Financial relationships between physicians and the pharmaceutical industry are common. This article evaluates the magnitude of the association between compensation and prescribing.
Keywords: Conflict of interest, Physicians, Antineoplastic agents, Practice patterns, Drug prescriptions, Drug industry
Abstract
Background.
Financial relationships between physicians and the pharmaceutical industry are common, but factors that may determine whether such relationships result in physician practice changes are unknown.
Materials and Methods.
We evaluated physician use of orally administered cancer drugs for four cancers: prostate (abiraterone, enzalutamide), renal cell (axitinib, everolimus, pazopanib, sorafenib, sunitinib), lung (afatinib, erlotinib), and chronic myeloid leukemia (CML; dasatinib, imatinib, nilotinib). Separate physician cohorts were defined for each cancer type by prescribing history. The primary exposure was the number of calendar years during 2013–2015 in which a physician received payments from the manufacturer of one of the studied drugs; the outcome was relative prescribing of that drug in 2015, compared with the other drugs for that cancer. We evaluated whether practice setting at a National Cancer Institute (NCI)‐designated Comprehensive Cancer Center, receipt of payments for purposes other than education or research (compensation payments), maximum annual dollar value received, and institutional conflict‐of‐interest policies were associated with the strength of the payment‐prescribing association. We used modified Poisson regression to control confounding by other physician characteristics.
Results.
Physicians who received payments for a drug in all 3 years had increased prescribing of that drug (compared with 0 years), for renal cell (relative risk [RR] 1.81, 95% confidence interval [CI] 1.58–2.07), CML (RR 1.22, 95% CI 1.08–1.39), and lung (RR 1.69, 95% CI 1.58–1.82), but not prostate (RR 0.97, 95% CI 0.93–1.02). Physicians who received compensation payments or >$100 annually had increased prescribing compared with those who did not, but NCI setting and institutional conflict‐of‐interest policies were not consistently associated with the direction of prescribing change.
Conclusion.
The association between industry payments and cancer drug prescribing was greatest among physicians who received payments consistently (within each calendar year). Receipt of payments for compensation purposes, such as for consulting or travel, and higher dollar value of payments were also associated with increased prescribing.
Implications for Practice.
Financial payments from pharmaceutical companies are common among oncologists. It is known from prior work that oncologists tend to prescribe more of the drugs made by companies that have given them money. By combining records of industry gifts with prescribing records, this study identifies the consistency of payments over time, the dollar value of payments, and payments for compensation as factors that may strengthen the association between receiving payments and increased prescribing of that company's drug.
Introduction
Financial relationships between physicians and the pharmaceutical industry are common in the U.S. The majority of U.S. physicians receive gifts and/or direct compensation from drug companies [1], totaling $6.48 billion in 2015 [2]. Financial relationships with industry are associated with changes in physicians’ interpretation of clinical trial results [3], clinical practice guideline recommendations [4], and prescribing of higher‐cost and/or brand‐name pharmaceuticals [5], [6], [7], [8], [9], [10], [11]. Although a causal effect of industry payments has not been demonstrated, these findings have given credence to public concern over a perceived influence of the pharmaceutical industry on medical practice.
Oncologists who have received money from a drug manufacturer tend to use more of that manufacturer's cancer drug(s) [12]. However, questions remain regarding which kinds of physician‐industry relationships have the greatest potential to influence physicians. Payments to physicians occur in many different forms, vary widely in financial value, and span different periods of time. Payments range from brief sponsored meals at promotional events to long‐term collaborative and consulting arrangements; it is unlikely that all types of payments have the same association with physician behavior. Additionally, physician practice setting may be an important factor. Academic physicians, such as those at National Cancer Institute (NCI)‐designated Comprehensive Cancer Centers, may have different kinds of relationships with industry or respond to them differently.
Because there has been little research describing the relationship between conflicts of interest (COIs) and physician behavior, such COI policies cannot be designed in an evidence‐based manner. A better understanding of what types of physician‐industry relationships are associated with differences in physician behavior will be important to inform the creation of evidenced‐based COI policies. This will help inform institutions considering important questions such as which types of physician‐industry relationships require increased scrutiny, or thresholds for maximum allowable industry payment amounts.
Therefore, the goal of this study was to evaluate several factors that might affect the magnitude of the association between receipt of financial payments from the pharmaceutical industry and oncologist prescribing, as well as the success of COI policies already in place. Specifically, we hypothesized that the consistency of physician‐industry payments over time, receipt of compensation payments, increased dollar value of payments, physician affiliation with an NCI‐designated Comprehensive Cancer Center, and less stringent institutional conflict‐of‐interest policies would strengthen the association between payments and prescribing.
Materials and Methods
Data Sources
We linked three large, publicly available data sets. The first was Open Payments, a transparency law requiring disclosure of all payments greater than $10 from U.S. drug manufacturers to physicians and teaching hospitals. Each record contained information regarding the industry payer and the physician recipient. Open Payments groups industry payments into three main categories: General Payments, Research Payments, and Ownership Interests. General Payments, which were the focus of this study, are further grouped into subcategories such as gifts, consulting or speaker fees, meals, travel, lodging, and education [13].
The second source was the Medicare Part D Public Use File, compiled by the Centers for Medicare and Medicaid Services (CMS) from prescription drug claims under Medicare Part D, a prescription drug benefit enrolling approximately 70% of Medicare‐eligible Americans [14]. For each provider, this file contained the number of claims filled for each prescription drug, by calendar year. To prevent reidentification, only drugs for which the provider had 10 or more claims in each calendar year are included [15].
For additional provider demographic data (gender, graduation year, practice size, institutional affiliation), the third source was Physician Compare, a CMS initiative to track physician performance and care quality [16].
Selection of Cancer Types
We analyzed cancer types for which several U.S. Food and Drug Administration‐approved, National Comprehensive Cancer Network (NCCN)‐recommended treatment options were available and on patent for the majority of the study period. We included orally administered agents with at least 10 unique physician prescribers within Medicare Part D during 2013–2015. Full description of the cancer type selection process is included in supplemental online data.
For each cancer, the Part D drugs used for its treatment constituted the choice set. The cancer types that met our inclusion criteria, and the associated choice sets, were as follows: renal cell cancer (axitinib, everolimus, pazopanib, sorafenib, sunitinib), prostate cancer (abiraterone, enzalutamide), chronic myeloid leukemia (CML; dasatinib, imatinib, nilotinib), and lung cancer (afatinib, erlotinib).
Physician Cohort Selection
Among the physicians identified as prescribers of any of the drugs of interest, we defined separate cancer‐specific cohorts. Physicians were included in a cancer‐specific cohort if they had 10 or more claims for one or more of the drugs in the choice set for that cancer type, during each year from 2013 to 2015. Individual physicians could be included in more than one cohort. Because imatinib is used to treat cancer types besides CML, to increase specificity for physicians treating CML, we required the CML cohort to have 10 or more associated claims for two or more drugs in the CML choice set in each year. Because some of the drugs in the renal cell cancer choice set (particularly sorafenib) also had indications for other cancer types, we attempted to apply similar requirements for the renal cell cancer cohort, but ultimately did not because of small sample size.
Exposure and Outcome Definitions
The primary physician‐level exposure for each drug was the number of calendar years during 2013–2015 in which the physician received one or more general payments from that drug's manufacturer (the manufacturer[s] associated with each drug can be found in supplemental online Table 1). The primary contrast was between physicians who never received payments from the manufacturer of a given drug and those who received payments in all 3 years.
The physician‐level outcome for each drug was the proportion of claims for the drug of interest among claims for all drugs in the relevant choice set, during 2015. For example, the outcome for abiraterone would be the number of claims for abiraterone, divided by the number of claims for both abiraterone and enzalutamide.
We used modified Poisson regression to estimate prescribing for each drug as a function of years of payment. Comparisons between groups of physicians were expressed as the relative risk (RR) of prescribing. In addition to drug‐specific models, we also ran aggregate models for each cancer type that included all drugs in the corresponding choice set; for the aggregate models, we used generalized estimating equations with a Poisson distribution, clustered at the level of the physician to account for repeated observations. In these models, the outcome was the relative risk of prescribing the drug produced by the manufacturer from which the physician had received payments (henceforth, the “paid drug”), among all drugs in that choice set. As payments from the manufacturer of imatinib have been found to be associated with decreased, rather than increased, use of imatinib [12], in the CML model, we included only dasatinib and nilotinib.
We weighted each physician by the total number of claims within the choice set for that cancer type. We controlled for potential confounders of the payment‐prescribing association, including physician gender, years since medical school graduation, practice size, overall prescribing volume (as a marker of patient volume across all cancer types), and affiliation with an NCI‐designated Comprehensive Cancer Center. In order to differentiate the contribution of receiving payments in more years from simply receiving more payments overall, we also controlled for the total dollar value of payments received for the paid drug during the study period.
To test whether our results were substantively affected by the temporal overlap between the payment exposure and prescribing outcome in 2015, we performed sensitivity analysis treating 2015 prescriptions as a function of the number of years of payments during 2013–2014 (zero, one, or two).
Subgroup and Dose‐Response Analyses
We evaluated the association between several physician characteristics and the strength of the observed payment‐prescribing association. For each characteristic, the primary contrast of interest was for those paid in 3 years versus those paid in 0 years.
The first characteristic was practice setting at an NCI‐designated Comprehensive Cancer Center (“NCI physicians”), ascertained by matching the NCI list to the Physician Compare‐derived hospital and/or institutional association for each physician. We evaluated for modification of the payment‐prescribing association using an additional set of models (one for each cancer type) with interaction terms between NCI affiliation and each stratum of the exposure variable.
The second characteristic was the type of payments received. We grouped general payment subcategories into those likely to represent short‐term educational interactions (Education, Food and Beverage, Charitable Contribution, Grant; “education payments”) and those likely to represent compensation for services the physician provided to the manufacturer (Compensation for Services, Consulting Fee, Current or Prospective Ownership or Investment Interest, Entertainment, Honoraria, Royalty or License, Travel and Lodging; “compensation payments”). We estimated the relative risk of prescribing separately for physicians who had received education payments only and those who had received compensation payments.
The third characteristic was the single‐year payment maximum a physician received. We grouped physicians according to the greatest dollar value received for the paid drug within a single calendar year: (a) never received ≥$100 from the manufacturer of the paid drug during any calendar year, (b) received ≥$100 but <$1,000, (c) received ≥$1,000 but <$5,000, (d) received ≥$5,000 but <$20,000, and (e) received ≥$20,000. We included the $5,000 and $20,000 cutoffs because they represent important COI thresholds for the NIH [17] and NCCN [18], respectively. We then estimated the relative risk of prescribing the paid drug separately for each group.
The fourth characteristic was the strength of the COI policy at the physician's institution. We defined institutional COI policy strength using data previously published by the Institute on Medicine as a Profession (IMAP) [19]. Our resulting measure of COI policy strength ranged from zero (least stringent) to six (most stringent); details regarding this measure are presented in supplemental online data. Among the subset of physicians at IMAP‐evaluated institutions, we asked (a) if more stringent COI policies were associated with a lower likelihood of receiving payments, and (b) if more stringent COI policies weakened the payment‐prescribing association.
Results
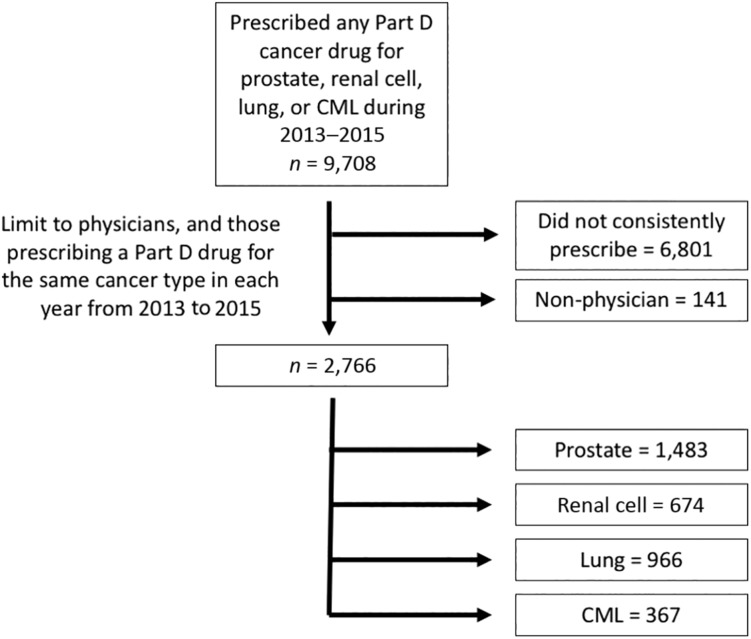
After removing duplicate records, physicians who had no claims for any of the 12 drugs of interest, and physicians who did not have sufficient prescribing to be included in any of the cancer‐specific cohorts, 2,766 physicians remained. Six hundred seventy‐four were included in the renal cell cancer cohort, 1,483 for prostate cancer, 367 for CML, and 966 for lung cancer (Fig. 1).
Figure 1.
Cohort selection.
Abbreviation: CML, chronic myeloid leukemia.
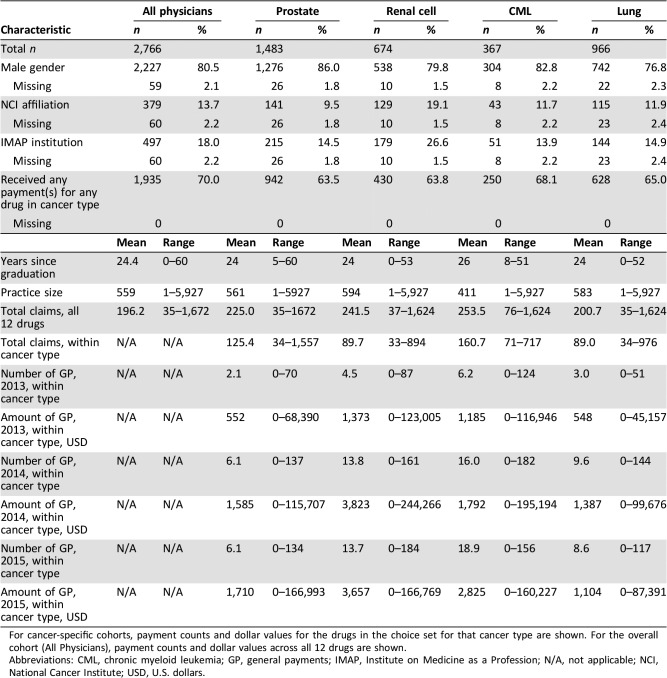
Of these physicians, 80.5% identified as male, 13.7% were affiliated with NCI centers, and 18.0% were in academic institutions evaluated by IMAP. There was a wide range in practice size, from single‐physician practices to a maximum size of 5,927 (Table 1).
Table 1. Physician characteristics.
For cancer‐specific cohorts, payment counts and dollar values for the drugs in the choice set for that cancer type are shown. For the overall cohort (All Physicians), payment counts and dollar values across all 12 drugs are shown.
Abbreviations: CML, chronic myeloid leukemia; GP, general payments; IMAP, Institute on Medicine as a Profession; N/A, not applicable; NCI, National Cancer Institute; USD, U.S. dollars.
Within each of the four cancer‐specific cohorts, the majority of physicians (range: 63.5–68.1%) had received at least one payment from the manufacturer of one of the drugs in the relevant choice set. During 2015, the final year of the study period, the average dollar value of payments received by physicians for drugs in their cancer type ranged from $1,104 (lung) to $3,657 (renal cell; Table 1). Physicians who received payment(s) from a manufacturer(s) during only 1 calendar year averaged $553 in total payments from that manufacturer(s), whereas those who received payments in all 3 years averaged $5,881 from that manufacturer(s) (supplemental online Fig. 1). The proportion of physicians who received only education payments was greater (per‐drug range: 26.1%–56.1%) than that of those who also received compensation payments (per‐drug range: 5.0%–12.3%; supplemental online Table 2).
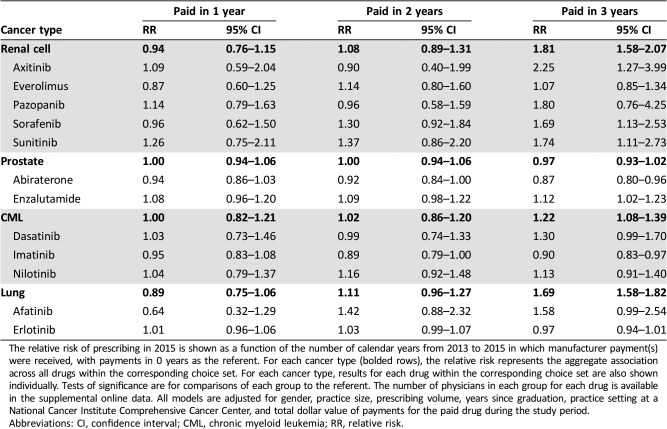
For three of the four cancer types, physicians who received payments in all 3 years had increased prescribing of the paid drug compared with physicians who did not receive payments: renal cell (RR: 1.81, 95% confidence interval [CI]: 1.58–2.07), CML (RR: 1.22, 95% CI: 1.08–1.39), and lung (RR: 1.69, 95% CI: 1.58–1.82). For renal cell cancer, CML, and lung cancer, only the physicians who received payments in all 3 years had statistically increased prescribing compared with 0 years; 1 year and 2 years did not (Table 2). This was also true of several drugs (sorafenib, sunitinib, axitinib, enzalutamide) when analyzed individually. For prostate cancer, there was no overall association between payments and prescribing (RR: 0.97, 95% CI: 0.93–1.02); physicians who received payments for enzalutamide in all 3 years prescribed more enzalutamide, whereas those who received payments for abiraterone unexpectedly prescribed less. Physicians who received payments for imatinib also prescribed less of that drug. Results were similar when excluding 2015 payments from the analysis (supplemental online Table 3).
Table 2. Number of years of payments.
The relative risk of prescribing in 2015 is shown as a function of the number of calendar years from 2013 to 2015 in which manufacturer payment(s) were received, with payments in 0 years as the referent. For each cancer type (bolded rows), the relative risk represents the aggregate association across all drugs within the corresponding choice set. For each cancer type, results for each drug within the corresponding choice set are also shown individually. Tests of significance are for comparisons of each group to the referent. The number of physicians in each group for each drug is available in the supplemental online data. All models are adjusted for gender, practice size, prescribing volume, years since graduation, practice setting at a National Cancer Institute Comprehensive Cancer Center, and total dollar value of payments for the paid drug during the study period.
Abbreviations: CI, confidence interval; CML, chronic myeloid leukemia; RR, relative risk.
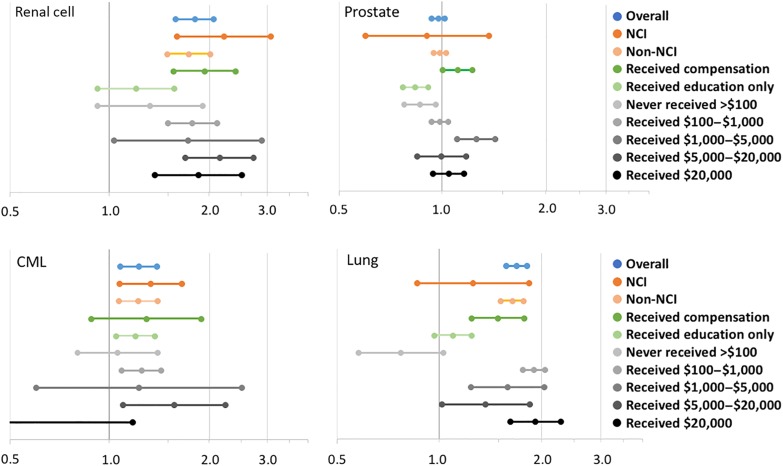
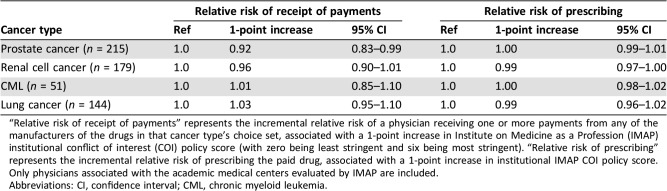
There was not a consistent relationship between NCI practice setting and either increased or decreased strength of the payment‐prescribing association (Fig. 2), and NCI practice setting was not a modifier of this association (not shown). Physicians who received compensation payments had greater prescribing of the paid drug compared with those who received education payments only (Fig. 2). Physicians who never received more than $100 within 1 calendar year had lower prescribing of the paid drug compared with those who received more than $100 in 1 or more years; above $100, there was not a consistent increase in prescribing at increasing payment thresholds. Among physicians at institutions evaluated by IMAP, the strength of institutional COI policy was not consistently associated with either lower risk of receiving payments or a weaker association between payments and prescribing (Table 3).
Figure 2.
Subgroup analysis. The relative risk (RR; and associated 95% confidence interval) of prescribing the paid drug for those who received payments in 3 years versus 0 years is shown within several subgroups. “Overall” represents the RR for 3 years versus 0 years in all physicians in that cancer type cohort, equivalent to the RR shown for the 3 years group in Figure 2. Higher (rightward) estimates represent greater likelihood of prescribing a drug after receiving payments from its manufacturer, and lower (leftward) estimates represent lower likelihood. Analyses for dollar amounts received refer to the highest value of general payments received within any single calendar year. All models are adjusted for gender, practice size, prescribing volume, and years since graduation. All models except those evaluating NCI practice setting are also adjusted for NCI practice setting; all models except those evaluating single‐year payment thresholds are also adjusted for the total dollar value of payments for the paid drug during the study period.
Abbreviations: CML, chronic myeloid leukemia; NCI, National Cancer Institute.
Table 3. Institutional conflict of interest policies.
“Relative risk of receipt of payments” represents the incremental relative risk of a physician receiving one or more payments from any of the manufacturers of the drugs in that cancer type's choice set, associated with a 1‐point increase in Institute on Medicine as a Profession (IMAP) institutional conflict of interest (COI) policy score (with zero being least stringent and six being most stringent). “Relative risk of prescribing” represents the incremental relative risk of prescribing the paid drug, associated with a 1‐point increase in institutional IMAP COI policy score. Only physicians associated with the academic medical centers evaluated by IMAP are included.
Abbreviations: CI, confidence interval; CML, chronic myeloid leukemia.
Discussion
Relationships between oncologists and the pharmaceutical industry—both the financial value of such relationships and instances of lack of transparent disclosure—have recently been highlighted in the public discourse [20], [21]. As a result, many institutions are reconsidering the design and enforcement of COI policies in order to balance productive industry collaboration with the desire to maintain transparency, produce unbiased research, provide optimal care for patients, and avoid public perception of misconduct. This study begins to illustrate the complexity of the relationship between industry payments and oncologist prescribing, beyond simply demonstrating that an association exists. The increased understanding of these factors resulting from this study should help to inform the management of physician‐industry relationships prospectively.
Among the specific set of oncology drugs we studied, the consistent receipt of payments over time appeared to be one important factor associated with increased prescribing. Even when controlling for the total dollar value of payments received, physicians who received payments consistently across 3 consecutive calendar years had greater prescribing of the paid drug. For renal cell cancer and CML, physicians who received payments in all 3 years had increased frequency of prescribing, whereas there was no association among those paid in 1 or 2 years. This is consistent with prior research finding a similar association with increased payment frequency [22]. If this association continues to be found in future research, it may support a greater emphasis in COI policies on long‐term relationships between physicians and a single company, relative to shorter‐term or intermittent relationships.
This study also found that the type of payment—specifically, compensation payments such as consulting fees and travel expenses, as opposed to education payments such as sponsored meals—appears to be associated with increased prescribing. When separating payments into these categories of “compensation” and “education,” we found that prescribing increases were largely concentrated among those physicians who received compensation payments, as opposed to education payments only. We note that this finding appears to differ from prior research that detected measurable prescribing changes in association with receipt of even a single sponsored meal, which were categorized as education payments in this study [9]. This difference may be explained by a relative lack of power in this study to detect small changes associated with single meals. However, as the association for compensation payments was strong, our results may support a greater emphasis on compensation payments in COI policies.
Additionally, the financial value of payments appeared to be important. When analyzing physicians with respect to the maximum dollar amount received within a single calendar year, those who received between $100 and $1,000 appeared to prescribe more than those who received less than this amount. However, there was not a consistent, additional difference in prescribing among physicians who received amounts greater than $1,000. The NIH requires disclosure of amounts greater than $5,000, whereas the National Comprehensive Cancer Network limits authors of its clinical practice guidelines to under $20,000 per year per company (and to $50,000 per year in total) [17], [18]. Taken together, our results suggest that an association between industry payments and physician prescribing behavior may exist at a dollar threshold significantly lower than those specified by these and other institutions.
It is important to interpret these findings in the context of the unexpected results regarding institutional COI policies. We did not find evidence that stronger institutional COI policies were associated with lower rates of industry payments to physicians at that institution, or with a reduced magnitude of prescribing changes among physicians at that institution who did receive payments. Although these results do not provide evidence of the effectiveness of institutional COI policies, we would not interpret them as conclusive evidence that such policies have not been effective—or that that could not be. It is possible that our metric of institutional COI policy stringency is not well correlated with how rigorously such policies are enforced in actuality. It is also possible that COI policies may need to be significantly more stringent before changes in the outcome measures studied herein would be observed.
Prior work reported a payment‐prescribing association among oncologists treating CML or renal cell cancer [12]. Using a different analytic model, our results are consistent with these findings, and find a similar association within lung cancer drugs. These results are consistent with an increasing body of literature finding an association between industry payments and physician prescribing behavior, across multiple medical specialties [22], [23], [24], [25], [26]. Our results are also consistent with the finding that an association between industry payments and physician prescribing may be absent for prostate cancer drugs [27]. The reason for the apparent difference between prostate cancer and other cancer types is an area for future study.
This study has several limitations. As Open Payments began in August 2013, we do not have payment data prior to that month. This precludes a payment “washout” period and may result in misclassification, as some physicians who received no payments in our study period may have done so prior to August 2013. The Medicare Part D Public Use File does not contain information about drug indication, so some of the claims included in our analysis may have been for other cancer types. The overlap of our exposure and outcome period in 2015 exposes the analysis to reverse causality, although we observed similar results when excluding 2015 payments (supplemental online Table 3). Our classification of general payments as either “education” or “compensation” payments was based on theory and has not previously been validated. In excluding physicians who did not have drug claims during each year, our results may not apply to physicians who have lower patient volume. In using Medicare data, our results may not apply to younger patients. We attributed treatment choices to the prescribing provider, which does not account for the possibility that the prescriber may be acting after the treatment decision or recommendation has already been made by a different provider. Our findings may not be generalizable to other cancer types, or to non‐oncology drugs.
Our study is strengthened by a conservative design. We included physicians who received payments from all of the different manufacturers in their cancer type; these physicians could make no net contribution to the overall payment‐prescribing association, as they could not have a relative increase in the prescribing of all associated drugs. All analyses (other than those evaluating payment thresholds) controlled for the total value of general payments; therefore, the observed associations are not explained by correlation with more (or larger) payments. This is particularly important with respect to the consistency‐of‐payment analysis; our findings predict greater prescribing of the paid drug when receiving payments in each year, compared with an equivalent dollar value of payments given within a single year.
Conclusion
The landscape surrounding physician relationships with industry continues to evolve. Public scrutiny of the industry role in cancer research and care delivery remains high. Ideally, institutions may increasingly be able to rely on research findings in order to construct informed, data‐driven policies to manage industry relationships within this changing environment.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was approved by the University of North Carolina Institutional Review Board on May 31, 2016 (number 16‐1594), as not constituting human subjects research. This research was partially supported by a National Research Service Award Post‐Doctoral Traineeship from the Agency for Healthcare Research and Quality (Grant 5T32 HS000032‐28) and by a Conquer Cancer Foundation Young Investigator Award. A.N.W. was supported by the National Center for Advancing Translational Sciences, National Institutes, Award Number KL2TR001438.
Author Contributions
Conception/design: Aaron P. Mitchell, Aaron N. Winn, Jennifer L. Lund, Stacie B. Dusetzina
Collection and/or assembly of data: Aaron P. Mitchell, Aaron N. Winn
Data analysis and interpretation: Aaron P. Mitchell, Aaron N. Winn
Manuscript writing: Aaron P. Mitchell, Aaron N. Winn, Jennifer L. Lund, Stacie B. Dusetzina
Final approval of manuscript: Aaron P. Mitchell, Aaron N. Winn, Jennifer L. Lund, Stacie B. Dusetzina
Disclosures
Jennifer L. Lund: GlaxoSmithKline (E [spouse]). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Campbell EG, Gruen RL, Mountford J et al. A national survey of physician‐industry relationships. N Engl J Med 2007;356:1742–1750. [DOI] [PubMed] [Google Scholar]
- 2.CMS. The Facts About Open Payments Data : 2015. Totals. Available at https://openpaymentsdata.cms.gov/summary. Accessed July 25, 2016.
- 3.Lerner TG, Miranda Mda C, Lera AT et al. The prevalence and influence of self‐reported conflicts of interest by editorial authors of phase III cancer trials. Contemp Clin Trials 2012;33:1019–1022. [DOI] [PubMed] [Google Scholar]
- 4.Tibau A, Bedard PL, Srikanthan A et al. Author financial conflicts of interest, industry funding, and clinical practice guidelines for anticancer drugs. J Clin Oncol 2015;33:100–106. [DOI] [PubMed] [Google Scholar]
- 5.Wazana A. Physicians and the pharmaceutical industry: Is a gift ever just a gift? JAMA 2000;283:373.10647801 [Google Scholar]
- 6.Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PloS One 2014;9:e110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spurling GK, Mansfield PR, Montgomery BD et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: A systematic review. PLoS Med 2010;7:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones R, Ornstein C. Matching industry payments to medicare prescribing patterns: An analysis. ProPublica. 2016. Available at https://static.propublica.org/projects/d4d/20160317‐matching‐industry‐payments.pdf?22. Accessed March 24, 2016. [Google Scholar]
- 9.DeJong C, Aguilar T, Tseng CW et al. Pharmaceutical industry‐sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med 2016;176:1114–1122. [DOI] [PubMed] [Google Scholar]
- 10.Perlis RH, Perlis CS. Physician payments from industry are associated with greater Medicare Part D prescribing costs. PLoS One 2016;11:e0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh JS, Franklin JM, Avorn J et al. Association of industry payments to physicians with the prescribing of brand‐name statins in Massachusetts. JAMA Intern Med 2016;176:763–768. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AP, Winn AN, Dusetzina SB. Pharmaceutical industry payments and oncologists’ selection of targeted cancer therapies in Medicare beneficiaries. JAMA Intern Med 2018;178:854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morain SR, Flexner C, Kass NE et al. Forecast for the Physician Payment Sunshine Act: Partly to mostly cloudy? Ann Intern Med 2014;161:915–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoadley J, Cubanski J, Neuman T. Medicare Part D at ten years: The 2015 marketplace and key trends, 2006‐2015. Kaiser Family Foundation, 2015. Available at http://kff.org/report‐section/medicare‐part‐d‐at‐ten‐years‐section‐1‐part‐d‐enrollment‐and‐plan‐availability/. Accessed July 22, 2016.
- 15.Medicare Provider Utilization and Payment Data : Part D Prescriber. Available at https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Medicare‐Provider‐Charge‐Data/Part‐D‐Prescriber.html. Accessed July 25, 2016.
- 16.Physician Compare National Downloadable File . Available at https://data.medicare.gov/Physician‐Compare/Physician‐Compare‐National‐Downloadable‐File/mj5m‐pzi6. Accessed August 27, 2017.
- 17.Responsibility of Applicants for Promoting Objectivity in Research for which PHS Funding is Sought (42 CFR Part 50, Subpart F). 2011. Available at https://grants.nih.gov/grants/policy/coi/index.htm. Accessed March 31, 2018.
- 18.NCCN Disclosure Policies and Potential Conflicts of Interest. Available at https://www.nccn.org/about/disclosure.aspx. Accessed March 31, 2018.
- 19.Chimonas S, Patterson L, Raveis VH et al. Managing conflicts of interest in clinical care: A national survey of policies at U.S. medical schools. Acad Med 2011;86:293–299. [DOI] [PubMed] [Google Scholar]
- 20.Chabner BA, Bates SE. Conflict of interest: An ethical firestorm with consequences for cancer research. The Oncologist 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer O. Leading US cancer hospital alters conflict of interest rules after controversies. BMJ 2018;363:k4151. [DOI] [PubMed] [Google Scholar]
- 22.Modi PK, Wang Y, Kirk PS et al. The receipt of industry payments is associated with prescribing promoted alpha‐blockers and overactive bladder medications. Urology 2018;117:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh P, Forman H, Adamson AS et al. Impact of industry payments on prescribing patterns for tumor necrosis factor inhibitors among Medicare beneficiaries. J Gen Intern Med 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morse E, Fujiwara RJT, Mehra S. Industry payments to physicians and prescriptions of brand‐name proton‐pump inhibitors. Otolaryngol Head Neck Surg 2019;160:70–76. [DOI] [PubMed] [Google Scholar]
- 25.Bandari J, Turner RM, Jacobs BL et al. The relationship of industry payments to prescribing behavior: A study of degarelix and denosumab. Urol Pract 2017;4:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse E, Fujiwara RJT, Mehra S. The association of industry payments to physicians with prescription of brand‐name intranasal corticosteroids. Otolaryngol Head Neck Surg 2018;159:442–448. [DOI] [PubMed] [Google Scholar]
- 27.Bandari J, Ayyash OM, Turner RM et al. The lack of a relationship between physician payments from drug manufacturers and Medicare claims for abiraterone and enzalutamide. Cancer 2017;123:4356–4362. [DOI] [PubMed] [Google Scholar]