Sertoli‐Leydig cell tumors (SLCT) represent less than 0.5% of ovarian tumors. Because of the rarity of this tumor, this study aimed to determine appropriate SLCT management strategies and indications for conservative surgery and adjuvant chemotherapy.
Keywords: Sertoli‐Leydig, Conservative surgery, Conservative treatment, Sex cord‐stromal tumors
Abstract
Background.
Sertoli‐Leydig cell tumors (SLCTs) represent less than 0.5% of ovarian tumors. Because of the rarity of this tumor and its peak in frequency at around 25 years of age, this study aimed to describe SLCT management strategies.
Objective.
The objective of this study was to determine the management (i.e., conservative surgery and adjuvant chemotherapy) of ovarian SLCTs.
Results.
This retrospective analysis included 23 patients treated for ovarian SLCTs. A centralized pathologic review of the tumors was conducted. Patients were referred to or treated in our institution for an ovarian SLCT between 1994 and 2015. The median age at diagnosis was 33 years (range, 4–82 years). According to the 2014 Federation of Gynecology and Obstetrics classification, tumors were classified as stage Ia (n = 15: well differentiated, n = 1; of intermediate differentiation, n = 8; undifferentiated, n = 4; and undefined, n = 2), stage Ib (n = 1), stage Ic1 (n = 5), stage IIb (n = 1), and stage IIIc (n = 1). Surgery was conservative in 13 patients (Ia, n = 7; Ib, n = 1; Ic1, n = 5) and radical in 10 patients (Ia, n = 8; IIb, n = 1; IIIc, n = 1). Seven patients received adjuvant chemotherapy with a cisplatin‐based regimen (Ia, n = 2; Ic1, n = 3; IIb, n = 1) or docetaxel + gemcitabine (IIIc, n = 1). Median follow‐up was 61 months (range, 15–252 months). Eight patients experienced a relapse (Ia, n = 2; Ib, n = 1; Ic1, n = 3; IIb, n = 1; IIIc, n = 1). Of these, six had at least one peritoneal carcinomatosis, and four died (Ic1, n = 2; IIb, n = 1; and Ia, n = 1). Two patients had a local relapse (one uterus and one ovary) and survived without disease after relapse treatment. The median time between the initial treatment and relapse was 28 months (range 9–70).
Conclusion.
Conservative surgery was safe for patients with stage Ia ovarian SLCTs. The place of conservative surgery for stage Ic1 remains to be defined. The best chemotherapy regimen remains to be defined.
Implications for Practice.
For stage Ia disease, conservative surgery (in women of reproductive age) was safe and effective for treating ovarian Seroli‐Leydig cell tumors. Adjuvant chemotherapy should be proposed for stage Ia when poor prognostic factors are present (poor differentiation, retiform pattern, or heterologous elements). For stage Ic1 and more severe stages, radical surgery and adjuvant chemotherapy should be considered. The combination of bleomycin, etoposide, and cisplatin was the most frequently used regimen, but the best chemotherapy regimen remains to be defined.
Introduction
Sertoli‐Leydig cell tumors (SLCTs) represent less than 0.5% of ovarian tumors. This group of tumors includes tumors in Sertoli and Leydig cells that are more or less differentiated, proliferating singly or in association. They occur in women aged between 1 and 84 years, and their frequency peaks in women aged 25 years [1], [2]. On the functional side, 50% of these tumors are accompanied by signs of hyperandrogenism, and they cause pseudo‐heterosexual puberty (virilization) in the child. The other 50% of cases include estrogen‐secreting and nonfunctional tumors. The latter types are typically discovered fortuitously, during an investigation of amenorrhea or sterility.
SLCTs are almost always unilateral (98% of cases). They are highly variable in size (2–35 cm), but they are often voluminous (13.5 cm on average). They exhibit a smooth external surface, and they are most often solid or semisolid. A capsular rupture is encountered in about 10% of cases, and ruptures are sometimes accompanied by ascites (4% of cases) [1], [2]. Histologically, SLCTs are characterized by the proliferation of Sertoli and Leydig cells in varying proportions. They are classified into three categories according to differentiation: (a) Well‐differentiated forms are characterized by Sertoli cells that form open or closed tubes without nuclear atypia or mitosis. Between the tube formations, Leydig cells are found in small clusters in a fine stroma. (b) Forms of intermediate differentiation are characterized by the presence of lobulated clusters of fusiform cells of ovarian stroma type. Some tubes with cellular atypia may be present. Mitosis activity is high, with around five mitoses per 10 large microscopic fields. Leydig cells are typically present at the periphery of the clusters or tumor. (c) Undifferentiated or sarcomatoid forms are characterized by a proliferation of cells that resemble cells of the primary gonadal stroma but without the lobulated aspect of intermediate differentiation. Mitotic activity is very high, exceeding 20 mitoses per 10 large microscopic fields. Retiform architecture and heterologous elements can be found in either undifferentiated or intermediately differentiated forms.
In immunohistochemistry, SLCTs are positive for vimentin, keratin, α inhibin, and calretinin. Expression levels are heterogeneous, and they can vary between tumor and stromal cells. From a genetic point of view, SLCTs are associated with a somatic mutation in the DICER‐1 gene. This mutation was found in approximately 60% of cases [3].
Because of the rarity of this tumor and the peak in frequency at around 25 years of age, this study aimed to determine appropriate SLCT management strategies and, in particular, indications for conservative surgery and adjuvant chemotherapy.
Materials and Methods
Patients with SLCTs referred to or treated in our institution between 1994 and 2015 were identified retrospectively. Patients were included when they met the following additional inclusion criteria:
A centralized pathologic review of the tumors by two expert pathologists according to the criteria of the 2014 World Health Organization (WHO) classification.
Molecular analysis: no FOXL2 mutation.
Immunohistochemical analysis: positive calretinin and α‐inhibin expression.
Surgical, histological, and follow‐up data available for analyzing the precise surgical procedures and their histological results.
We defined conservative surgery as the preservation of the uterus and at least part of one ovary. We defined radical surgery as the removal of both adnexa and the uterus, or both adnexa when the patient had a medical history of hysterectomy. Complete peritoneal surgical staging was defined as an analysis of peritoneal cytology (at least), multiple peritoneal biopsies, an omentectomy, or omental biopsies. Tumors were typed according to the 2014 WHO classification criteria. Moreover, we noted the degree of differentiation, the specific differentiation pattern (e.g., a retiform pattern), and the presence of heterologous elements. Tumors were staged according to the 2014 Federation of Gynecology and Obstetrics (FIGO) staging system, which included three new classes of stage Ic disease [4]. Molecular analysis of the DICER‐1 mutation was performed in difficult cases to confirm the diagnosis of SLCT.
Results
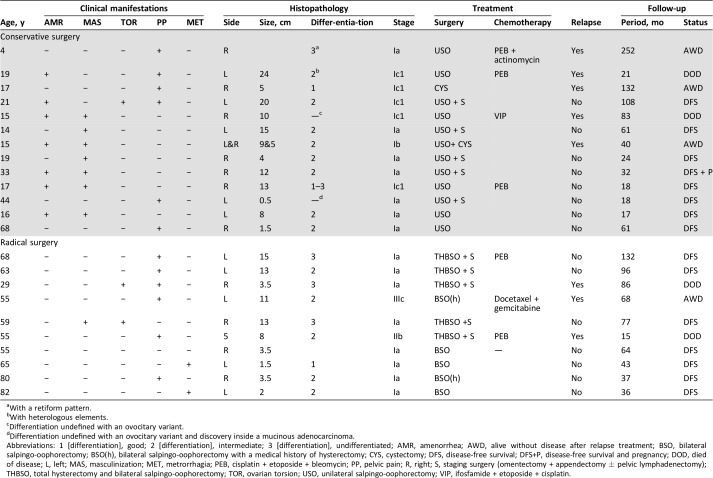
Twenty‐three patients fulfilled the inclusion criteria. The characteristics of these 23 patients are detailed in Table 1. The median age was 33 years (range 4–82 years). Eight patients were postmenopausal. The median tumor size was 8.5 cm (range 0.5–24 cm). The tumor FIGO stages were Ia (n = 15), Ib1 (n = 1), Ic1 (n = 5), IIb (n = 1), and IIIc (n = 1).
Table 1. Patient characteristics.
With a retiform pattern.
With heterologous elements.
Differentiation undefined with an ovocitary variant.
Differentiation undefined with an ovocitary variant and discovery inside a mucinous adenocarcinoma.
Abbreviations: 1 [differentiation], good; 2 [differentiation], intermediate; 3 [differentiation], undifferentiated; AMR, amenorrhea; AWD, alive without disease after relapse treatment; BSO, bilateral salpingo‐oophorectomy; BSO(h), bilateral salpingo‐oophorectomy with a medical history of hysterectomy; CYS, cystectomy; DFS, disease‐free survival; DFS+P, disease‐free survival and pregnancy; DOD, died of disease; L, left; MAS, masculinization; MET, metrorrhagia; PEB, cisplatin + etoposide + bleomycin; PP, pelvic pain; R, right; S, staging surgery (omentectomy + appendectomy ± pelvic lymphadenectomy); THBSO, total hysterectomy and bilateral salpingo‐oophorectomy; TOR, ovarian torsion; USO, unilateral salpingo‐oophorectomy; VIP, ifosfamide + etoposide + cisplatin.
Eight patients exhibited androgenic manifestations. Fifteen patients had no endocrine manifestations but underwent radiologic exams because of postmenopausal hemorrhage (n = 2), postmenopausal pelvic pain (n = 6), amenorrhea and/or pelvic pain (n = 6), or a systematic radiologic exam (n = 1).
Five patients had undifferentiated tumors, 13 had tumors of intermediate differentiation, 2 had well‐differentiated tumors, and 3 had tumors with undefined differentiation (2 of 3 had ovocitary variants).
Thirteen patients (median age, 17 years; range, 4–68 years) received conservative surgeries. The median tumor size was 10 cm (range 0.5–24 cm). The FIGO stages of these 13 patients were Ia (n = 7), Ib (n = 1), and Ic1 (n = 5).
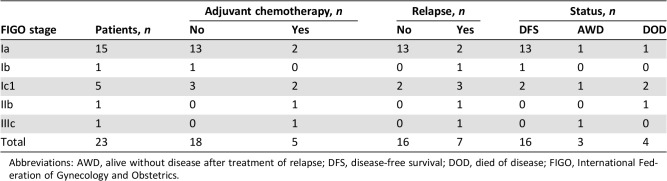
Seven patients received adjuvant chemotherapy (n = 6 received cisplatin‐based regimens, and one received a docetaxel + gemcitabine regimen). The FIGO stages of these seven patients were stage Ia (n = 2), stage Ic1 (n = 2), stage IIb (n = 1), and stage IIIc (n = 1). Table 2 shows the details of the adjuvant chemotherapies according to FIGO stage and tumor evolution.
Table 2. Adjuvant chemotherapy according to FIGO stage and evolution.
Abbreviations: AWD, alive without disease after treatment of relapse; DFS, disease‐free survival; DOD, died of disease; FIGO, International Federation of Gynecology and Obstetrics.
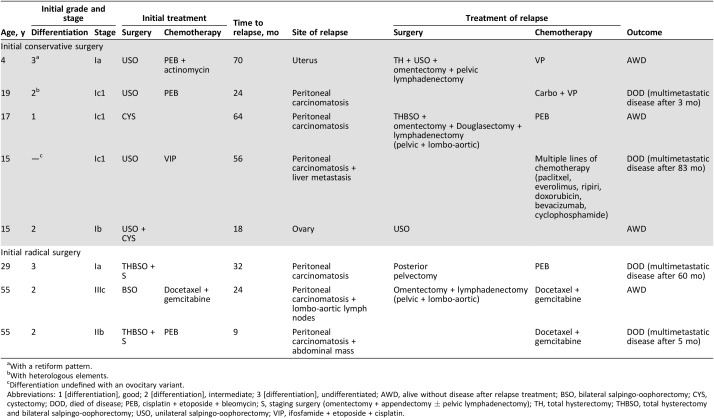
We observed eight relapses (stage Ia, n = 2; Ib, n = 1; Ic1, n = 3; IIb, n = 1; IIIc, n = 1). The median delay before a relapse was 28 months (range, 9–70 months). The characteristics of relapses are detailed in Table 3. Recurrent disease was found in five and three patients from the conservative surgery and radical surgery groups, respectively. Only two of these patients experienced limited recurrences; one was located in the ovary (initially stage Ib) and one was located in the uterus (initially stage Ia with adjuvant chemotherapy). The other six patients had peritoneal carcinomatosis (one patient had liver metastasis, and one patient had metastatic lymph nodes). Of these six patients, all received chemotherapy, and four underwent complete cytoreductive surgery before chemotherapy. Four of these six patients died (Table 2).
Table 3. Details of patients with relapses.
With a retiform pattern.
With heterologous elements.
Differentiation undefined with an ovocitary variant.
Abbreviations: 1 [differentiation], good; 2 [differentiation], intermediate; 3 [differentiation], undifferentiated; AWD, alive without disease after relapse treatment; BSO, bilateral salpingo‐oophorectomy; CYS, cystectomy; DOD, died of disease; PEB, cisplatin + etoposide + bleomycin; S, staging surgery (omentectomy + appendectomy ± pelvic lymphadenectomy); TH, total hysterectomy; THBSO, total hysterectomy and bilateral salpingo‐oophorectomy; USO, unilateral salpingo‐oophorectomy; VIP, ifosfamide + etoposide + cisplatin.
Of the 15 patients with stage Ia disease, 2 with grade 3 tumors experienced recurrences: 1 in the conservative surgery group and 1 in the radical surgery group. Of the five patients with stage Ic disease (all in the conservative surgery group), three experienced recurrences (two had received adjuvant chemotherapy), and two died. The two patients with a stage II or more severe disease experienced recurrences, and one died. In the conservative surgery group, one pregnancy was observed.
Discussion
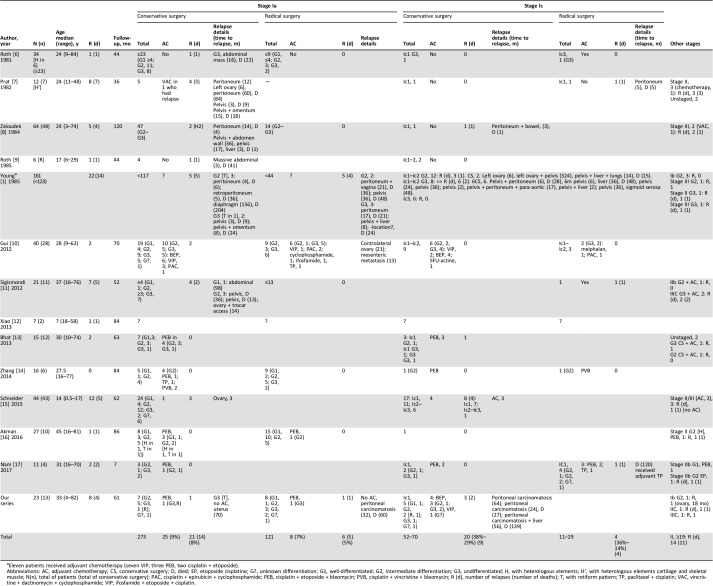
This study raised the complex question of how SLTCs should be managed, and we focused on two topics: the role of conservative surgery and the indications for adjuvant chemotherapy. The present study included 23 cases, and, to our knowledge, it is the only series to describe a centralized pathologic review of these tumors by two expert pathologists. This point is crucial because of the difficult diagnosis of this type of tumor [5]. Because of the rarity of SLTCs, we found few studies that focused on these questions in the literature and little data. Table 4 shows all the series we found in the literature [1], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]. The role of conservative surgery remains debated, and an evaluation was crucial because the peak frequency of SLCT occurs in young women of reproductive age.
Table 4. Review of series with Sertoli‐leydig cell tumors.
Eleven patients received adjuvant chemotherapy (seven VIP, three PEB, two cisplatin + etoposide).
Abbreviations: AC, adjuvant chemotherapy; CS, conservative surgery; D, died; EP, etoposide cisplatine; G?, unknown differentiation; G1, well‐differentiated; G2, intermediate differentiation; G3, undifferentiated; H, with heterologous elements; H’, with heterologous elements cartilage and skeletal muscle; N(n), total of patients (total of conservative surgery); PAC, cisplatin + epirubicin + cyclophosphamide; PEB, cisplatin + etoposide + bleomycin; PVB, cisplatin + vincristine + bleomycin; R (d), number of relapses (number of deaths); T, with retiform pattern; TP, paclitaxel + cisplatin; VAC, vincristine + dactinomycin + cyclophosphamide; VIP, ifosfamide + etoposide + cisplatin.
Our results suggested that, for stage Ia disease, conservative surgery should be proposed in children and in women of reproductive age. The difficulty in managing stage Ia is determining whether to use an adjuvant treatment. Table 4 shows that the risk of relapse for stage Ia was around 7% (27/394), but the risk of death in case of relapse was an impressive 70% (19/27). However, Table 4 also shows that the rate of relapse was relatively similar, regardless of the type of surgery (8% in the conservative surgery group and 3% in the radical surgery group). In our series, 2 of the 15 patients with stage Ia disease experienced a relapse (peritoneal carcinomatosis) and died of the disease. These two patients had undifferentiated tumors, and one had a retiform pattern. One of these patients received conservative surgery and adjuvant platinum‐based chemotherapy, and the other received radical surgery without adjuvant chemotherapy. The prognosis of SLCTs is known to be correlated with the FIGO stage, but prognosis also depends on tumor differentiation, the presence of heterologous elements, and the presence of a retiform pattern [1]. European Society for Medical Oncology (ESMO) guidelines identified poor differentiation and the presence of heterologous elements as indicators of a poor prognosis [18]. Schneider et al. showed that, in addition to those two prognostic factors, the presence of a retiform pattern was a third indicator of a poor prognosis [15]. The ESMO guidelines published in 2012 recommended that, for all stage I disease (without distinguishing between stages Ia and Ic), adjuvant chemotherapy should be considered in cases of poor differentiation and/or heterologous elements [18]. In 2014, the Study Group on Pediatric Rare Tumors described a series of 44 young patients with pediatric SLCT (median age 13 years) and confirmed that the differentiation grade, heterologous elements, and a retiform pattern were prognostic factors [14]. However, it can be challenging to administer adjuvant treatment for SLCT because of the lack of a standard. We found that the most frequently used first‐line adjuvant regimen was the combination of bleomycin, etoposide, and cisplatin (BEP) as shown in Table 4. Other regimens included ifosfamide, etoposide, and cisplatin, particularly for children [12].
For stage Ic disease, the analysis was complicated because of the lack of information regarding the specific Ic stage. According to the new FIGO classification, stage Ic has been broken down into three substages: Ic1, Ic2, and Ic3 [4]. However, in general, stage Ic has been correlated with a high risk of relapse (around 30 %) and a high risk of death (around 54%; Table 4). In our series, five patients had stage Ic disease (exclusively Ic1), and all received conservative surgery. Three patients received adjuvant chemotherapy. Of the five patients, three experienced a relapse (peritoneal carcinomatosis), and two died. The key message from those results was that, when treating young patients with a suspected ovarian mass, it is crucial to operate with extreme caution, particularly when there are signs of hyperandrogenism, to avoid a rupture (e.g., perform an oophorectomy rather than a cystectomy). Indeed, Young et al. identified the rupture as a poor prognostic factor [1]. The second message that arose from these data concerned the role of conservative surgery. Indeed, one explanation for the poor prognosis associated with stage Ic disease could be related to the preservation of the ovary, which raises the question of the safety of conservative surgery. Alternatively, the poor prognosis might be related to the natural history of SLCT or both ovary preservation and the natural history. However, adjuvant cisplatin‐based chemotherapy was indicated in all patients with stage Ic disease with an undifferentiated tumor, with or without a retiform pattern, and with or without heterologous elements [15], [18].
The prognosis of advanced‐stage disease (stage II and more severe) is poor; advanced stages are associated with a high rate of death. In our series, the two patients with advanced‐stage disease experienced relapses with peritoneal carcinomatosis, and one died from the disease. The second patient survived to a follow‐up of 44 months without disease. Table 2 shows that we found 19 patients with advanced‐stage disease. Of these, 14 experienced a relapse, and 11 died. Advanced‐stage disease or relapse may be managed with surgery (macroscopically complete, when possible), chemotherapy, radiotherapy, and combinations of these treatments. The best treatment remains to be defined. A few ongoing phase II trials are currently testing drugs for treating advanced SLCT, such as paclitaxel (Gynecologic Oncology Group NCT00006227) or paclitaxel with carboplatin (Gynecologic Oncology Group NCT01042522). Indeed, Brown et al., in a retrospective study of 44 patients with sex cord‐stromal tumors of the ovary, proposed that taxanes with platinum might serve as an alternative to BEP. Those authors argued that this chemotherapy regimen seemed to be active with less toxicity than other chemotherapies. Unfortunately, that series included only granulosa cell tumors and two unclassified tumors but no SLCTs [19]. Brown et al. reported the efficacy and safety of bevacizumab in a phase II trial of the Gynecologic Oncology Group in 36 patients with recurrent sex cord‐stromal tumors of the ovary. In that study, 32 patients had granulosa cell tumors, and 4 had unclassified sex cord‐stromal tumors [20]. Therefore, further studies are needed to investigate the efficacy of bevacizumab in SLCT and to determine the best chemotherapy regimen for SLTC.
Conclusion
Our results suggested that, for stage Ia disease, conservative surgery (in women of reproductive age) was safe and effective for treating ovarian SLCT. The place of adjuvant chemotherapy for stage Ia with poor prognostic factors (poor differentiation, retiform pattern, or heterologous elements) remains to be defined. For stage Ic1, we need more data to suggest safely the place of conservative surgery. The combination of bleomycin, etoposide, and cisplatin was the most frequently used regimen, but the best chemotherapy regimen remains to be defined.
Author Contributions
Conception/design: Sebastien Gouy, Philippe Morice
Provision of study material or patients: Sebastien Gouy, Alexandra Arfi, Catherine Genestie
Collection and/or assembly of data: Sebastien Gouy, Alexandra Arfi
Data analysis and interpretation: Sebastien Gouy, Alexandra Arfi, Amandine Maulard, Patricia Pautier, Enrica Bentivegna, Alexandra Leary, Cyrus Chargari, Catherine Genestie, Philippe Morice
Manuscript writing: Sebastien Gouy, Philippe Morice
Final approval of manuscript: Sebastien Gouy, Alexandra Arfi, Amandine Maulard, Patricia Pautier, Enrica Bentivegna, Alexandra Leary, Cyrus Chargari, Catherine Genestie, Philippe Morice
Disclosures
Sebastien Gouy: Roche (C/A); Alexandra Leary: GamaMabs Pharma (C/A), AstraZeneca, Gristone, Clovis (SAB), GamaMabs Pharma, AstraZeneca, Roche, Merus, Clovis (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Young RH, Scully RE. Ovarian Sertoli‐Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol 1985;9:543–69. [DOI] [PubMed] [Google Scholar]
- 2.Young RH. Sex cord‐stromal tumors of the ovary and testis: Their similarities and differences with consideration of selected problems. Mod Pathol 2005;18 (suppl 2):S81–S98. [DOI] [PubMed] [Google Scholar]
- 3.Goulvent T, Ray‐Coquard I, Borel S et al. DICER1 and FOXL2 mutations in ovarian sex cord‐stromal tumours: A GINECO Group study. Histopathology 2016;68:279–285. [DOI] [PubMed] [Google Scholar]
- 4.Prat J; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124:1–5. [DOI] [PubMed] [Google Scholar]
- 5.Liggins CA, Ma LT, Schlumbrecht MP. Sertoli‐Leydig cell tumor of the ovary: A diagnostic dilemma. Gynecol Oncol Rep 2015;15:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth LM, Anderson MC, Govan AD et al. Sertoli‐Leydig cell tumors: A clinicopathologic study of 34 cases. Cancer 1981;48:187–197. [DOI] [PubMed] [Google Scholar]
- 7.Prat J, Young RH, Scully RE. Ovarian Sertoli‐Leydig cell tumors with heterologous elements. II. Cartilage and skeletal muscle: A clinicopathologic analysis of twelve cases. Cancer 1982;50:2465–2475. [DOI] [PubMed] [Google Scholar]
- 8.Zaloudek C, Norris HJ. Sertoli‐Leydig tumors of the ovary. A clinicopathologic study of 64 intermediate and poorly differentiated neoplasms. Am J Surg Pathol 1984;8:405–418. [DOI] [PubMed] [Google Scholar]
- 9.Roth LM, Slayton RE, Brady LW et al. Retiform differentiation in ovarian Sertoli‐Leydig cell tumors. A clinicopathologic study of six cases from a Gynecologic Oncology Group study. Cancer 1985;55:1093–1098. [DOI] [PubMed] [Google Scholar]
- 10.Gui T, Cao D, Shen K et al. A clinicopathological analysis of 40 cases of ovarian Sertoli‐Leydig cell tumors. Gynecol Oncol 2012;127:384–389. [DOI] [PubMed] [Google Scholar]
- 11.Sigismondi C, Gadducci A, Lorusso D et al. Ovarian Sertoli‐Leydig cell tumors. A retrospective MITO study. Gynecol Oncol 2012;125:673–676. [DOI] [PubMed] [Google Scholar]
- 12.Xiao H, Li B, Zuo J et al. Ovarian Sertoli‐Leydig cell tumor: A report of seven cases and a review of the literature. Gynecol Endocrinol 2013;29:192–195. [DOI] [PubMed] [Google Scholar]
- 13.Bhat RA, Lim YK, Chia YN et al. Sertoli‐Leydig cell tumor of the ovary: Analysis of a single institution database. J Obstet Gynaecol Res 2013;39:305–310. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HY, Zhu JE, Huang W et al. Clinicopathologic features of ovarian Sertoli‐Leydig cell tumors. Int J Clin Exp Pathol 2014;7:6956–6964. [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider DT, Orbach D, Cecchetto G et al. Ovarian Sertoli Leydig cell tumours in children and adolescents: An analysis of the European Cooperative Study Group on Pediatric Rare Tumors (EXPeRT). Eur J. Cancer 2015;51:543–550. [DOI] [PubMed] [Google Scholar]
- 16.Akman L, Ertas IE, Gokcu M et al. Ovarian Sertoli‐Leydig cell tumors: A multicenter long‐term clinicopathological analysis of 27 patients. J Cancer Res Ther 2016;12:290–294. [DOI] [PubMed] [Google Scholar]
- 17.Nam SM, Kim JW, Eoh KJ et al. A novel clinicopathological analysis of early stage ovarian Sertoli‐Leydig cell tumors at a single institution. Obstet Gynecol Sci 2017;60:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo N, Peiretti M, Garbi A et al.; ESMO Guidelines Working Group. Non‐epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2012;23 (suppl 7):vii20–vii26. [DOI] [PubMed] [Google Scholar]
- 19.Brown J, Shvartsman HS, Deavers MT et al. The activity of taxanes in the treatment of sex cord‐stromal ovarian tumors. J Clin Oncol 2004;22:3517–3523. [DOI] [PubMed] [Google Scholar]
- 20.Brown J, Brady WE, Schink J et al. Efficacy and safety of bevacizumab in recurrent sex cord‐stromal ovarian tumors: Results of a phase 2 trial of the Gynecologic Oncology Group. Cancer 2014;120:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]