Abstract
Intramuscular fat is considered a potential factor that is associated with meat quality in animal production and insulin resistance in humans. N6-methyladenosine (m6A) modification of mRNA plays an important role in regulating adipogenesis. However, the effects of m6A on the adipogenesis of intramuscular preadipocytes and associated mechanisms remain unknown. Here, we performed m6A sequencing to compare m6A methylome of the longissimus dorsi muscles (LDMs) between Landrace pigs (lean-type breed) and Jinhua pigs (obese-type breed with higher levels of intramuscular fat). Transcriptome-wide m6A profiling of porcine LDMs was highly conserved with humans and mice. Furthermore, we identified a unique methylated gene in Jinhua pigs named mitochondrial carrier homology 2 (MTCH2). The m6A levels of MTCH2 mRNA were reduced by introducing a synonymous mutation, and adipogenesis test results showed that the MTCH2 mutant was inferior with regard to adipogenesis compared with the MTCH2 wild-type. We then found that MTCH2 protein expression was positively associated with m6A levels, and an YTH domain family protein 1–RNA immunoprecipitation–quantitative PCR assay indicated that MTCH2 mRNA was a target of the YTH domain family protein 1. This study provides comprehensive m6A profiles of LDM transcriptomes in pigs and suggests an essential role for m6A modification of MTCH2 in intramuscular fat regulation.—Jiang, Q., Sun, B., Liu, Q., Cai, M., Wu, R., Wang, F., Yao, Y., Wang, Y., Wang, X. MTCH2 promotes adipogenesis in intramuscular preadipocytes via an m6A-YTHDF1–dependent mechanism.
Keywords: mRNA m6A, porcine model, intramuscular fat, meRIP-seq, longissimus dorsi muscle
Intramuscular fat (IMF) in humans has negative effects on health, closely related to insulin resistance and obesity (1, 2). Conversely, it is the main factor that positively affects gustatory qualities of meat, including flavor, water-holding capacity, and tenderness (3). It is important to elucidate the molecular mechanisms underlying IMF deposition because of its importance to human health and the meat industry.
Adipogenesis of intramuscular preadipocytes has been determined via genetics (4), epigenetics, and transcriptomic mechanisms (5, 6). Epigenetic imprinting through DNA methylation (7–9), microRNAs (10, 11), and noncoding RNAs (12) play important roles in preadipocytes adipogenesis. N6-methyladenosine (m6A) is the most prevalent chemical modification in eukaryotic mRNA (13), and it is installed by the methyltransferase complex mainly consisting of the methyltransferase-like (METTL)3–METTL14–WTAP core, which are essential components with catalytic and structural functions, respectively (14–16). However, this process could be reversed by fat mass and obesity-associated protein (FTO) (17) or AlkBH5 (18). In addition, the YTH domain family (YTHDF) proteins YTHDF1-3 (19–21) and YTHDC1 (22) are believed to modulate m6A residues on mRNAs, thus regulating various aspects of RNA metabolism such as mRNA transcription (20, 23), splicing (22), and stability (19). Previous studies reported that m6A modification down-regulates adipogenesis in porcine adipocytes (24), and it may affect adipogenesis by regulating alternative RNA splicing (25). Whether m6A modification is a driving force behind adipogenesis in intramuscular preadipocytes remains largely unknown.
Pigs (Sus scrofa domesticus) are a common mammalian model because they share many physiologic similarities to humans, including metabolic diseases such as diabetes and obesity (26). Moreover, pig breeds vary in weight, growth rate, and reproductive performance, and these characters are useful for comparative studies (27). Here, we used the Jinhua pig, a fatty, local Chinese breed prized for its high-quality meat, from which the famous Jinhua ham is made (28). It has a strong aerobic and adipogenic metabolism, which is believed to contribute to its desirable meat (29). In contrast, the Landrace pig is a Danish breed known for being lean. The differences between these 2 breeds make them ideal for studying m6A modifications in intramuscular adipogenesis. The present study compared whole transcriptome-wide m6A profiles from samples taken from the longissimus dorsi muscles (LDMs) of Jinhua and Landrace pigs to determine the effects of m6A on the adipogenesis of intramuscular preadipocytes.
MATERIALS AND METHODS
Animal samples and phenotypes
Sample collection and experiments were performed according to the guidelines for the care and use of experimental animals established by the Ministry of Agriculture of China. A total of 4 male Jinhua pigs and 4 male Landrace pigs were slaughtered at 6 mo of age via a conventional and humane procedure. The pigs were killed by electrocution and were immediately hoisted for bleeding. LDM tissues from the last rib of the back were collected and immersed in liquid nitrogen and were then stored at −80°C for the extraction of total RNA. All procedures were approved by the Committee on Animal Care and Use and Committee on the Ethics of Animal Experiments of Zhejiang University (ZJU2015-458-09).
RNA extraction and real-time quantitative PCR
Total RNA was extracted by using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions, and 2 µg of total RNA was reversed transcribed. The primer sequences are shown in Supplemental Table S2, and a SYBR Green Kit (Roche, Clifton, NJ, USA) with a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) was used to complete the quantitative PCR (qPCR) reactions. The 2−ΔΔCt method was used to quantify the mRNA expression of each gene relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
RNA m6A quantification using HPLC–tandem mass spectrometry
mRNA was isolated from total RNA by using a Dynabeads mRNA Purification Kit (Thermo Fisher Scientific), and rRNA contaminants were removed by using a RiboMinus Eukaryote Kit (Thermo Fisher Scientific). Subsequently, mRNA was digested into nucleosides by using nuclease P1 and alkaline phosphatase and was then filtered with a 0.22-µm filter. The amount of m6A was measured according to HPLC–tandem mass spectrometry, following the published procedure (14). Quantification was performed by using the standard curve obtained from pure nucleoside standards that were run with the same batch of samples. The ratio of m6A to A was calculated based on the calibrated concentrations.
Methylated RNA immunoprecipitation library construction and sequencing
m6A-specific methylated RNA immunoprecipitation (meRIP) with next-generation sequencing was performed as previously described (13). Briefly, random mRNA fragments (∼200 nt) were generated by using an RNA fragmentation reagent (Thermo Fisher Scientific), and samples were then incubated for 2 h at 4°C with anti-m6A pAb (Synaptic Systems GmbH, Göttingen, Germany). The mixture was immunoprecipitated by incubation with Protein A beads (Bio-Rad, Hercules, CA, USA) at 4°C for 2 h. After extensive washing, bound RNA was eluted from the beads with m6A (MilliporeSigma, Burlington, MA, USA) and precipitated with ethanol. The TruSeq Stranded mRNA Sample Prep Kit (Illumina, San Diego, CA, USA) was used to construct libraries from immunoprecipitated and input RNA. The libraries were subjected to single-end sequencing on an Illumina HiSeq 4000 System.
Isolation of intramuscular preadipocytes and cell culture
To investigate the role of mitochondrial carrier homology 2 (MTCH2) in lipid accumulation in muscle, we first isolated intramuscular preadipocytes to determine whether MTCH2 functioned in lipid accumulation, and when it works. The LDM of 3-d-old Duroc-Landrace-Yorkshire piglets were separated under sterile conditions. Visible connective tissue was removed and finely minced. Intramuscular preadipocytes were isolated based on previous studies (11, 30, 31). In brief, muscle tissues were digested in a digestion buffer consisting of 1 mg/ml collagenase type I (Thermo Fisher Scientific) in a shaking water bath for 1.5 h at 37°C. The digested sample was filtered aseptically through 80- and 200-µm nylon mesh filters to isolate cells. The filtered cells were then washed 3 times with DMEM/F12 via centrifugation at 1500 rpm for 5 min. Cells were seeded in growth medium that consisted of DMEM/F12 medium with 10% fetal bovine serum (Thermo Fisher Scientific) with penicillin (100 U/ml) and streptomycin (100 U/ml). After 1 h, cells were rinsed with DMEM/F12 medium to remove unadhered cells, and the adhered cells consisted of pure intramuscular preadipocytes. The timescale of intramuscular preadipocyte differentiation is shown in Supplemental Fig. S1A. After the cells reached 100% confluence for 2 d, adipogenesis was induced with differentiation medium, which consisted of growth medium, 5 μg/ml (872 nM) insulin, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine (MilliporeSigma), for 2 d to induce differentiation. The medium was then changed to growth medium with 5 μg/ml insulin, and samples were induced for another 4 d to maintain differentiation. Differentiation continued until d 8 when lipid droplets were observed. Subsequently, 293T cells were cultured in high-glucose DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin in a 5% CO2/95% air incubator.
Plasmids, cloning, and short interfering RNA analyses
The porcine MTCH2 (ENSSSCT00000014452) coding sequence (CDS) was synthesized with a FLAG tag (5′-GACTACAAGGACGATGATGACAAG-3′) at the N terminus and cloned into the PCDH-CMV-MCS-EF1-CopGFP-T2A-puro expression plasmid (System Biosciences, Palo Alto, CA, USA) to generate the wild-type construct (MTCH2-WT). Nucleotide 9 of the MTCH2 CDS sequence was mutated from cytosine to thymine (5′-GGACG-3′→5′-GGATG-3′) by using a Q5 Site-Directed Mutagenesis Kit (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instructions. Porcine YTHDF1 cDNA was generated via PCR and cloned into the pFLAG-CMV2 expression plasmid. Porcine FTO overexpression plasmid CMV-pFTO was used by Wang et al. (24). The following short interfering RNA sequences were synthesized by GenePharma (Shanghai, China): siMTCH2-F, 5ʹ-CGAGUUAUCAAGGAGACAACU-3ʹ (forward) and siMTCH2-R, 5ʹ-UUGUCUCCUUGAUAACUCGGU-3ʹ (reverse); siYTHDF1-F, 5ʹ-UUAGUAUCCUGUCCUUUUGUU-3ʹ (forward) and siYTHDF1-R, 5ʹ-CAAAAGGACAGGAUACUAAAG-3ʹ (reverse).
m6A immunoprecipitation and measurement of m6A MTCH2 mRNA levels
m6A immunoprecipitation experiments were performed as previously described (32). Briefly, 24 h after transfection, RNA from 293T cells was chemically sheared into 200-nt fragments, and 200 μg of total RNA was subjected to immunoprecipitation by using affinity-purified mouse polyclonal anti-m6A antibodies. Bound m6A RNA fragments were isolated with Trizol reagent and analyzed by using real-time qPCR. The primer sequences used to amplify the m6A peak region were as follows: pMTCH2-m6A-F, 5′-GGCTGCGTCTGTCTGTC-3′ and pMTCH2-m6A-R, 5′-GATGAGCACCTTCACGTACA-3′.
Measurement of MTCH2 mRNA stability
Cells were transfected with either the MTCH2-WT or the MTCH2-Mut plasmid for 24 h and were then treated with vehicle or actinomycin D (MilliporeSigma) at a concentration of 5 µg/ml to inhibit global mRNA transcription. The samples were collected at 0, 3, and 6 h to assess degradation. RNA was extracted by using TRIzol reagent and was then reverse transcribed as described earlier. The mRNA transcript levels of interest were detected by using qPCR.
RNA immunoprecipitation and Western blotting
RNA immunoprecipitation (RIP) and Western blotting were performed as previously described (19). Porcine intramuscular preadipocytes overexpressing the FLAG-YTHDF1 peptide were collected with a cell lifter (two 15-cm plates for each) and were concentrated via centrifugation at 400 g for 5 min. The cell pellet was resuspended in 2 V of lysis buffer (150 mM KCl, 10 mM HEPES, 2 mM EDTA, 0.5% Nonidet P-40, 0.5 mM DTT, 1:100 protease inhibitor cocktail, and 400 U/ml RNase inhibitor) and incubated on ice for 10 min. Cell debris was removed via centrifugation at 15,000 g for 15 min at 4°C, and the supernatant was further cleared by passage through a 0.45 μm membrane syringe filter. A 50 μl aliquot of cell lysate was saved as input, and the remaining sample was incubated with anti-FLAG magnetic beads (MilliporeSigma) in ice-cold NT2 buffer [200 mM NaCl, 50 mM HEPES (pH 7.6), 2 mM EDTA, 0.05% Nonidet P-40, 0.5 mM DTT, and 200 U/ml RNase inhibitor] for 4 h at 4°C. The beads were washed 8 times and were saved as the immunoprecipitated fraction, 2 μg of which was loaded on a gel along with 10 μg of the input (corresponding to ∼1% of overall input). Antibodies against FLAG (A8592; MilliporeSigma), YTHDF1 (ab99080; Abcam, Cambridge, MA, USA), and MTCH2 (16888-1-AP; Proteintech, Rosemont, IL, USA) were used for Western blotting.
Oil Red O staining and dye extraction analysis
After 8 d of adipogenic stimulation, the culture medium was discarded, and the cells were washed 3 times with PBS (Nanjing KeyGen BioTech, Nanjing, China). Thereafter, the cells were fixed with cold 10% neutral-buffered formalin for 1 h and were then rinsed twice with 60% isopropanol. Subsequently, cells were stained with Oil Red O (0.35 g, 60% isopropanol; MilliporeSigma) for 2 h, and the cells were exhaustively rinsed with water. Oil Red O dye was extracted from the stained cells with isopropanol for 20 min, and a quantitative analysis was performed by using a SpectraMax Microplate Luminometer (Molecular Devices, San Jose, CA, USA).
m6A data analysis
Reads were aligned to the reference genome (Sscrofa10.2/susScr3) using Tophat (v.2.0.14; The Center for Computational Biology, Johns Hopkins University, Baltimore, MD, USA). Gene structure annotations were downloaded from Ensembl release 78 (http://dec2014.archive.ensembl.org/index.html) (Sscrofa10.2/susScr3). In total, 27 million mapped reads were sampled from each IP and input experiment. After filtering out low-quality data, >76.5% of IP reads uniquely mapped to the Sus scrofa reference genome (Supplemental Table S1). The longest isoform was used if multiple isoforms were detected. The m6A peak calling method was modified from Dominissini et al. (32). To call m6A peaks, the longest isoform of each pig gene was scanned by using a 100 bp sliding window with a 10 bp step. To reduce bias from potential inaccurate gene structure annotation and the arbitrary usage of the longest isoform, windows with read counts <1/20 of the top window in both m6A immunoprecipitation and input samples were excluded. For each gene, thread counts in each window were normalized by the median count of all windows of that gene. Fisher’s exact test was used to identify the differential windows between IP and input samples. The window was called positive if the false discovery rate was <1% and the log2 (enrichment score) was ≥1. Overlapping positive windows were merged. The following 4 numbers were calculated to obtain the enrichment score of each peak (or window): 1) read counts of the IP sample in the current peak/window; 2) median read counts of the IP sample in all 100 bp windows of the current mRNA; 3) read counts of the input sample in the current peak/window; and 4) median read counts of the input sample in all 100 bp windows of the current mRNA. The enrichment score of each window was calculated as (a × d)/(b × c). Homer (33) was used to search motifs in each set of m6A peaks. Gene expression analysis using Cufflinks (v.2.2.1; The Center for Computational Biology at Johns Hopkins University) was used to calculate the fragments per kilobase of transcript per million mapped reads of each gene to represent mRNA expression levels (34).
Statistical analysis
The data are shown as means se. All statistical analyses were performed by using SPSS v.22 (IBM, Armonk, NY, USA). Significant differences between all groups were analyzed by using a 1-way ANOVA. Values of P < 0.05 were considered significant.
Data deposition
The high-throughput data used in this study were deposited in the Gene Expression Omnibus database (National Center for Biotechnology Information, Bethesda, MD, USA; GSE113237).
RESULTS
m6A is negatively related to fat deposition in muscle tissue
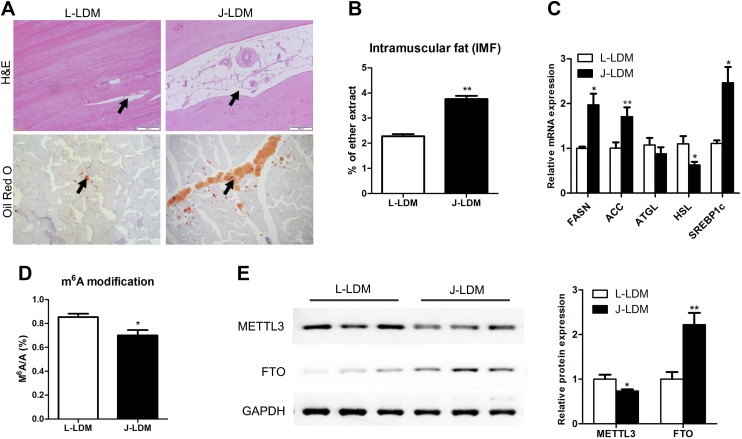
The LDM of Jinhua (J-LDM) pigs had a higher IMF content than that of Landrace (L-LDM) pigs (P < 0.01) (Fig. 1A, B). Accordingly, genes related to triglyceride synthesis and fatty acid transport were more highly expressed in J-LDM than in L-LDM, whereas the lipolysis gene HSL exhibited the opposite trend (Fig. 1C). The m6A/A ratio of the LDM from the 2 pig breeds was compared by using HPLC coupled with a triple quadrupole tandem mass analyzer. The ratio, which varied over a range of 0.6–0.9%, was higher for L-LDM than for J-LDM (Fig. 1D). Conversely, the expression levels of demethylase FTO were lower in L-LDM than in J-LDM, whereas the levels of methyltransferase METTL3 showed the opposite trend (Fig. 1E). Our results are consistent with previous studies of 3T3-L1 cells (25) and pig adipocytes (24) in that m6A levels decreased during adipogenesis. This outcome suggests that m6A of mRNA in LDM is negatively associated with fat deposition in skeletal muscle.
Figure 1 .
Jinhua pigs have higher IMF content and lower levels of m6A modification than Landrace pigs. A) Hematoxylin and eosin (H&E) (top) and Oil Red O (bottom) staining of longitudinal sections of LDM in Landrace and Jinhua pigs (black arrows point to the adipocytes). B) IMF contents in LDMs of Landrace and Jinhua pigs; n = 4. C) qPCR analysis of adipogenesis-related gene expression in LDMs of Landrace and Jinhua pigs. Expression levels are shown as fold change relative to the control (GAPDH); n = 4. D) m6A level in LDMs from Landrace and Jinhua pigs; n = 4. E) Protein expression of FTO and METTL3 in LDMs of Landrace and Jinhua pigs, as determined by immunoblotting. The expression levels were normalized to that of GAPDH; n = 3. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 (Student’s t test).
MeRIP with next-generation sequencing analysis
The meRIP with next-generation sequencing analysis of L-LDM and J-LDM yielded high-confidence m6A peaks within thousands of coding transcripts (common peaks and transcripts from 2 biologic replicates) (Table 1). We detected >2978 m6A transcripts among the 17,279 expressed transcripts in the L-LDM samples, and 3301 m6A transcripts were found among the 17,258 expressed transcripts in the J-LDM samples. Based on this information, we estimated that the pig LDM transcriptome contains ∼1.75–1.82 m6A peaks per modified gene and 0.31–0.34 m6A peaks per actively expressed transcript. These results are comparable to findings from pig (35), human (13), mouse (32), and plant (36) transcriptomes, which exhibit ∼1.5 m6A peaks per transcript.
TABLE 1.
Number of m6A peaks detected in the LDM of Jinhua and Landrace pig breeds
| Breed | Sample | Total transcripts (n) | Total m6A transcripts (n) | Total m6A sites | Total m6A sites/m6A transcript | Total m6A sites/transcript |
|---|---|---|---|---|---|---|
| Landrace | LDM-1 | 16,626 | 3798 | 6471 | 1.7 | 0.39 |
| LDM-2 | 15,966 | 3647 | 6176 | 1.69 | 0.39 | |
| LDM | 17,279 | 2978 | 5404 | 1.82 | 0.31 | |
| Jinhua | LDM-1 | 16,432 | 3863 | 6433 | 1.67 | 0.39 |
| LDM-2 | 16,292 | 4468 | 7733 | 1.73 | 0.47 | |
| LDM | 17,258 | 3301 | 5780 | 1.75 | 0.34 |
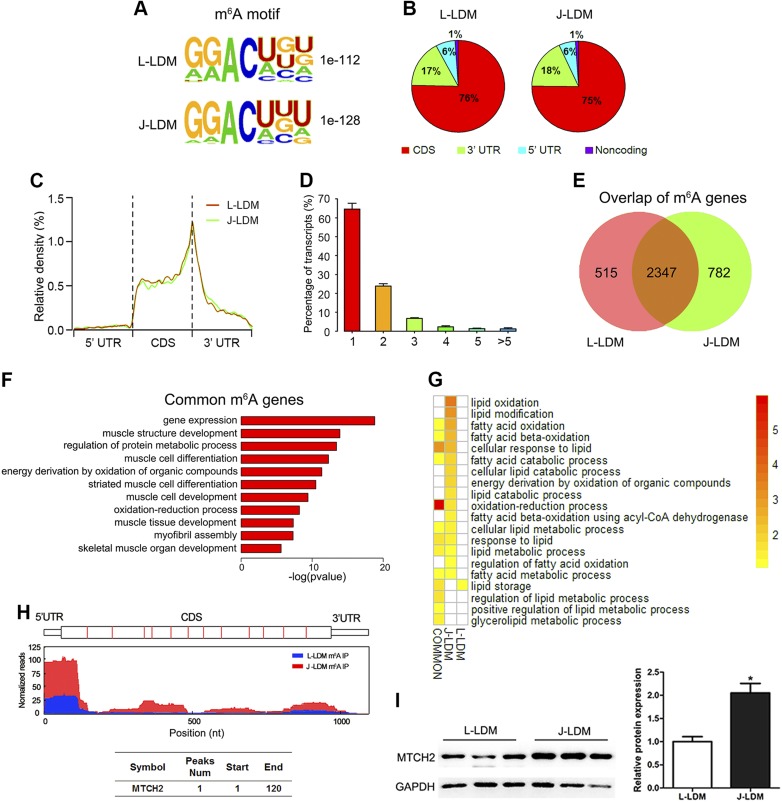
We searched for consensus motifs and identified the GGACU sequence (Fig. 2A), which is conserved in the published consensus motif RRACH (where R = A/G, A = m6A, and H = A/C/G) (13, 32, 36). To confirm the preferential localization of m6A in transcripts, m6A peaks were categorized based on gene annotations and nonoverlapping segments as CDS (75–76% of total peaks), 5′-UTR (∼6% of total peaks), and 3′-UTR (17–18% of total peaks) (Fig. 2B). An analysis of the relative positions of m6A peaks in mRNAs revealed that these were mainly near stop codons or close to the beginning of 3′-UTRs (Fig. 2C), which is consistent with patterns identified in other mammals (13, 32). The number of m6A peaks varied from 1 to 16 in individual genes, whereas nearly 80% of methylated transcripts harbored only 1 or 2 m6A peaks. Furthermore, <10% contained ≥4 peaks (Fig. 2D); this ratio is comparable to that previously reported in humans (5.5%) (32) but lower than that in Arabidopsis thaliana (17%) (36). These results indicate that RNA m6A properties are conserved between pigs and other species (32).
Figure 2 .
LDM m6A methylome profiles of Jinhua and Landrace pigs. A) Sequence motifs in m6A peaks identified by using Homer software. B) Pie chart depicting the fraction of m6A peaks in 4 transcript segments. C) Enrichment of m6A peaks along transcripts. Each transcript was divided into 3 parts: 5′-UTR, CDS, and 3′-UTR. D) Percentage of m6A-methylated mRNAs with different numbers of m6A peaks. E) Overlap of m6A peaks from L-LDM and J-LDM samples. F) Gene ontology (GO) enrichment analysis of common methylated genes in L-LDM and J-LDM samples. G) GO enrichment analysis of unique and common methylated genes in L-LDM and J-LDM samples involved in lipid-related functions. Different colors represent GO term enrichment values, and the white color indicates that the term is not significantly enriched. H) Peaks indicate the relative abundance of m6A sites in MTCH2 mRNA. The y-axis shows the sequence read number, red boxes represent exons, the red line represents L-LDM IP samples, and the blue line represents J-LDM IP samples. I) Relative expression levels of MTCH2 protein in LDMs of Landrace and Jinhua pigs; n = 3. Data are expressed as means ± sem. *P < 0.05 (Student’s t test).
Biologic pathways associated with m6A-modified genes
We identified 2474 genes that were common to L-LDM and J-LDM (Fig. 2E), along with 504 and 827 genes that were uniquely methylated in L-LDM and J-LDM, respectively. To predict the functions associated with m6A-modified genes, we conducted a gene ontology enrichment analysis, and the results indicate that common methylated genes were mainly involved in gene regulation, muscle structure development, and muscle differentiation (Fig. 2F). In contrast, L-LDM unique m6A genes were associated with artery morphogenesis and development (Supplemental Table S3). However, J-LDM m6A genes were associated with RNP complex biogenesis and cellular catabolic processes. These results suggest that m6A modification is tissue specific and that m6A-related genes vary between breeds.
We selected lipid metabolism–related genes from the set of methylated genes unique in L-LDM or J-LDM by categorizing them according to lipid-associated terms. We found that most of the m6A-modified lipid metabolism genes were involved in oxidation reduction and lipid oxidation. No lipid metabolism genes were unique to L-LDM (Fig. 2G), suggesting that methylation of oxidative genes is essential for lipid metabolism. On the basis of the relative high expression levels of the top 10 m6A modification J-LDM unique methylated gene–related oxidation, we chose MTCH2 (Fig. 2H) for further analysis as a candidate m6A gene in the regulation of intramuscular adipogenesis. Recent studies implicated MTCH2 in lipid accumulation and oxidation in mouse muscles (37) and Caenorhabditis elegans (38). In our study, we found that this gene exhibits relatively higher protein expression compared with L-LDM (P < 0.01) (Fig. 2I). We therefore suggest that m6A modification of MTCH2 may play an important role in lipid deposition in muscle tissue.
MTCH2 promotes adipogenesis of preadipocytes in porcine muscles
We checked the protein expression of MTCH2 during adipogenesis and found an increase in protein expression in the first 2 d after methylisobutylxanthine, dexamethasone, and insulin (MDI) induction (Supplemental Fig. S1B) with a slight uptrend until d 6, which suggests that MTCH2 may be involved in adipogenesis of intramuscular preadipocytes. Short interfering RNA–mediated MTCH2 knockdown of intramuscular preadipocytes decreased lipid accumulation (Supplemental Fig. S1C–F). However, enforced MTCH2 expression in intramuscular preadipocytes significantly enhanced lipid accumulation (Supplemental Fig. S1G–I), indicating that MTCH2 promoted adipogenesis of intramuscular preadipocytes.
m6A enhances MTCH2 protein expression and promotes adipogenesis
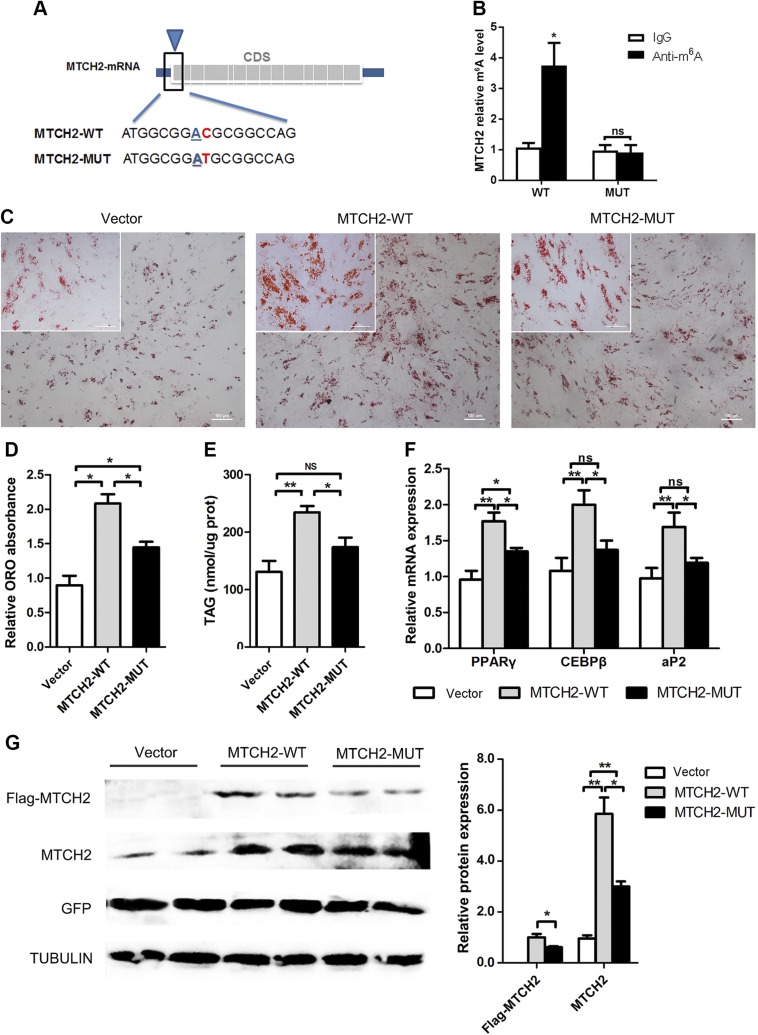
We investigated the effects of m6A modification of MTCH2 on adipogenesis in intramuscular preadipocytes. First, we searched for m6A modification sites in MTCH2, and a search of the sequence corresponding to the conserved m6A motif, RRACH, revealed 2 putative m6A sites in the first 120 nt of MTCH2 located at position 13 of the 5ʹ-UTR (5ʹ-ACGTGATCTGGGACCGGC-3ʹ) and position 8 of the CDS (5ʹ-ATGGCGGACGCGGCCAG-3ʹ) (Fig. 3A). Here, we introduced synonymous mutation at the putative m6A site on CDS (MTCH2-Mut) and then evaluated the m6A level of MTCH2-WT and MTCH2-Mut by using meRIP-qPCR. As expected, the m6A level decreased in MTCH2-Mut compared with MTCH2-WT (P < 0.01), indicating that the predicted site was modified by m6A (Fig. 3B). Intramuscular preadipocytes were transfected with MTCH2-WT and MTCH2-Mut, and the results indicate that MTCH2-Mut markedly decreased adipogenesis compared with that of MTCH2-WT (Fig. 3C–F).
Figure 3 .
m6A modification of MTCH2 promotes adipogenesis in intramuscular preadipocytes by enhancing protein expression. A) Synonymous mutations in the MTCH2 CDS. B) Real-time qPCR analysis of immunoprecipitated m6A in 293T cells using MTCH2 PCR primer for amplification. C) Oil Red O staining of intramuscular preadipocytes 6 d after MDI induction (upper-left corner, high magnification). D) Relative absorption of Oil Red O (ORO) staining. E) Triglyceride content. F) Real-time qPCR analysis of adipogenic genes. G) Flag and MTCH2 protein expression in vector, MTCH2-WT, and MTCH2-Mut groups after 1 d of MDI induction. Each column represents the means ± sem. Ns, not significant. *P < 0.05, **P < 0.01 (1-way ANOVA).
To further investigate how m6A affects MTCH2 expression, protein levels were evaluated during adipogenesis. We found that MTCH2 protein expression was higher in MTCH2-WT than in MTCH2-Mut (Fig. 3G), thus explaining the greater lipid accumulation in the former.
m6A enhances MTCH2 translation via an YTHDF1-dependent pathway
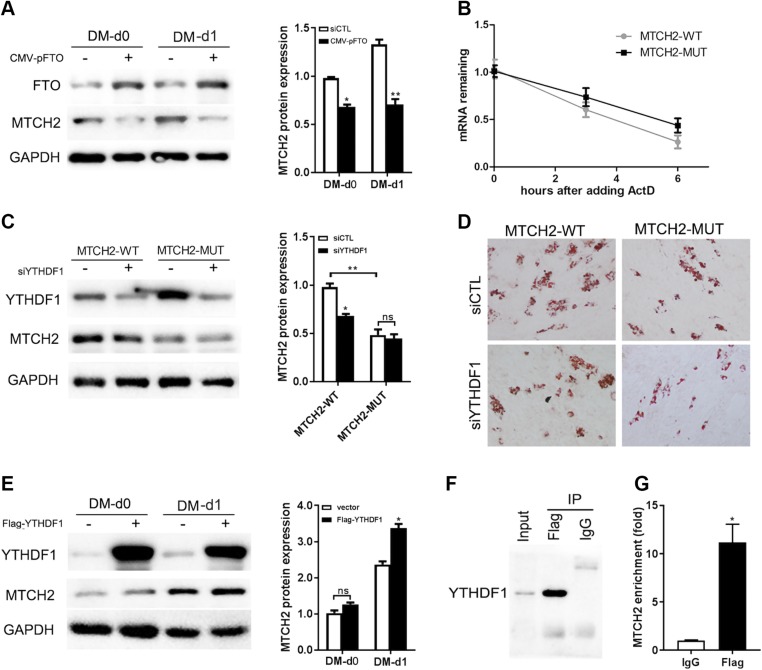
Overexpression of FTO suppressed the expression of MTCH2 in the first 2 d after MDI induction (Fig. 4A), suggesting that m6A modification may increase MTCH2 expression in adipogenesis. Because m6A reader protein YTHDF (19, 20) reportedly affects protein expression, we evaluated whether and how these proteins functioned here. There was no difference in the half-life of mRNA between the MTCH2-WT and MTCH2-Mut groups (Fig. 4B), suggesting that YTHDF2 does not modulate MTCH2 mRNA stability. To determine whether YTHDF1 regulates MTCH2 expression, siYTHDF1 was introduced to knock down YTHDF1 in both MTCH2-WT and MTCH2-Mut. Western blot results showed a significant decrease in MTCH2 expression after YTHDF1 knockdown in MTCH2-WT, whereas no significant change could be found in the Mut group (Fig. 4C). Oil Red O staining also correlated with the expression of MTCH2 in the 4 groups (Fig. 4D). Overexpression of YTHDF1 and induction of adipogenesis resulted in an increase in MTCH2 protein expression in the first day of adipogenic differentiation (Fig. 4E), suggesting that YTHDF1 regulates MTCH2 expression during adipogenesis in intramuscular preadipocytes. To confirm whether YTHDF1 functions as a direct m6A reader of MTCH2, we conducted RIP-qPCR experiments with lysates of porcine intramuscular preadipocytes overexpressing YTHDF1. Precipitation with the anti-FLAG antibody–enriched MTCH2 mRNA compared with the IgG control (Fig. 4F, G) suggests that YTHDF1 directly binds to the MTCH2 mRNA, thus partly explaining the higher protein expression detected in MTCH2-WT. Together, these findings suggest that m6A modification of porcine MTCH2 promotes adipogenesis by enhancing YTHDF1-dependent mRNA translation.
Figure 4 .
MTCH2 CDS mediates translation via a YTHDF1-dependent pathway. A) MTCH2 protein levels after FTO overexpression for 48 h (MD-d0) or MDI-induced for 24 h (DM-d1). B) Half-life of MTCH2 mRNA in cells transfected with MTCH2-WT vs. MTCH2-Mut. C) MTCH2 protein levels in MTCH2-WT or MTCH2-Mut with YTHDF1 knockdown after MDI induction for 24 h. D) Oil Red O staining of cell samples from C after adipogenic induction for 8 d. E) MTCH2 protein expression after YTHDF1 overexpression for 48 h (MD-d0) or MDI induction for 24 h (DM-d1). F) Western blot analysis of YTHDF1 in input and FLAG-RIP groups. G) Real-time qPCR analysis of relative MTCH2 expression levels in input and FLAG-RIP groups. Each column represents the mean ± sem. Ns, not significant. *P < 0.05, **P < 0.01.
DISCUSSION
Compared with Landrace pigs, the Jinhua breed exhibits high levels of fat in skeletal muscles; comparing the 2 breeds, therefore, can provide insight into the mechanisms underlying fat deposition in muscle. To this end, we conducted mRNA m6A methylome profiling of porcine skeletal muscle tissue, and the results indicate that m6A peaks are mainly enriched near stop codons and 3′-UTRs. Moreover, these peaks were primarily found in the highly conserved sequence motif GGACU, and this pattern was similar to the mRNA m6A distribution pattern observed in humans and mice (13, 32).
Our results revealed that highly methylated transcripts in the LDM of both breeds were mainly associated with muscle differentiation and development, suggesting that m6A modification is tissue specific and may play an important role in muscle-related functions. Previous studies have indicated that development and lipid metabolism differ in adipocytes from subcutaneous and intramuscular sources (39, 40). Although m6A reportedly regulates adipogenesis in 3T3-L1 cell lines (25) and porcine subcutaneous adipocytes (24), whether m6A affects IMF deposition in a different way remains unknown. In the present study, we found that the number of m6A genes associated with lipid metabolism was higher in J-LDM than in L-LDM. In addition, these genes were mainly enriched in the oxidation process, suggesting that m6A modification may regulate genes associated with oxidation through a posttranscriptional mechanism to influence intramuscular preadipocyte adipogenesis.
MTCH2 was found to be uniquely methylated in J-LDM. This gene was localized to the outer membrane of the mitochondria and had a relatively high expression in adipose tissue. Numerous independent genome-wide association studies have shown that MTCH2 is an obesity susceptibility gene (41, 42), and its protein level is significantly higher in obese individuals compared with lean individuals (43). However, the underlying mechanisms affecting fat deposition remain largely unknown. Recent studies have reported that MTCH2 regulates lipid accumulation in mouse muscle and that its deficiency leads to an increase in whole-body energy utilization and protection against diet-induced obesity (37). In addition, MTCH2 overexpression enhanced lipid accumulation in C. elegans by inhibiting the transcriptional activation of estrogen receptor 1 (38). In the present study, we found that MTCH2 mRNA and protein levels were higher in Jinhua pigs compared with those in Landrace pigs, and that MTCH2 protein was positively associated with adipogenesis in porcine intramuscular preadipocytes in the first 2 d after MDI induction. MTCH2 expression could be hindered by FTO overexpression, indicating that MTCH2 protein expression was positively associated with m6A levels during adipogenesis.
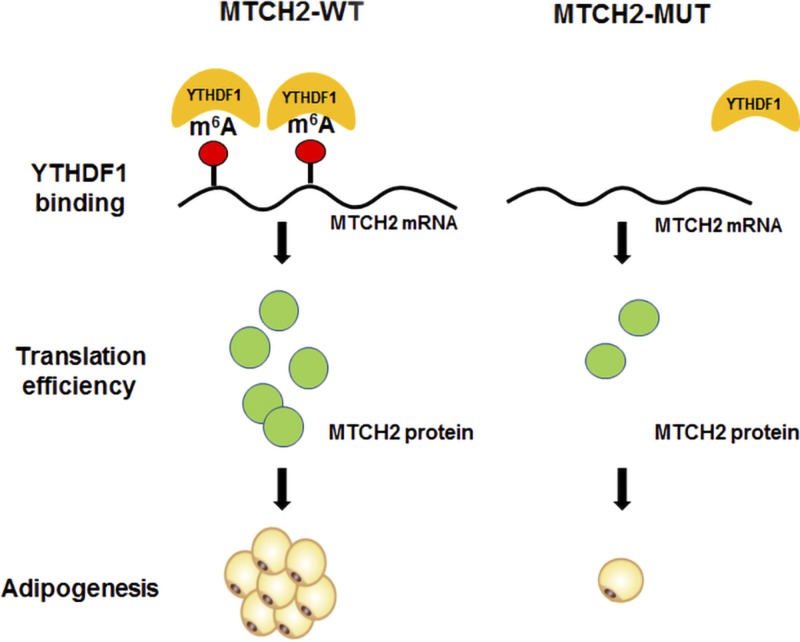
m6A peaks are believed to function by recruiting ≥1 binding protein to the transcript for various functions. For instance, eIF3 directly binds to 5′UTR m6A modification–mediated cap-independent translation (44). YTHDF1 was shown to enhance translational efficiency (20), YTHDF3 also modulates translation (23), and YTHDF2 mediates m6A-associated mRNA degradation by recruiting the CC chemokine receptor 4-negative regulator of transcription complex to trigger mRNA deadenylation (45), with subsequent translocation to P-bodies (19). We presumed that m6A peaks primarily act to recruit YTHDF1 protein during adipogenesis. The results showed that knockdown of YTHDF1 to decrease MTCH2 protein occurs only in the MTCH2-WT group but not in the synonymous m6A site-mutated ones. Overexpression of YTHDF1 increased MTCH2 protein levels after MDI induction. The direct interaction between YTHDF1 and MTCH2 mRNA was revealed by RIP experiments. Moreover, previous studies examining the role of YTHDF1 and YTHDF2 in HeLa cells revealed that MTCH2 is a target of the former but not of the latter (20). Thus, multiple lines of evidence indicate that YTHDF1 directly targets MTCH2 transcripts to enhance translation of the protein in an m6A-dependent manner in intramuscular preadipocytes (Fig. 5).
Figure 5 .
MTCH2 promotes intramuscular adipogenesis in an m6A-YTHDF1–dependent mechanism. MTCH2 could promote adipogenesis in muscles in an m6A-dependent manner, which enhanced MTCH2 protein expression via YTHDF1-dependent translation promotion.
To the best of our knowledge, this study is the first to uncover the function of m6A modification in IMF deposition, and m6A-mediated MTCH2 translation relied on the m6A reader protein YTHDF1. Furthermore, our study provides novel insights into the molecular mechanisms underlying intramuscular adipogenesis and paves the way to develop more effective novel therapeutic strategies to modulate fat deposition.
CONCLUSIONS
Intramuscular, subcutaneous, and visceral preadipocytes reportedly differ during differentiation (39, 40, 46); whether m6A of MTCH2 could also promote adipogenesis of adipocytes in other depots of the body should be investigated.
The m6A core motif GGAC in the CDS of MTCH2 is conserved in human and mouse cDNA (Supplemental Fig. S2), providing the molecular basis for m6A-mediated MTCH2 expression efficiency during intramuscular adipogenesis in human and mouse. Strategies targeting MTCH2 can be used to control lipid deposition in muscles, which could have both clinical implications for the treatment of obesity and economic value in livestock breeding for human consumption.
The interesting finding that m6A of MTCH2 promotes adipogenesis offers insights into better understanding the novel mechanisms regulating IMF deposition. Based on the compared transcriptome-wide m6A map of porcine muscle, more genes and pathways associated with m6A modification could be discovered in the future.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the Special Fund for Cultivation and Breeding of New Transgenic Organism (Grant 2014ZX0800949B), the National Natural Science Foundation of China (Grants 31572413 and 31630075), and the Natural Science Foundation of Zhejiang Province (Grant LZ17C1700001). The authors declare no conflicts of interest.
Glossary
- CDS
coding sequence
- FTO
fat mass and obesity-associated
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IMF
intramuscular fat
- J-LDM
Jinhua-longissimus dorsi muscle
- LDM
longissimus dorsi muscle
- L-LDM
Landrace-LDM
- m6A
N6-methyladenosine
- MDI
methylisobutylxanthine, dexamethasone, and insulin
- meRIP
methylated RNA immunoprecipitation
- METTL
methyltransferase-like
- MTCH2
mitochondrial carrier homology 2
- Mut
mutant
- qPCR
quantitative PCR
- RIP
RNA immunoprecipitation
- WT
wild type
- YTHDF
YTH domain family
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Wang and X. Wang conceived and designed the study; Q. Jiang, Q. Liu, M. Cai, R. Wu, F. Wang, and Y. Yao performed the research and acquired the data; B. Sun, Q. Jiang, and X. Wang performed data analyses; Q. Jiang wrote the manuscript; and all authors read, edited, and agreed upon the final version of the manuscript.
REFERENCES
- 1.Hilton, T. N., Tuttle, L. J., Bohnert, K. L., Mueller, M. J., Sinacore, D. R. (2008) Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys. Ther. 88, 1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vettor, R., Milan, G., Franzin, C., Sanna, M., De Coppi, P., Rizzuto, R., Federspil, G. (2009) The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Endocrinol. Metab. 297, E987–E998 [DOI] [PubMed] [Google Scholar]
- 3.Dodson, M. V., Jiang, Z., Chen, J., Hausman, G. J., Guan, L. L., Novakofski, J., Thompson, D. P., Lorenzen, C. L., Fernyhough, M. E., Mir, P. S., Reecy, J. M. (2010) Allied industry approaches to alter intramuscular fat content and composition in beef animals. J. Food Sci. 75, R1–R8 [DOI] [PubMed] [Google Scholar]
- 4.Zhao, S. M., Li, W. Z., Pan, H. B., Huang, Y., Yang, M. H., Wei, H. J., Gao, S. Z. (2012) Expression levels of candidate genes for intramuscular fat deposition in two Banna mini-pig inbred lines divergently selected for fatness traits. Genet. Mol. Biol. 35, 783–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodhi, S. S., Park, W. C., Ghosh, M., Kim, J. N., Sharma, N., Shin, K. Y., Cho, I. C., Ryu, Y. C., Oh, S. J., Kim, S. H., Song, K. D., Hong, S. P., Cho, S. A., Kim, H. B., Jeong, D. K. (2014) Comparative transcriptomic analysis to identify differentially expressed genes in fat tissue of adult Berkshire and Jeju Native Pig using RNA-seq. Mol. Biol. Rep. 41, 6305–6315 [DOI] [PubMed] [Google Scholar]
- 6.Wang, Z., Li, Q., Chamba, Y., Zhang, B., Shang, P., Zhang, H., Wu, C. (2015) Identification of genes related to growth and lipid deposition from transcriptome profiles of pig muscle tissue. PLoS One 10, e0141138; erratum: 12, e0172930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, Y. Z., Sun, J. J., Zhang, L. Z., Li, C. J., Womack, J. E., Li, Z. J., Lan, X. Y., Lei, C. Z., Zhang, C. L., Zhao, X., Chen, H. (2014) Genome-wide DNA methylation profiles and their relationships with mRNA and the microRNA transcriptome in bovine muscle tissue (Bos taurine). Sci. Rep. 4, 6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, S., Shen, L., Xia, Y., Yang, Q., Li, X., Tang, G., Jiang, Y., Wang, J., Li, M., Zhu, L. (2016) DNA methylation landscape of fat deposits and fatty acid composition in obese and lean pigs. Sci. Rep. 6, 35063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang, Y., Ma, C., Sun, Y., Li, Y., Kang, L., Jiang, Y. (2017) Dynamic transcriptome and DNA methylome analyses on longissimus dorsi to identify genes underlying intramuscular fat content in pigs. BMC Genomics 18, 780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, H., Zheng, Y., Wang, G., Li, H. (2013) Identification of microRNA and bioinformatics target gene analysis in beef cattle intramuscular fat and subcutaneous fat. Mol. Biosyst. 9, 2154–2162 [DOI] [PubMed] [Google Scholar]
- 11.Chen, F. F., Xiong, Y., Peng, Y., Gao, Y., Qin, J., Chu, G. Y., Pang, W. J., Yang, G. S. (2017) miR-425-5p inhibits differentiation and proliferation in porcine intramuscular preadipocytes. Int. J. Mol. Sci. 18, 2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, T., Zhang, X., Han, K., Zhang, G., Wang, J., Xie, K., Xue, Q., Fan, X. (2017) Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. PLoS One 12, e0172389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer, K. D., Saletore, Y., Zumbo, P., Elemento, O., Mason, C. E., Jaffrey, S. R. (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J., Yue, Y., Han, D., Wang, X., Fu, Y., Zhang, L., Jia, G., Yu, M., Lu, Z., Deng, X., Dai, Q., Chen, W., He, C. (2014) A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, P., Doxtader, K. A., Nam, Y. (2016) Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell 63, 306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorci, M., Ianniello, Z., Cruciani, S., Larivera, S., Ginistrelli, L. C., Capuano, E., Marchioni, M., Fazi, F., Fatica, A. (2018) METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 9, 796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia, G., Fu, Y., Zhao, X., Dai, Q., Zheng, G., Yang, Y., Yi, C., Lindahl, T., Pan, T., Yang, Y. G., He, C. (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885–887; erratum: 8, 1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng, G., Dahl, J. A., Niu, Y., Fedorcsak, P., Huang, C. M., Li, C. J., Vågbø, C. B., Shi, Y., Wang, W. L., Song, S. H., Lu, Z., Bosmans, R. P., Dai, Q., Hao, Y. J., Yang, X., Zhao, W. M., Tong, W. M., Wang, X. J., Bogdan, F., Furu, K., Fu, Y., Jia, G., Zhao, X., Liu, J., Krokan, H. E., Klungland, A., Yang, Y. G., He, C. (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, X., Lu, Z., Gomez, A., Hon, G. C., Yue, Y., Han, D., Fu, Y., Parisien, M., Dai, Q., Jia, G., Ren, B., Pan, T., He, C. (2014) N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, X., Zhao, B. S., Roundtree, I. A., Lu, Z., Han, D., Ma, H., Weng, X., Chen, K., Shi, H., He, C. (2015) N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi, H., Wang, X., Lu, Z., Zhao, B. S., Ma, H., Hsu, P. J., Liu, C., He, C. (2017) YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 27, 315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao, W., Adhikari, S., Dahal, U., Chen, Y. S., Hao, Y. J., Sun, B. F., Sun, H. Y., Li, A., Ping, X. L., Lai, W. Y., Wang, X., Ma, H. L., Huang, C. M., Yang, Y., Huang, N., Jiang, G. B., Wang, H. L., Zhou, Q., Wang, X. J., Zhao, Y. L., Yang, Y. G. (2016) Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519; erratum: 925 [DOI] [PubMed] [Google Scholar]
- 23.Li, A., Chen, Y. S., Ping, X. L., Yang, X., Xiao, W., Yang, Y., Sun, H. Y., Zhu, Q., Baidya, P., Wang, X., Bhattarai, D. P., Zhao, Y. L., Sun, B. F., Yang, Y. G. (2017) Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 27, 444–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, X., Zhu, L., Chen, J., Wang, Y. (2015) mRNA m6A methylation downregulates adipogenesis in porcine adipocytes. Biochem. Biophys. Res. Commun. 459, 201–207 [DOI] [PubMed] [Google Scholar]
- 25.Zhao, X., Yang, Y., Sun, B. F., Shi, Y., Yang, X., Xiao, W., Hao, Y. J., Ping, X. L., Chen, Y. S., Wang, W. J., Jin, K. X., Wang, X., Huang, C. M., Fu, Y., Ge, X. M., Song, S. H., Jeong, H. S., Yanagisawa, H., Niu, Y., Jia, G. F., Wu, W., Tong, W. M., Okamoto, A., He, C., Rendtlew Danielsen, J. M., Wang, X. J., Yang, Y. G. (2014) FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 24, 1403–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters, E. M., Wells, K. D., Bryda, E. C., Schommer, S., Prather, R. S. (2017) Swine models, genomic tools and services to enhance our understanding of human health and diseases. Lab Anim. (NY) 46, 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassols, A., Costa, C., Eckersall, P. D., Osada, J., Sabrià, J., Tibau, J. (2014) The pig as an animal model for human pathologies: a proteomics perspective. Proteomics Clin. Appl. 8, 715–731 [DOI] [PubMed] [Google Scholar]
- 28.Wu, T., Zhang, Z., Yuan, Z., Lo, L. J., Chen, J., Wang, Y., Peng, J. (2013) Distinctive genes determine different intramuscular fat and muscle fiber ratios of the longissimus dorsi muscles in Jinhua and landrace pigs. PLoS One 8, e53181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo, J., Shan, T., Wu, T., Zhu, L. N., Ren, Y., An, S., Wang, Y. (2011) Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J. Anim. Sci. 89, 185–191 [DOI] [PubMed] [Google Scholar]
- 30.Zhao, C., Chen, X., Wu, W., Wang, W., Pang, W., Yang, G. (2016) MAT2B promotes adipogenesis by modulating SAMe levels and activating AKT/ERK pathway during porcine intramuscular preadipocyte differentiation. Exp. Cell Res. 344, 11–21 [DOI] [PubMed] [Google Scholar]
- 31.Peng, Y., Chen, F. F., Ge, J., Zhu, J. Y., Shi, X. E., Li, X., Yu, T. Y., Chu, G. Y., Yang, G. S. (2016) miR-429 inhibits differentiation and promotes proliferation in porcine preadipocytes. Int. J. Mol. Sci. 17, 2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominissini, D., Moshitch-Moshkovitz, S., Schwartz, S., Salmon-Divon, M., Ungar, L., Osenberg, S., Cesarkas, K., Jacob-Hirsch, J., Amariglio, N., Kupiec, M., Sorek, R., Rechavi, G. (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 [DOI] [PubMed] [Google Scholar]
- 33.Heinz, S., Benner, C., Spann, N., Bertolino, E., Lin, Y. C., Laslo, P., Cheng, J. X., Murre, C., Singh, H., Glass, C. K. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trapnell, C., Williams, B. A., Pertea, G., Mortazavi, A., Kwan, G., van Baren, M. J., Salzberg, S. L., Wold, B. J., Pachter, L. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, X., Sun, B., Jiang, Q., Wu, R., Cai, M., Yao, Y., Liu, Q., Shi, H., Feng, J., Wang, Y. (2018) mRNA m6A plays opposite role in regulating UCP2 and PNPLA2 protein expression in adipocytes. [E-pub ahead of print] Int. J. Obes. (Lond) 10.1038/s41366-018-0027 [DOI] [PubMed] [Google Scholar]
- 36.Luo, G. Z., MacQueen, A., Zheng, G., Duan, H., Dore, L. C., Lu, Z., Liu, J., Chen, K., Jia, G., Bergelson, J., He, C. (2014) Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 5, 5630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzaglo-Azriel, L., Kuperman, Y., Tsoory, M., Zaltsman, Y., Shachnai, L., Zaidman, S. L., Bassat, E., Michailovici, I., Sarver, A., Tzahor, E., Haran, M., Vernochet, C., Gross, A. (2016) Loss of muscle MTCH2 increases whole-body energy utilization and protects from diet-induced obesity. Cell Reports 14, 1602–1610; erratum: 18, 1335–1336 [DOI] [PubMed] [Google Scholar]
- 38.Rottiers, V., Francisco, A., Platov, M., Zaltsman, Y., Ruggiero, A., Lee, S. S., Gross, A., Libert, S. (2017) MTCH2 is a conserved regulator of lipid homeostasis. Obesity (Silver Spring) 25, 616–625 [DOI] [PubMed] [Google Scholar]
- 39.Zhang, G. H., Lu, J. X., Chen, Y., Zhao, Y. Q., Guo, P. H., Yang, J. T., Zang, R. X. (2014) Comparison of the adipogenesis in intramuscular and subcutaneous adipocytes from Bamei and Landrace pigs. Biochem. Cell Biol. 92, 259–267 [DOI] [PubMed] [Google Scholar]
- 40.Yamada, T., Kawakami, S., Nakanishi, N. (2010) Fat depot-specific differences in angiogenic growth factor gene expression and its relation to adipocyte size in cattle. J. Vet. Med. Sci. 72, 991–997 [DOI] [PubMed] [Google Scholar]
- 41.Wang, J., Mei, H., Chen, W., Jiang, Y., Sun, W., Li, F., Fu, Q., Jiang, F. (2012) Study of eight GWAS-identified common variants for association with obesity-related indices in Chinese children at puberty. Int. J. Obes. 36, 542–547 [DOI] [PubMed] [Google Scholar]
- 42.Bernhard, F., Landgraf, K., Klöting, N., Berthold, A., Büttner, P., Friebe, D., Kiess, W., Kovacs, P., Blüher, M., Körner, A. (2013) Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia 56, 311–322 [DOI] [PubMed] [Google Scholar]
- 43.Kulyté, A., Rydén, M., Mejhert, N., Dungner, E., Sjölin, E., Arner, P., Dahlman, I. (2011) MTCH2 in human white adipose tissue and obesity. J. Clin. Endocrinol. Metab. 96, E1661–E1665 [DOI] [PubMed] [Google Scholar]
- 44.Meyer, K. D., Patil, D. P., Zhou, J., Zinoviev, A., Skabkin, M. A., Elemento, O., Pestova, T. V., Qian, S. B., Jaffrey, S. R. (2015) 5′ UTR m(6)A promotes cap-independent translation. Cell 163, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du, H., Zhao, Y., He, J., Zhang, Y., Xi, H., Liu, M., Ma, J., Wu, L. (2016) YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 7, 12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chau, Y. Y., Bandiera, R., Serrels, A., Martínez-Estrada, O. M., Qing, W., Lee, M., Slight, J., Thornburn, A., Berry, R., McHaffie, S., Stimson, R. H., Walker, B. R., Chapuli, R. M., Schedl, A., Hastie, N. (2014) Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 16, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.