Abstract
Background
Keratoconus is a degenerative condition of the cornea that profoundly affects vision and vision‐specific quality of life. The axial cornea thins and protrudes, resulting in irregularity and, eventually, scarring of the cornea. There are multiple options available for treating keratoconus. Intrastromal corneal ring segments are small, crescent‐shaped plastic rings that are placed in the deep, peripheral corneal stroma in order to flatten the cornea. They are made of polymethylmethacrylate (PMMA). The procedure does not involve corneal tissue nor does it invade the central optical zone. Intrastromal corneal ring segments are approved for use when contact lenses or spectacles are no longer adequate.
Objectives
To evaluate the effectiveness and safety of intrastromal corneal ring segments as a treatment for keratoconus.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2018, Issue 1); Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Health Sciences Literature Database (LILACS); ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not implement any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 25 January 2018.
Selection criteria
Two review authors independently assessed records from the electronic searches to identify randomized controlled trials (RCTs). Disagreements were resolved by discussion.
Data collection and analysis
We planned for two authors to independently review full‐text reports, using standard methodological procedures expected by Cochrane.
Main results
We found no RCTs comparing intrastromal corneal ring segments with spectacles or contact lenses.
Authors' conclusions
In the absence of eligible RCTs to review, no conclusions can be drawn.
Plain language summary
Intrastromal corneal ring segments for keratoconus
What is the aim of this review? The aim of this Cochrane Review was to evaluate the effectiveness and safety of intrastromal corneal ring segments as treatment for keratoconus. Intrastromal corneal ring segments are small, crescent‐shaped plastic rings that are placed in the cornea (the clear, front surface of the eye) to treat keratoconus. Keratoconus is a worsening disease of the eye in which the normally round cornea bulges into a cone‐like shape with irregular surface, causing distorted vision.Studies that evaluated uncorrected vision with intrastromal corneal rings were searched for, and no randomized controlled trials (RCTs) that fit the protocol's inclusion criteria were identified.
Key message In the absence of eligible RCTs on this topic, the effectiveness and safety of intrastromal corneal ring segments as treatment for keratoconus is uncertain.
what was studied in this review? It was important to evaluate the effectiveness and safety of intrastromal corneal ring segments as treatment for keratoconus, a rare and progressive disease of the cornea. Keratoconus often affects both eyes, causing protrusion of the eyes leading to the outer surface becoming irregular and distorted. It can sometimes lead to scarring of the cornea, resulting in blurry vision, even with visual correction. When contact lenses or spectacles are no longer effective enough at correcting vision, intrastromal corneal ring segments are used.
What are the main results of this review? We found no studies meeting our inclusion criteria, and therefore no conclusion could be drawn regarding the effectiveness and safety of intrastromal corneal ring segments as treatment for people with keratoconus. Studies are needed that compare people who undergo intrastromal corneal ring segments as treatment for keratoconus to those individuals who did not receive intrastromal corneal ring segments.
How up‐to‐date is this review? The review authors searched for studies that have been up to 25 January 2018.
Background
Description of the condition
Keratoconus is a relatively rare, degenerative disease of the cornea, the clear, front surface of the eye (Zadnik 1996). It is bilateral, asymmetric, and progressive. The cornea thins, steepens, and protrudes; and its outer surface becomes irregular, distorted, and sometimes even scarred, resulting in blurry vision, even with visual correction.
In the United States, the best estimate of keratoconus incidence is 2 per 100,000 people per year; prevalence is 54.5 per 100,000 (Kennedy 1986). Because the disease is rare, other worldwide incidence and prevalence rates have never been rigorously assessed. The onset of keratoconus generally occurs in the teenage years or 20s. It presents initially as blurred or distorted distance vision and can be difficult to distinguish from myopia or astigmatism, or both. With time, the corneal surface becomes irregular. Techniques that assess corneal shape and topography (surface characteristics) help with the diagnosis. It has been associated with many other conditions, both eye‐related (other corneal dystrophies, allergic conjunctivitis) and general, such as atopic diseases (hay fever, dermatitis), Down syndrome, and connective tissue disorders (Krachmer 1984).
Keratoconus is slowly progressive with gradual loss in visual acuity, especially low‐contrast visual acuity, even with best visual correction (Davis 2006). Likewise, the corneal curvature worsens, gradually steepening, in association with decreasing best‐corrected visual acuity (McMahon 2006). Younger age at onset is generally believed to be associated with faster progression and worse outcomes, including the need for surgery (Barr 2006; Gordon 2006).
Although much research has been done, the cause of keratoconus remains unknown, but is probably a combination of genetics and environmental influences (Rabinowitz 1998).
Description of the intervention
There are a variety of treatments for keratoconus, including spectacles, contact lenses, corneal collagen cross‐linking, and corneal surgery. Spectacles are generally the first optical treatment, and are used early in the disease course to correct myopia and astigmatism. When vision with spectacles is no longer adequate, rigid gas permeable contact lenses become the mainstay of optical treatment; they correct the cornea's irregular surface, but only while the contact lenses are worn (Zadnik 1996).
Rigid gas permeable contact lenses are not prescribed for patients with keratoconus to attempt to flatten the cornea permanently. Corneal surgery, corneal collagen cross‐linking, and intrastromal corneal rings attempt to treat the underlying disease rather than just managing the visual symptoms. Corneal surgery (either penetrating keratoplasty or deep anterior lamellar keratoplasty) actually removes the irregular, opaque cornea, either partially or completely. Corneal collagen cross‐linking uses ultraviolet light and riboflavin eyedrops to strengthen the collagen fibers in the cornea (Wollensak 2003). The effectiveness and safety of corneal collagen cross‐linking is examined in a separate Cocrhrane review (Sykakis 2015).
Intrastromal corneal ring segments (also referred to as INTACS, Ferrara rings, Kerarings, or corneal implants) were approved by the Food and Drug Administration in the United States, through an Humanitarian Device Exemption in 2004, for use in patients with keratoconus whose corneas are not scarred, when spectacles and contact lenses no longer provide adequate visual correction. They are small, thin, arc‐shaped pieces of plastic that are inserted in the stroma of the cornea during a brief outpatient procedure, under topical anesthesia (Rabinowitz 2013). The expectation is for modest corneal flattening (two to three diopters) with a modest improvement in visual acuity (two to three lines on a visual acuity chart) (Rabinowitz 2013).
How the intervention might work
The insertion of the corneal implants is thought to result in corneal flattening and reduction of the myopia (nearsightedness) and astigmatism that accompany keratoconus and adversely affect vision. A tunnel is created in the corneal stroma, either with a steel dissector or with a femtosecond laser, and the rings are inserted (an example is shown in Figure 1). In the case of INTACS, the clear optical zone between the two implants is larger than the pupil to prevent optical distortions postoperatively. The flattening is mechanical and does not affect the underlying biochemical abnormalities in keratoconus, so there are limits to how much flattening can be expected, how much the vision may improve, and how long the positive effects of the flattening may last. The rings can be removed, so the procedure is reversible in theory, but severe complications such as perforation of the cornea and severe corneal infection have been reported (Rabinowitz 2013).
1.
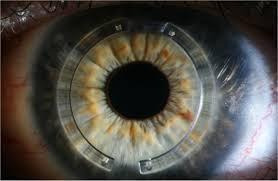
Example of intrastromal corneal rings implanted in the eye.
Why it is important to do this review
Although rare, keratoconus has a marked negative effect on patients' quality of life. One report equates the vision‐specific quality of life in keratoconus with that of much older patients with age‐related macular degeneration (Kymes 2004). Patients with keratoconus experience blurry vision, dependence on uncomfortable contact lenses, and even the prospect of legal blindness and invasive corneal procedures, from a relatively young age. The systematic evaluation of a possibly viable, minimally invasive, therapeutic alternative that could be better than contact lenses would be valuable to patients afflicted with this potentially visually‐disabling disease.
Objectives
To evaluate the effectiveness and safety of intrastromal corneal ring segments as a treatment for keratoconus.
Methods
Criteria for considering studies for this review
Types of studies
We had planned to include randomized controlled trials (RCTs). If outcomes from RCTs were not available, we had planned to discuss findings from other study designs, such as cohort studies and case series.
Types of participants
We had planned to include participants with keratoconus, and to exclude any participants with non‐keratoconic ectasia, e.g. post‐laser‐assisted in situ keratomileusis (LASIK). We planned to consider keratoconus as defined by the included studies, and to document whether corneal topography data and slit‐lamp data were used for diagnosis.
Types of interventions
We had planned to include studies that compared intrastromal corneal ring segments with spectacles or contact lenses. We planned to include intrastromal corneal ring segments with or without photorefractive keratectomy (although its use in keratoconus is controversial and decidedly non‐standard), and with or without corneal collagen cross‐linking. We had planned to include any type of ring studied (e.g., INTACS versus Ferrara).
Types of outcome measures
Outcomes will assess variables associated with keratoconus disease progression.
Primary outcomes
The primary outcome for this review, uncorrected distance visual acuity (UCVA) at three months, will be assessed as both a dichotomous outcome and a continuous outcome. Our primary outcome for comparison of treatments would have been:
uncorrected distance visual acuity (UCVA) in the study eye at 12 months after intervention.
This would have been considered as:
the proportion with UCVA 20/40 or better in the study eye; and
the mean change in UCVA from baseline in the study eye, measured on the Early Treatment in Diabetic Retinopathy Study (ETDRS) chart or equivalent.
Secondary outcomes
Secondary outcomes of interest, assessed at three, six, 12, and 24 months, included:
UCVA in the study eye at three, six, and 24 months, measured as: 1) the proportion with UCVA 20/40 or better; and 2) the mean change in UCVA from baseline;
best‐corrected distance visual acuity (BCVA) in the study eye, measured as: 1) the proportion with BCVA of 20/40 or better; and 2) the mean change in BCVA from baseline;
corneal curvature in the study eye (mean change in diopters);
corneal thickness in the study eye (mean change in mm);
refractive error in the study eye (mean change of spherical equivalent in diopters);
contact lens tolerance (yes/no, or scale, as reported by study, categorized by type of contact lens worn); and
surgeons' experience with intrastromal corneal rings.
We intended to document and report adverse events reported by the included studies. Specific adverse events of interest included:
penetration of the ring(s) into the anterior chamber;
corneal infection;
migration or extrusion of the ring(s);
other corneal complications, e.g. corneal abrasion, corneal scarring; and
loss of one or more lines of BCVA.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. There were no restrictions on language or year of publication. The electronic databases were last searched on 25 January 2018.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 25 January 2018) (Appendix 1)
MEDLINE Ovid (1946 to 25 January 2018) (Appendix 2)
Embase.com (1947 to 25 January 2018) (Appendix 3)
PubMed (1948 to 25 January 2018) (Appendix 4)
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 25 January 2018) (Appendix 5)
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov; searched 25 January 2018) (Appendix 6)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 25 January 2018) (Appendix 7)
Searching other resources
We had planned to search the reference lists of included studies and use the Science Citation Index to identify potentially relevant studies that cited included studies. We did not search conference proceedings specifically for the purposes of this review, as RCTs presented at these meetings are searched by the Cochrane Eyes and Vision Group and included in CENTRAL.
Data collection and analysis
Selection of studies
Two review authors independently assessed the eligibility of all records identified by the searches, beginning with titles and abstracts. Each author classified each record as (1) definitely relevant, (2) possibly relevant, or (3) definitely not relevant, according to the Criteria for considering studies for this review. We obtained full‐text copies of each record classified as either (1) definitely relevant, or (2) possibly relevant.
Two authors independently assessed the full‐text report(s) of studies and classified each study as (a) include, (b) unclear, or (c) exclude. We resolved discrepancies at each stage by consensus. We documented all studies excluded after assessment of the full‐text report and the reasons for exclusion. We had planned to contact study investigators for studies classified as unclear for additional information to determine eligibility. If no response was received after four weeks, we planned to classify the reference based on the information available. For articles written in languages not read by the review authors, we will request assistance by colleagues to assess the studies for eligibility, and to translate the study information when needed.
Data extraction and management
The following methods will apply to future updates of this review, assuming we identify eligible studies to include.
Two authors will independently extract data using data extraction forms developed by the Cochrane Eyes and Vision Group, and modified for the specific purposes of this review. We will extract the following study characteristics for each included study: participants, interventions, outcomes, and funding sources. One review author will enter the data into Review Manager 5 (Review Manager 2014), and a second review author will verify the data entered. We will resolve discrepancies by discussion. We will contact study investigators to request missing data. If no response is received after four weeks, we will document that data were not reported and will report the information available.
Assessment of risk of bias in included studies
Two review authors will independently assess the risk of bias in studies, according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Discrepancies between authors will be resolved through discussion.
We will consider the following parameters when assessing risk of bias in randomized trials:
selection bias (random sequence generation, adequacy of allocation concealment);
performance bias (masking of participants and study personnel);
detection bias (masking of outcome assessors);
attrition bias (completeness of follow‐up, reasons for missing data);
reporting bias (i.e. selective outcome reporting); and
other potential sources of bias (such as funding source).
We will assess each included study for each parameter as having a low risk of bias, a high risk of bias, or an unclear risk of bias (insufficient information to permit judgment of low or high risk, or impact on risk of bias unclear). We will contact the study investigators when study details are unclear or when additional information would facilitate making an assessment. If no response is received after four weeks, we will assess the risk of bias based on the information available.
Measures of treatment effect
We will assess primary and secondary dichotomous outcomes as risk ratios with 95% confidence intervals. We also will report adverse events as risk ratios with 95% confidence intervals, when data are available.
We will report continuous outcomes as mean differences (with 95% confidence intervals) in the mean changes from baseline between groups, or comparing pre‐intrasomal corneal rings results to post‐intrasomal corneal rings results. When mean changes from baseline are not available, we will calculate the mean differences (with 95% confidence intervals) based on mean values at a follow‐up time point, assuming baseline values between groups were distributed uniformly. When distributions are skewed, we will report the median and interquartile ranges, whenever sufficient data are available.
Unit of analysis issues
The unit of analysis will be the participant (i.e. one eye per participant). For studies in which both eyes of a single participant were included and analyzed separately, we will report whether appropriate adjustments for within‐person correlation of outcomes were performed.
Dealing with missing data
When data are missing or incomplete, we will contact study authors for additional information. If no response is received after four weeks, we will use the information available and document missing data. We will not employ imputation methods for missing data for the purposes of this review.
Assessment of heterogeneity
We will assess clinical, methodological, and statistical heterogeneity among included studies. We will assess clinical heterogeneity based on the characteristics of the participants, interventions, and outcomes of the included studies. We will consider risk of bias when assessing methodological heterogeneity. We will use the I2 statistic to examine statistical heterogeneity. We will consider an I2 value greater than 60% to indicate substantial statistical heterogeneity. When substantial statistical heterogeneity is present, we will not conduct meta‐ analyses; instead, we will report the study results independently.
Assessment of reporting biases
We will examine reporting biases at the individual study level (i.e. selective outcome reporting) and review level (i.e. publication bias). We will assess selective outcome reporting for each included study by comparing study outcomes prespecified in study protocols, or clinical trial registrations, with those that were reported. We will examine publication bias based on the symmetry of funnel plots when ten or more studies are included in a meta‐analysis.
Data synthesis
When non‐substantial statistical heterogeneity is detected, we will combine results in a meta‐analysis. We will use a random‐effects model for meta‐analyses that include three or more studies. We will use a fixed‐effect model when there are fewer than three studies. We will calculate the summary risk ratio with 95% confidence interval for dichotomous outcomes, and the summary mean difference between groups with 95% confidence interval for continuous outcomes. We will document study results that are not included in a meta‐analysis as narrative summaries.
Subgroup analysis and investigation of heterogeneity
When sufficient data are available, we will conduct subgroup analyses based on whether patients received additional therapy (e.g. participants who received intrastromal corneal ring segments only and participants who received intrastromal corneal ring segments plus photorefractive keratectomy).
Sensitivity analysis
When sufficient data are available, we will conduct sensitivity analyses to examine the impact of excluding unpublished studies, industry‐funded studies, and studies assessed as having a high risk of bias, for any 'Risk of bias' parameter.
Results
Description of studies
Results of the search
The electronic search performed on 25 January 2018 provided 636 unique records (Figure 2). From these 636 records, we classified 19 studies as possibly relevant. Upon reviewing these 19 records, we excluded them all. The majority of ineligible RCTs in this review were excluded because they compared types of intrastromal ring segments, rather than comparing intrastromal ring segments with spectacles or contact lenses. We did not find any ongoing trials.
2.
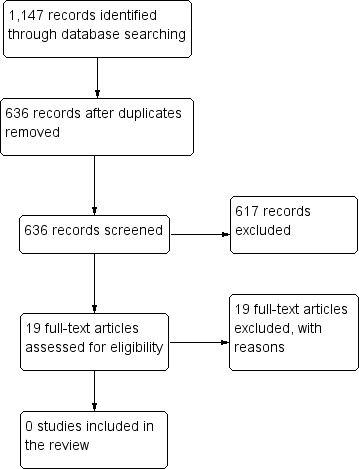
Study flow diagram.
Included studies
There are no eligible included studies in this review.
Excluded studies
We excluded 19 studies after reviewing the full‐text reports (see Characteristics of excluded studies).
Risk of bias in included studies
There were no trials eligible for inclusion to assess for risk of bias.
Effects of interventions
We have no information on effects of interventions as there were no eligible included trials.
Discussion
We found no RCTs that fit the inclusion criteria for this review. Keratoconus is relatively rare, which may explain the lack of relevant RCTs. Although the condition is rare, it has negative impacts on patients' quality of life. Clinical trials with this comparison are needed to inform patients and healthcare providers of the comparative benefits or harms of instrastromal ring segment implantation.
Authors' conclusions
Implications for practice.
Because we found no eligible RCTs, ophthalmologists have no evidence to consider the benefits and harms of instrastromal ring segment implantation relative to spectacles or contact lenses. Ophthalmologists therefore have no research evidence for recommendations of either treatment over the other.
Implications for research.
As evident by this review, RCTs comparing spectacles and contact lenses with instrastromal ring segments are needed. Contact lenses and spectacles are traditionally the first method for treating keratoconus. When these are no longer adequate, patients are recommended for corneal cross‐linking or surgical interventions.
Acknowledgements
We acknowledge the Cochrane Eyes and Vision Group (CEV) Information Specialist, Lori Rosman, for developing the search strategy for this review. We thank Joseph Barr, Barbara Hawkins, and the CEV editors for comments to the protocol. We are grateful to the following peer reviewers for their time and comments on the review: Melissa Daluvoy (Duke University), Vishal Jhanji (University of Pittsburgh), and Shannon Shoaf.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Keratoconus] explode all trees #2 keratocon* #3 #1 or #2 #4 (cornea* near/2 implant*) #5 (cornea* near/2 ring*) #6 (Intrastromal near/2 ring*) #7 (Intracorneal near/2 ring*) #8 (Intacs or Keraring* or "Kera ring" or Ferrara*) #9 (Stromal near/2 implant*) #10 #4 or #5 or #6 or #7 or #8 or #9 #11 #3 and #10
Appendix 2. MEDLINE Ovid search strategy
1. exp Keratoconus/ 2. keratocon*.tw. 3. or/1‐2 4. (cornea* adj2 implant*).tw. 5. (cornea* adj2 ring*).tw. 6. (Intrastromal adj2 ring*).tw. 7. (Intracorneal adj2 ring*).tw. 8. (Intacs or Keraring* or "Kera ring" or Ferrara*).tw. 9. (Stromal adj2 implant*).tw. 10. or/4‐9 11. 3 and 10
Appendix 3. Embase.com search strategy
#1 'keratoconus'/exp #2 keratocon*:ab,ti,kw #3 #1 OR #2 #4 'intrastromal corneal ring segment'/exp #5 (cornea* NEAR/2 implant*):ab,ti,kw #6 (cornea* NEAR/2 ring*):ab,ti,kw #7 (intrastromal NEAR/2 ring*):ab,ti,kw #8 (intracorneal NEAR/2 ring*):ab,ti,kw #9 intacs:ab,ti,kw OR keraring*:ab,ti,kw OR 'kera ring':ab,ti,kw OR ferrara*:ab,ti,kw #10 (stromal NEAR/2 implant*):ab,ti,kw #11 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #12 #3 AND #11
Appendix 4. PubMed search strategy
#1 keratocon*[tiab] NOT Medline[sb] #2 (cornea*[tiab] AND implant*[tiab]) NOT Medline[sb] #3 (cornea*[tiab] AND ring*[tiab]) NOT Medline[sb] #4 (Intrastromal[tiab] AND ring*[tiab]) NOT Medline[sb] #5 (Intracorneal[tiab] AND ring*[tiab]) NOT Medline[sb] #6 (Intacs[tiab] OR Keraring*[tiab] OR "Kera ring"[tiab] OR Ferrara*[tiab]) NOT Medline[sb] #7 (Stromal[tiab] AND implant*[tiab]) NOT Medline[sb] #8 #2 OR #3 OR #4 OR #5 OR #6 OR #7 #9 #1 AND #8
Appendix 5. LILACS search strategy
(keratocon$ OR Queratocono OR Ceratocone OR MH:C11.204.627$) AND (implant$ OR ring$ OR Intacs OR Keraring$ OR "Kera ring" OR Ferrara$)
Appendix 6. ClinicalTrials.gov search strategy
Keratoconus AND (ring OR implant OR Intacs)
Appendix 7. WHO ICTRP search strategy
Keratoconus AND ring OR Keratoconus AND implant OR Keratoconus AND Intacs OR Keratoconus AND Keraring OR Keratoconus AND Ferrara
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Tuwairqi 2017 | Wrong comparison intervention |
| Birnbaum 2011 | Wrong patient population |
| Carrasquillo 2007 | Wrong patient population |
| Coskunseven 2009 | Wrong study design |
| Elsaftawy 2015 | Wrong comparison intervention |
| Ertan 2006 | Wrong comparison intervention |
| Ganesh 2013 | Wrong comparison intervention |
| Greenstein 2014 | Wrong comparison intervention |
| Hashemi 2014 | Wrong patient population |
| Hashemian 2014 | Wrong comparison intervention |
| Hosny 2015 | Wrong comparison intervention |
| IRCT2014022516738N1 | Wrong comparison intervention |
| Jabbarvand 2014 | Wrong comparison intervention |
| Kubaloglu 2010a | Wrong comparison intervention |
| Kubaloglu 2010b | Wrong comparison intervention |
| Mulet 2010 | Wrong intervention |
| NCT01869517 | Wrong comparison intervention |
| Ossma‐Gomez 2006 | Wrong intervention |
| Sousa 2006 | Wrong study design |
Contributions of authors
KZ designed and wrote the protocol. KL assisted with writing the protocol. SM and KL screened the studies for inclusion. All authors contributed to drafting the review and will be responsible for future updates.
Sources of support
Internal sources
No sources of support supplied
External sources
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
None known.
New
References
References to studies excluded from this review
Al‐Tuwairqi 2017 {published data only}
- Al‐Tuwairqi WS, Osuagwa UL, Razzouk H, AlHarbi A, Ogbuehi KC. Clinical evaluation of two types of intracorneal ring segments (ICRS) for keratoconus. International Opthalmology 2017;37:1185‐98. [DOI: 10.1007/s10792-016-0385-2] [DOI] [PubMed] [Google Scholar]
Birnbaum 2011 {published data only}
- Birnbaum F, Schwartzkopff J, Böhringer D, Reinhard T. The intrastromal corneal ring in penetrating keratoplasty ‐ long‐term results of a prospective randomized study. Cornea 2011;30(7):780‐3. [DOI] [PubMed] [Google Scholar]
Carrasquillo 2007 {published data only}
- Carrasquillo KG, Rand J, Talamo JH. Intacs for keratoconus and post‐LASIK ectasia: mechanical versus femtosecond laser‐assisted channel creation. Cornea 2007;26(8):956‐62. [DOI] [PubMed] [Google Scholar]
Coskunseven 2009 {published data only}
- Coskunseven E, Jankov MR, Hafezi F, Atun S, Arslan E, Kymionis GD. Effect of treatment sequence in combined intrastromal corneal rings and corneal collagen crosslinking for keratoconus. Journal of Cataract and Refractive Surgery 2009;35(12):2084‐91. [DOI] [PubMed] [Google Scholar]
Elsaftawy 2015 {published data only}
- Elsaftawy HS, Ahmed MH, Saif MY, Mousa R. Sequential intracorneal ring segment implantation and corneal transepithelial collagen cross‐linking in keratoconus. Cornea 2015;34(11):1420‐6. [DOI] [PubMed] [Google Scholar]
Ertan 2006 {published data only}
- Ertan A, Colin J. Intacs for keratoconus: comparison of mechanical vs. femtolaser channel dissection. American Academy of Opthalmology; 2006 Nov 11‐14; Las Vegas (NV). 2006:Abstract page 191.
Ganesh 2013 {published data only}
- Ganesh S, Shetty R, D'Souza S, Ramachandran S, Kurian M. Intrastromal corneal ring segments for management of keratoconus. Indian Journal of Opthalmology 2013;61(8):451‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenstein 2014 {published data only}
- Greenstein SA, Hersh PS. Symmetric vs. asymmetric intracorneal ring segment placement with adjunctive corneal collagen crosslinking for keratoconus and corneal ectasia. Investigative Opthalmology and Visual Science 2015;55(13):4227. [Google Scholar]
Hashemi 2014 {published data only}
- Hashemi H, Gholaminejad A, Amanzadeh K, Hashemi M, Khabazkhoob M. Single‐segment and double‐segment INTACS for post‐LASIK ectasia. Acta Medica Iranica 2014;52(9):681‐6. [PubMed] [Google Scholar]
Hashemian 2014 {published data only}
- Hashemian MN, Zare MA, Mohammadpour M, Rahimi F, Fallah MR, Panah FK. Outcomes of single segment implantation of conventional Intacs versus Intacs SK for keratoconus. Journal of Opthalmic and Vision Research 2014;9(3):305‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosny 2015 {published data only}
- Hosny M, El‐Mayah E, Sidky MK, Anis M. Femtosecond laser‐assisted implantation of complete versus incomplete rings for keratoconus treatment. Clinical Opthalmology 2015;9:121‐7. [DOI: 10.2147/OPTH.S73855] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2014022516738N1 {published data only}
- IRCT2014022516738N1. Comparison between Myoring versus Keraring 355 degree implantation for treatment of keratoconus: a randomised, double‐blind, two parallel‐groups clinical trial. en.irct.ir/trial/15568 (first received 4 May 2015).
Jabbarvand 2014 {published data only}
- Jabbarvand M, Hashemi H, Mohammadpour M, Khojasteh H, Khodaparast M, Hashemian H. Implantation of a complete intrastromal corneal ring at 2 different stromal depths in keratoconus. Cornea 2014;33(2):141‐4. [DOI] [PubMed] [Google Scholar]
Kubaloglu 2010a {published data only}
- Kubaloglu A, Cinar Y, Sari E, Koytak A, Ozdemir B, Özertürk Y. Comparison of 2 intrastromal corneal ring segment models in the management of keratoconus. Journal of Cataract and Refractive Surgery 2010;36(6):978‐85. [DOI] [PubMed] [Google Scholar]
Kubaloglu 2010b {published data only}
- Kubaloglu A, Sari ES, Cinar Y, Cingu K, Koytak A, Coşkun E, Özertürk Y. Comparison of mechanical and femtosecond laser tunnel creation for intrastromal corneal ring segment implantation in keratoconus: prospective randomized clinical trial. Journal of Cataract and Refractive Surgery 2010;36(9):1556‐61. [DOI] [PubMed] [Google Scholar]
Mulet 2010 {published data only}
- Mulet ME, Pérez‐Santonja JJ, Ferrer C, Alió JL. Microbial keratitis after intrastromal corneal ring segment implantation. Journal of Refractive Surgery 2010;26(5):364‐9. [DOI] [PubMed] [Google Scholar]
NCT01869517 {published data only}
- NCT01869517. Myoring versus Keraring implantation for keratoconus. clinicaltrials.gov/ct2/show/NCT01869517 (first received 5 June 2013).
Ossma‐Gomez 2006 {published data only}
- Ossma‐Gomez I, Galvis A. Intrastromal ring segments vs. penetrating keratoplasty in keratoconus patients: results of a randomized clinical trial. American Academy of Opthalmology; 2006 Nov 11‐14; Las Vegas (NV). 2006:Abstract page 181.
Sousa 2006 {published data only}
- Sousa LB, Grupenmacher L, Dalfre LT, Andrade TB. Comparison among intra‐estromal ring implant and automated anterior lamellar keratoplasty techniques in the treatment of keratoconus patients. Investigative Ophthalmology and Visual Science 2006; Vol. 47:ARVO E‐abstract 1322.
Additional references
Barr 2006
- Barr JT, Wilson BS, Gordon MO, Rah MJ, Riley C, Kollbaum PS, et al. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea 2006;25(1):16‐25. [DOI] [PubMed] [Google Scholar]
Davis 2006
- Davis LJ, Schechtman KH, Wilson BS, Rosenstiel CE, Riley CH, Libassi DP, et al. Longitudinal changes in visual acuity in keratoconus. Investigative Ophthalmology and Visual Science 2006;47(2):489‐500. [DOI] [PubMed] [Google Scholar]
Gordon 2006
- Gordon MO, Steger‐May K, Szczotka‐Flynn L, Riley C, Joslin CE, Weissman BA, et al. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. American Journal of Ophthalmology 2006;142(6):923‐30. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Kennedy 1986
- Kennedy RH, Bourne WM, Dyer JA. A 48‐year clinical and epidemiologic study of keratoconus. American Journal of Ophthalmology 1986;101(3):267‐73. [DOI] [PubMed] [Google Scholar]
Krachmer 1984
- Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Survey of Ophthalmology 1984;28(4):293‐322. [DOI] [PubMed] [Google Scholar]
Kymes 2004
- Kymes SM, Walline JJ, Zadnik K, Gordon MO, Collaborative Longitudinal Evaluation of Keratoconus study group. Quality of life in keratoconus. American Journal of Ophthalmology 2004;138(4):527‐35. [DOI] [PubMed] [Google Scholar]
McMahon 2006
- McMahon TT, Edrington TB, Szczotka‐Flynn L, Olafsson HE, Davis LJ, Schechtman KH, et al. Longitudinal changes in corneal curvature in keratoconus. Cornea 2006;25(3):296‐305. [DOI] [PubMed] [Google Scholar]
Rabinowitz 1998
- Rabinowitz YS. Keratoconus. Survey of Ophthalmology 1998;42(4):297‐319. [DOI] [PubMed] [Google Scholar]
Rabinowitz 2013
- Rabinowitz YR. INTACS for keratoconus and ectasia after LASIK. International Ophthalmology Clinics 2013;53(1):27‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sykakis 2015
- Sykakis E, Karim R, Evans JR, Bunce C, Amissah‐Arthur KN, Patwary S, McDonnell PJ, Hamada S. Corneal collagen cross‐linking for treating keratoconus. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010621.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wollensak 2003
- Wolensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet‐a‐induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology 2003;135(5):620‐7. [DOI] [PubMed] [Google Scholar]
Zadnik 1996
- Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Investigative Ophthalmology and Visual Science 1996;39(13):2537‐46. [PubMed] [Google Scholar]
References to other published versions of this review
Zadnik 2014
- Zadnik K, Lindsley K. Intrastromal corneal ring segments for treating keratoconus. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011150] [DOI] [PMC free article] [PubMed] [Google Scholar]


