This review highlights the etiology, assessment, and management of unintentional weight loss in patients with pancreatic cancer and provides recommendations for best supportive care.
Keywords: Pancreatic cancer, Weight loss, Cachexia, Anorexia, Malabsorption, Supportive care
Abstract
Unintentional weight loss in patients with pancreatic cancer is highly prevalent and contributes to low therapeutic tolerance, reduced quality of life, and overall mortality. Weight loss in pancreatic cancer can be due to anorexia, malabsorption, and/or cachexia. Proper supportive care can stabilize or reverse weight loss in patients and improve outcomes. We review the literature on supportive care relevant to pancreatic cancer patients, and offer evidence‐based recommendations that include expert nutritional assessment, counseling, supportive measures to ensure adequate caloric intake, pancreatic enzyme supplementation, nutritional supplement replacement, orexigenic agents, and exercise. Pancreatic Cancer Action Network‐supported initiatives will spearhead the dissemination and adoption of these best supportive care practices.
Implications for Practice.
Weight loss in pancreatic cancer patients is endemic, as 85% of pancreatic cancer patients meet the classic definition of cancer cachexia. Despite its significant prevalence and associated morbidity, there is no established approach to this disease entity. It is believed that this is due to an important knowledge gap in understanding the underlying biology and lack of optimal treatment approaches. This article reviews the literature regarding pancreas cancer‐associated weight loss and establishes a new framework from which to view this complex clinical problem. An improved approach and understanding will help educate clinicians, improve clinical care, and provide more clarity for future clinical investigation.
摘要
无意识的体重减轻在胰腺癌患者中十分常见,可以导致治疗耐受性低、降低生活质量以及总死亡率。胰腺癌患者的体重减轻可能归因于厌食症、吸收障碍和/或恶病质。适当的支持性治疗可以让患者的体重减轻状况稳定下来或发生逆转,同时还能改进预后。我们查阅了与胰腺癌患者相关的支持性治疗文献并提出了基于证据的建议,包含专家营养评估、咨询服务、确保充足的热量摄入的支持性措施、胰酶补充、营养补充剂替换、开胃药物和锻炼。胰腺癌行动网络支持的各项举措将带头宣传并采用此类最佳支持性治疗实践。
实践意义:由于 85% 的胰腺癌患者符合癌症恶病质的经典定义,所以,胰腺癌患者的体重减轻很常见。尽管它具有显著的流行率和相关病损率,但是,我们没有确定的方法来治疗此类疾病。人们认为,这是由于在理解潜在的生物学方面存在重要的知识差距和缺乏最佳的治疗方法。本文查阅了有关胰腺癌相关体重减轻的文献,并建立了一个可供人们查看此类复杂的临床问题的全新框架。改进的方法和认识将有助于教育临床医生、改善临床治疗并为未来的临床研究提供更清晰的信息。
Prevalence, Classification, and Significance
Weight loss (WL) is highly prevalent among patients with pancreatic cancer (PC) [1], [2], as up to 85% of patients present with WL at diagnosis [3]. Nearly 50% of those with early‐stage and locally advanced disease exhibit preoperative WL [4], and 80% will develop progressive WL after diagnosis. There are no approved medical treatments for PC‐associated weight loss (PAWL), and commonly used orexigenic agents that stimulate appetite have no definite benefit [5]. Therefore, progressive WL is one of the most distressing and intractable features of PC [6].
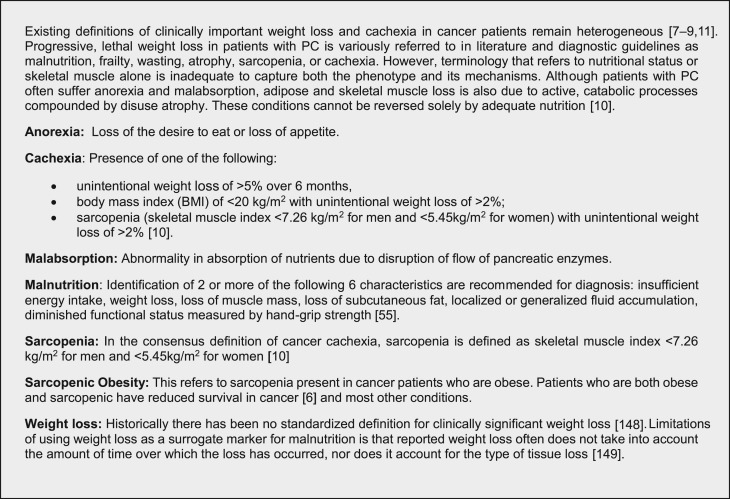
Assessment of clinically significant WL in PC is complex and hindered by a lack of standardized definitions and therapeutic strategies [7], [8], [9]. The etiologies can be categorized as due to anorexia, malabsorption, and/or cachexia (Fig. 1). Clinically impactful WL in PC is frequently described as cachexia, and classification systems for WL in cancer patients are usually concerned with cancer cachexia. Cachexia is a multifactorial syndrome characterized by progressive involuntary WL, loss of skeletal muscle mass with or without adipose loss, and systemic inflammation [10]. Despite an international consensus statement in 2011 regarding cachexia [10], definitions of clinically important WL in cancer patients remain unclear and heterogeneous [11].
Figure 1.
Definitions and classifications.
Abbreviation: PC, pancreatic cancer.
WL in PC patients has predictive and prognostic implications. WL and cachexia predict poor outcomes in all PC stages [4], [9], [12], [13]. Most patients with PC will die with cachexia, and it's estimated that ~30% will die of cachexia [6], [9], [10], [11]. Decreased lean body mass and/or weight are predictors for toxicity [14], [15], mortality [16], postoperative infections, length of hospitalization, and therapeutic intensity [4], [9], [14], [16], [17], [18], [19], [20], [21]. WL also correlates with shorter progression‐free survival (PFS) and overall survival (OS), decreased response to chemotherapy, lower quality of life (QOL), and declining performance status [3], [10]. In addition, obesity is a known risk factor for the development of PC [22], [23], [24], [25], and its presence at the time of diagnosis is an independent predictor of survival [26]. Patients with low muscle mass and high body mass index (BMI), a condition now described as sarcopenic obesity, exhibit worse outcomes in PC and many other diseases [6], [27], [28].
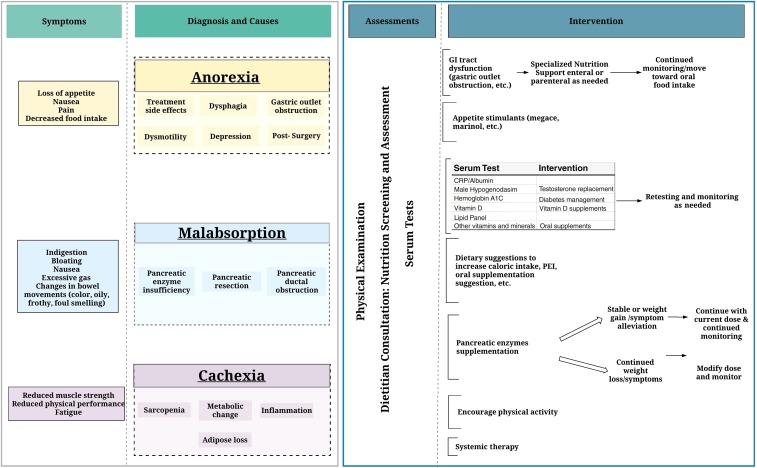
Optimal approaches to the diagnosis and management of PC weight loss and cachexia are understudied and poorly understood; however, nutritional intervention can improve QOL and OS in advanced PC [29], [30], [31]. Recognizing and treating malnutrition in advanced cancer early in the treatment process is essential for improving outcomes [28], [32]. Furthermore, nutritional support in PC is complicated and influenced by many factors including tumor response, diabetes, pain management, altered physiology, and other comorbidities. In this review, we highlight the etiology, assessment, and management of PAWL, provide best supportive care recommendations based on current knowledge (Fig. 2), and bring attention to the gaps.
Figure 2.
Clinically significant weight loss (see Table 1 for interpretation).
Abbreviations: CRP, c‐reactive protein; GI, gastrointestinal; PEI, pancreatic exocrine insufficiency.
Supportive Care Working Group Consensus Process
The Supportive Care Working Group (SCWG) was formed to establish best supportive care practices across the Precision Promise Consortium (PPC) and to help foster supportive care research. The SCWG consists of scientists, physicians, and a dietician with expertise in pancreatic cancer, nutrition, exercise, cachexia, survivorship, and palliative care. The SCWG compiled all evidence by searching PubMed articles (from 1977 to 2017) on PAWL and other relevant topics. Keywords searched included weight loss, cachexia, anorexia, malabsorption, sarcopenia, pancreatic exocrine insufficiency, malabsorption, pancreatic enzyme replacement therapy, nutrition, and exercise. The group convened quarterly over an 18‐month period and corresponded via teleconference bimonthly. The group initially presented the evidence and consensus to the PPC and Steering Committee (SC) in January 2017. The feedback was considered by the SCWG, integrated, and presented back to the PPC and SC in August 2017. Additional feedback was integrated, and the final consensus is presented here.
Pathogenesis (Causes of PAWL)
We recommend categorizing PAWL into three causes: anorexia, malabsorption/pancreatic exocrine insufficiency, and cachexia/sarcopenia.
Anorexia
Anorexia is defined as loss of the desire to eat and is mediated by the inability of the hypothalamus to respond to energy deficit signals [28]. Pain, fatigue, depression, dysmotility, constipation, chemosensory disturbances (changes in smell and taste), vomiting, early satiety, and loss of appetite all contribute to anorexia [10], [33], [34], [35], [36]. An important aspect of anorexia is the negative social impact including changes in social encounters and relationships and a sense of helplessness, anxiety, and distress [37], [38]. Patients commonly report distress related to dietary intake and gut symptoms including difficulties with gas, belching, bloating, pain/discomfort, and diarrhea leading to WL [39]. Additionally, current chemotherapy regimens, including gemcitabine/nab‐paclitaxel [40], FOLIFIRINOX [41], and nanoliposomal irinotecan/5FU [42], can cause nausea, vomiting, indigestion, diarrhea, and mouth sores [43] and thus contribute to lower food intake and reduced nutrition.
Malabsorption/Pancreatic Exocrine Insufficiency
Pancreatic exocrine insufficiency (PEI), a common complication of PC, occurs when the pancreas is unable to maintain normal digestive function (secretion of proteases, lipase, and amylase), resulting in malabsorption and malnutrition. PEI negatively impacts QOL [44], [45] and is associated with poor survival [44], [45], [46]. Additionally, markers of malabsorption and exocrine secretion impairment, such as fecal elastase‐1, are strongly correlated with poor survival in PC [46].
Symptoms of malabsorption include increased abdominal bloating or discomfort, excessive gas causing burping or flatulence, indigestion, nausea, delayed gastric emptying/early satiety, changes in bowel movements—increased frequency, light color, floating, frothy, oily, and/or foul smelling, and WL despite intake meeting estimated caloric needs.
Cachexia and Sarcopenia
Cachexia results from a complex interaction between tumor, host, and therapy [34], [47] characterized by progressive wasting of skeletal muscle mass and to a lesser extent adipose tissue, occurring even before WL becomes apparent (precachexia) [28], [48]. Preclinical models indicate that chemotherapy and targeted agents can cause cachexia through mitogen‐activated protein kinase‐dependent muscle atrophy, mitochondrial depletion, and muscle weakness leading to disuse atrophy [49]. Despite the high prevalence and potential for mortality, PC‐associated cachexia is diagnosed and treated in a minority of patients [5], as there is no established treatment [50]. The stages of cachexia occur across a spectrum starting with “precachexia” characterized by WL <5%, then “cachexia” associated with systemic inflammation, and ending with “refractory cachexia defined by a loss of body reserves and rapidly deteriorating nutritional status [10]. Not all patients will progress through all the stages, and no predictive biomarkers have been identified to distinguish patients who will progress [10].
WL is our current measure of cachexia. However, advances in body composition assessment techniques indicate skeletal muscle and adipose tissue may behave independently during WL [51]. In obese patients with cancer, muscle wasting is common and obscured [51]; 40% of PC patients who were found overweight/obese at the time of diagnosis exhibited muscle wasting [6]. Consequently, sarcopenic obesity is linked to functional status, survival, chemotherapy toxicity, and poor outcomes following surgery [52], [53].
Assessment of PAWL
The morbidity and mortality associated with PAWL compels a comprehensive approach to its assessment and management and should be tailored to each individual. We recommend that all patients be screened for WL, receive clinical and nutritional screening, be assessed for PEI, and receive serum testing. Body composition measurements should be considered in the research setting.
Screening for WL
It is important that clinicians assess and record the weight of a patient at presentation and prior to diagnosis. A quick review of WL history can illuminate the extent of PAWL. Assessment of WL over time is one of the standardized diagnostic characteristics used to identify and document adult malnutrition as recommended by the Academy of Nutrition and Dietetics [54] and the American Society for Parenteral and Enteral Nutrition. Any patient who demonstrates moderate or severe WL and/or evidence of malnutrition or malnutrition risk by these criteria should be directed to a registered dietitian for a more extensive assessment and intervention as described below. The consensus guidelines for interpretation of WL in adults is outlined in Table 1 [55].
Table 1. Interpretation of adult weight loss.
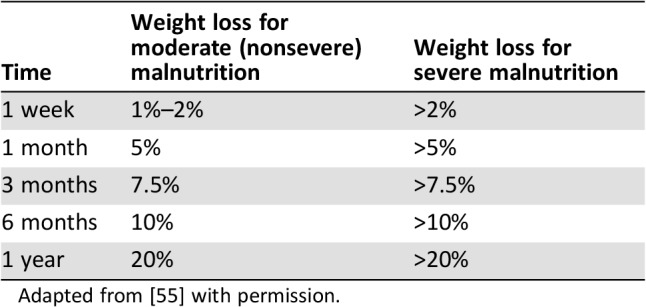
Adapted from [55] with permission.
Nutrition Screening and Clinical Assessment
Nutrition screening using a validated tool allows for identification and referral of at‐risk patients to a registered dietitian for complete nutrition assessment and intervention. Screening should be completed on all patients before the start of treatment and at regular intervals such as weekly during radiotherapy, with each chemotherapy visit, and at each surveillance visit [54], [56], [57].
Two nutritional screening tools have been vetted for both inpatient and outpatient use: the malnutrition screening tool (MST) and the patient‐generated subjective global assessment (PG‐SGA). The MST is a quick, valid, and reliable screening tool. It consists of two questions regarding appetite and recent unintentional WL and has demonstrated high sensitivity and specificity for identification of individuals with or at risk of malnutrition [58]. The PG‐SGA is a valid and reliable assessment tool to identify and triage malnourished patients with cancer in both the inpatient and outpatient setting. The abridged PG‐SGA forgoes the physical examination used in the PG‐SGA and is a practical, informative, and valid tool for use in the outpatient oncology setting for detecting malnutrition [59].
We recommend that patients are screened using one of these tools; those deemed to be at risk for malnutrition should then be referred to a registered dietitian for nutrition assessment. The services of a registered dietitian are important for the early identification of malnutrition and anticipation of nutritional impact on symptoms to help patients maintain weight, stay on their intended treatment, and maintain or improve QOL over the course of their disease.
A full nutrition assessment involves the following elements: obtaining a food history, evaluation of anthropometric measurements, review of medical history, biochemical data, medical tests and procedures, and completion of a nutrition‐focused physical assessment [60]. Routine assessment of food intake should include patient estimate of overall food intake and 24‐hour food recall [34]. An oncology‐focused assessment also involves reviewing the oncologic treatment plan with the goal to determine current and anticipated nutrition issues [54], [57], [61]. This information is all assimilated and used to formulate a plan to address existing and potential influences on malnutrition.
Assessment for PEI
Although there are several tests to diagnose pancreatic exocrine insufficiency, their clinical application and use is variable [62], [63]. Definitive identification of PEI in PC patients is challenging; thus, PEI is usually a clinical diagnosis. Direct measures of gastric and duodenal contents such as a secretin‐cerulein or secretin‐pancreozymin tests are expensive, invasive, and only available at specialized centers [64]. Other tests such as fecal fat test, fecal elastase, and coefficient of fat absorption (CFA) are more clinically available but may be cumbersome [64], [65], [66]. Steatorrhea is classically defined as at least 7 grams of fecal fat over 24 hours, in the context of a 72‐hour stool test while on 100 grams of fat daily [67]. However, the stool collection and associated diet is inconvenient and difficult for patients. Therefore, fecal elastase measurements are often used to diagnose PEI [67], [68], [69]. Fecal elastase is less sensitive (72%) than CFA collection but quite specific (90%). In PC patients, studies have shown a prevalence of PEI varying from 50%–89% when defined by low fecal elastase [46], [70], [71]. Expert opinion is that fecal elastase is appropriate for unresectable patients but is insufficiently sensitive to detect mild‐moderate PEI and unsuitable for postpancreaticoduodenectomy patients [72]. Regardless, in standard PC care, these tests are not routinely performed and the diagnosis is established clinically and based on patients’ history and reported symptoms (unintentional WL, change in stool, bloating after meals, etc.). Patients should be clinically assessed for PEI upon diagnosis, at the start of treatment, and on a regular basis throughout the course of their treatment.
Serum Tests
There is no widely adopted serologic evaluation of patients with PAWL. However, WL in cancer patients has been associated with markers of inflammation, low levels of serum testosterone in men, and vitamin deficiencies. A more comprehensive serologic evaluation of patients who present with PAWL will likely improve supportive care.
Measuring serum inflammation in PC patients with a Glasgow Prognostic Score (GPS) has shown prognostic significance in several studies [73], [74], and is predictive of surgical outcomes [75], [76]. The GPS and other markers of inflammation are consistently associated with WL and cachexia in cancer patients [77], [78]. GPS is usually scored 0, 1, or 2 by providing 1 point each for c‐reactive protein (CRP) >10 and Albumin <3.5. The GPS was recently studied as a predictive factor for response to a novel therapeutic in advanced PC [79]. Recent studies have also suggested that CRP:Albumin ratio and neutrophil:lymphocyte ratio also have prognostic validity [80], [81]. In routine practice, this score could help identify patients at high risk for PAWL and cachexia and could promote early intervention.
Male hypogonadism (MH) is a common complication in advanced cancer [82] and prominent in cancer patients [83] with cachexia [84], occurring in more than 70% of these patients [85]. Hypogonadism in cancer cachexia is associated with decreased strength, poor nutritional status [86], decreased performance status, depression, and decreased survival [87], [88]. In a randomized study in cancer patients with fatigue and MH, testosterone replacement improved fatigue and performance status but not QOL [89]. Despite the high prevalence of MH in cancer patients and associated morbidity and mortality, there are no established guidelines for the diagnosis and management of MH in this population [90]. Consensus from the supportive care literature supports the practical application of testosterone replacement in symptomatic patients with MH [91].
There has been little published material regarding serum assessment for vitamin and mineral deficiencies in patients with PC. Most available literature is related to the post‐pancreatic resection setting [92] or in the broader topic of PEI from all causes (chronic pancreatitis and cystic fibrosis included) [62]. After resection, reported nutritional deficiencies include fat‐soluble vitamins A, D, and E, vitamins B12 and B6, iron, zinc, selenium, biotin, and copper. None of these can be considered diagnostic of malnutrition; however, vitamin D deficiency has been reported in patients with PC and may contribute to poor outcomes [93], [94], [95]. These findings were concluded from retrospective analysis, and it is not clear whether vitamin D supplementation improves outcomes. We recommend that vitamin D deficiency should be evaluated and supplemented per physician discretion.
Body Composition Measurements
Muscle volumes can be measured and sarcopenia diagnosed using several modalities: computed tomography (CT) or magnetic resonance imaging (MRI); appendicular skeletal muscle index obtained from dual energy x‐ray absorptiometry (DEXA); mid‐upper‐arm muscle area by anthropometry; and whole‐body fat‐free mass index determined by bioimpedance analysis [10], [96], [97], [98]. As CT is commonly used in the diagnosis and treatment of PC, this modality has been used to estimate sarcopenia radiographically by total psoas muscle area and radiodensity [96]. However, psoas muscle measurements do not reflect whole‐body muscle; thus, measurements of a whole‐body slice at the level of lumbar vertebrae are superior [99]. Morphometric analysis of diagnostic CT or MRI slices can be used to calculate whole‐body skeletal muscle and adipose tissue, to assess muscle quality, and to detect presence of myosteatosis. Currently, this can be done using dedicated commercial software such as Sliceomatic or ImageJ. Skeletal muscle and adipose tissue can behave independently during cancer progression and therapy [51], [100], and there may be value in measuring these separate compartments in the research setting.
Intervention for PAWL
Role of the Registered Dietitian
Dietitians play a central role in the successful management of PAWL. Nutrition interventions for PAWL have improved weight [30], QOL, and outcomes [29], [60], [101]. Dietitians can provide essential dietary suggestions, identify PEI, and provide recommendations for oral nutritional supplementation and specialized nutrition support [102]. Dietitians may also provide more detailed advice on diet and pancreatic enzyme supplementation for management of symptoms of PEI [103], [104], [105], [106].
Pancreatic Enzyme Replacement Therapy
Pancreatic enzyme replacement therapy (PERT) has demonstrated benefit for PC patients with PEI. Two randomized controlled trials have evaluated the impact of PERT among patients with PEI due to chronic pancreatitis or surgery. These studies demonstrated an increase in fat absorption, reduced stool frequency, improved stool consistency [107], [108], [109], and symptom improvement [109]. A previous double‐blinded trial of 21 patients with unresectable PC with obstructive jaundice in whom a biliary stent was placed showed that patients randomized to PERT had improved fat absorption and that WL was prevented compared with those who were randomized to placebo [110].
In a retrospective, nonrandomized study of 66 unresectable PC patients receiving PERT with nutritional counseling and palliative care or standard palliative care without PERT, median survival of patients receiving PERT was longer than that of those with standard palliative care (301 days vs. 89 days) [111]. More recently, a randomized study showed an increase in OS in patients randomized to taking enzymes but did not reach statistical significance because of a low enrollment rate [112]. Additionally, a prospective study of unresectable patient receiving chemotherapy showed improved changes in BMI and increased OS of patients who received PERT compared with historical controls [113].
In a retrospective, nonrandomized study of 66 unresectable PC patients receiving PERT with nutritional counseling and palliative care or standard palliative care without PERT, median survival of patients receiving PERT was longer than that of those with standard palliative care (301 days vs. 89 days).
These clinical trials evaluating PERT used doses ranging from 40,000 to 72,000 units of lipase for meals and 20,000–36,000 units of lipase per snack. The maximum effective dose is unclear, guidelines vary, and dosing recommendations for PC are not currently indicated on U.S. Food and Drug Administration labels. Enzymes may be dosed based on body weight, fat content of diet, clinical symptoms, degree of steatorrhea present, or general per meal/snack guidelines. The recommended starting dose per meal is 500 lipase units per kg; dosing should be modified as appropriate and should be tailored to individuals as needed to reflect variations in meal sizes and fat content of the diet, clinical symptoms, and the degree of steatorrhea present. Dosages should not exceed 10,000 lipase units per kilogram body weight per day with a maximum of 2,500 lipase units per kilogram per meal [64]. Patients should be instructed to take enzymes at the start of the meal, and if taking multiple capsules, pills should be taken at intervals at the start and then throughout the meal [114]. A study using the Pancreatic Cancer Action Network's Patient Registry indicated that in patients taking PERT appropriately, symptoms such as a feeling of indigestion and WL were significantly improved [115].
Oral Nutrition Supplements
If patients show signs of deficiency, have prolonged inadequate intake, or have prolonged malabsorption, clinicians should consider evaluation for deficiencies including vitamins A, D, E, B12, and B6 as well as iron, zinc, selenium, biotin, and copper. Supplements should be recommended for replacement of known deficiencies in the diet or serum levels. Serum values may be rechecked approximately 3 months after attempted repletion. For individuals with inadequate intake of calories and/or protein, nutrition supplement drinks may be recommended as part of the nutrition care plan. Unfortunately, there have been few studies investigating the vitamin deficiencies associated with PEI and the benefit of oral nutrition supplements.
Specialized Nutrition Support
Specialized Nutrition Support (SNS) is the use of enteral or parenteral nutrition. Both American and European guidelines for nutrition support recommend against routine use of SNS for oncology patients [116], [117]. However SNS may be necessary for patients with a dysfunction of the gastrointestinal tract such as gastric outlet obstruction or who are severely malnourished and will be undergoing major surgery [116], [117], [118]. If SNS is needed, enteral feeding is preferred because of lower risk of infections or other complications [119], [120]. In randomized studies, enteral feeding has shown benefit over other forms of artificial nutrition in patients following a pancreaticoduodenectomy [121].
Parenteral nutrition may improve weight [122] and maintain or improve performance status in selected patients, but it is not routinely recommended. Careful evaluation should be employed to identify those patients who would benefit and to avoid indiscriminate use of artificial nutrition [123], especially in cases of futile care and at end of life. Benefits may be limited to the perioperative setting for patients who are unable to tolerate oral nutrition, although there is a need for more explicit criteria for use [121], [124].
Parenteral nutrition may improve weight and maintain or improve performance status in selected patients, but it is not routinely recommended. Careful evaluation should be employed to identify those patients who would benefit and to avoid indiscriminate use of artificial nutrition, especially in cases of futile care and at end of life.
Pharmacologic Interventions
Advances in the understanding of etiology of cachexia are leading to pharmaceutical interventions aimed at targets upstream (inhibiting systemic inflammation) or downstream (blocking catabolic pathways, stimulating anabolic pathways, and reducing anorexia) [51].
Appetite stimulants are frequently prescribed for patients with PAWL. Progestogens, such as megestrol acetate and corticosteroids, are used as orexigenics [5], [125], [126], [127]. Their mechanism of action is also through inhibition of cytokines (including interleukin‐6 and tumor necrosis factor) and de‐repression of appetite. Anecdotal evidence suggests cannabis improves appetite and food intake in patients with PAWL, and synthetic delta‐9‐tetrahydrocannabinol, dronabinol, is approved for use in acquired immunodeficiency syndrome‐related cachexia and emesis from chemotherapy. However, insufficient data exist for prescribing cannabis for cachexia [128], and a randomized study in advanced cancer patients demonstrated superiority of megesterol acetate over dronabinol [129]. Novel agents for appetite stimulation include grehlin and grehlin mimetics, notably anamorelin, for which significant increase in lean body mass was observed in non‐small cell lung cancer patients from two phase III trials [130], [131].
Nonsteroidal anti‐inflammatory (NSAID) agents, thalidomide, and omega‐3 fatty acids modulate cytokine production, thereby inhibiting inflammation. Previous studies of these agents have not shown conclusive benefit, and their routine use is not recommended. Agents targeting the cytokine activin and the related muscle growth inhibitor myostatin have also been evaluated, without report of benefit to date (NCT01433263 and NCT01505530]. Newer therapies include monoclonal antibodies against cytokines, including interleukin‐1 alpha (NCT03207724) [132]. Combination therapy with NSAID, nutritional supplementation, dietary consultation, and exercise showed benefit in a multicenter, randomized phase II trial of patients with lung or pancreatic cancer, and has now moved to phase III (MENAC, NCT02330926). Investigations of many novel agents are ongoing; however, there are currently no standard systemic therapies for PAWL or PC cachexia [133].
Exercise
Physical activity (PA) and exercise could benefit patients with PAWL. In cancer patients, PA has been shown to improve health‐related QOL and symptom burden. PA resulted in improved treatment tolerance [134], [135] and is associated with improved PFS and OS [136], suggesting that the benefit of PA is not confined to disease‐free survivors.
Gradual resistance training and aerobic exercise can also maintain and restore lean muscle mass [137]. In addition, prescribed exercise resulted in improved appetite and general well‐being in patients with advanced cancer receiving palliative care [138].
PA interventions have been deemed safe and feasible in PC patients [139] and may attenuate cachexia by modulating insulin sensitivity and inflammation [140], [141], [142]. Progressive postoperative exercise programs have also been demonstrated to be feasible, resulting in lower levels of fatigue and pain and improved perception of general health [143]. In advanced disease, a home‐based walking intervention was associated with increased endurance and improved symptom burden, with patients increasing their average duration of moderate‐intensity exercise [144]. Given the potential for high symptom burden and poor survival outcomes in PC, Groupe Cooperateur Multidisciplinaire en Oncologie has initiated a large randomized trial of adapted physical activity in PC [139]. This study will formally evaluate the benefits of aerobic and resistance exercises on fatigue and health‐related QOL, but also investigate the effects of intervention on nutritional status, insulin resistance, cancer treatment tolerance, and survival.
One hundred fifty minutes per week of moderate‐intensity aerobic exercise and at least two sessions of resistance training per week is the recommended physical activity for cancer patients and survivors [145], [146], [147]. The optimal form and duration of exercise is unclear, and recommendations should be tailored to an individual's current fitness level, treatment‐related late/long‐term toxicities, and comorbidities. Referral to physical therapy or other exercise professionals to guide initiation and engagement in physical activity may improve success and thus should be considered for all patients [145], [146], [147].
Consensus Statement and Recommendation (Fig. 3)
Figure 3.
Consensus recommendations for pancreatic cancer‐associated weight loss.
Slowing WL in patients with PC should improve quality and length of life both directly through maintaining function and indirectly through enhancing treatment delivery and response. In many cases, favorable response to therapy will translate to overall improvement in symptoms and QOL. We recommend all patients who have significant WL and/or evidence of malnutrition be directed to a registered dietician for a more extensive evaluation. As needed, patients should be provided pancreatic enzyme replacement therapy, correction of hormonal and micronutrient deficiencies, and oral nutrition supplementation. Appetite stimulants should be considered. Patients should be warned against inactivity, and moderate‐intensity exercise should be encouraged and supported. Measures to optimize appetite, nutritional intake, micronutrients, and anabolic potential, while reducing disuse and catabolism, should be implemented and integrated as part of treatment planning as thoughtfully and aggressively as antitumor therapy.
Acknowledgments
We thank Merle Witter for assistance of the preparation of the manuscript. This work was supported by the Pancreatic Cancer Action Network, NIH grants R01 CA122596 and R01 CA194593, the Lustgarten Foundation, Veterans Administration Merit Award I01 BX004177, and the Lilly Endowment, Inc. At the time this manuscript was written, The Precision Promise Consortium was composed of the following members: Andrew Hendifar, Principal Investigator; Richard Tuli, Co‐PI (Cedars‐Sinai Medical Center); Brian Wolpin, Principal Investigator; Manuel Hidalgo; David Ryan (Dana Farber/Harvard Cancer Center); Sunil Hingorani, Principal Investigator; Elena Gabriela Chiorean; Andrew Coveler (Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance/University of Washington); Eileen O'Reilly, Principal Investigator; Vinod Balachandran (Memorial Sloan Kettering Cancer Center); Diane Simeone (Perlmutter Cancer Center/NYU Langone Health); Andrew Lowy, Principal Investigator; Paul Fanta; Razelle Kurzrock; Tony Reid (University of California, San Diego); Andrew Ko, Principal Investigator; Eric Collisson; Margaret Tempero (University of California, San Francisco); Hedy Kindler, Principal Investigator (University of Chicago); Thomas George, Principal Investigator; Jose Trevino (University of Florida); Robert Vonderheide, Principal Investigator; Gregory Beatty (University of Pennsylvania); Vincent Picozzi, Principal Investigator; Margaret Mandelson (Virginia Mason Medical Center); Andrea Wang‐Gillam, Principal Investigator; William Hawkins (Washington University); and the Precision Promise Steering Committee (Julie Fleshman, Pancreatic Cancer Action Network; Manuel Hidalgo, Dana Farber Cancer Institute; Sunil Hingorani, Fred Hutchinson Cancer Research Center; Anirban Maitra, MD Anderson Cancer Center; Victoria Manax, Pancreatic Cancer Action Network; Lynn Matrisian, Pancreatic Cancer Action Network; Vincent Picozzi, Virginia Mason Medical Center; Diane Simeone, Perlmutter Cancer Center/NYU Langone Health).
Contributor Information
Andrew E. Hendifar, Email: andrew.hendifar@cshs.org.
Collaborators: on behalf of the Precision Promise Consortium, Andrew Hendifar, Richard Tuli, Brian Wolpin, Manuel Hidalgo, David Ryan, Sunil Hingorani, Elena Gabriela Chiorean, Andrew Coveler, Eileen O'Reilly, Vinod Balachandran, Diane Simeone, Andrew Lowy, Paul Fanta, Razelle Kurzrock, Tony Reid, Andrew Ko, Eric Collisson, Margaret Tempero, Hedy Kindler, Thomas George, Jose Trevino, Robert Vonderheide, Gregory Beatty, Vincent Picozzi, Margaret Mandelson, Andrea Wang‐Gillam, William Hawkins, Julie Fleshman, Manuel Hidalgo, Sunil Hingorani, Anirban Maitra, Victoria Manax, Lynn Matrisian, Vincent Picozzi, and Diane Simeone
Author Contributions
Conception/design: Andrew E. Hendifar, Maria Q.B. Petzel, Teresa A. Zimmers, Crystal S. Denlinger, Lynn M. Matrisian, Vincent J. Picozzi, Lola Rahib
Collection and/or assembly of data: Andrew E. Hendifar, Maria Q.B. Petzel, Teresa A. Zimmers, Crystal S. Denlinger, Lynn M. Matrisian, Vincent J. Picozzi, Lola Rahib
Data analysis and interpretation: Andrew E. Hendifar, Maria Q.B. Petzel, Teresa A. Zimmers, Crystal S. Denlinger, Lynn M. Matrisian, Vincent J. Picozzi, Lola Rahib
Manuscript writing: Andrew E. Hendifar, Maria Q.B. Petzel, Teresa A. Zimmers, Crystal S. Denlinger, Lynn M. Matrisian, Vincent J. Picozzi, Lola Rahib
Final approval of manuscript: Andrew E. Hendifar, Maria Q.B. Petzel, Teresa A. Zimmers, Crystal S. Denlinger, Lynn M. Matrisian, Vincent J. Picozzi, Lola Rahib
Disclosures
The authors indicated no financial relationships.
References
- 1.Bye A, Jordhoy MS, Skjegstad G et al. Symptoms in advanced pancreatic cancer are of importance for energy intake. Support Care Cancer 2013;21:219–227. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci LM, Bell D, Thornton J et al. Nutritional status of patients with locally advanced pancreatic cancer: A pilot study. Support Care Cancer 2011;19:1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendifar A, Chang J, Huang B et al. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J Gastrointest Oncol 2018;9:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann J, Heiligensetzer M, Krakowski‐Roosen H et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193–1201. [DOI] [PubMed] [Google Scholar]
- 5.Hendifar AE, Co Tan CR, Yaffee P et al. Evaluating outcomes of pancreatic cancer patients with cachexia. J Clin Oncol 2014;32(suppl 15):e15208a. [Google Scholar]
- 6.Tan BH, Birdsell LA, Martin L et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–6979. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann J, Ketterer K, Marsch C et al. Pancreatic cancer related cachexia: Influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer 2009;9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann J, Buchler MW, Friess H et al. Cachexia in patients with chronic pancreatitis and pancreatic cancer: Impact on survival and outcome. Nutr Cancer 2013;65:827–833. [DOI] [PubMed] [Google Scholar]
- 9.Pausch T, Hartwig W, Hinz U et al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152(3 suppl 1):S81–S88. [DOI] [PubMed] [Google Scholar]
- 10.Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 11.Martin L, Senesse P, Gioulbasanis I et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 12.Hendifar A, Osipov A, Khanuja J et al. Influence of body mass index and albumin on perioperative morbidity and clinical outcomes in resected pancreatic adenocarcinoma. PLoS One 2016;11:e0152172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng P, Hyder O, Firoozmand A et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali R, Baracos VE, Sawyer MB et al. Lean body mass as an independent determinant of dose‐limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 2016;5:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prado CM, Baracos VE, McCargar LJ et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 16.Dalal S, Hui D, Bidaut L et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: A pilot study. J Pain Symptom Manage 2012;44:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kazemi‐Bajestani SM, Mazurak VC, Baracos V. Computed tomography‐defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2–10. [DOI] [PubMed] [Google Scholar]
- 18.Cousin S, Hollebecque A, Koscielny S et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs 2014;32:382–387. [DOI] [PubMed] [Google Scholar]
- 19.Di Sebastiano KM, Yang L, Zbuk K et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: The relationship with diabetes and anaemia. Br J Nutr 2013;109:302–312. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard TJ, Lawson‐McLean A, Fearon KC. Nutritional predictors of postoperative outcome in pancreatic cancer (Br J Surg 2011; 98: 268‐274). Br J Surg 2011;98:1032; author reply 1032–1033. [DOI] [PubMed] [Google Scholar]
- 21.Kanda M, Fujii T, Kodera Y et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011;98:268–274. [DOI] [PubMed] [Google Scholar]
- 22.Arslan AA, Helzlsouer KJ, Kooperberg C et al. Anthropometric measures, body mass index, and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium (PanScan). Arch Intern Med 2010;170:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aune D, Greenwood DC, Chan DS et al. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non‐linear dose‐response meta‐analysis of prospective studies. Ann Oncol 2012;23:843–852. [DOI] [PubMed] [Google Scholar]
- 24.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta‐analysis of prospective studies. Int J Cancer 2007;120:1993–1998. [DOI] [PubMed] [Google Scholar]
- 25.Michaud DS, Giovannucci E, Willett WC et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 26.Yuan C, Bao Y, Wu C et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol 2013;31:4229–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper AB, Slack R, Fogelman D et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol 2015;22:2416–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laviano A, Meguid MM, Inui A et al. Therapy insight: Cancer anorexia‐cachexia syndrome—when all you can eat is yourself. Nat Clin Pract Oncol 2005;2:158–165. [DOI] [PubMed] [Google Scholar]
- 29.Davidson W, Ash S, Capra S et al. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr 2004;23:239–247. [DOI] [PubMed] [Google Scholar]
- 30.Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy—A pilot study. Support Care Cancer 2005;13:270–274. [DOI] [PubMed] [Google Scholar]
- 31.Richter E, Denecke A, Klapdor S et al. Parenteral nutrition support for patients with pancreatic cancer—Improvement of the nutritional status and the therapeutic outcome. Anticancer Res 2012;32:2111–2118. [PubMed] [Google Scholar]
- 32.Vashi PG, Dahlk S, Popiel B et al. A longitudinal study investigating quality of life and nutritional outcomes in advanced cancer patients receiving home parenteral nutrition. BMC Cancer 2014;14:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uomo G, Gallucci F, Rabitti PG. Anorexia‐cachexia syndrome in pancreatic cancer: Recent development in research and management. JOP 2006;7:157–162. [PubMed] [Google Scholar]
- 34.Bruera E, Sweeney C. Cachexia and asthenia in cancer patients. Lancet Oncol 2000;1:138–147. [DOI] [PubMed] [Google Scholar]
- 35.Wigmore SJ, Plester CE, Richardson RA et al. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer 1997;75:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holly EA, Chaliha I, Bracci PM et al. Signs and symptoms of pancreatic cancer: A population‐based case‐control study in the San Francisco Bay area. Clin Gastroenterol Hepatol 2004;2:510–517. [DOI] [PubMed] [Google Scholar]
- 37.Hopkinson JB. The emotional aspects of cancer anorexia. Curr Opin Support Palliat Care 2010;4:254–258. [DOI] [PubMed] [Google Scholar]
- 38.Splinter TA. Cachexia and cancer: A clinician's view. Ann Oncol 1992;3(suppl 3):25–27. [DOI] [PubMed] [Google Scholar]
- 39.Gooden HM, White KJ. Pancreatic cancer and supportive care—Pancreatic exocrine insufficiency negatively impacts on quality of life. Support Care Cancer 2013;21:1835–1841. [DOI] [PubMed] [Google Scholar]
- 40.Von Hoff DD, Ervin T, Arena FP et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conroy T, Desseigne F, Ychou M et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 42.Wang‐Gillam A, Li CP, Bodoky G et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine‐based therapy (NAPOLI‐1): A global, randomised, open‐label, phase 3 trial. Lancet 2016;387:545–557. [DOI] [PubMed] [Google Scholar]
- 43.Nitta H, Baba H, Sugimori K et al. Chemotherapy‐induced nausea and vomiting in patients with hepatobiliary and pancreatic cancer treated with chemotherapy: A prospective observational study by the CINV study group of Japan. Anticancer Res 2016;36:1929–1935. [PubMed] [Google Scholar]
- 44.Damerla V, Gotlieb V, Larson H et al. Pancreatic enzyme supplementation in pancreatic cancer. J Support Oncol 2008;6:393–396. [PubMed] [Google Scholar]
- 45.Park JW, Jang JY, Kim EJ et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br J Surg 2013;100:1064–1070. [DOI] [PubMed] [Google Scholar]
- 46.Partelli S, Frulloni L, Minniti C et al. Faecal elastase‐1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis 2012;44:945–951. [DOI] [PubMed] [Google Scholar]
- 47.Inui A. Recent development in research and management of cancer anorexia‐cachexia syndrome [in Japanese]. Gan To Kagaku Ryoho 2005;32:743–749. [PubMed] [Google Scholar]
- 48.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013;10:90–99. [DOI] [PubMed] [Google Scholar]
- 49.Barreto R, Waning DL, Gao H et al. Chemotherapy‐related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 2016;7:43442–43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gullett NP, Mazurak VC, Hebbar G et al. Nutritional interventions for cancer‐induced cachexia. Curr Probl Cancer 2011;35:58–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fearon KC, Baracos VE. Cachexia in pancreatic cancer: New treatment options and measures of success. HPB (Oxford) 2010;12:323–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prado CM, Lieffers JR, McCargar LJ et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 53.Pecorelli N, Carrara G, De Cobelli F et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg 2016;103:434–442. [DOI] [PubMed] [Google Scholar]
- 54.Academy of nutrition and dietetics oncology expert work group: Nutrition and the adult oncology patient. Chicago, IL, 2013. www.andeal.org. Accessed December 9, 2016.
- 55.White JV, Guenter P, Jensen G et al. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275–283. [DOI] [PubMed] [Google Scholar]
- 56.Cushen SJ, Power DG, Ryan AM. Nutrition assessment in oncology. Top Clin Nutr 2015;30:103–119. [Google Scholar]
- 57.Levin R. Nutrition risk screening and assessment of the oncology patient In: M Leser, N Ledesma, Bergerson S, Truillo E. eds. Oncology Nutrition for Clinical Practice. Chicago: Oncology Nutrition Dietetic Practice Group, 2013:25–32. [Google Scholar]
- 58.Ferguson M, Capra S, Bauer J et al. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999;15:458–464. [DOI] [PubMed] [Google Scholar]
- 59.Gabrielson DK, Scaffidi D, Leung E et al. Use of an abridged scored Patient‐Generated Subjective Global Assessment (abPG‐SGA) as a nutritional screening tool for cancer patients in an outpatient setting. Nutr Cancer 2013;65:234–239. [DOI] [PubMed] [Google Scholar]
- 60.Mueller C, Compher C, Ellen DM. A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr 2011;35:16–24. [DOI] [PubMed] [Google Scholar]
- 61.Huhmann MB, August DA. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) clinical guidelines for nutrition support in cancer patients: Nutrition screening and assessment. Nutr Clin Pract 2008;23:182–188. [DOI] [PubMed] [Google Scholar]
- 62.Lindkvist B, Phillips ME, Domínguez‐Muñoz JE. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology 2015;15:589–597. [DOI] [PubMed] [Google Scholar]
- 63.Tseng DS, Molenaar IQ, Besselink MG et al. Pancreatic exocrine insufficiency in patients with pancreatic or periampullary cancer: A systematic review. Pancreas 2016;45:325–330. [DOI] [PubMed] [Google Scholar]
- 64.Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: Present and future. Clin Exp Gastroenterol 2011;4:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dominguez‐Munoz JE. Pancreatic exocrine insufficiency: Diagnosis and treatment. J Gastroenterol Hepatol 2011;26(suppl 2):12–16. [DOI] [PubMed] [Google Scholar]
- 66.Dominguez‐Munoz JE. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency: When is it indicated, what is the goal and how to do it? Adv Med Sci 2011;56:1–5. [DOI] [PubMed] [Google Scholar]
- 67.Roberts IM, Poturich C, Wald A. Utility of fecal fat concentrations as screening test in pancreatic insufficiency. Dig Dis Sci 1986;31:1021–1024. [DOI] [PubMed] [Google Scholar]
- 68.Naruse S, Ishiguro H, Ko SB et al. Fecal pancreatic elastase: A reproducible marker for severe exocrine pancreatic insufficiency. J Gastroenterol 2006;41:901–908. [DOI] [PubMed] [Google Scholar]
- 69.Lankisch PG, Schmidt I, Konig H et al. Faecal elastase 1: Not helpful in diagnosing chronic pancreatitis associated with mild to moderate exocrine pancreatic insufficiency. Gut 1998;42:551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez MM, Newcomer AD, Moertel CG et al. Assessment of weight loss, food intake, fat metabolism, malabsorption, and treatment of pancreatic insufficiency in pancreatic cancer. Cancer 1983;52:346–352. [DOI] [PubMed] [Google Scholar]
- 71.Sikkens EC, Cahen DL, de Wit J et al. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol 2014;48:e43–e46. [DOI] [PubMed] [Google Scholar]
- 72.Bartel MJ, Asbun H, Stauffer J et al. Pancreatic exocrine insufficiency in pancreatic cancer: A review of the literature. Dig Liver Dis 2015;47:1013–1020. [DOI] [PubMed] [Google Scholar]
- 73.Shimoda M, Katoh M, Kita J et al. The Glasgow Prognostic Score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy 2010;56:501–506. [DOI] [PubMed] [Google Scholar]
- 74.Glen P, Jamieson NB, McMillan DC et al. Evaluation of an inflammation‐based prognostic score in patients with inoperable pancreatic cancer. Pancreatology 2006;6:450–453. [DOI] [PubMed] [Google Scholar]
- 75.Jamieson NB, Denley SM, Logue J et al. A prospective comparison of the prognostic value of tumor‐ and patient‐related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol 2011;18:2318–2328. [DOI] [PubMed] [Google Scholar]
- 76.La Torre M, Nigri G, Cavallini M et al. The Glasgow Prognostic Score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol 2012;19:2917–2923. [DOI] [PubMed] [Google Scholar]
- 77.McMillan DC, Preston T, Watson WS et al. Relationship between weight loss, reduction of body cell mass and inflammatory response in patients with cancer. Br J Surg 1994;81:1011–1014. [DOI] [PubMed] [Google Scholar]
- 78.Staal‐van den Brekel AJ, Dentener MA, Schols AM et al. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 1995;13:2600–2605. [DOI] [PubMed] [Google Scholar]
- 79.Hurwitz HI, Uppal N, Wagner SA et al. Randomized, double‐blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 2015;33:4039–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Z, Jin K, Guo M et al. Prognostic value of the CRP/Alb ratio, a novel inflammation‐based score in pancreatic cancer. Ann Surg Oncol 2017;24:561–568. [DOI] [PubMed] [Google Scholar]
- 81.Wu M, Guo J, Guo L, et al. The C‐reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525–12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strasser F, Palmer JL, Schover LR et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: A pilot study. Cancer 2006;107:2949–2957. [DOI] [PubMed] [Google Scholar]
- 83.Fuoco D, di Tomasso J, Boulos C et al. Identifying nutritional, functional, and quality of life correlates with male hypogonadism in advanced cancer patients. Ecancermedicalscience 2015;9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dev R, Hui D, Del Fabbro E et al. Association between hypogonadism, symptom burden, and survival in male patients with advanced cancer. Cancer 2014;120:1586–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burney BO, Hayes TG, Smiechowska J et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab 2012;97:E700–E709. [DOI] [PubMed] [Google Scholar]
- 86.Garcia JM, Li H, Mann D et al. Hypogonadism in male patients with cancer. Cancer 2006;106:2583–2591. [DOI] [PubMed] [Google Scholar]
- 87.Del Fabbro E, Hui D, Nooruddin ZI et al. Associations among hypogonadism, C‐reactive protein, symptom burden, and survival in male cancer patients with cachexia: A preliminary report. J Pain Symptom Manage 2010;39:1016–1024. [DOI] [PubMed] [Google Scholar]
- 88.Bilir C, Engin H, Can M et al. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol 2015;32:56. [DOI] [PubMed] [Google Scholar]
- 89.Del Fabbro E, Garcia JM, Dev R et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: A preliminary double‐blind placebo‐controlled trial. Support Care Cancer 2013;21:2599–2607. [DOI] [PubMed] [Google Scholar]
- 90.Bhasin S, Cunningham GR, Hayes FJ et al. Testosterone therapy in men with androgen deficiency syndromes: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–2559. [DOI] [PubMed] [Google Scholar]
- 91.Dev R, Bruera E, Del Fabbro E. When and when not to use testosterone for palliation in cancer care. Curr Oncol Rep 2014;16:378. [DOI] [PubMed] [Google Scholar]
- 92.Armstrong T, Walters E, Varshney S et al. Deficiencies of micronutrients, altered bowel function, and quality of life during late follow‐up after pancreaticoduodenectomy for malignancy. Pancreatology 2002;2:528–534. [DOI] [PubMed] [Google Scholar]
- 93.McGovern EM, Lewis ME, Niesley ML et al. Retrospective analysis of the influence of 25‐hydroxyvitamin D on disease progression and survival in pancreatic cancer. Nutr J 2016;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Loon K, Owzar K, Jiang C et al. 25‐Hydroxyvitamin D levels and survival in advanced pancreatic cancer: Findings from CALGB 80303 (Alliance). J Natl Cancer Inst 2014;106:dju185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuan C, Qian ZR, Babic A et al. Prediagnostic plasma 25‐hydroxyvitamin D and pancreatic cancer survival. J Clin Oncol 2016;34:2899–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care 2009;3:269–275. [DOI] [PubMed] [Google Scholar]
- 97.Di Sebastiano KM, Mourtzakis M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl Physiol Nutr Metab 2012;37:811–821. [DOI] [PubMed] [Google Scholar]
- 98.Simons JP, Schols AM, Westerterp KR et al. The use of bioelectrical impedance analysis to predict total body water in patients with cancer cachexia. Am J Clin Nutr 1995;61:741–745. [DOI] [PubMed] [Google Scholar]
- 99.Rutten IJG, Ubachs J, Kruitwagen RFPM et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nattenmuller J, Wochner R, Muley T et al. Prognostic impact of CT‐quantified muscle and fat distribution before and after first‐line‐chemotherapy in lung cancer patients. PLoS One 2017;12:e0169136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vashi P, Popiel B, Lammersfeld C et al. Outcomes of systematic nutritional assessment and medical nutrition therapy in pancreatic cancer. Pancreas 2015;44:750–755. [DOI] [PubMed] [Google Scholar]
- 102.Ottery F. Supportive nutritional management of the patient with pancreatic cancer. Oncology (Williston Park) 1996;10:26–32. [PubMed] [Google Scholar]
- 103.Phillips ME. Pancreatic exocrine insufficiency following pancreatic resection. Pancreatology 2015;15:449–455. [DOI] [PubMed] [Google Scholar]
- 104.Sikkens EC, Cahen DL, van Eijck C et al. The daily practice of pancreatic enzyme replacement therapy after pancreatic surgery: A northern European survey: Enzyme replacement after surgery. J Gastrointest Surg 2012;16:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toouli J, Biankin AV, Oliver MR et al. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust 2010;193:461–467. [DOI] [PubMed] [Google Scholar]
- 106.Optimal care pathway for people with pancreatic cancer. Available at http://www.cancervic.org.au/downloads/health‐professionals/optimal‐care‐pathways/Optimal_care_pathway_for_people_with_pancreatic_cancer.pdf. Accessed August 4, 2016.
- 107.Whitcomb DC, Lehman GA, Vasileva G et al. Pancrelipase delayed‐release capsules (CREON) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: A double‐blind randomized trial. Am J Gastroenterol 2010;105:2276–2286. [DOI] [PubMed] [Google Scholar]
- 108.Gubergrits N, Malecka‐Panas E, Lehman GA et al. A 6‐month, open‐label clinical trial of pancrelipase delayed‐release capsules (Creon) in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Aliment Pharmacol Ther 2011;33:1152–1161. [DOI] [PubMed] [Google Scholar]
- 109.Safdi M, Bekal PK, Martin S et al. The effects of oral pancreatic enzymes (Creon 10 capsule) on steatorrhea: A multicenter, placebo‐controlled, parallel group trial in subjects with chronic pancreatitis. Pancreas 2006;33:156–162. [DOI] [PubMed] [Google Scholar]
- 110.Bruno MJ, Haverkort EB, Tijssen GP et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998;42:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dominguez‐Muñoz JE, Nieto L, Iglesias‐Garcia J. Pancreatic enzyme replacement therapy and nutritional advice are associated with longer survival in patients with unresectable pancreatic cancer (PC). Pancreas 2013;42:1347–1347. [Google Scholar]
- 112.Zdenkowski N, Radvan G, Pugliese L et al. Treatment of pancreatic insufficiency using pancreatic extract in patients with advanced pancreatic cancer: A pilot study (PICNIC). Support Care Cancer 2017;25:1963–1971. [DOI] [PubMed] [Google Scholar]
- 113.Saito T, Hirano K, Isayama H et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: A prospective cohort study. Pancreas 2017;46:341–346. [DOI] [PubMed] [Google Scholar]
- 114.Dominguez‐Munoz JE, Iglesias‐Garcia J, Iglesias‐Rey M et al. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: A randomized, three‐way crossover study. Aliment Pharmacol Ther 2005;21:993–1000. [DOI] [PubMed] [Google Scholar]
- 115.Rahib L, Westermann A, Barkin JA et al. Frequency of appropriate use of pancreatic enzyme replacement therapy (PERT) and symptomatic response in pancreatic cancer patients. Abstract presented at: World Congress of Gastroenterology at ACG2017; 2017; Orlando, FL. [Google Scholar]
- 116.Arends J, Bachmann P, Baracos V et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. [DOI] [PubMed] [Google Scholar]
- 117.August DA, Huhmann MB. A.S.P.E.N. clinical guidelines: Nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472–500. [DOI] [PubMed] [Google Scholar]
- 118.Huhmann MB, August DA. Perioperative nutrition support in cancer patients. Nutr Clin Pract 2012;27:586–592. [DOI] [PubMed] [Google Scholar]
- 119.Chow R, Bruera E, Chiu L et al. Enteral and parenteral nutrition in cancer patients: A systematic review and meta‐analysis. Ann Palliat Med 2016;5:30–41. [DOI] [PubMed] [Google Scholar]
- 120.Cotogni P. Enteral versus parenteral nutrition in cancer patients: Evidences and controversies. Ann Palliat Med 2016;5:42–49. [DOI] [PubMed] [Google Scholar]
- 121.Lassen K, Coolsen MM, Slim K et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced recovery after surgery (ERAS®) society recommendations. Clin Nutr 2012;31:817–830. [DOI] [PubMed] [Google Scholar]
- 122.Pelzer U, Arnold D, Govercin M et al. Parenteral nutrition support for patients with pancreatic cancer. Results of a phase II study. BMC Cancer 2010;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruggeri EA, Agostini F, Fettucciari L et al. Home artificial nutrition in advanced cancer patients. Tumori 2013;99:218–224. [DOI] [PubMed] [Google Scholar]
- 124.Bozzetti F, Mariani L. Perioperative nutritional support of patients undergoing pancreatic surgery in the age of eras. Nutrition 2014;30:1267–1271. [DOI] [PubMed] [Google Scholar]
- 125.Paulsen Ø, Klepstad P, Rosland JH et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: A randomized, placebo‐controlled, double‐blind trial. J Clin Oncol 2014;32:3221–3228. [DOI] [PubMed] [Google Scholar]
- 126.Tchekmedyian N, Tait N, Moody M et al. High‐dose megestrol acetate: A possible treatment for cachexia. JAMA 1987;257:1195–1198. [PubMed] [Google Scholar]
- 127.Ruiz Garcia V, López‐Briz E, Carbonell Sanchis R et al. Megestrol acetate for treatment of anorexia‐cachexia syndrome. Cochrane Database Syst Rev 2013;28:CD004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reuter SE, Martin JH. Pharmacokinetics of cannabis in cancer cachexia‐anorexia syndrome. Clin Pharmacokinet 2016;55:807–812. [DOI] [PubMed] [Google Scholar]
- 129.Jatoi A, Windschitl HE, Loprinzi CL et al. Dronabinol versus megestrol acetate versus combination therapy for cancer‐associated anorexia: A North Central Cancer Treatment Group study. J Clin Oncol 2002;20:567–573. [DOI] [PubMed] [Google Scholar]
- 130.Argilés JM, López‐Soriano FJ, Stemmler B et al. Novel targeted therapies for cancer cachexia. Biochem J 2017;474:2663–2678. [DOI] [PubMed] [Google Scholar]
- 131.Temel JS, Abernethy AP, Currow DC et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. [DOI] [PubMed] [Google Scholar]
- 132.Hong DS, Hui D, Bruera E et al. MABp1, a first‐in‐class true human antibody targeting interleukin‐1alpha in refractory cancers: An open‐label, phase 1 dose‐escalation and expansion study. Lancet Oncol 2014;15:656–666. [DOI] [PubMed] [Google Scholar]
- 133.Tan CR, Yaffee PM, Jamil LH et al. Pancreatic cancer cachexia: A review of mechanisms and therapeutics. Front Physiol 2014;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heldens AF, Bongers BC, de Vos‐Geelen J et al. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. Eur J Surg Oncol 2016;42:1322–1330. [DOI] [PubMed] [Google Scholar]
- 135.Mishra SI, Scherer RW, Snyder C et al. Are exercise programs effective for improving health‐related quality of life among cancer survivors? A systematic review and meta‐analysis. Oncol Nurs Forum 2014;41:E326–E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guercio BJ, Venook AP, Niedzwiecki D et al. Associations of physical activity with survival and progression in metastatic colorectal cancer: Results from CALGB 80405 (Alliance). J Clin Oncol 2017;35:659–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muscaritoli M, Molfino A, Lucia S et al. Cachexia: A preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit Rev Oncol Hematol 2015;94:251–259. [DOI] [PubMed] [Google Scholar]
- 138.Pyszora A, Budzynski J, Wojcik A et al. Physiotherapy programme reduces fatigue in patients with advanced cancer receiving palliative care: Randomized controlled trial. Support Care Cancer 2017;25:2899–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neuzillet C, Vergnault M, Bonnetain F et al. Rationale and design of the adapted physical activity in advanced pancreatic cancer patients (APACaP) GERCOR (Groupe Cooperateur Multidisciplinaire en Oncologie) trial: Study protocol for a randomized controlled trial. Trials 2015;16:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gould DW, Lahart I, Carmichael AR et al. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J Cachexia Sarcopenia Muscle 2013;4:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maddocks M, Murton AJ, Wilcock A. Therapeutic exercise in cancer cachexia. Crit Rev Oncog 2012;17:285–292. [DOI] [PubMed] [Google Scholar]
- 142.Pedrinolla A, Ardigo LP, Salvagno GL et al. Inflammatory response to exercise in a pancreatic‐cancer patient: A case report. Pancreat Disord Ther 2016;6:176. [Google Scholar]
- 143.Yeo TP, Burrell SA, Sauter PK et al. A progressive postresection walking program significantly improves fatigue and health‐related quality of life in pancreas and periampullary cancer patients. J Am Coll Surg 2012;214:463–475; discussion 475–467. [DOI] [PubMed] [Google Scholar]
- 144.Denlinger CS, Hall MJ, Cohen SJ et al. Physical activity intervention for patients with advanced pancreatic cancer. J Clin Oncol 2017;35:217–217.28056205 [Google Scholar]
- 145.Rock CL, Doyle C, Demark‐Wahnefried W et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243–274. [DOI] [PubMed] [Google Scholar]
- 146.Schmitz KH, Courneya KS, Matthews C et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409–1426. [DOI] [PubMed] [Google Scholar]
- 147.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology ‐ Survivorship. 2017. http://www.jnccn.org/content/15/9/1140.long. Accessed July 6, 2017.
- 148.Hébuterne X, Lemarié E, Michallet M et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38(2):196–204. [DOI] [PubMed] [Google Scholar]
- 149.Ali R, Baracos VE, Sawyer MB, Bianchi, L et al. Lean body mass as an independent determinant of dose‐limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 2016;5(4):607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]