Abstract
Lessons Learned.
Non‐small‐cell lung cancer (NSCLC) represents 85% of lung cancer in elderly patients.
In the present study performed in the 36 elderly subjects with epidermal growth factor receptor (EGFR) T790M mutation‐positive NSCLC, osimertinib 80 mg demonstrated statistically significant improvement in the objective response rate, which was comparable to those in the nonelderly population.
Osimertinib appears to be an effective and safe treatment option in elderly patients with advanced NSCLC with EGFR mutation; further research in larger scale is warranted.
Background.
Previous findings suggest the possibility of relatively safe use of osimertinib for patients with T790M‐positive non‐small‐cell lung cancer (NSCLC), with few serious adverse events for the elderly in comparison with conventional endothelial growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), and with an antitumor effect.
Methods.
This phase II study was performed to prospectively investigate the efficacy and safety of osimertinib for elderly patients aged ≥75 years with ineffective prior EGFR TKI treatment or with recurrence in T790M EGFR TKI resistance mutation‐positive NSCLC.
Results.
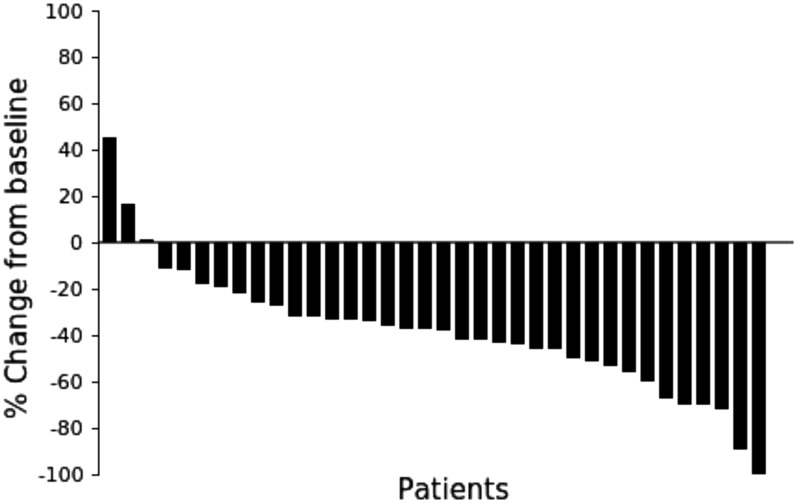
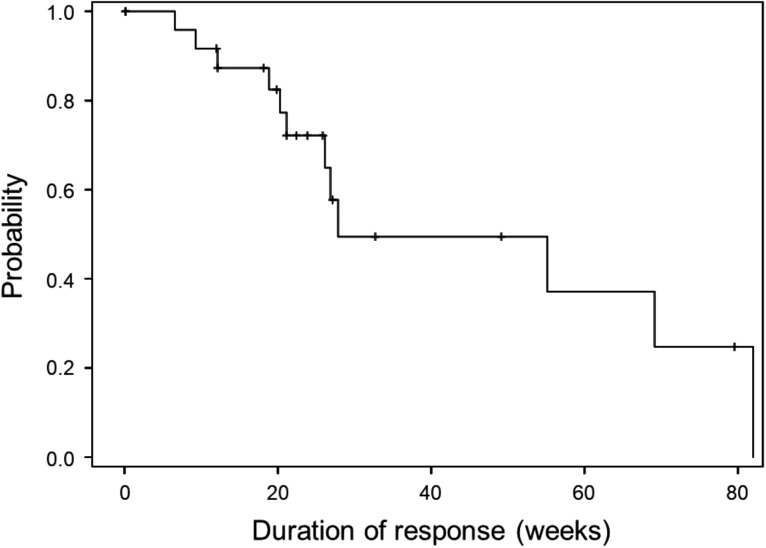
A total of 36 patients were included in the analyses. Among the 36 subjects, 63.9% were female, with mean age of 79.9 years. The objective response rate (ORR) was 58.3% (95% confidence interval [CI], 42.2%–72.9%), demonstrating statistically significant efficacy of osimertinib (p = .0017). The median duration of response (DOR) was 27.9 weeks (95% CI, 21.1–82.0). Complete response (CR) and partial response (PR) were 2.8% and 55.6%, respectively. Disease control rate (DCR) was 97.2%. A waterfall plot revealed that 33 (91.6%) subjects exhibited tumor shrinkage during treatment, including 12 of 14 subjects who had stable disease (SD). All adverse events were not reason for discontinuation of the study drug.
Conclusion.
Osimertinib may be an effective and safe treatment option in elderly patients with advanced NSCLC with EGFR mutation.
Abstract
经验获取
• 非小细胞肺癌 (NSCLC) 在老年患者肺癌中占 85%。
• 在本次于 36 名表皮生长因子受体 (EGFR) T790M 突变阳性 NSCLC 老年受试者中实施的研究中, 奥希替尼80 mg在客观缓解率方面展示出统计学方面的显著改善,这可以与非老年群体中的结果相媲美。
• 对于患有 EGFR 突变的晚期 NSCLC 老年患者,奥希替尼似乎是一种有效且安全的治疗方案;有必要进一步实施大规模研究。
摘要
背景。既往研究结果表明,在治疗 T790M‐阳性非小细胞肺癌 (NSCLC) 患者时可以相对安全地使用奥希替尼,与传统的表皮生长因子受体 (EGFR) 酪氨酸激酶抑制剂 (TKI) 相比,这种治疗方法在老年患者中产生的重度不良反应较少,且具有抗肿瘤效果。
方法。本次 II 期研究旨在前瞻性地调查奥希替尼在既往经 EGFR TKI 治疗无效或出现 T790M EGFR TKI 耐药性突变‐阳性 NSCLC 复发且年龄 ≥75 岁的老年患者中的疗效和安全性。
结果。一共有 36 名患者被纳入分析。在 36 名受试者中,63.9% 的患者为女性,平均年龄为 79.9 岁。客观缓解率 (ORR) 为 58.3% [95% 置信区间 (CI),42.2%–72.9%],显示出奥希替尼在统计学方面的显著疗效 (p = 0.001 7)。中位缓解持续时间 (DOR) 为 27.9 周(95% CI,21.1–82.0)。完全缓解 (CR) 和部分缓解 (PR) 分别为 2.8% 和 55.6%。疾病控制率 (DCR) 为 97.2%。据瀑布图显示,33 名受试者 (91.6%) 在治疗期间出现肿瘤退缩,包括 14 名受试者中的 12 名受试者出现疾病稳定 (SD)。所有不良反应均不是停用研究药物的原因。
结论。在患有 EGFR 突变的晚期 NSCLC 老年患者中,奥希替尼可能是一种有效且安全的治疗方案。
Discussion
Lung cancer is the most common malignancy, accounting for 12.9% of all new cancer diagnoses, and the most common cause of cancer deaths [1]. Median age at diagnosis of lung cancer has been reported to be 63–70 years [2], [3], [4], [5], [6], and NSCLC represents 85% of lung cancer in elderly patients [7], [8], [9]. Treatment of elderly patients requires particular care for several reasons. Elderly patients typically have more comorbidities than younger patients, requiring concurrent management. Physiological functions decline with aging, such that some patients tolerate therapy more poorly. Furthermore, concurrent noncancer therapies may interfere with the metabolism of chemotherapeutic drugs. Afatinib, a second‐generation EGFR TKI, showed a proliferation‐inhibitory effect on a lung cancer cell strain that had T790M mutation [10]; however, as for safety in the elderly, the medical evidence is not yet clear, unlike that for the first‐generation EGFR TKI.
Osimertinib, a third‐generation EGFR TKI that irreversibly inhibits EGFR, demonstrates inhibitory activity against tumor cells harboring sensitizing EGFR mutations and also selective inhibitory activity against tumor cells harboring the TKI‐resistant mutation T790M [11], and is a first‐line treatment for EGFR mutation‐positive NSCLC [12]. The grade ≥3 adverse events reported in patients receiving osimertinib as a first‐line treatment in the AURA study [12], AURA extension study [13], and AURA2 study [14] were rash (2%, 1%, and 1%, respectively) and diarrhea (3%, 1%, and 1%, respectively). These results suggest the possibility of relatively safe use of osimertinib with few serious adverse events for the elderly in comparison with conventional EGFR TKIs.
Figure 1.
Waterfall plot of maximum changes in tumor size (diameter) from baseline in individual patients during the treatment.
In the present study, which included 36 patients with EGFR T790M mutation‐positive NSCLC with ineffective prior EGFR TKI treatment or with recurrence, the ORR was 58.3% (95% CI, 42.2%–72.9%), the DOR was 27.9 weeks (95% CI, 21.1–82.0), and the DCR was 97.2% with osimertinib 80 mg administration. Because the lower limit of the estimated CI exceeded a threshold of 35%, a statistically significant improvement in the ORR was demonstrated. The ORR in the present study performed in elderly patients was comparable to that in the nonelderly population [13], [14], [15]. In addition, a waterfall plot revealed that 33 (91.6%) subjects exhibited tumor shrinkage during treatment, including 12 of 14 subjects who had SD.
There were no fatal events and no adverse events that required dose reduction or discontinuation of the study drug. The results of previous findings and the present study suggest that osimertinib may be an effective and safe treatment option in elderly patients with advanced NSCLC with EGFR mutation.
Trial Information
- Disease
Lung cancer – NSCLC
- Stage of Disease/Treatment
Metastatic/advanced
- Prior Therapy
More than two prior regimens
- Type of Study ‐ 1
Phase II
- Type of Study ‐ 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoint
Toxicity
- Additional Details of Endpoints or Study Design
- Study design: This study was a single‐arm, open‐label trial. The rationale and design of the present study have been published elsewhere [16].
- Inclusion criteria: The inclusion criteria were (a) histologically or cytologically confirmed stage IIIB–IV NSCLC and postoperative recurrence; (b) recurrence after a response better than SD or not effective (less than SD) observed in best overall response according to RECIST after treatment with EGFR TKI (e.g., erlotinib, gefitinib, afatinib) for NSCLC or (c) NSCLC harboring activating EGFR mutations with or without T790M mutation; (d) able to accept treatment with oral medicine; (e) at least one measurable lesion according to RECIST criteria; (f) Eastern Cooperative Oncology Group (ECOG) performance status 0–1; (g) in principle, capable of participating in this study for at least A 2‐week hospital stay or corresponding management; (h) ≥75 years of age (at the time of enrollment); (i) bone marrow, hepatic, and renal functions confirmed normal within 14 days prior to enrollment according to clinical test standards (white blood cell count ≥3,000/mm3 to ≤12,000/mm3, neutrophil count ≥1,500/mm3, platelet count ≥100,000/mm3, hemoglobin ≥9.0 g/dL, aspartate aminotransferase, alanine aminotransferase ≤100 IU/L, total bilirubin ≤1.5 mg/dL, creatinine ≤2.0 mg/dL, or SpO2 [room air] ≥90%); (j) life expectancy of at least 3 months; (k) prior treatment having passed the planned starting point of administration (for chemotherapy, ≥4 weeks have passed since the final treatment of prior chemotherapy; for EGFR TKI, the next day after the last administration; for radiation therapy in the case of radiation to the chest, ≥12 weeks have passed since the day of final radiation treatment; for radiation therapy in the case of radiation to other than the chest, ≥2 weeks have passed since the day of final radiation treatment; for an operation or treatment, including chest drainage, ≥4 weeks have passed since the day of final operation or treatment); and (l) having provided written informed consent to participate in the study.
- Exclusion criteria: The exclusion criteria were (a) treatment history of osimertinib or other third‐generation EGFR TKI; (b) pulmonary disorders such as idiopathic pulmonary fibrosis, interstitial pneumonia, pneumoconiosis, active radiation pneumonitis, and drug‐induced pneumonia; (c) infectious disorders requiring intravenous injection of antibacterial drugs or antimycotics; (d) inability to swallow oral medications; (e) currently receiving (or unable to stop use prior to receiving the first dose of study treatment) medications or herbal supplements known to be potent inhibitors of cytochrome P450 3A4 (CYP3A4; at least 1 week prior) or potent inducers of CYP3A4 (at least 3 weeks prior; all patients avoided concomitant use of any medications, herbal supplements, and/or ingestion of foods with known inducer or inhibitory effects on CYP3A4); (f) currently receiving checkpoint inhibitor; (g) patients with certain cardiac criteria (mean resting corrected QT interval [using Fridericia formula] >470 ms; any clinically important abnormalities in rhythm, conduction, or morphology of resting electrocardiogram [e.g., complete left‐bundle branch block, third‐degree heart block, second‐degree heart block]; any factors that increase the risk of QTc prolongation or risk of arrhythmic events such as heart failure, hypokalemia, congenital long QT syndrome, family history of long QT syndrome, unexplained sudden death under 40 years of age in first‐degree relatives, or any concomitant medication known to prolong the QT interval), (h) those who are pregnant, nursing, or possibly pregnant; (i) symptomatic brain metastases; (j) active double cancer; (k) patients with uncontrollable diabetes mellitus; (l) complications of clinical concern (such as uncontrollable cardiac disease, severe cardiac arrhythmia requiring medical treatment, sustained serious diarrhea); (m) regarded as unsuitable for this study by the investigators.
- Ethics: The study was conducted in accordance with ethical principles originating in the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, Brazil, October 2013), Ethical Guidelines for Medical and Health Research Involving Human Subjects (December 22, 2014), and Ethical Guidelines for Human Genome/Gene Analysis Research (March 29, 2001). The study received ethical approval from the Ethics Committee of Kyoto Prefectural University of Medicine, Kyoto, Japan (number ERB‐C‐630‐3, the last edition version 3, December 21, 2016). The trial was subject to the supervision and management of the Ethics Committee. All patients provided written informed consent.
- Endpoints: The primary endpoint was ORR defined as the proportion of subjects whose best overall response was either CR or PR. Secondary endpoints were DCR defined as the percentage of patients who have achieved CR, PR, or SD, and safety. Depth of response (DpR), defined as maximum percent change in tumor reduction from baseline, was calculated for each subject and plotted with response status (waterfall plot). Grade and frequency of each adverse event and laboratory abnormality were recorded.
- Statistical analysis: For the primary endpoint (ORR), response rate and its two‐sided 95% CI were calculated using Wilson's method. When the lower limit of the estimated CI exceeded a threshold of 35%, statistical significance was decided. Disease control rate (DCR) and its 2‐sided 95% CI were calculated (Wilson's method).
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working Name
Osimertinib
- Company Name
AstraZeneca
- Drug Type
Small molecule
- Drug Class
EGFR
- Dose
Milligrams (mg) per flat dose
- Route
Oral (p.o.)
- Schedule of Administration
Osimertinib 80 mg OD tablet was orally administered until progressive disease occurred or a criterion for discontinuation was met.
Patient Characteristics
- Number of Patients, Male
13
- Number of Patients, Female
23
- Stage
IIIB: 1, IV: 25, Postoperative recurrence: 10
- Age
Median (range): 79.9 years
- Number of Prior Systemic Therapies
Median (range): Not collected
- Performance Status: ECOG
-
0 — 8
1 — 28
2 — 0
3 — 0
Unknown — 0
- Other
- A total of 36 patients were enrolled at 21 study centers. All 36 patients were included in the efficacy and safety analyses. Among the 36 subjects, 63.9% were female, with mean age of 79.9 years; 97.2% of the subjects had adenocarcinoma, 69.4% were at stage IV, and 27.8% had postoperative recurrent NSCLC. All patients were harboring EGFR T790M mutation: 61.1% had exon 19 deletion, and 30.6% had L858R mutation.
- Cancer Types or Histologic Subtypes
Adenocarcinoma, 35; Combined SCLC with adenocarcinoma, 1
Primary Assessment Method
- Title
ORR
- Number of Patients Enrolled
36
- Number of Patients Evaluable for Toxicity
36
- Number of Patients Evaluated for Efficacy
36
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 1
- Response Assessment PR
n = 20
- Response Assessment SD
n = 14
- Response Assessment PD
n = 1
- Response Assessment OTHER
n = 0
- Outcome Notes
The ORR was 58.3% (95% CI, 42.2%–72.9%), demonstrating statistically significant efficacy of osimertinib (p = 0.0017). The median of the DOR was 27.9 weeks (95% CI, 21.1–82.0). CR and PR were 2.8% and 55.6%, respectively. DCR was 97.2%. The waterfall plot shows that 33 (91.6%) subjects exhibited tumor shrinkage during treatment, including 12 of 14 subjects who had SD. DpR ≥50% was achieved in 11 (30.5%) subjects and ≥30% in 26 (72.2%) subjects.
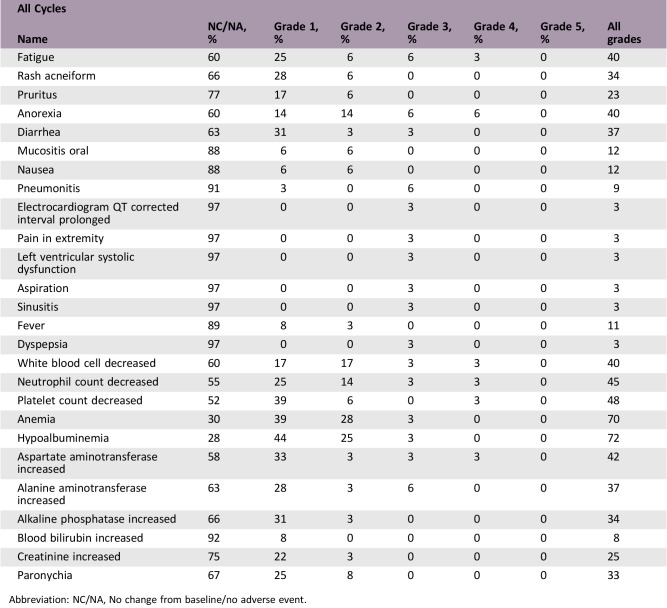
Adverse Events
Abbreviation: NC/NA, No change from baseline/no adverse event.
- Adverse Events Legend
Adverse events reported in 10% or more cases were fatigue (38.9%), decreased appetite (38.9%), diarrhea (36.1%), rash (33.3%), paronychia (33.3%), pruritus (22.2%), oral mucositis (11.1%), nausea (11.1%), and fever (11.1%).
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Active and should be pursued further
To the authors’ knowledge, this is the first prospective study to examine the efficacy and safety of osimertinib in elderly patients with epidermal growth factor receptor (EGFR) mutation T790M‐positive non‐small‐cell lung cancer (NSCLC) with disease progression on prior treatment. In the AURA phase II extension study performed in patients with EGFR mutation T790M‐positive advanced NSCLC and progression after EGFR tyrosine kinase inhibitor (TKI) treatment [13], the objective response rate (ORR) was 62% (95% confidence interval [CI], 54%–68%), and in the AURA 2 phase II and AURA 3 phase III studies in patients after NSCLC progression on frontline EGFR TKI, ORR was 51%–71% [14], [15]. In comparison, the ORR of docetaxel, which is the standard treatment for elderly patients in Japanese guidelines, has been reported to be 22.7% in a controlled study with vinorelbine and 41.2% in a study of combined carboplatin and pemetrexed in elderly Japanese patients [17], [18]. Based on these findings, the expected response rate and threshold response rate are estimated to be 60% and 35%, respectively. Assuming a two‐sided significance level of 5% and a power of 80%, 31 subjects were required. Considering dropouts, 35 subjects were to be enrolled in the study. In the present study, ORR was 58.3% (95% CI, 42.2%–72.9%), duration of response was 27.9 weeks (95% CI, 21.1–82.0), and the disease control rate was 97.2% with osimertinib 80 mg administration in 36 elderly subjects with EGFR T790M‐positive NSCLC. Because the lower limit of the estimated CI exceeded a threshold of 35%, statistically significant improvement in the ORR was demonstrated. The ORR in the present study performed in elderly patients was comparable to those in the nonelderly population. In addition, waterfall plot revealed that 33 (91.6%) subjects exhibited tumor shrinkage during treatment, including 12 of 14 subjects who had stable disease.
The most common side effects observed in the AURA phases I–II and AURA 2 studies were gastrointestinal symptoms (diarrhea, 47%; nausea, 22%; and decreased appetite, 21%), followed by dermatologic side effects (rash, 40%; dry skin; and pruritus), and a fatal event was reported as being possibly drug‐related. Hyperglycemia and QT prolongation were seen in 2% and 4%, respectively, which required no dose reduction [19]. In the present study, as for the gastrointestinal symptoms, decreased appetite was observed in more patients compared with those in the AURA studies (38.9% vs. 21%); however, diarrhea (36.1% vs. 47%) and nausea (11.1% vs. 22%) were less frequent. Among these, grade ≥3 events were decreased appetite (11.1%) and diarrhea (2.8%). The most frequent dermatological symptom, rash, was less frequent in the present study versus that of the AURA studies (33.3% vs. 40%), followed by paronychia in 33.3%, but neither of these was grade ≥3. There were no fatal events and no adverse events that required dose reduction or discontinuation of the study drug.
A recently conducted retrospective study compared the frequency of adverse events associated with osimertinib between elderly (aged ≥75 years) and nonelderly (aged <75 years) patients with advanced NSCLC with EGFR T790M mutation [20]. Comparison was also performed between the initial EGFR TKI treatment before osimertinib administration in the same patient cohort. The results showed that the only grade ≥2 event that was significantly more frequent in the elderly group compared with the nonelderly group was paronychia (16.6% vs. 1.6%; p = .04), and the maximum grade of EGFR TKI‐related adverse events associated with osimertinib in the elderly group was significantly lower than that of the initial EGFR TKI treatment (p = .03).
To obtain conclusive results, further studies in a larger elderly population is warranted.
Taking together the results of previous findings and the present study, osimertinib appears to be an effective and safe treatment option in elderly patients with advanced NSCLC with EGFR mutation.
Figure
Figure 2.
Kaplan‐Meier curve shows the duration of response, defined as the time from the achievement of a response to progression, among 26 responders. The median of the duration of response was 27.9 weeks (95% confidence interval, 21.1–82.0 weeks).
Acknowledgments
We thank all of the patients who participated in this study, as well as their families. We also thank the Clinical Research Support Center Kyushu for managing the study. This study was funded by AstraZeneca (externally sponsored scientific research number ESR‐15‐11419).
Contributed equally
Footnotes
ClinicalTrials.gov Identifier: UMIN000022553
Sponsor(s): AstraZeneca
Principal Investigator: Junji Uchino
IRB Approved: Yes
Disclosures
Junji Uchino: AstraZeneca, Eli Lilly Japan K.K. (RF); Minoru Fukuda: AstraZeneca (H, RF); Masaki Fujita: AstraZeneca (H); Koichi Takayama: Chugai‐Roche Co., Ono Pharmaceutical Co. (RF), AstraZeneca, Chugai‐Roche Co., MSD‐Merck Co., Eli Lilly Co., Boehringer‐Ingelheim Co., DaiichiSankyo Co. (H); Kenichi Yoshimura: AstraZeneca, Chugai (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2915;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Lebitasy MP, Hédelin G, Purohit A et al. Progress in the management and outcome of small‐cell lung cancer in a French region from 1981–1994. Br J Cancer 2001;85:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregor A, Thomson CS, Brewster DH et al. Management and survival of patients with lung cancer in Scotland diagnosed in 1995: Results of a national population based study. Thorax 2001;56:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foeglé J, Hédelin G, Lebitasy MP et al. Non‐small‐cell lung cancer in a French department, (1982–1997): Management and outcome. Br J Cancer 2005;92:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchon F, Grivaux M, Collon T et al. Epidemiologic of primary bronchial carcinoma management in the general French hospital centres [in French]. Rev Mal Respir 2002;19:727–734. [PubMed] [Google Scholar]
- 6.Jennens RR, Giles GG, Fox RM. Increasing underrepresentation of elderly patients with advanced colorectal or non‐small‐cell lung cancer in chemotherapy trials. Intern Med J 2006;36:216–220. [DOI] [PubMed] [Google Scholar]
- 7.Owonikoko TK, Ragin CC, Belani CP et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570–5577. [DOI] [PubMed] [Google Scholar]
- 8.Piquet J, Blandhon F, Grivaux M et al. Primary lung cancer in elderly subjects in France [in French]. Rev Mal Respir 2004;21:8570–8578. [PubMed] [Google Scholar]
- 9.Quoix E, Monnet I, Scheid P et al. Management and outcome of French elderly patients with lung cancer: An IFCT survey [in French]. Rev Mal Respir 2010;27:421–430. [DOI] [PubMed] [Google Scholar]
- 10.Sos ML, Rode HB, Heynck S et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res 2010;70:868–874. [DOI] [PubMed] [Google Scholar]
- 11.Cross DAE, Ashton SE, Ghiorghiu S et al. AZD9291, an irreversible EGFR TKI overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramalingam SS, Yang JC, Lee CK et al. Osimertinib as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer. J Clin Oncol 2018;36:841–849. [DOI] [PubMed] [Google Scholar]
- 13.Yang JCH, Ahn MJ, Kim W et al. Osimertinib in pretreated 790m‐positive advanced non‐small‐cell lung cancer: AURA Study Phase II extension component. J Clin Oncol 2017;35:1288–1296. [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Tsai CM, Shepherd F et al. AZD9291 in pre‐treated T790M positive advanced NSCLC: AURA2 phase II study. J Thorac 2015;10:S320. [Google Scholar]
- 15.Mok TS, Wu YL, Ahn MJ et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med 2017;376:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchino J, Nakao A, Tamiya N et al. Treatment rationale and design of the SPIRAL study: A phase II trial of osimertinib in elderly epidermal growth factor receptor T790M‐positive nonsmall‐cell lung cancer patients who progressed during prior EGFR‐TKI treatment. Medicine 2018;97:e11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudoh S, Takeda K, Nakagawa K et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non‐small‐cell lung cancer: Results of the West Japan Thoracic Oncology Group trial (WJOG 9904). J Clin Oncol 2006;24:3657–3663. [DOI] [PubMed] [Google Scholar]
- 18.Tamiya M, Tamiya A, Kaneda H et al. A phase II study of pemetrexed plus carboplatin followed by maintenance pemetrexed as first‐line chemotherapy for elderly patients with advanced non‐squamous non‐small cell lung cancer. Med Oncol 2016;33:2. [DOI] [PubMed] [Google Scholar]
- 19.Saad N, Poudel A, Basnet A et al. Epidermal growth factor receptor T790M mutation‐positive metastatic non‐small‐cell lung cancer: Focus on osimertinib (AZD9291). Onco Targets Ther 2017;10:1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuta H, Uemura T, Yoshida T et al. Efficacy and safety data of osimertinib in elderly patients with NSCLC who harbor the EGFR T790M mutation after failure of initial EGFR‐TKI treatment. Anticancer Res 2018;38:5231–5237. [DOI] [PubMed] [Google Scholar]