Abstract
The prevalence of obesity increases with age in humans and in rodents. Age‐related obesity is characterized by leptin resistance and associated with heightened risk of metabolic disorders. However, the effect of leptin resistance per se has been difficult to disentangle from other effects of aging. Here we demonstrate that celastrol, a natural phytochemical that was previously shown to act as a leptin sensitizer, induces weight loss in aged animals, but not in young controls. Celastrol reduces food intake and lowers fasting glucose without affecting energy expenditure. Unexpectedly, administration of celastrol just before the dark period disrupted circadian rhythms of sleep and activity. This regimen was also associated with loss of lean mass an outcome that would not be desirable in elderly patients. Adjusting the timing of celastrol administration by 12 hr, to the beginning of the light period, avoided interference with circadian rhythms while retaining the reductions in body weight and adiposity. Thus, targeting leptin signaling is an effective strategy to ameliorate age‐associated weight gain, and can profoundly impact circadian rhythms.
1. INTRODUCTION
The aging population is rising worldwide, with aged individuals 65 years or older projected to represent more than 20% of the population in the United States by 2035 (Mathus‐Vliegen, 2012). Obesity becomes increasingly more prevalent with age, and is a major risk factor for many conditions including cardiovascular disease, hypertension, stroke, diabetes, dyslipidemia, cognitive decline, and mortality (Chung, Kang, Lee, Lee & Lee, 2013; Dominguez & Barbagallo, 2016; Han & Lean, 2016; Saag & Choi, 2006). In young individuals, the hormone leptin plays a key role in maintaining energy balance and body weight. Leptin is secreted by adipose tissue in proportion to its mass to relay information on peripheral energy stores to the central nervous system (Ahima, Saper, Flier & Elmquist, 2000; Frederich et al., 1995). Binding of leptin to its receptor expressed in different sites within the brain can suppress food intake and increase energy expenditure, thus serving as a negative feedback on energy storage (Ovesjö, Gamstedt, Collin & Meister, 2001; Balthasar et al., 2004; Scott, Williams, Rossi, Lee & Elmquist, 2011; Billes, Simonds & Cowley, 2012; Rezai‐Zadeh et al., 2014; Li, Kelly, Heiman, Greengard & Friedman 2015). Aging in humans and rodents is characterized by an expansion of adipose mass in middle age that is not resolved, despite increased circulating leptin levels (Justesen et al., 2001; Kotani et al., 1994; Kuk, Saunders, Davidson & Ross, 2009; Kyle et al., 2001; Muzumdar et al., 2008; Shek & Scarpace, 2000; Visser et al., 2003). The inability of elevated leptin to bring about appropriate metabolic and endocrine outcomes, including the decrease in food intake and body weight, is termed leptin resistance (Carter, Caron, Richard & Picard, 2013; Gabriely, Xiao Hui, Yang, Rossetti & Barzilai, 2002; Ma et al., 2002; Myers et al., 2012; Pétervári et al., 2014). The underlying mechanism of leptin resistance with age is debated, and has alternately been attributed to disruption of leptin transport across the blood‐brain barrier, decreased leptin receptor expression, feedback inhibition of leptin signaling by downstream effectors such as SOCS3 and TCPTP, or chronic ER stress and inflammation (Bigford, Bracchi‐Ricard, Nash & Bethea, 2012; Fernández‐Galaz et al., 2001, 2002; González‐Rodríguez et al., 2012; Martínez, Duran‐Aniotz, Cabral‐Miranda, Vivar & Hetz, 2017; Peralta, Carrascosa, Gallardo, Ros & Arribas, 2002; Rostás et al., 2016; Scarpace, Matheny & Tümer, 2001). Although improved hypothalamic leptin signaling correlates with the amelioration of age‐associated obesity in several models (Fernández‐Galaz et al., 2002; Sasaki et al., 2014; Yang et al., 2012), the contribution of leptin resistance per se has been difficult to ascertain.
Celastrol, a phytochemical isolated from the thunder god vine (Tripterygium Wilfordi) was recently identified as a leptin sensitizer based on its ability to suppress food intake and reduce body weight in diet‐induced obese mice, but not in lean mice or in obese mice with genetically disrupted leptin signaling (Liu, Lee, Hernandez, Mazitschek & Ozcan, 2015). Thus, its effects are contingent upon elevated plasma leptin levels and leptin receptor expression. We hypothesized that celastrol might also be an effective strategy to restore leptin sensitivity and body weight homeostasis in aged mice, which, like obese mice, display hyperleptinemia and leptin resistance. We report that celastrol ameliorates leptin resistance in aged mice and decreases body weight, but unexpectedly had adverse effects on the circadian rhythms of locomotor activity and sleep when administered prior to the lights off active period. Switching the time of drug delivery to just before the onset of the light period avoided the circadian effects while still maintaining the reductions in food intake and body weight. Therefore, celastrol is effective in treating age‐related obesity, but the time of delivery has a profound impact on the outcome of treatment.
2. RESULTS
2.1. Celastrol ameliorates age‐associated leptin resistance
Aged mice show an increase in body weight and fat mass without any change in lean mass (Figure 1a), as previously reported (Houtkooper et al., 2011). Consistent with the increased adipose mass, aged mice display higher plasma leptin concentrations (Figure 1b). A single injection of recombinant leptin tended to decrease body weight and food intake, in young but not in aged mice (Figure 1c,d), consistent with previous reports that aged mice are leptin resistant (Fernández‐Galaz et al., 2002; Gabriely et al., 2002). Next, we determined the ability of celastrol to reestablish leptin signaling in aged mice. We found that 2 days of celastrol pre‐treatment sensitized aged mice to leptin's action on food intake and body weight (Figure 1e,f). Importantly, the difference in leptin sensitivity between young and aged mice was completely abolished after celastrol treatment.
Figure 1.
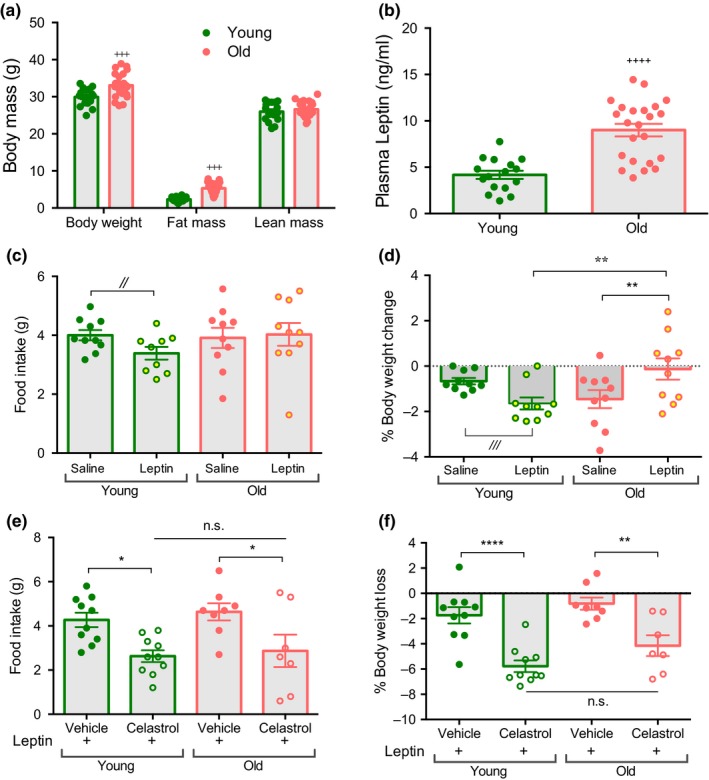
Celastrol restores leptin sensitivity in aged mice. (a) Body composition of young (4 month) and old (18 month) male mice. n = 16–23. (b) Plasma leptin of young (4 month) and old (18 month) mice. n = 16–23. (c) 24 hr food intake measurement during saline (average of 5 d) and leptin injection in young (4 month) and old (21 month) mice. n = 9–10. (d) % body weight change during saline (average of 5 d) and leptin injection in young (5 month) and old mice (21 month). n = 9–10. (e) Food intake of vehicle or celastrol treated mice after 24 hr of leptin injection in young (6 month) and old (22 month) mice. n = 7–10. (f) % Body weight change of vehicle or celastrol treated mice after 24 hr of leptin injection in young (6 month) and old (22 month) mice. n = 7–10. All data are presented as mean ± SEMs. *p < 0.05, **p < 0.01, +++ p < 0.005, ++++ p < 0.0001, //,///indicated comparisons are non‐significant when corrected for multiple comparisons (ANOVA) but are nominally significant by Student's t‐test (// p < 0.05, /// p < 0.005)
2.2. Celastrol decreases body weight in aged mice by reducing food intake
To assess the effect of celastrol on body weight homeostasis young and aged mice were intraperitoneally injected with vehicle or celastrol for 5 days. We found that aged mice lost ~11.5% of their initial body weight with celastrol treatment (Figure 2a). Celastrol treatment decreased both fat mass and lean mass in aged mice (Figure 2b,c). On the other hand, celastrol did not cause a significant change in body weight, fat or lean mass in young mice, as previously reported (Liu, Lee, Hernandez, Mazitschek, Ozcan, 2015). This difference is most likely attributable to the lower circulating leptin concentrations in young, leptin sensitive animals. Celastrol lowered fasting glucose in both young and old mice and reduced the absolute area under the glucose curve during an insulin tolerance test (ITT) in aged mice (Supporting information Figure S1a‐b). However, the effect of celastrol on ITT is no longer significant when the data are plotted as percent change (Supporting information Figure S1c‐d), suggesting that celastrol may in part lower glucose through mechanisms independent of insulin sensitivity per se. Celastrol treatment significantly decreased food intake during the dark period, with a much more pronounced effect in aged mice (Figure 2d). Food intake correlates with water intake in both lean and obese rodents (Fitzsimons & Le Magnen, 1969; Strominger, 1947). Consistently, we found that celastrol reduced water consumption in aged mice more than in young mice (Supporting information Figure S2a). In contrast, energy expenditure was unaffected by celastrol treatment in both age groups (Figure 2e,f). RER was decreased only in celastrol treated aged mice, consistent with reduced food intake (Figure 2g). Thus, our data establish that a decrease in food intake in the absence of any overt change in energy expenditure leads to body weight loss in celastrol treated old mice.
Figure 2.
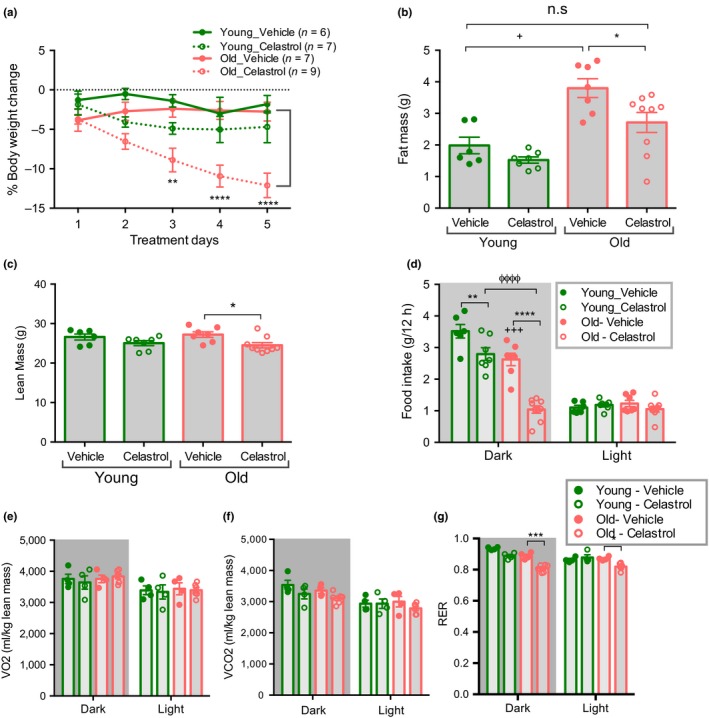
Celastrol decreases food intake and body weight in aged mice. (a) % Body weight change in 4 month young and 18–20 month aged mice treated with either vehicle or celastrol. n = 6–9. (b) Fat mass of mice treated with vehicle or celastrol for 5 d as in (a). n = 6–9. (c) Lean mass of mice treated with vehicle or celastrol for 5 d as in (a). n = 6–9. (d) Average food intake during 5 days of vehicle or celastrol treatment as in (a). n = 6–9. (e) Average VO2 normalized to lean mass during 5 days of vehicle or celastrol treatment as in (a). n = 4–6. (f) Average VCO2 normalized to lean mass during 5 days of vehicle or celastrol treatment as in (a). n = 4–6. (g) Average RER during 5 days of vehicle or celastrol treatment as in (a). n = 4–6. All data are presented as mean ± SEMs. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001, ANOVA comparison between vehicle and celastrol treated group; + p < 0.05, +++ p < 0.005, ++++ p < 0.0001 ANOVA between vehicle treated young and old mice, ϕϕϕϕ p < 0.0001 ANOVA between celastrol treated young and old mice
2.3. Celastrol treatment disrupts circadian rhythms of locomotor activity and sleep
Locomotor activity, sleep/wake architecture and circadian rhythms decline with age (Houtkooper et al., 2011; Naidoo et al., 2011; Wimmer et al., 2013). Accordingly, we observed reduced locomotor activity in aged mice (Figure 3a,b). Celastrol treatment significantly decreased total locomotor activity in both young and aged mice.
Figure 3.
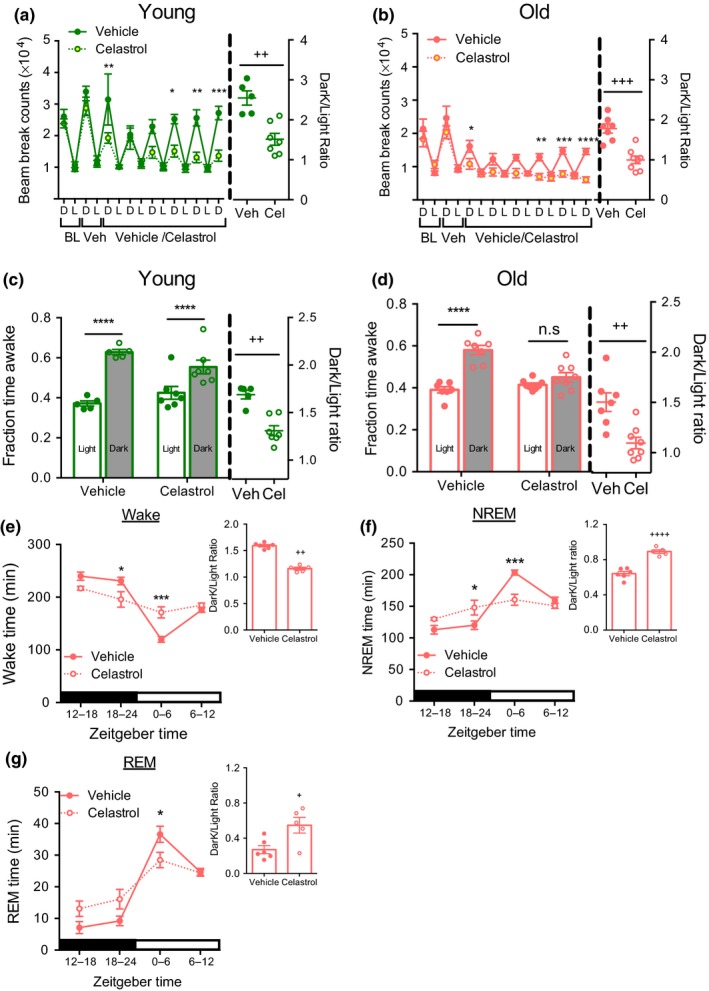
Celastrol treatment disrupts circadian activity and sleep pattern. (a) Beam break counts (left) and dark to light counts ratio (right) of young mice treated with vehicle or celastrol as in Figure 2a. n = 5–7. (b) Beam break counts (left) and dark to light counts ratio (right) of old mice treated with vehicle or celastrol as in Figure 2a. n = 7–8. (c) Fraction wake time (left) and dark:light ratio of young mice in (a) determined using beam break counts as in Figure 2a. n = 5–7. (d) Fraction wake time (left) and dark:light ratio of old mice as treated in Figure 2a determined by beam break counts. n = 7–8. (e) Wake time (left) and dark light ratio (right) as recorded by EEG in aged (20 month) mice treated with cealstrol for Figure 4d. n = 5–6. (f) NREM sleep (left) and dark light ratio (right) measured as in (d). n = 5–6. (g) REM sleep (left) and dark light ratio (right) recorded as in (d). n = 5–6. All data are presented as mean±SEMs. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001, ANOVA comparison between vehicle and celastrol treated groups; + p < 0.05, ++ p < 0.005, +++ p < 0.001 and ++++ p < 0.0001, unpaired t‐test between vehicle and celastrol treatment
Strikingly, the circadian rhythm of activity was significantly altered after celastrol treatment in both age groups. Liu et al. (2015) also reported decrease in locomotor activity in young lean and diet‐ induced obese mice treated with celastrol, and suggested that this might reflect reduced food‐ seeking behavior. Given that we and others have shown that locomotor activity can predict sleep patterns (Brown, Hasan, Foster & Peirson, 2017; Pack et al., 2007), we chose to examine the alternative hypothesis that celastrol affects the circadian timing of sleep. Specifically, we have previously established that a period of immobility as estimated by lack of beam break for >40 s is an accurate predictor of sleep (Pack et al., 2007). We used this parameter to determine total sleep and wake time, and estimated diurnal ratio to measure changes in circadian rhythm. Based on this analysis, the vehicle‐treated young and aged mice displayed increased wake time during the active dark period compared to the inactive light period (Figure 3c,d). Paralleling the effect on activity, the circadian pattern of sleep/wake cycles was disrupted in mice treated with celastrol in both of the age groups. Celastrol had more pronounced effects in aged mice, resulting in complete loss of the circadian pattern of wake time. To formally confirm the effect of celastrol on sleep we next performed electroencephalography (EEG) measurements in aged mice. Reduced body weight and food intake were observed in response to celastrol after EEG instrumentation as in non‐instrumented mice (Supporting information Figure S2b‐c). EEG measurements confirmed that celastrol decreased wake time during the dark period while increasing it during light period, resulting in a flattening of circadian rhythms of sleep (Figure 3e). Furthermore, the decrease in wakefulness in the dark period paralleled an increase in both total NREM sleep and REM bouts (Figure 3F,G and Table 1).
Table 1.
Electroencephalography (EEG) recording data of aged mice treated with vehicle or celastrol
| Vehicle | Celastrol | Significance | |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| Wake | |||
| Number of bouts | |||
| 24‐hr | 446.5 ± 21.9 | 437.2 ± 11.2 | ns |
| Light | 230.8 ± 15.0 | 211.6 ± 15.6 | ns |
| Dark | 215.7 ± 14.4 | 225.6 ± 9.2 | ns |
| Bout length | |||
| 24‐hr | 104.1 ± 4.9 | 105.5 ± 2.6 | ns |
| Light | 78.32 ± 4.6 | 103.7 ± 10.2 | ns (p < 0.086) |
| Dark | 134.3 ± 10.8 | 110.3 ± 5.2 | ns (p < 0.1) |
| NREM | |||
| Number of bouts | |||
| 24‐hr | 447.5 ± 22.0 | 437.4 ± 10.8 | ns |
| Light | 231.3 ± 14.7 | 212.0 ± 15.4 | ns |
| Dark | 216.2 ± 14.7 | 225.4 ± 8.9 | ns |
| Bout length | |||
| 24‐hr | 80.9 ± 4.1 | 81.2 ± 3.9 | ns |
| Light | 96.1 ± 6.1 | 90.2 ± 7.4 | ns |
| Dark | 65.6 ± 3.5 | 74.6 ± 4.8 | ns |
| REM | |||
| Number of bouts | |||
| 24‐hr | 89.2 ± 7.7 | 106.2 ± 12.8 | ns |
| Light | 69.8 ± 5.4 | 63.2 ± 5.3 | ns |
| Dark | 19.3 ± 4.2 | 43.0 ± 8.8 | a |
| Bout length | |||
| 24‐hr | 53.1 ± 2.4 | 47.2 ± 2.5 | ns |
| Light | 53.4 ± 2.6 | 50.8 ± 2.2 | ns |
| Dark | 54.5 ± 4.0 | 41.9 ± 3.1 | ns (p < 0.1) |
p < 0.05, repeated measures two way ANOVA following Sidak post‐hoc test.
Taken together, our finding suggests that celastrol adversely affects circadian rhythms of activity and sleep.
2.4. Celastrol delivery at ZT23 abrogates age‐associated obesity
Our results thus far show that celastrol negatively influences sleep and wake, by decreasing wake time during their active period and increasing wake time during their inactive period (Figure 3). We reasoned that if celastrol provided a circadian cue, then shifting the time of delivery might reverse its effect on circadian rhythms. Notably, a circadian rhythm has been described for plasma leptin, peaking before the onset of the light period and falling over the course of the day (Ahima, Prabakaran & Flier, 1998; Sukumaran, Jusko, DuBois & Almon, 2011). Injection at ZT23 (before the onset of the light period and 12 hr later than in the previous study) decreased body weight and adipose mass in aged mice (Figure 4a,b), similar to the effect at ZT11 injection. In contrast to ZT11 injection, ZT23 injection did not significantly decrease lean mass in aged mice (Figure 4c). Indirect calorimetry confirmed that the weight loss induced by ZT23 injection was primarily driven by a decrease in food intake and RER without any change in energy expenditure in aged mice (Figure 4d‐g, Supporting information Figure S2d).
Figure 4.
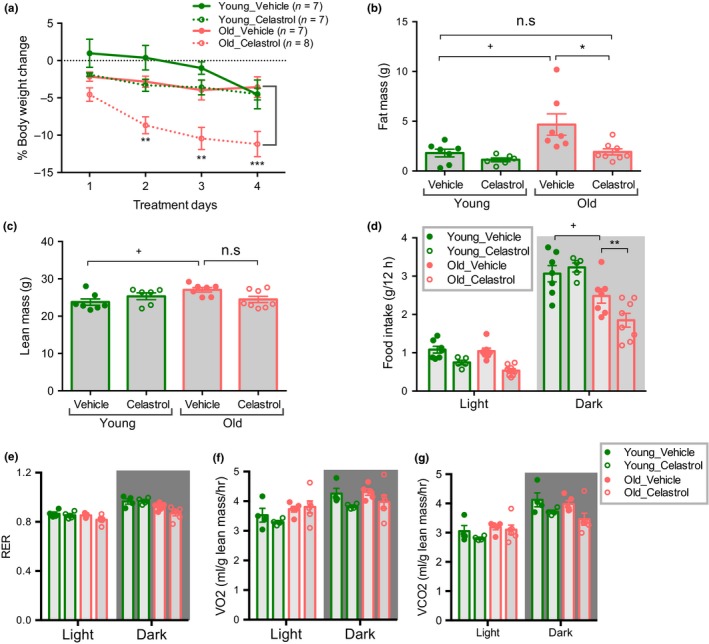
Celastrol delivery at ZT23 ameliorates age‐associated obesity. (a) % Body weight change in 4 month young and 18–20 month old mice treated with either vehicle or celastrol. n = 6–8. (b) Fat mass of mice treated with vehicle or celastrol for 4 d as in (a). n = 6–8. (c) Lean mass of mice treated with vehicle or celastrol for 4 d as in (a). n = 6–8. (d) Average food intake during 4 days of vehicle or celastrol treatment as in (a). n = 5–8. (e) Average VO2 normalized to lean mass during 4 days of vehicle or celastrol treatment as in (a). n = 4–6. (f) Average VCO2 normalized to lean mass during 4 days of vehicle or celastrol treatment as in (a). n = 4–6. (g) Average RER during 4 days of vehicle or celastrol treatment as in (a). n = 4–6. All data are presented as mean ± SEMs. *p < 0.05, **p < 0.01, ***p < 0.001, ANOVA comparison between vehicle and celastrol treated groups; + p < 0.05, ANOVA between vehicle treated young and aged mice
2.5. Celastrol delivery at ZT23 restores circadian rhythms of activity and sleep
To test whether the shift in circadian timing of celastrol delivery could ameliorate its effects on sleep and activity patterns, we performed beam break analysis. Mice injected with celastrol at ZT23 retained the pattern of increased activity during the dark period as compared to the light period similar to vehicle‐treated controls (Figure 5a,b), although a modest reduction in activity during the dark period was still detected. Calculating wake epochs demonstrates that the circadian pattern of the wake period was preserved by ZT23 injection (Figure 5c,d).
Figure 5.
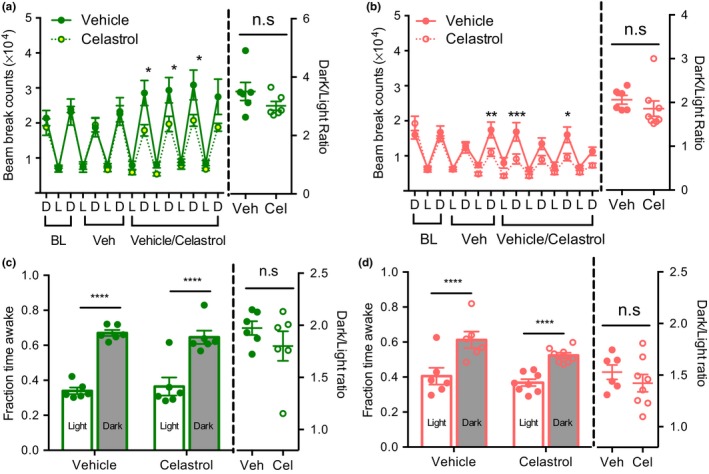
Celastrol injection at ZT23 restores circadian pattern of behavior. (a) Beam break counts (left) and dark to light counts ratio (right) of 4 month young mice treated with vehicle or celastrol. n = 6. (b) Beam break counts (left) and dark to light counts ratio (right) of 18–20 month old mice treated with vehicle or celastrol. n = 6–8. (c) Average fraction wake time (left) and dark:light ratio determined by beam break counts of 4 month young mice during 4 days of vehicle or celastrol treatment as in (a). n = 6. (d) Average fraction wake time (left) and dark:light ratio determined by beam break counts of 18–20 month old mice during 4 days of vehicle or celastrol treatment as in (b). n = 6–8. All data are presented as mean ± SEMs. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ANOVA comparison of vehicle and celastrol treated groups; p > 0.05 non‐significant (n.s.), unpaired t‐test between vehicle and celastrol treatment
Collectively, our data demonstrates that the circadian timing of celastrol delivery can have a profound effect on the circadian rhythms of activity and sleep.
3. DISCUSSION
Aging is characterized by an increase in obesity and disruption of metabolic homeostasis, driving intense interest in identifying small molecules that can reverse these effects. The naturally occurring phytochemical celastrol was recently reported to cause weight loss in diet‐ induced obese mice by restoring leptin sensitivity and thereby allowing hyperleptinemia to appropriately suppress food intake. In contrast, the body weights of lean mice with lower leptin levels were not affected. In this study, we determined that age‐related obesity, which involves milder leptin resistance and hyperleptinemia, is also treatable with celastrol. We report that celastrol restores leptin sensitivity, suppresses food intake and lowers body weight selectively in aged mice. Unexpectedly, we found that celastrol injection at ZT11 disrupts the circadian rhythms of locomotor activity and sleep, and that this can be avoided by shifting the timing of administration by 12 hours to ZT23. Thus, celastrol holds promise in the treatment of age‐ related obesity, but requires careful attention to circadian timing of treatment.
We found that intraperitoneal injection of celastrol (200 μg/kg/day) decreases body weight almost exclusively by suppressing food intake in aged mice. Our findings are consistent with previous reports on celastrol‐induced weight loss in DIO mice receiving a similar dosing regimen (Hu et al., 2017; Liu et al., 2015; Zhang et al., 2017). Central leptin signaling was shown to mediate the effect of celastrol on food intake (Liu et al., 2015).
Although the mechanism connecting celastrol to leptin signaling remains elusive, Hu et al., (2017) recently demonstrated that celastrol can bind Nurr77 and induce interaction with TRAF2 to stimulate autophagy and reduce inflammation. They further demonstrate that Nurr77 is required for celastrol‐induced weight loss in vivo and speculate that improved leptin sensitivity might be a consequence of reduced hypothalamic inflammation. Interestingly, celastrol administration at higher doses in the diet (1–3 mg/kg/day) was reported to increase energy expenditure by stimulating thermogenesis and oxygen consumption in adipose tissue and skeletal muscle (Ma et al., 2015). This effect was dependent on an HSF1‐PGC1α pathway that was found to act independently from food intake (Ma et al., 2015). Together, these studies suggest that celastrol treatment can influence different aspects of energy balance to alleviate obesity, depending on the dose and/or route of delivery. It will be interesting in future studies to determine how aging and celastrol treatment affect expression of leptin and its receptor, as well as downstream signaling in the hypothalamus versus peripheral tissues.
We and others have previously shown decreased locomotor activity and fragmented sleep‐wake architecture in aged mice (Houtkooper et al., 2011; Naidoo et al., 2011; Wimmer et al., 2013). Nevertheless, aged mice maintain a higher level of activity during the active dark period compared to the inactive light period. Similarly, aged mice exhibit longer wake time in the dark period, and sleep time during the light period. Our study suggests that administering celastrol just before the dark period disrupts circadian patterns of activity and sleep in both young and aged mice. However, we were able to overcome this effect by switching the time of injection to the light period. We do not believe that celastrol is acting primarily as a somnogen because of the delayed onset of the sleep/wake differences (Figure 3). Interestingly, this fits with the known circadian rhythm of leptin concentration, which peaks during late dark phase (Ahima et al., 1998; Sukumaran et al., 2011). We hypothesize that sensitizing to leptin at the time of its natural peak may be less disruptive to circadian rhythms, and that further optimization of the timing might even strengthen them, though this idea remains to be formally tested. It also remains possible that the effects of celastrol on circadian rhythms could be independent from leptin. The importance of delivering drugs at the optimum time within 24 hr biological rhythms to maximize their efficacy and tolerability is well recognized, specifically in the field of cancer therapeutics (Ballesta, Innominato, Dallmann, Rand & Lévi, 2017; Lévi & Okyar, 2011). Our study reemphasizes the need to assess optimal drug delivery time of celastrol (and possibly leptin) in clinical trials to avoid adverse side effects.
Celastrol, the most potent bioactive material in Tripterygium Wilfordi has been extensively tested in the treatment of cancer, lupus, amyotrophic lateral sclerosis, and Alzheimer's disease in rodent models (Cascão, Fonseca & Moita, 2017). However, the clinical relevance of celastrol is currently restricted by insolubility, bioavailability and narrow therapeutic window. Thus, further studies are required to completely understand the wide range of physiological and behavioral effects of celastrol before this compound reaches human therapy. In summary, we demonstrate that celastrol improves leptin sensitivity and ameliorates age‐associated obesity in mice. We further establish that the circadian timing of celastrol delivery impacts the behavioral rhythms of activity and sleep.
4. MATERIALS AND METHODS
4.1. Animal use and care
Animal experiments were conducted in accordance with guidelines of University of Pennsylvania Institutional Animal Care and Use Committee. Mice were maintained under 12‐hr light/dark cycles at ~21°C and either fed a standard lab chow (Rodent Diet 5010, LabDiet). Young (4–6 month) and Old (18–22 month) male mice were obtained from National Institute of Aging. Mice were sacrificed by cervical dislocation and tissues were harvested and frozen in liquid nitrogen and stored at −80°C until use.
4.2. Celastrol administration
Experiments were conducted in either in home cage or metabolic cage. Mice were acclimatized to single housing and vehicle (0.6% DMSO in 10% captisol) injection for 1–4 days and then intraperitoneally injected with vehicle or Celastrol (100–200 μg/kg body weight) for 4–6 days. Injections were carried out either before the onset of dark period at ZT11 (Figure 1, 2, 3 and Supporting information Figure S1) or onset of light period at ZT23 (Figures 4 and 5). Body weight and food intake were recorded daily at the time of injection.
4.3. Leptin sensitivity assay
Mice were singly housed in their home cage and intraperitoneally injected with saline at ZT11 for 5 days and monitored for body weight and food intake. On day 6 mice were intraperitoneally injected with leptin (4 mg/kg body weight). For leptin sensitivity after celastrol treatment, mice were injected with either vehicle or celastrol (200 μg/kg body weight) for 2 days. On the 3rd day mice were injected with either vehicle or celastrol 1 hr before leptin injection (5 mg/kg body weight). Body weight and food intake were manually tracked 24 hr after injection.
4.4. Metabolic studies
Energy balance after celastrol treatment was monitored using comprehensive lab animal monitoring system (CLAMS) (Columbus Instruments, Columbus, OH). Mice were acclimatized to metabolic cage (1–2 d) and vehicle injection (1–2 d) and then injected with either vehicle or celastrol. Food intake, energy expenditure, RER and activity is presented as an average of 4 d during vehicle or celastrol treatment.
4.5. Body composition
Body composition was measured by nuclear magnetic resonance imaging (EchoMRI, Echo Medical Systems, Houston, USA).
4.6. Insulin tolerance test
For insulin tolerance test mice were fasted for 6 hr and injected intraperitoneally with human insulin (0.75 IU/kg body weight). Blood glucose was measured periodically using one touch glucometer for 2 hr following injection.
4.7. EEG sleep recording
Sleep recordings were conducted as previously described (Perron, Pack & Veasey, 2015). Briefly, silver wire electrodes were soldered to gold sockets and push fit into 6‐channel plastic holders. Mice were anesthsized under isoflurane, and holes were drilled in frontoparietal areas where electrodes were inserted; EMG reference electodes were placed in nuchal muscles. After 1 week of recovery, mice were acclimated to tether cables for 1 week prior to any sleep/wake recordings. Data was acquired with Neurodata amplifier systems, exported to .edf files, and scored manually by an experience investigator blinded to experimental condition. Sleep/wake recordings were measured on Day 0 (prior to celestral treatment) and Day 4 of daily celestral injections. Total amount of wake, NREM sleep, and REM sleep, as well as sleep/wake fragmentation analysis, was calculated in MATLAB.
4.8. Statistical analysis
Data are expressed as mean ± SEM, of sample size n. One‐way or two‐way ANOVA was used with Tukey's or Sidak post‐hoc test for comparisons of three or more groups. Student's t‐test was used for two group comparisons or to determine nominal significance. p < 0.05 was considered to be significant.
Supporting information
ACKNOWLEDGMENTS
We thank all the members of the Baur lab for positive feedback and suggestions. We thank Cole S Purdy, Denise M DeFelice and Xiao Yin for technical assistance. We thank the University of Pennsylvania Diabetes Research Center (P30‐DK19525) for the use of the Mouse Phenotyping, Physiology and Metabolism Cores. All authors declare that they have no conflicts of interest.
Chellappa K, Perron IJ, Naidoo N, Baur JA. The leptin sensitizer celastrol reduces age‐associated obesity and modulates behavioral rhythms. Aging Cell. 2019;18:e12874 10.1111/acel.12874
Funding information
This work was supported by grants from the National Institutes of Aging (R01 AG043483 to J.A.B and P01 AG17628 to N.N) and the National Institute of Diabetes and Digestive, and Kidney Diseases (R01 DK098656 to J.A.B).
Contributor Information
Nirinjini Naidoo, Email: naidoo@pennmedicine.upenn.edu.
Joseph A. Baur, Email: baur@pennmedicine.upenn.edu
REFERENCES
- Ahima, R. S. , Prabakaran, D. , Flier, J. S. (1998) Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. Journal of Clinical Investigation. 101, 1020–1027. 10.1172/JCI1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima, R. S. , Saper, C. B. , Flier, J. S. , & Elmquist, J. K. (2000). Leptin regulation of neuroendocrine systems. Frontiers in Neuroendocrinology, 21, 263–307. 10.1006/frne.2000.0197 [DOI] [PubMed] [Google Scholar]
- Ballesta, A. , Innominato, P. F. , Dallmann, R. , Rand, D. A. , & Lévi, F. A. (2017). Systems chronotherapeutics. Pharmacological Reviews, 69, 161–199. 10.1124/pr.116.013441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar, N. , Coppari, R. , McMinn, J. , Liu, S. M. , Lee, C. E. , Tang, V. , … Lowell, B. B. (2004). Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron, 42, 983–991. 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bigford, G. E. , Bracchi‐Ricard, V. C. , Nash, M. S. , & Bethea, J. R. (2012). Alterations in mouse Hypothalamic adipokine gene expression and leptin signaling following chronic spinal cord injury and with advanced age. PLoS ONE, 7, e41073 10.1371/journal.pone.0041073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billes, S. K. , Simonds, S. E. , & Cowley, M. A. (2012). Leptin reduces food intake via a dopamine D2 receptor‐dependent mechanism. Molecular Metabolism, 1, 86–93. 10.1016/j.molmet.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. A. , Hasan, S. , Foster, R. G. , & Peirson, S. N. (2017). COMPASS: Continuous open mouse phenotyping of activity and sleep status. Wellcome Open Research, 1, 2 10.12688/wellcomeopenres.9892.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, S. , Caron, A. , Richard, D. , & Picard, F. (2013). Role of leptin resistance in the development of obesity in older patients. Clinical Interventions in Aging, 8, 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascão, R. , Fonseca, J. E. , & Moita, L. F. (2017). Celastrol: A spectrum of treatment opportunities in chronic diseases. Frontiers in Medicine, 4, 69 10.3389/fmed.2017.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J. Y. , Kang, H. T. , Lee, D. C. , Lee, H. R. , & Lee, Y. J. (2013). Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Archives of Gerontology and Geriatrics, 56, 270–278. 10.1016/j.archger.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Dominguez, L. J. , & Barbagallo, M. (2016). The biology of the metabolic syndrome and aging. Current Opinion in Clinical Nutrition and Metabolic Care, 19, 5–11. 10.1097/MCO.0000000000000243 [DOI] [PubMed] [Google Scholar]
- Fernández‐Galaz, C. , Fernández‐Agulló, T. , Campoy, F. , Arribas, C. , Gallardo, N. , Andrés, A. , … Carrascosa, J. M. (2001). Decreased leptin uptake in hypothalamic nuclei with ageing in Wistar rats. Journal of Endocrinology, 171, 23–32. [DOI] [PubMed] [Google Scholar]
- Fernández‐Galaz, C. , Fernández‐Agulló, T. , Pérez, C. , Peralta, S. , Arribas, C. , Andrés, A. , … Ros, M. (2002). Long‐term food restriction prevents ageing‐associated central leptin resistance in wistar rats. Diabetologia, 45, 997–1003. [DOI] [PubMed] [Google Scholar]
- Fitzsimons, T. J. , & Le Magnen, J. (1969). Eating as a regulatory control of drinking in the rat. Journal of Comparative and Physiological Psychology, 67(3), 273–283. 10.1037/h0026772 [DOI] [PubMed] [Google Scholar]
- Frederich, R. C. , Hamann, A , Anderson, S , Löllmann, B , Lowell, BB , Flier, J. S. (1995). Leptin levels reflect body lipid content in mice: Evidence for diet‐ induced resistance to leptin action. Nature Medicine, 1, 1311–1314. 10.1038/nm1295-1311 [DOI] [PubMed] [Google Scholar]
- Gabriely, I. , Xiao Hui, M. , Yang, X. M. , Rossetti, L. , & Barzilai, N. (2002). Leptin resistance during aging is independent of fat mass. Diabetes, 51, 1016–1021. 10.2337/diabetes.51.4.1016 [DOI] [PubMed] [Google Scholar]
- González‐Rodríguez, A. , Más‐Gutierrez, J. A. , Mirasierra, M. , Fernandez‐Pérez, A. , Lee, Y. J. , Ko, H. J. , … Valverde, A. M. (2012). Essential role of protein tyrosine phosphatase 1B in obesity‐induced inflammation and peripheral insulin resistance during aging. Aging Cell, 11, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, T. S. , & Lean, M. E. (2016). A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovascular Disease, 5, 2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper, R. H. , Argmann, C. , Houten, S. M. , Cantó, C. , Jeninga, E. H. , Andreux, P. A. , … Auwerx, J. (2011). The metabolic footprint of aging in mice. Scientific Reports, 1, 134 10.1038/srep00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M. , Luo, Q. , Alitongbieke, G. , Chong, S. , Xu, C. , Xie, L. , … Zhang, X. K. (2017). Celastrol‐Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Molecular Cell, 66, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justesen, J. , Stenderup, K. , Ebbesen, E. N. , Mosekilde, L. , Steiniche, T. , & Kassem, M. (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology, 2, 165–171. [DOI] [PubMed] [Google Scholar]
- Kotani, K. , Tokunaga, K. , Fujioka, S. , Kobatake, T. , Keno, Y. , Yoshida, S. , … Matsuzawa, Y. (1994). Sexual dimorphism of age‐related changes in whole‐body fat distribution in the obese. International Journal of Obesity and Related Metabolic Disorders, 18, 207–212. [PubMed] [Google Scholar]
- Kuk, J. L. , Saunders, T. J. , Davidson, L. E. , & Ross, R. (2009). Age‐related changes in total and regional fat distribution. Ageing Research Reviews, 8, 339–348. 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Kyle, U. G. , Genton, L , Hans, D , Karsegard, L , Slosman, DO , Pichard, C (2001). Age‐related differences in fat‐free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. European Journal of Clinical Nutrition, 55, 663–672. 10.1038/sj.ejcn.1601198 [DOI] [PubMed] [Google Scholar]
- Lévi, F. , & Okyar, A. (2011). Circadian clocks and drug delivery systems: impact and opportunities in chronotherapeutics. Expert Opinion on Drug Delivery, 8, 1535–1541. 10.1517/17425247.2011.618184 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Kelly, L. , Heiman, M. , Greengard, P. , & Friedman, J. M. (2015). Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell Metabolism., 22, 1059–1067. 10.1016/j.cmet.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Lee, J. , Hernandez, M. A. S. , Mazitschek, R. , & Ozcan, U. (2015). Treatment of obesity with celastrol. Cell, 161, 999–1011. 10.1016/j.cell.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X. H. , Muzumdar, R. , Yang, X. M. , Gabriely, I. , Berger, R. , & Barzilai, N. (2002). Aging is associated with resistance to effects of leptin on fat distribution and insulin action. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 57, B225–B231. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Xu, L. , Alberobello, A. T. , Gavrilova, O. , Bagattin, A. , Skarulis, M. , … Mueller, E. . (2015). Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1‐PGC1α transcriptional axis. Cell Metabolism, 22, 695–708. 10.1016/j.cmet.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Martínez, G. , Duran‐Aniotz, C. , Cabral‐Miranda, F. , Vivar, J. P. , & Hetz, C. (2017). Endoplasmic reticulum proteostasis impairment in aging. Aging Cell, 16, 615–623. 10.1111/acel.12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathus‐Vliegen, E. M. H. , Obesity Management Task Force of the European Association for the Study of Obesity . (2012). Prevalence, pathophysiology, health consequences andtreatment options of obesity in the elderly: A guideline. Obesity Facts, 5, 460–483. 10.1159/000341193 [DOI] [PubMed] [Google Scholar]
- Muzumdar, R. , Allison, DB , Huffman, DM , Ma, X , Atzmon, G , Einstein, FH , … Barzilai, N (2008). Visceral adipose tissue modulates mammalian longevity. Aging Cell, 7, 438–440. 10.1111/j.1474-9726.2008.00391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, M. G. Jr , Heymsfield, S. B. , Haft, C. , Kahn, B. B. , Laughlin, M. , Leibel, R. L. , … Yanovski, J. A. (2012). Challenges and opportunities of defining clinical leptin resistance. Cell Metabolism, 15, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo, N. , Zhu, J. , Zhu, Y. , Fenik, P. , Lian, J. , Galante, R. , & Veasey, S. (2011). Endoplasmic reticulum stress in wake‐active neurons progresses with aging. Aging Cell, 10, 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovesjö, M. L. , Gamstedt, M. , Collin, M. , & Meister, B. (2001). GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. Journal of Neuroendocrinology. 13, 505–516. 10.1046/j.1365-2826.2001.00662.x [DOI] [PubMed] [Google Scholar]
- Pack, A. I. , Galante, R. J. , Maislin, G. , Cater, J. , Metaxas, D. , Lu, S. , … Peters, L. L. (2007). Novel method for high‐throughput phenotyping of sleep in mice. Physiological Genomics, 28, 232–238. [DOI] [PubMed] [Google Scholar]
- Peralta, S. , Carrascosa, J. M. , Gallardo, N. , Ros, M. , & Arribas, C. (2002). Ageing increases SOCS‐3 expression in rat hypothalamus: Effects of food restriction. Biochemical and Biophysical Research Communications, 296, 425–428. 10.1016/S0006-291X(02)00906-3 [DOI] [PubMed] [Google Scholar]
- Perron, I. J. , Pack, A. I. , & Veasey, S. (2015). Diet/energy balance affect sleep and wakefulness independent of body weight. Sleep, 38, 1893–1903. 10.5665/sleep.5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétervári, E. , Rostás, I. , Soós, S. , Tenk, J. , Mikó, A. , Füredi, N. , … Balaskó, M. (2014). Age versus nutritional state in the development of central leptin resistance. Peptides, 56, 59–67. [DOI] [PubMed] [Google Scholar]
- Rezai‐Zadeh, K. , Yu, S. , Jiang, Y. , Laque, A. , Schwartzenburg, C. , Morrison, C. D. , … Münzberg, H. (2014). Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Molecular Metabolism., 3, 681–693. 10.1016/j.molmet.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostás, I. , Tenk, J. , Mikó, A. , Füredi, N. , Soós, S. , Solymár, M. , … Balaskó, M. (2016). Age‐related changes in acute central leptin effects on energy balance are promoted by obesity. Experimental Gerontology, 85, 118–127. [DOI] [PubMed] [Google Scholar]
- Saag, K. G. , & Choi, H. (2006). Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Research and Therapy, 8(Suppl 1), S2 10.1186/ar1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T. , Kikuchi, O. , Shimpuku, M. , Susanti, V. Y. , Yokota‐Hashimoto, H. , Taguchi, R. , … Kitamura, T. (2014). Hypothalamic SIRT1 prevents age‐associated weight gain by improving leptin sensitivity in mice. Diabetologia, 57, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpace, P. J. , Matheny, M. , & Tümer, N. (2001). Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience, 104, 1111–1117. 10.1016/S0306-4522(01)00142-7 [DOI] [PubMed] [Google Scholar]
- Scott, M. M. , Williams, K. W. , Rossi, J. , Lee, C. E. , & Elmquist, J. K. (2011). Leptin receptor expression in hindbrain Glp‐1 neurons regulates food intake and energy balance in mice. Journal of Clinical Investigation. 121, 2413–2421. 10.1172/JCI43703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek, E. W. , & Scarpace, P. J. (2000). Resistance to the anorexic and thermogenic effects of centrally administrated leptin in obese aged rats. Regulatory Peptides, 92, 65–71. 10.1016/S0167-0115(00)00151-8 [DOI] [PubMed] [Google Scholar]
- Strominger, J. L. (1947). The relation between water intake and food intake in normal rats and in rats with hypothalamic hyperphasia. Yale Journal of Biology and Medicine, 19, 279–288. [PMC free article] [PubMed] [Google Scholar]
- Sukumaran, S. , Jusko, W. J. , DuBois, D. C. , & Almon, R. R. (2011). Mechanistic modeling of the effects of glucocorticoids and circadian rhythms on adipokine expression. Journal of Pharmacology and Experimental Therapeutics, 337, 734–746. 10.1124/jpet.111.179960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, M. , Pahor, M. , Tylavsky, F. , Kritchevsky, S. B. , Cauley, J. A. , Newman, A. B. , … Harris, T. B. (2003). One‐ and two‐year change in body composition as measured by DXA in a population‐based cohort of older men and women. Journal of Applied Physiology, 94, 2368–2374. [DOI] [PubMed] [Google Scholar]
- Wimmer, M. E. , Rising, J. , Galante, R. J. , Wyner, A. , Pack, A. I. , & Abel, T. (2013). Aging in mice reduces the ability to sustain sleep/wake states. PLoS ONE, 8, e81880 10.1371/journal.pone.0081880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S. B. , Tien, A. C. , Boddupalli, G. , Xu, A. W. , Jan, Y. N. , & Jan, L. Y. (2012). Rapamycin ameliorates age‐dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron, 75, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Geng, C. , Liu, X. , Li, M. , Gao, M. , Liu, X. , … Chang, Y. (2017). Celastrol ameliorates liver metabolic damage caused by a high‐fat diet through Sirt1. Molecular Metabolism, 6, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


