Immune checkpoint inhibitors are associated with a unique profile of adverse events that can affect many organ systems. One of them is immune‐mediated pneumonitis — an uncommon adverse event that might be life threatening. Up to date, no consensus exists regarding immunotherapy rechallenge in these cases. This article evaluates the clinical and radiological characteristics of patients with metastatic melanoma who experienced recurrent immune‐related pneumonitis with or without treatment rechallenge.
Keywords: Immune checkpoint inhibitors, Immune‐related adverse events, Pneumonitis, Recurrent pneumonitis
Abstract
Introduction.
Immune checkpoint inhibitors (ICIs) have changed the oncologic landscape in the past few years. Alongside impressive antitumor responses, new novel immune‐related adverse events (irAEs) have emerged; pneumonitis is an irAE that can potentially be fatal and necessitates a proper management. No consensus exists regarding steroid treatment duration or drug rechallenge options. Our study describes the clinical and radiological course of melanoma patients diagnosed with immune‐related pneumonitis that has recurred because of rechallenge attempt or despite complete treatment discontinuation (unprovoked).
Materials and Methods.
The study population was composed of patients with metastatic melanoma who were treated with anti‐programmed cell death 1 (PD‐1) as monotherapy or in combination with anti‐cytotoxic T lymphocyte antigen‐4 and who were diagnosed with immune‐related pneumonitis. For recurrent cases after clinical and radiological resolution, we explored the differences from cases with no recurrence.
Results.
Nineteen out of 386 (4.8%) patients treated with ICI were diagnosed with pneumonitis. Median age was 66 years, and 53% were male. Compared with single‐agent nivolumab, patients treated with ipilimumab‐nivolumab combination presented with an earlier onset (27.5 vs. 10.3 weeks, respectively, p = .015) and had higher grades of severity. After complete resolution, rechallenge was attempted in seven patients; three of them had recurrent pneumonitis. Three other patients experienced recurrent pneumonitis despite complete discontinuation of the drug (unprovoked by rechallenge). The latter were characterized with an earlier onset of the first pneumonitis compared with those who did not experience recurrence (median, 12.4 vs. 26.4 weeks) and a shorter course of steroid treatment at first episode (median, 5.1 vs. 10 weeks). Recurrent cases were generally more severe than the first episode.
Conclusion.
Unprovoked recurrent pneumonitis is a new, poorly reported entity that requires further investigation. Our observations suggest that cases of pneumonitis that present early in the course of immunotherapy treatment may recur despite treatment discontinuation, thus necessitating closer monitoring and a longer course of steroid treatment.
Implications for Practice.
This article sheds light on a poorly described immune‐related adverse event: recurrent pneumonitis despite complete discontinuation of immunotherapy (unprovoked), in patients with advanced melanoma.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the oncologic landscape in the past few years. Impressive antitumor responses have been reported, especially in melanoma, hematologic malignancies, and tumors with high microsatellite instability, as well as in non‐small cell lung cancer, renal cell carcinoma, bladder cancer, and head and neck squamous cell carcinoma. The anti‐cytotoxic T lymphocyte antigen (CTLA)‐4 monoclonal antibody, ipilimumab, was the first immune checkpoint inhibitor approved by the U.S. Food and Drug Administration (FDA) for the treatment of metastatic melanoma [1], [2]. Not long after, agents that block the programmed cell death (PD)‐1 protein (pembrolizumab and nivolumab) [3], [4], [5] and the PD‐ligand‐1 protein (atezolizumab, durvalumab, and avelumab) were also approved by the FDA for various cancer indications after showing efficacy and safety profiles superior to standard therapy [6], [7], [8]. A combination of ipilimumab and nivolumab was approved for the treatment of both metastatic melanoma and renal cell carcinoma (RCC), demonstrating improved response rate, progression free survival [9], [10], [11], and, for RCC, also overall survival over standard of care treatment [12].
ICIs are associated with a unique profile of adverse events, called immune‐related adverse events (irAEs). irAEs can affect a wide spectrum of organ systems, often several organs simultaneously. Immune‐mediated pneumonitis is an inflammatory insult to lung parenchyma [13], [14], [15], [16], [17], [18], [19] that may lead to a permanent treatment discontinuation and was documented as a cause of treatment‐related death [20], [21], [22], [23]. It is an uncommon adverse event, with an incidence of 1%–4% in patients treated with single‐agent anti‐PD‐1 [11], [22] and higher with dual checkpoint blockade (anti‐PD‐1 and anti‐CTLA‐4), reaching up to 10.6%. A pooled analysis on selected immune‐related adverse events (AEs) in ipilimumab‐nivolumab‐treated patients with melanoma reports a pneumonitis incidence of 6.9% all grade and 1.3% grade 3–4, resulting in permanent treatment discontinuation in 2.2% of patients [24].
The onset of pneumonitis during the course of treatment depends on the regimen; it typically develops 2.8 months following single‐agent anti‐PD‐1 administration, and earlier with ipilimumab‐nivolumab combination at 9.4 weeks (range, 3.7–20.6) [24]. The clinical presentation is variable. Most cases present no symptoms and the diagnosis is radiological (grade 1). Some present mild to moderate symptoms such as dyspnea, cough, and chest pain and are treated with oral corticosteroids in an outpatient setting (grade 2), whereas others require hospitalization, intraveneous (IV) corticosteroids, and other immune‐modulators (grades 3–4) [25], [26], [27], [28].
The optimal immunosuppressive choice, doses, and duration have not been fully studied. Usually, corticosteroids are continued until complete symptom resolution, with a slow tapering down over at least 4 weeks in order to prevent pneumonitis flare‐up. Furthermore, there is no consensus regarding treatment rechallenges. There have been reports of recurrent pneumonitis in patients in whom drug rechallenge was attempted after symptoms had resolved and corticosteroid treatment was concluded [29]. Those recurrent cases were of a higher grade of severity, yet were still manageable with a longer course of corticosteroid treatment. There is even less data in the literature on cases of recurrent pneumonitis despite complete discontinuation of immunotherapy and proper corticosteroid treatment duration (unprovoked recurrent pneumonitis).
The aim of this study was to evaluate the clinical and radiological characteristics of metastatic melanoma patients experiencing recurrent immune‐related pneumonitis, whether provoked by treatment rechallenge, or unprovoked.
Subjects, Materials, and Methods
Patients
The study population was composed of patients with metastatic melanoma who were treated with anti‐PD‐1 as monotherapy or in combination with anti‐CTLA‐4 at the Ella Lemelbaum Institute for Immuno‐Oncology between May 2013 and August 2017 and had a recorded diagnosis of pneumonitis related to immunotherapy. The diagnosis of immune‐related pneumonitis was based on typical clinical features and on new typical imaging changes such as ground glass opacities in chest computed tomography (CT) scan. Some patients were diagnosed based only on new typical radiologic changes, without respiratory symptoms. None of the patients presented with fever >38.3°C or productive cough, suggestive of pneumonia. Because all cases had complete resolution after steroid treatment, there was no need to further rule out infectious causes or to perform invasive procedures such as bronchoalveolar lavage of lung biopsy [27], [28]. Clinical severity of pneumonitis was classified according to the common terminology criteria for adverse events (CTCAE) version 4.03. For each patient, the following data were collected: demographics, current and past treatment regimens and exposure, nonpneumonitis irAE (the affected system and its grade of severity), and tumor response to treatment according to routine radiologic evaluations; clinical features of pneumonitis collected and analyzed were time of onset, predominating symptoms, severity, treatment, and outcomes (clinical and radiologic). Recurrent pneumonitis was defined as an additional episode of pneumonitis, after the previous one had a complete clinical and radiological resolution. Recurrent pneumonitis cases were further subcategorized as either provoked by treatment renewal or unprovoked.
Imaging
A radiological review of all serial CT scans of patients with pneumonitis was performed by an experienced chest radiologist, blinded to the clinical data. Scans prior to the diagnosis of pneumonitis, at diagnosis, and all subsequent scans were evaluated. Description of the radiologic characteristics was done using classification methods previously published [30]. Briefly, this method included the dominant radiographic pattern, distribution and topography of parenchymal damage, and a severity assessment according to the involved lung volume (mild <25%, moderate 25%–50%, severe >50%). Cases of recurrent pneumonitis were similarly described. Patterns and severity of recurrent events were compared with the first event.
Statistical Analysis
Descriptive statistics were used to describe patient characteristics and clinical as well as radiological features of their pneumonitis. For quantitative variables, we utilized median values and their relative ranges, especially in cases with right skewed distribution. For nominal variables we utilized frequencies. Differences among quantitative variables were evaluated using the nonparametric Mann‐Whitney U test because of small sample sizes. For similar reasons, the Fisher's exact test was used to evaluate differences among categorical variables. Statistical significance was defined as p ≤ .05 level, and all tests were two‐sided.
Ethics
This single‐center, retrospective medical records study was approved by the Institutional Review Board of the Sheba Medical Center (4387‐17‐SMC).
Results
General Clinical Features
We identified 386 patients with metastatic melanoma who were treated with ICIs, of whom 336 patients (87.2%) were treated with monotherapy anti‐PD‐1 mAb, and 50 patients (12.7%) were treated with the combination ipilimumab‐nivolumab. Nineteen patients (4.8%) developed immune‐related pneumonitis; of these, 14 were treated with a single‐agent anti‐PD‐1 mAb (4 with nivolumab and 10 with pembrolizumab), and 5 received the combination ipilimumab‐nivolumab. This reflects an incidence rate of 4.1% and 10% for monotherapy and combination therapy, respectively. No specific risk factor was identified, because 94.7% of the study population did not have an underlying lung condition, and only one patient was a past smoker. Among the patients who developed pneumonitis, in 12 (63%) it occurred during first‐line treatment, in 6 (31.6%) it occurred during second‐line treatment, and in one patient, during third‐line of treatment. Seven of the 19 patients (37%) developed pneumonitis as their sole AE, whereas 12 (63%) patients had at least one additional irAE: rash (4), vitiligo (4), psoriasis (2), hepatitis (2), nephritis (2), colitis (1), arthritis (1), thyroiditis (1), parotitis (1), or diabetes (1). Most of the pneumonitis cases (69%, 13 patients) were of grade 1–2. The overall response rate to immunotherapy in these patients was 58%, with disease control rate of 79%. For further basic clinical characteristics, treatment, and outcomes see Table 1.
Table 1. Patient and treatment characteristics.
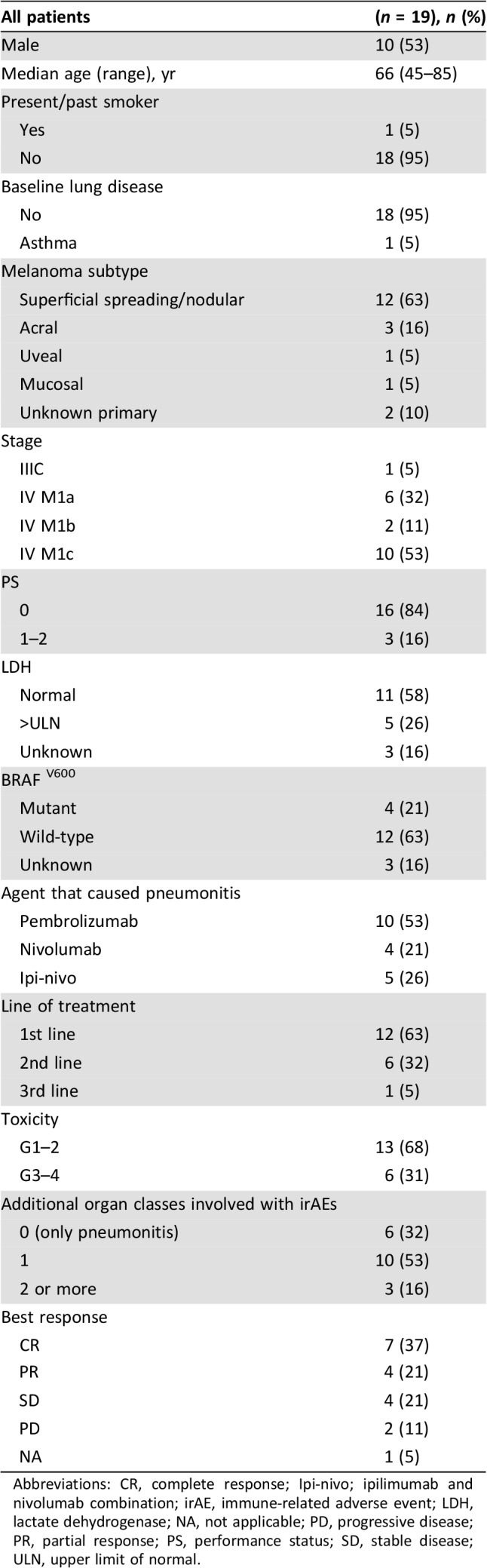
Abbreviations: CR, complete response; Ipi‐nivo; ipilimumab and nivolumab combination; irAE, immune‐related adverse event; LDH, lactate dehydrogenase; NA, not applicable; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease; ULN, upper limit of normal.
Clinical Features of Pneumonitis
Median onset of pneumonitis for all 19 patients was at 18.1 weeks. In patients treated with anti‐PD‐1 monotherapy, the onset was after a median of 27.5 weeks of treatment, with no significant difference in onset between nivolumab (26.5 weeks) and pembrolizumab (27.5 weeks). However, the onset was significantly earlier in patients treated with the combination ipilimumab‐nivolumab (10.3 weeks, p = .015). The diagnosis of pneumonitis was exclusively radiological in 7 asymptomatic patients (37%) whereas 10 patients (53%) presented with mild to moderate symptoms such as cough and shortness of breath. Two patients (10.5%) presented with severe hypoxemic pneumonitis.
In addition to treatment discontinuation, 14 patients (73.6%) were also treated with corticosteroids until clinical and radiological resolution; 12 were treated orally—9 patients at the dose of 1 mg/kg and 5 at the dose of 0.5 mg/kg; the 2 patients with severe pneumonitis were hospitalized for IV corticosteroids. Median time on corticosteroid treatment was 6 weeks for patients treated with anti‐PD‐1 and 13.6 weeks for patients treated with ipilimumab‐nivolumab combination (p = .49). Three patients in the ipilimumab‐nivolumab group could not taper down treatment: two because of spinal cord compression caused by overt and ultimately fatal disease progression, and one because of respiratory exacerbations; this patients has remained asymptomatic on 2.5 mg of prednisolone for the past two years. None of the patients required other immunomodulators or further evaluation of other etiologies with invasive procedures. For further basic clinical details of pneumonitis, see Table 2. The severity of the pneumonitis, expressed by CTCAE grading, did not correlate with response to the antineoplastic treatment.
Table 2. Clinical characteristics of pneumonitis according to treatment.
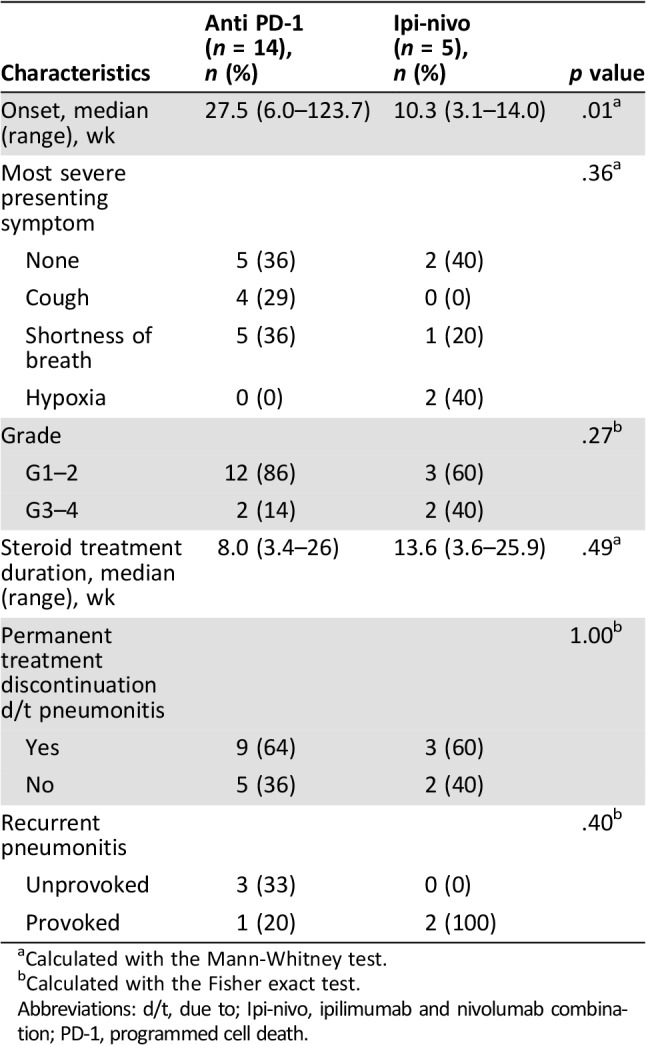
Calculated with the Mann‐Whitney test.
Calculated with the Fisher exact test.
Abbreviations: d/t, due to; Ipi‐nivo, ipilimumab and nivolumab combination; PD‐1, programmed cell death.
Imaging Characteristics
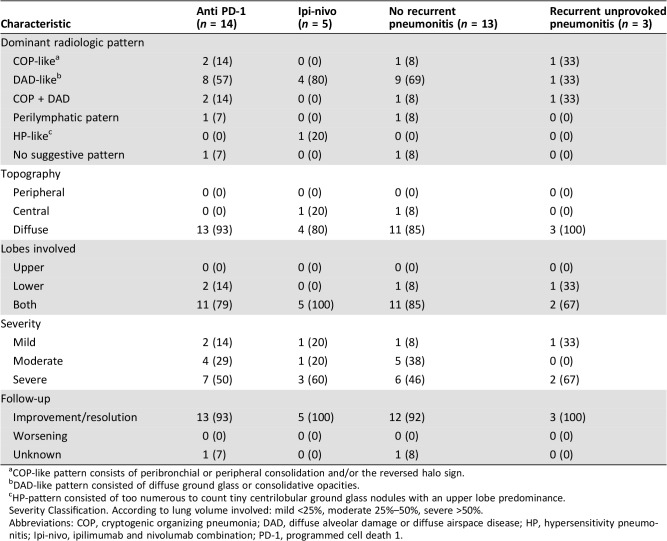
The most common radiologic pattern on CT was the diffuse alveolar damage‐like pattern, which consisted of diffuse ground glass or consolidative opacities. This was seen in 57% of patients treated with anti‐PD‐1 and in 80% of patients treated with the combination ipilimumab‐nivolumab. In the majority of patients (80%–93%), the topography of lung damage was diffuse rather than peripheral or central, involving both upper and lower lobes. Fifty three percent of patients had CT finding defined as severe according to the radiologic classification of severity. Radiologic improvement or complete resolution of pneumonitis was verified in subsequent scans in 18 (95%) patients (an example in Fig. 1). Postpneumonitis follow‐up scans were not available for one patient (5%), yet a clinical improvement was documented in his medical records. Full radiologic characteristics are detailed in Table 3.
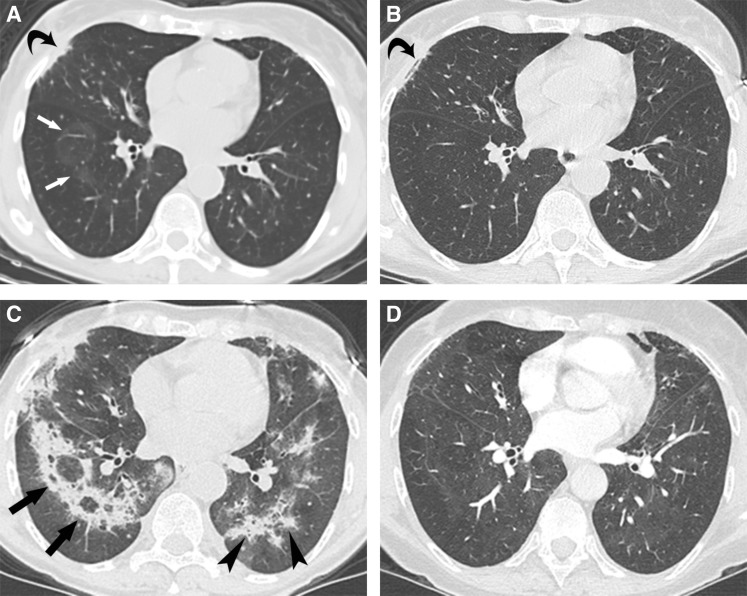
Figure 1.
Computed tomography (CT) scans at the level of the right pulmonary vein of a 70‐year‐old patient with unprovoked recurrent pneumonitis after nivolumab treatment. (A): At the time of clinical symptoms of grade 2 pneumonitis, 22 weeks after treatment initiation, nodular opacities with a ring of soft tissue and clear center (the reversed halo sign) appeared, an imaging finding that is typical for cryptogenic organizing pneumonia (arrow). This was classified as mild changes, affecting less than 25% of lung parenchyma. Curved arrow points at radiation changes from axillary radiotherapy delivered 20 years earlier. (B): A CT scan at the same level 5 weeks after (A) following steroid therapy shows resolution of the pulmonary opacities. Clinical symptoms of pneumonitis resolved and thus steroids were discontinued. (C): A CT scan performed 8 weeks following (B), with recurrence of pneumonitis symptoms, now grade 3, shows extensive consolidations, some in the reversed halo pattern (arrow) and others in a peribronchial distribution (arrowhead), both typical imaging findings in cryptogenic organizing pneumonia. This was classified as severe changes, affecting more than 50% of the lung parenchyma. (D): A CT scan performed 14 weeks following (C), after 14 weeks of steroid treatment, shows resolution of the pulmonary opacities. Clinical symptoms resolved as well.
Table 3. Radiological characteristics.
COP‐like pattern consists of peribronchial or peripheral consolidation and/or the reversed halo sign.
DAD‐like pattern consisted of diffuse ground glass or consolidative opacities.
HP‐pattern consisted of too numerous to count tiny centrilobular ground glass nodules with an upper lobe predominance.
Severity Classification. According to lung volume involved: mild <25%, moderate 25%–50%, severe >50%.
Abbreviations: COP, cryptogenic organizing pneumonia; DAD, diffuse alveolar damage or diffuse airspace disease; HP, hypersensitivity pneumonitis; Ipi‐nivo, ipilimumab and nivolumab combination; PD‐1, programmed cell death 1.
Three out of the 19 patients with pneumonitis had radiotherapy to axillary nodes several months prior to the diagnosis of pneumonitis (specifically 6, 8, and 18 months); all three were treated with 48Gy in 20 fractions of 2.4Gy each; however, only a small portion of the lung was included within the radiation field—calculated V20 values were 9%, 18%, and 25%.
In all three cases, postradiation changes were noticed on CT scans and were limited to radiation fields, whereas the radiological diagnosis of immune‐related pneumonitis was multifocal, involving also nonirradiated lung volumes. The temporal relation to immunotherapy fits the diagnosis of immunotherapy induced pneumonitis, although the pathogenetic contribution of a recall radiation‐induced pneumonitis, although extremely rare, cannot be fully excluded.
Recurrence of Pneumonitis
All 19 patients diagnosed with pneumonitis had completely recovered with complete clinical and radiological resolution. Rechallenge of immunotherapy was attempted in seven patients. In six of them, rechallenge was decided by the treating physician, based on clear clinical benefit from immunotherapy and only mild to moderate grade pneumonitis. One patient achieved a near‐complete response, three experienced partial response, and two experienced disease stabilization. In the seventh patient, the decision to rechallenge despite grade 4 pneumonitis was made by a multidisciplinary team because of high disease burden with no other therapeutic option.
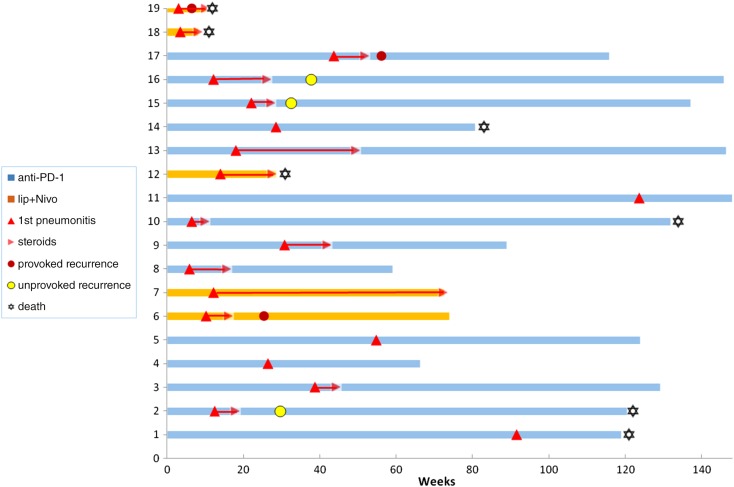
After a median follow‐up of 14 months (18.8 for anti‐PD‐1, and 7 for combination) six patients (31.6%) experienced recurrence of pneumonitis. In three patients (15.8%), the recurrence was attributed to the reintroduction of immunotherapy (one patient with pembrolizumab, one with nivolumab, and one with combination ipilimumab‐nivolumab), and in the other three patients (15.8%), the pneumonitis recurred despite complete discontinuation of immunotherapy (unprovoked). Of the three patients with previous radiation to the axilla, one had developed recurrent pneumonitis, contralateral to the irradiated axilla, which we attribute to the reintroduction of pembrolizumab. The severity of the recurrent pneumonitis was generally higher than in the first event, with two patients experiencing grade 3 and one experiencing grade 5. All recurrent episodes of pneumonitis were treated with corticosteroids; four patients were treated with oral corticosteroids and two required hospitalization and IV steroids, one of whom was hospitalized with severe respiratory distress secondary to reintroduction of nivolumab, which was fatal. This patient suffered from high disease burden, and his first event of pneumonitis was also severe (grade 4). The remaining five patients had complete resolution of pneumonitis and did not require other immunomodulators. One patient, however, experienced a second unprovoked recurrence of pneumonitis, 2 months after completion of prednisone treatment. This second recurrence was treated with IV steroids with a slower tapering down, resulting once again in complete resolution.
In terms of imaging characteristics, all recurrent cases presented with worsening of the same radiographic pattern of the first event. No specific imaging pattern distinguished the recurrent cases from the nonrecurrent ones (Table 3).
The subset of patients who experienced unprovoked recurrent pneumonitis (3 patients) was further characterized (see Table 4): in all three, the first event of pneumonitis was related to monotherapy anti‐PD‐1 and occurred relatively early, at 12.4 weeks, whereas in the 13 patients who did not develop a second pneumonitis, the first event tended to present later, at a median of 26.4 weeks (p = .08). The duration of steroid treatment in the first event was shorter in that group (median 5.1 weeks), whereas the patients who did not experience recurrence were treated for a longer period (median 10 weeks). There were no differences in the number of additional organ classes affected by immune‐related adverse events between the two groups, and the severity of the first event of pneumonitis did not seem to correlate with the probability of developing an unprovoked recurrent event. The three patients developed the unprovoked pneumonitis at a median of 9.6 weeks after completion of steroid weaning, with a higher severity compared with the first event. See Figure 2 for individual patient depiction of onset of pneumonitis and recurrences. Among those three cases, tumor responses to antineoplastic therapy were as follows: one of the patients was treated with nivolumab, and after 23 weeks treatment was permanently interrupted because of pneumonitis. She achieved a complete response and is still in remission as of the writing of this paper (27 months) with no active treatments; the second patient was treated with nivolumab for 16 weeks, which was also permanently interrupted for pneumonitis. This patient also achieved a complete response, still maintained (30 months) with no active treatments; the third patient was treated with pembrolizumab and experienced progressive disease after 12 weeks of treatment. He then achieved a longstanding near‐complete response for 24 months on BRAF and MEK inhibitors.
Table 4. Clinical characteristics of patients with or without recurrent pneumonitis.
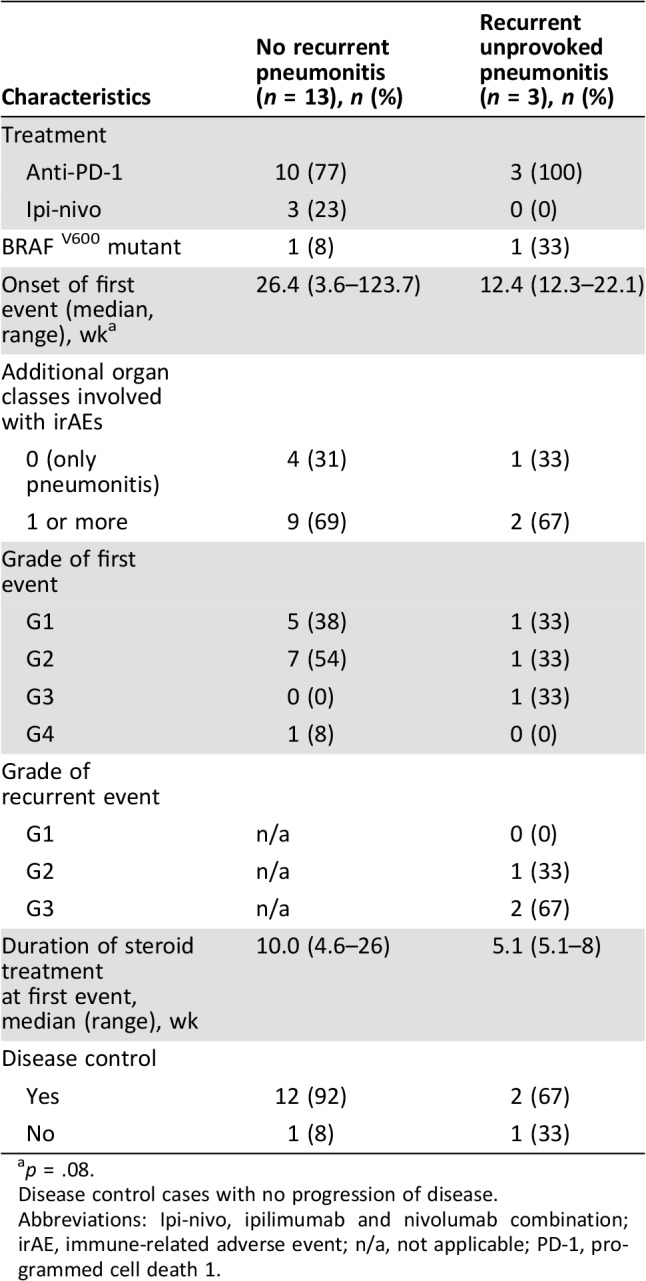
p = .08.
Disease control cases with no progression of disease.
Abbreviations: Ipi‐nivo, ipilimumab and nivolumab combination; irAE, immune‐related adverse event; n/a, not applicable; PD‐1, programmed cell death 1.
Figure 2.
Clinical courses: swimmers plot. Individual clinical courses of patients with pneumonitis, from initiation of treatment that provoked pneumonitis, to last follow‐up or death. In most patients there is sufficient follow‐up time that minimizes the possibility of the occurrence of successive pneumonitis flare.
Abbreviations: Ipi + Nivo, ipilimumab and nivolumab combination; PD‐1, programmed cell death 1.
Discussion
This report describes six patients who experienced recurrent pneumonitis after complete resolution of initial pneumonitis; three of them were unprovoked and occurred in the absence of immunotherapy rechallenge. Here we detail the clinical and imaging course of this entity.
All three patients were initially treated with anti‐PD‐1. Compared with those who had not experienced recurrent pneumonitis, their first episode of pneumonitis tended to occur earlier in the course of treatment (12.4 vs. 26.4 weeks). We did not find any predictors for pneumonitis recurrence; the severity of clinical pneumonitis and the nonpneumonitis irAE, as well as the imaging pattern of pneumonitis, was similar in both patient groups: those with and those without recurrent pneumonitis. The median duration of corticosteroid treatment was numerically shorter (5.1 vs. 10 weeks) in patients with pneumonitis recurrence, but this difference was not statistically significant. The true incidence for developing recurrent pneumonitis was not assessed in our study, as some of the patients died shortly after their initial pneumonitis or had a short follow‐up. However, the majority of our cohort had long‐term close clinical and imaging follow‐up (Fig. 2).
All three patients with unprovoked recurrences were successfully retreated with steroids, yet one experienced a second recurrence 2 months after completion of prednisone treatment. This second recurrence was treated with IV steroids with a slower tapering down, resulting once again in complete resolution.
Nishino et al. reported on two similar cases: one case of a lung cancer patient treated with anti‐PD‐1 and diagnosed with pneumonitis at week 15 [31]. After a total of 8 weeks on corticosteroids treatment, resolution was documented, but within 4 weeks of steroid treatment cessation the patient experienced a recurrent pneumonitis in the absence of drug rechallenge. As in our cohort, radiographic changes were similar to those seen in the first event. The second case [30] is of a patient with lymphoma who developed pneumonitis on combination ipilimumab‐nivolumab and, on rechallenge, with nivolumab monotherapy developed a recurrent episode after two doses. He was treated with a 2 month course of prednisone with subsequent improvement, yet 1 month after, the patient again developed pneumonitis, this time unprovoked by nivolumab retreatment. He was treated with prednisone for 2.7 months, but 2 weeks after completion of prednisone taper, he experienced a third flare, which this time responded to a longer course of steroid treatment.
This unique event of “pneumonitis flare” can be theoretically related to two combined factors—the host and the insulting agent. The first represents a particularly susceptible lung parenchyma, supported by an earlier occurrence of first event of pneumonitis. However, we did not observe any particular respiratory risk factor or tendency to a higher severity grade of pneumonitis. We also did not observe a higher extent of involvement of other organ systems with immune‐related adverse events. A higher parenchymal vulnerability to insults is, nevertheless, found in patients with lung cancer who seem to experience more severe cases of pneumonitis at an earlier presentation, compared with patients with melanoma [13]. It remains to be investigated whether patients with lung cancer experience more recurrent events of pneumonitis caused by preexisting smoking‐related lung damage as compared with patients with no such susceptibility. The second factor is the long half‐life of anti‐PD‐1 agents (27 days for nivolumab, 27 days for pembrolizumab), which, combined with the particular vulnerability of certain patients to immunotherapy, implies the need for a longer course of steroid treatment in order to prevent a flare‐up.
Conclusion
Because of the small number of our cohort, it would be difficult to identify specific predictive factors for immune‐related pneumonitis recurrences in the absence of drug reintroduction, yet we believe that cases of pneumonitis who present early in the course of immunotherapy treatment are at higher risk of pneumonitis recurrence. Therefore, in these cases, a longer course of steroid treatment with a slower tapering down should be considered. Finally, a careful follow‐up with a high index of suspicion for respiratory and constitutive symptoms or imaging changes is warranted, not only in patients with drug rechallenge but also in patients who do not undergo a rechallenge attempt.
Contributor Information
Nethanel Asher, Email: nethanel.asher@sheba.health.gov.il.
Gal Markel, Email: gal.markel@sheba.health.gov.il.
Author Contributions
Conception/design: Nethanel Asher, Gal Markel
Provision of study material or patients: Nethanel Asher, Guy Ben‐Betzalel, Yael Steinberg‐Silman, Jacob Schachter, Gal Markel
Collections and/or assembly of data: Nethanel Asher, Yael Steinberg‐Silman
Data analysis and interpretation: Nethanel Asher, Edith M. Marom, Erez Nissim Baruch, Gal Markel
Manuscript writing: Nethanel Asher, Edith M. Marom, Erez Nissim Baruch, Gal Markel
Final approval of manuscript: Gal Markel
Disclosures
Nethanel Asher: Bristol‐Myers Squibb, Merck Sharp & Dohme (H, other‐travel support); Ronnie Shapira‐Frommer: Merck Sharp & Dohme, Bristol‐Myers Squibb (H); Gal Markel: Novartis (RF), 4c BioMed (E), Novartis, Merck Sharp & Dohme, Bristol‐Myers Squibb Medison, Roche (H), 4c BioMed, Famewave, Biond Biologics (OI), 4c BioMed, Famewave (IP), Biond Biologics (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Robert C, Thomas L, Bondarenko I et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): A randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J, Ribas A, Long GV et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open‐label phase 3 study (KEYNOTE‐006). Lancet 2017;390:1853–1862. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration . Approved Drugs: Atezolizumab (TECENTRIQ). https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525780.htm. Accessed December 20, 2017.
- 7.Food and Drug Administration . Approved Drugs: Avelumab (BAVENCIO). https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm547965.htm. Accessed December 29, 2017.
- 8.Food and Drug Administration . Approved Drugs: Durvalumab (Imfinzi). https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555930.htm. Accessed December 29, 2017.
- 9.Hodi FS, Chesney J, Pavlick AC et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2‐year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2046;17:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long GV, Atkinson V, Cebon JS et al. Standard‐dose pembrolizumab in combination with reduced‐dose ipilimumab for patients with advanced melanoma (KEYNOTE‐029): An open‐label, phase 1b trial. Lancet Oncol 2017;18:1202–1210. [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal‐cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Hong D, Zhang X et al. PD‐1 inhibitors increase the incidence and risk of pneumonitis in cancer patients in a dose‐independent manner: A meta‐analysis. Sci Rep 2017;7:44173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuzi S, Tavora F, Cruz M et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor‐related pneumonitis. Cancer Manag Res 2017;9:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaunay M, Cadranel J, Lusque A et al. Immune‐checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017;50:1700050. [DOI] [PubMed] [Google Scholar]
- 16.Khunger M, Rakshit S, Pasupuleti V et al. Incidence of pneumonitis with use of programmed death 1 and programmed death ligand 1 inhibitors in non‐small cell lung cancer: A systematic review and meta‐analysis of trials. Chest 2017;152:271–281. [DOI] [PubMed] [Google Scholar]
- 17.Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Possick JD. Pulmonary toxicities from checkpoint immunotherapy for malignancy. Clin Chest Med 2017;38:223–232. [DOI] [PubMed] [Google Scholar]
- 19.Shannon VR. Pneumotoxicity associated with immune checkpoint inhibitor therapies. Curr Opin Pulm Med 2017;23:305–316. [DOI] [PubMed] [Google Scholar]
- 20.Gettinger SN, Horn L, Gandhi L et al. Overall survival and long‐term safety of nivolumab (anti‐programmed death 1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 22.Topalian SL, Sznol M, McDermott DF et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti–PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sznol M, Ferrucci PF, Hogg D et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017;35:3815–3822. [DOI] [PubMed] [Google Scholar]
- 25.Sznol M, Postow MA, Davies MJ et al. Endocrine‐related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–76. [DOI] [PubMed] [Google Scholar]
- 26.Weber JS, Postow M, Lao CD et al. Management of adverse events following treatment with anti‐programmed death‐1 agents. The Oncologist 2016;21:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haanen JBAG, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28(suppl 4):iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 28.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spain L, Walls G, Messiou C et al. Efficacy and toxicity of rechallenge with combination immune checkpoint blockade in metastatic melanoma: A case series. Cancer Immunol Immunother 2017;66:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino M, Ramaiya NH, Awad MM et al. PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin Cancer Res Off 2016;J Am Assoc Cancer Res 22:6051–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino M, Chambers ES, Chong CR et al. Anti‐PD‐1 inhibitor‐related pneumonitis in non‐small cell lung cancer. Cancer Immunol Res 2016;4:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]