size, Oncotype DX recurrence score, and time period (before vs. after intervention). Because stage was redundant with tumor size and nodal status and was not independently associated with outcomes, it was not included in the multivariate model. Patients whose nodal status was unknown were excluded from the model (n = 34). Adjusted odds ratios (ORs), 95% confidence intervals (CIs), and p values were calculated to ascertain the strength of association between each variable and receipt of chemotherapy. All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of Patients with and Without Oncotype DX Ordered
The pre‐intervention cohort included 266 patients treated at our center prior to reflex criteria implementation. In this group, 28.9% (n = 77) had Oncotype DX ordered and 71.1% (n = 189) did not (Fig. 1). The post‐intervention cohort included 232 patients who underwent Oncotype DX testing, among the 680 patients with HR+/HER2− cancers who were most appropriate for Oncotype DX testing (34.1% of patients). There was no statistical difference in the proportion of patients who had Oncotype DX testing in the pre‐ versus post‐intervention settings (p = .12). Among the postintervention patients, 63.8% (n = 148) met reflex criteria, whereas 36.2% (n = 84) had testing ordered by their medical oncologist.
The differences in characteristics between patients who had Oncotype DX ordered compared with those who did not in the combined pre‐ and post‐intervention cohorts are shown in Table 1. Age <50 years and 50–65 years (70.8% and 68.8% tested vs. 41.2% in those age >65 years, p < .0001) and private insurance (72.6% vs. 35.5% tested, p < .0001) were associated with Oncotype DX testing. Stage II tumors, grade 2 or 3 disease, tumors >2 cm, and patients with 1–3 positive lymph nodes were more likely to have Oncotype DX ordered (p < .001). Race, single versus multifocal cancer, and LVI were not significantly associated with Oncotype DX ordering.
Table 1. Characteristics of patients with and without Oncotype DX ordered (n = 498)a .

Patients without Oncotype DX ordered are from the pre‐intervention cohort only. Patients with Oncotype DX ordered are combined from pre‐ and post‐intervention cohorts.
p values generated from chi‐square analyses.
Bolded values are statistically significant (p < .05).
Node status and stage was unknown for 34 women.
Abbreviation: LVI, lymphovascular invasion.
Factors Associated with Oncotype DX Ordering Before and After Intervention
Age (≤65 years), private insurance, and having multiple foci of cancer were all significantly associated with increased Oncotype DX ordering in the post‐intervention versus pre‐intervention cohorts (Table 2). No significant difference in the distribution of Oncotype DX recurrence scores was observed between the pre‐ and post‐intervention cohorts (p = .27). Patient race, cancer stage, grade, nodal status, LVI, and tumor size were not associated with a significant change in Oncotype DX ordering between pre‐ and post‐intervention cohorts.
Table 2. Characteristics of all patients with Oncotype DX ordered before and after intervention (n = 309).

p values generated from chi‐square analyses.
Bolded values are statistically significant (p < .05).
Thirty‐four women did not undergo axillary lymph node dissection, so node positivity and stage are unknown.
Abbreviation: LVI, lymphovascular invasion.
Factors Associated with Chemotherapy Receipt After Oncotype DX Testing
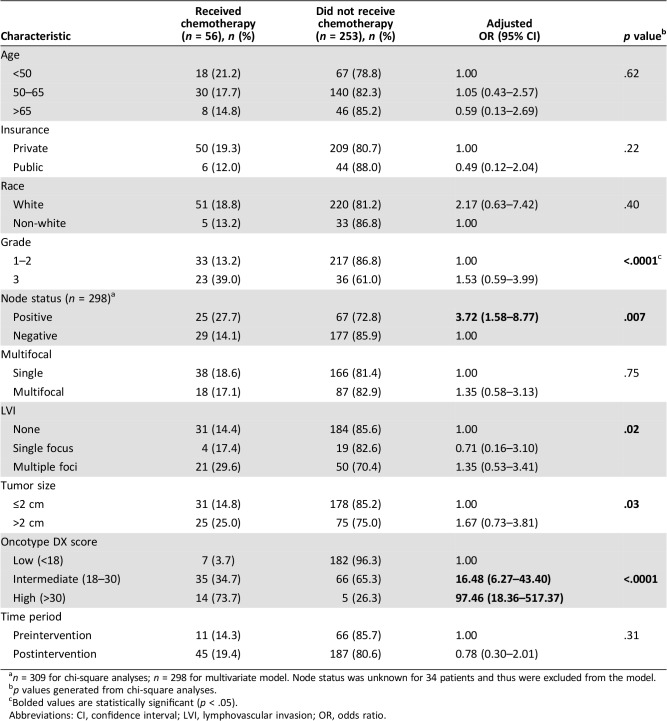
The multivariate analysis in Table 3 highlights factors associated with adjuvant chemotherapy receipt among all patients who had Oncotype DX ordered. Among the 309 patients with Oncotype DX ordered, 56 (18.1%) received adjuvant chemotherapy, including 11 (14.3%) patients from the pre‐intervention cohort and 45 (19.4%) patients from the post‐intervention cohort (p = .31). Patients with node‐positive disease (OR = 3.72, 95% CI = 1.58–8.77) and intermediate (18–30) and high (>30) Oncotype DX recurrence scores (OR = 16.48, 95% CI = 6.27–43.30; OR = 97.46, 95% CI = 18.36–517.37) were significantly more likely to receive chemotherapy. No difference was observed in the proportion of patients who received chemotherapy in the pre‐ or post‐intervention settings (p = .31). Additionally, in the pre‐intervention cohort, 16.9% of patients who did not have Oncotype DX ordered received chemotherapy (Fig. 1). Notably, among the 309 patients who had Oncotype DX ordered, 72.8% of patients with positive lymph nodes and 61.0% of patients with grade 3 cancers did not receive chemotherapy.
Table 3. Multivariate logistic regression evaluating factors associated with chemotherapy receipt after Oncotype DX testing (n = 298)a .
n = 309 for chi‐square analyses; n = 298 for multivariate model. Node status was unknown for 34 patients and thus were excluded from the model.
p values generated from chi‐square analyses.
Bolded values are statistically significant (p < .05).
Abbreviations: CI, confidence interval; LVI, lymphovascular invasion; OR, odds ratio.
Among the 11 pre‐intervention patients who received chemotherapy after Oncotype DX ordering, 54.5% (n = 6) received doxorubicin + cyclophosphamide (AC), 27.3% (n = 3) received docetaxel + cyclophosphamide (TC), and 18.2% (n = 2) received doxorubicin + cyclophosphamide + paclitaxel (ACT). Among the 45 post‐intervention patients who received chemotherapy after Oncotype DX ordering, 17.8% (n = 8) received AC, 44.4% (n = 20) received TC, 33.3% (n = 15) received ACT, and 4.4% (n = 2) received an alternate regimen (cyclophosphamide+methotrexate+5‐FU and carboplatin + paclitaxel).
Discussion
In this contemporary analysis of 498 patients in a single, high‐volume comprehensive cancer center, we examined factors associated with Oncotype DX ordering and adjuvant chemotherapy administration before and after implementation of reflex criteria for Oncotype DX testing. Because of our prespecified reflex criteria, patients ≤65 years of age and those with private insurance were more likely to have Oncotype DX ordered after intervention versus before intervention. Interestingly, among patients who underwent Oncotype DX testing, recurrence score was the factor most significantly associated with chemotherapy use, suggesting that once this test is sent, it is often the deciding factor in chemotherapy decisions over any other patient or tumor variable. This is consistent with a large, prospective study in the Netherlands that demonstrated that the use of gene expression profiling changed chemotherapy treatment decisions in 51% of patients who underwent testing [16]. Additionally, in the recent TAILORx study, which demonstrated that patients with small, node‐negative tumors with moderate risk Oncotype DX recurrence scores can safely forego chemotherapy [17], further highlighting the power of gene expression profiling in chemotherapy decision‐making and the advantage of a streamlined ordering process to make efficient and informed clinical decisions. Our results confirm the utility of reflex criteria testing, as we previously established that this intervention results in shorter times to chemotherapy initiation for those receiving treatment [15], and our current work presented here demonstrates the importance of genomic assay testing in chemotherapy decision‐making, with most women being spared chemotherapy.
Reassuringly, similar proportions of patients received adjuvant chemotherapy before and after the intervention. These results demonstrate that our multidisciplinary consensus criteria for Oncotype DX ordering are reflective of our center's clinical practice, appropriately capturing the patient population for whom oncologists would have otherwise ordered Oncotype DX testing, but doing so faster. This will be particularly important as Oncotype DX becomes increasingly pivotal in cancer staging [6]. Moreover, the percentage of patients who received Oncotype DX testing before and after implementation of reflex criteria was not significantly different, demonstrating that use of reflex criteria did not lead to overuse of testing. Rather, our findings suggest that the selected testing criteria aided clinicians in more efficiently providing care that reflects their clinical judgment. A recent study at an NCI‐designated cancer center also found that implementing guideline‐directed early Oncotype DX ordering maintained a stable chemotherapy rate, and found that overall patient costs were not appreciably different before and after their intervention, further supporting that reflex criteria are an effective method to streamline Oncotype DX ordering [18].
Of note, the chemotherapy rate observed in patients with Oncotype DX testing was lower in our study versus previously published reports [3], [19]. This likely represents an evolving practice away from chemotherapy use for most patients with HR+/HER2− disease, as comfort with Oncotype DX testing improves and the benefits of chemotherapy are increasingly felt to be limited to a small proportion of these patients. An example of this was observed in our model of chemotherapy receipt. Although positive nodal status was significantly associated with receiving adjuvant chemotherapy, 72.8% of patients with node‐positive disease who had Oncotype DX ordered did not receive chemotherapy. This suggests that Oncotype DX may also be a valuable tool in sparing many lymph node‐positive patients from chemotherapy, as suggested by some guidelines [20]. Clinical practice will be informed by the forthcoming results of the “RxPONDER” trial (Rx for Positive Node, Endocrine Responsive Breast Cancer; NCT01272037), which seeks to prospectively evaluate the utility of Oncotype DX testing in node‐positive disease and the benefits of chemotherapy.
Prior studies have also attempted to target the patients most likely to benefit from Oncotype DX testing using clinical and demographic criteria [21], [22], [23], [24]. Kim et al. developed a model that predicted Oncotype DX risk category in 52.5% of cases and then modeled how ordering practices might change after broad implementation of their prediction strategy [21]. They projected that, if widely implemented, their risk prediction model could identify a group of patients for whom Oncotype DX ordering was likely to contribute information beyond standard clinicopathologic data. However, there is very little literature studying the outcome of implementing such prediction strategies into clinical practice, and we are unaware of any literature that has evaluated the criteria used for reflex or early testing strategies.
Through our study, we identified potential ways to revise reflex criteria for Oncotype DX ordering in our practice. When comparing patients who had Oncotype DX testing before and after intervention, patients with multifocal cancers were more likely to have Oncotype DX ordered after intervention, a factor not addressed by reflex criteria, thus highlighting a variable that providers found important in treatment decisions and a possible area where reflex criteria could be broadened. In addition, among patients with grade 3 cancers who underwent Oncotype DX testing, 61.0% did not receive chemotherapy. Increasing the use of reflex testing in the setting of high‐grade cancers (where the majority will have low or intermediate scores [25]) is an area of active discussion within our center.
We recognize several limitations to this study. First, this was a single‐center, retrospective study, and we acknowledge that practice patterns may not be widely generalizable. However, we feel that implementation of reflex testing and its outcomes thus far are of larger interest and could have broader implications if the time to chemotherapy initiation could be influenced on a wider scale. Second, because the number of patients receiving chemotherapy was low, despite a large sample size, results for some subgroups yielded wide confidence intervals and some categories were collapsed, such as grade, because of small sample sizes. However, the factors that were significant, such as high Oncotype DX recurrence scores, had markedly positive ORs, still providing valuable information regarding the role of these variables in chemotherapy initiation. Third, we could not ascertain whether recurrence and survival rates changed between the pre‐ and post‐intervention cohorts and/or if chemotherapy was administered appropriately, due to the short follow‐up times available with our recent implementation of the reflex ordering process; however, we will examine this in future studies. Finally, we recognize that our inclusion criteria for the pre‐ and post‐intervention cohorts were not identical, given that some of the patients in the pre‐intervention cohort did not have Oncotype DX testing. However, we were still able to examine factors associated with chemotherapy receipt in all patients included.
Conclusion
Despite these limitations, we demonstrated that implementing multidisciplinary consensus reflex criteria for Oncotype DX ordering maintains a stable Oncotype DX ordering rate and chemotherapy rate, mirroring what we observe in our specific clinical practice, while decreasing treatment delays due to additional testing [15]. Our reflex criteria appropriately capture a population of women who would likely have had Oncotype DX ordered by their providers and for whom the results of this test are predicted to influence management. This intervention serves as a potential model for other large integrated, multidisciplinary oncology centers to implement institutional processes to target the patient population most likely to benefit from genomic assay testing, while mitigating treatment‐delays.
Acknowledgments
We thank all the patients who contributed to our database.
Footnotes
For Further Reading: Maria Vittoria Dieci, Valentina Guarneri, Tommaso Giarratano et al. First Prospective Multicenter Italian Study on the Impact of the 21‐Gene Recurrence Score in Adjuvant Clinical Decisions for Patients with ER Positive/HER2 Negative Breast Cancer. The Oncologist 2018;23:297–305.
Implications for Practice: This study shows that, although a high proportion of patients were recommended to receive endocrine treatment alone before knowing the recurrence score (RS) assay, the RS test further contributed in sparing chemotherapy for some of these patients, especially in case of the N1 stage or for patients enrolled at referral centers. These data highlight the need for further work in collaboration with health authorities and companies in order to define strategies for the implementation of the use of RS testing in clinical practice in the Italian setting.
Author Contributions
Conception/design: Katya Losk, Tari A. King, Nancy U. Lin, Mehra Golshan, Craig A. Bunnell, Rachel A. Freedman
Provision of study material or patients: Katya Losk, Tari A. King, Nancy U. Lin, Mehra Golshan, Stephen Pochebit, Craig A. Bunnell, Rachel A. Freedman
Collection and/or assembly of data: Kelsey H. Natsuhara, Katya Losk, Tari A. King, Nancy U. Lin, Kristen Camuso, Stephen Pochebit, Craig A. Bunnell, Rachel A. Freedman
Data analysis and interpretation: Kelsey H. Natsuhara, Katya Losk, Tari A. King, Nancy U. Lin, Kristen Camuso, Mehra Golshan, Jane E. Brock, Craig A. Bunnell, Rachel A. Freedman
Manuscript writing: Kelsey H. Natsuhara, Katya Losk, Tari A. King, Nancy U. Lin, Kristen Camuso, Mehra Golshan, Stephen Pochebit, Jane E. Brock, Craig A. Bunnell, Rachel A. Freedman
Final approval of manuscript: Kelsey H. Natsuhara, Katya Losk, Tari A. King, Nancy U. Lin, Kristen Camuso, Mehra Golshan, Stephen Pochebit, Jane E. Brock, Craig A. Bunnell, Rachel A. Freedman
Disclosures
Katya Losk: Genomic Health (H); Tari A. King: Genomic Health (H); Nancy U. Lin: Genentech, Cascadia (RF); Mehra Golshan: Abbvie (C/A); Rachel A. Freedman: Eisai, Puma (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Paik S, Shak S, Tang G et al. A multigene assay to predict recurrence of tamoxifen‐treated, node‐negative breast cancer. N Engl J Med 2004;351:2817–2826. [DOI] [PubMed] [Google Scholar]
- 2.Gradishar WJ, Anderson BO, Balassanian R et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:324–354. [DOI] [PubMed] [Google Scholar]
- 3.Hassett MJ, Silver SM, Hughes ME et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 2012;30:2218‐2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2015;26(suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 5.Harris L, Fritsche H, Mennel R et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287–5312. [DOI] [PubMed] [Google Scholar]
- 6.Giuliano AE, Connolly JL, Edge SB et al. Breast cancer‐Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290–303. [DOI] [PubMed] [Google Scholar]
- 7.Kip M, Monteban H, Steuten L. Long‐term cost‐effectiveness of Oncotype DX(R) versus current clinical practice from a Dutch cost perspective. J Comp Eff Res 2015;4:433–445. [DOI] [PubMed] [Google Scholar]
- 8.Katz G, Romano O, Foa C et al. Economic impact of gene expression profiling in patients with early‐stage breast cancer in France. PLoS One 2015;10:e0128880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry NL, Somerfield MR, Krop IE. Role of patient and disease factors in adjuvant systemic therapy decision making for early‐stage, operable breast cancer: American Society of Clinical Oncology endorsement of Cancer Care Ontario Guideline Recommendations Summary. J Oncol Pract 2016;12:482–484. [DOI] [PubMed] [Google Scholar]
- 10.Losk K, Vaz‐Luis I, Camuso K et al. Factors associated with delays in chemotherapy initiation among patients with breast cancer at a comprehensive cancer center. J Natl Compr Canc Netw 2016;14:1519–1526. [DOI] [PubMed] [Google Scholar]
- 11.Liederbach E, Sisco M, Wang C et al. Wait times for breast surgical operations, 2003‐2011: A report from the National Cancer Data Base. Ann Surg Oncol 2015;22:899–907. [DOI] [PubMed] [Google Scholar]
- 12.Bleicher RJ, Ruth K, Sigurdson ER et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol 2016;2:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saint‐Jacques N, Younis T, Dewar R et al. Wait times for breast cancer care. Br J Cancer 2007;96:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandergrift JL, Niland JC, Theriault RL et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst 2013;105:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losk K, Freedman RA, Lin NU et al. Implementation of surgeon‐initiated gene expression profile testing (Onco type DX) among patients with early‐stage breast cancer to reduce delays in chemotherapy initiation. J Oncol Pract 2017;13:e815–e820. [DOI] [PubMed] [Google Scholar]
- 16.Kuijer A, Straver M, den Dekker B et al. Impact of 70‐gene signature use on adjuvant chemotherapy decisions in patients with estrogen receptor‐positive early breast cancer: Results of a prospective cohort study. J Clin Oncol 2017;35:2814–2819. [DOI] [PubMed] [Google Scholar]
- 17.Sparano JA, Gray RJ, Makower DF et al. Adjuvant chemotherapy guided by a 21‐gene expression assay in breast cancer. N Engl J Med 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzimitrowicz H, Mougalian S, Storms S et al. Impacts of early guideline‐directed 21‐gene recurrence score testing on adjuvant therapy decision making. J Oncol Pract 2017;13:e1012–e1020. [DOI] [PubMed] [Google Scholar]
- 19.Friese CR, Li Y, Bondarenko I et al. Chemotherapy decisions and patient experience with the recurrence score assay for early‐stage breast cancer. Cancer 2017;123:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology, Breast Cancer (Version 3.2017). Available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 25, 2018.
- 21.Kim HS, Umbricht CB, Illei PB et al. Optimizing the use of gene expression profiling in early‐stage breast cancer. J Clin Oncol 2016;34:4390–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner BM, Skinner KA, Tang P et al. Use of modified Magee equations and histologic criteria to predict the Oncotype DX recurrence score. Mod Pathol 2015;28:921–931. [DOI] [PubMed] [Google Scholar]
- 23.Gage MM, Rosman M, Mylander WC et al. A validated model for identifying patients unlikely to benefit from the 21‐gene recurrence score assay. Clin Breast Cancer 2015;15:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein ME, Dabbs DJ, Shuai Y et al. Prediction of the Oncotype DX recurrence score: Use of pathology‐generated equations derived by linear regression analysis. Mod Pathol 2013;26:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik S, Tang G, Shak S et al. Gene expression and benefit of chemotherapy in women with node‐negative, estrogen receptor‐positive breast cancer. J Clin Oncol 2006;24:3726–3734. [DOI] [PubMed] [Google Scholar]