Abstract
Context
Atypical antipsychotics (AAs) are the first-line treatments for schizophrenia, schizoaffective disorder and bipolar disorder. However, they are now extensively utilized as off label in a myriad of diseases despite their frequently serious metabolic side-effects and hyperprolactinemia.
Objective
The purpose of our study was to observe long-term (one year) prolactin level change in first episode schizophrenia patients treated with one of the four AAs: olanzapine, quetiapine, amisulpride, ziprasidone.
Design
This study is an analysis of the prolactin level associated with the atypical antipsychotics used in European First Episode Schizophrenia Trial (EUFEST) study.
Subjects and Methods
Seventy-three first episode schizophrenia patients from the 113 patients, randomized to one of the four AAs treatment arms. Prolactin level was obtained at baseline, 6 and 12 months for all the four AAs. Analyses have been done for each antipsychotic separately for each sex.
Results
For the male patients neither of the four antipsychotics have been associated with a statistically significant increase of prolactin level in the entire study (p>0.05). In case of the female patients, treatment with olanzapine (p=.021) and ziprasidone (p=.005) has been associated with a decrease of prolactin level in one year compared with baseline.
Conclusions
In both men and women, the administration of these four AAs is not associated with the increase of prolactin levels, moreover, in women’s case, there is a reduction of prolactin values at administration of Olanzapine and Ziprasidone. These results are optimistic, suggesting that long term administration of these antipsychotics is safe regarding prolactin level.
Keywords: antipsychotics, hyperprolactinemia, psychosis
INTRODUCTION
Atypical antipsychotics (AAs) (1) represent a class of drugs used mainly for the treatment of serious mental illnesses. They are the first-line treatments for psychosis, schizophrenia, schizoaffective disorder and bipolar disorder (2). However, clinical use of AAs constantly increased since their introduction in the ‘90’s, and these drugs are now extensively utilized regardless of sometimes scarce scientific proof (3) as off label in a myriad of diseases: attention-deficit/hyperactivity disorder, anxiety, dementia in elderly patients, depression, eating disorders, insomnia, obsessive-compulsive disorder, personality disorder, posttraumatic stress disorder, substance abuse disorders, and Tourette’s syndrome. Data obtained by market research firm IMS Health showed that in 2011 AAs were prescribed to a staggering number of 3.1 million Americans at a cost of $18.2 billion (4). In the same year, two AAs represented the fifth and sixth best-selling prescription drugs in the United States (4). Judicious use of antipsychotics in severe mental illnesses represents a cornerstone of overall management of these diseases. On long term, schizophrenic patients treated with antipsychotics have a better quality of life, a reduced number of hospitalization and a lower number of relapses (1), reported to untreated schizophrenic patients. Also, untreated schizophrenic patients have an increased mortality compared to treated schizophrenic patients (1).
In contrast with typical first-generation antipsychotics, atypicals are less likely to cause extrapyramidal symptoms (EPS) and tardive dyskinesia (5).
In fact, an AA is most accurately and simply described as one antipsychotic that produces minimal EPS at clinically effective doses (6), even though this is rather an oversimplification. However, compared to older typical antipsychotics, AA may cause more frequently serious metabolic side effects such as significant weight gain, dyslipidaemia and sometimes diabetes mellitus (7). Head-to-head comparisons of atypical antipsychotics lead to the conclusion that atypicals are not a homogenous class (8) and this is also true regarding their chemical structure, atypicals belonging to four chemical groups (9). While it is generally accepted that AAs are rather equally efficacious (10) they largely differ between them by side effects. Therefore, clinical guidelines suggest choosing an AA mainly by its side effects (2).
AAs side effects are: gastrointestinal (nausea, vomiting, constipation), extrapyramidal side effects (EPS), Neuroleptic Malignant Syndrome, seizures, sedation, hypotension, QTc prolongation, anticholinegic, haematological, liver enzyme abnormalities, hyperprolactinemia, the most frequent side effects being: weight gain, diabetes mellitus, dyslipidaemia, metabolic syndrome (11, 12).
Hyperprolactinemia is associated with amenorrhea, galactorrhoea, hirsutism, gynecomastia, impotence, loss of libido, and infertility (13). Long- term hyperprolactinemia may induce a state of chronic hypogonadism, which may increase the risk for osteoporosis and bones fractures (14). Data about increasing risk of breast cancer in females with hyperprolactinemia are questionable (15). Further complicating the issue is the fact that several studies reported an increase in prolactin levels in naïve schizophrenic patients (16).
However, it should also to be noted that many patients with high prolactin levels may have no symptoms (17).
Prolactin (18) is a peptide hormone secreted mainly by lactotropic cells of the pituitary (usually secreted in pulse, but phasic too in response to eating, mating, oestrogen treatment, ovulation and nursing) which main function is to induce lactation.
Beyond its role in lactation, prolactin plays an essential role in metabolism, regulation of the immune system and pancreatic development.
Prolactin is formed by lactotropic pituitary cells and acts on prolactin receptors: these are located in the woman’s breast, in the ovary, in testis, sexually independent in the CNS, and in adipose tissue and several other organs.Prolactin regulates milk production and supports the functions of the corpus luteum. Prolactin plays a role in the establishment of mother/child relations (18).
Different from other pituitary hormones that are almost entirely controlled by various releasing factors, prolactin secretion is controlled essentially by an inhibiting factor, dopamine (19). However, prolactin is also partially regulated by thyrotropin-releasing hormone (TRH) through an extracellular signal-regulated kinase (20) and ghrelin, a peptide hormone involved in metabolic homeostasis (21), which stimulates prolactin secretion by a direct action on the pituitary somatomammotroph cells. Prolactin release is also activated during the stress response.
Pituitary prolactin secretion is regulated by endocrine neurons in the hypothalamus (22). The most important ones are the neurosecretory tuberoinfundibulum (TIDA) neurons of the arcuate nucleus that secrete dopamine. Dopamine, therefore represents the Prolactin Inhibitory Hormone, and by its action on the D2 receptors of lactotrophs inhibits prolactin secretion. Thyrotropin-releasing hormone has an opposite stimulatory effect on prolactin release, as also have vasoactive intestinal polypeptide (VIP) and oxytocin. Nevertheless, prolactin is the only adreno-hypophyseal hormone whose principal control is inhibitory.
Dopamine tonically inhibits prolactin release. Prolactin activates prolactin receptors leading to increased dopamine synthesis and release, which in turn inhibits prolactin release.
Serotonin counterbalances the dopamine-inhibitory function via thyrotropin-releasing hormone, acting by HTR2A or HTR2C serotonin receptor (23).
Antipsychotics induce hyperprolactinemia by the blockade of D2 receptors on the lactotroph cells thus preventing inhibition of prolactin secretion (24). As a group, AA cause a smaller elevation of the PRL plasma levels than the typical antipsychotics (25, 26).
The explanation for the more limited elevation of PRL levels could lie in their greater specificity and stronger 5-HT2AR blockade and also in their faster dissociation from D2 which, in turn, results in a lesser blockade of the dopaminergic receptors (24).
The information concerning the increased prolactin secondary to AAs treatment is not at all as large as the data about the metabolic syndrome generated by the treatment with AAs. Moreover, the data is rather scarce on medium and long term AAs treatment. Likewise, that is exactly the data we need (data on the prolactin level on long term AAs use) because AAs treatment in severe mental illnesses is most often long term, if not lifelong.
Due to the risks associated with long-term increase in prolactin level, it is very important to understand exactly how is the dynamics of prolactin level in patients treated chronically with AAs.
Some older studies conducted in schizophrenic patients treated with typical antipsychotics suggest that in time, after an initial increase, prolactin level tends to plateau or even decreases (27).
More recent studies (28) seem to indicate that prolactin increased initially after the treatment with AAs (in this case Olanzapine) and afterwards developed tolerance over time. Yet, in case of risperidone, research (29) indicates that the phenomenon of prolactin tolerance did not appear, and, after a rapid increase usually during the first months, the prolactin level remained high. However, not all studies sustain this conclusion (30).
Data for amisulpride looks quite contradictory, with one study (31) reporting 100% patients developing hypeprolactinemia at 12 months while other study (32) indicating that, after an initial increase at 8-week, at 12 months the prolactin level decreased, yet remaining higher compared to the baseline.
Most studies suggest that quetiapine does not interfere with prolactin level or is slightly interfering (22% of patients developing hyperprolactinemia) (33). On long term patients treated with AAs tend to have a peak of prolactin plasma level at 6 months followed by a decrease (which did not yet attain baseline level). The results were similar for quetiapine and ziprasidone (34). Yet another study did not show significant change in prolactin level after administration of ziprasidone (35).
Regarding gender, lot of data seem to indicate that female patients are more at risk to develop hyperprolactinemia when exposed to antipsychotics compared to male patients (36).
The purpose of our paper is to observe time dynamics of prolactin in first episode schizophrenic patients treated with 4 different AAs (olanzapine, quetiapine, amisulpride, ziprasidone).
There are several ways to look into this data. One very interesting approach would have been to look at the 1-year dynamics of prolactin level of all patients (females and males) treated with AA’ (considered as a single unitary group). That would have been offered a bigger view about this topic and the statistic power would also have been far larger. However, we decided to look on the 1-year dynamics of prolactin level separately for females and males and for each AA’ in part. That is because as we have seen AAs are not a unitary group (8, 9) and because there is an important difference between females and males regarding AAs propensity to increase prolactin level. Even though our group of AAs is comprised of only 4 antipsychotics: olanzapine, quetiapine, amisulpride and ziprasidone there seems to be enough differences between these drugs (37) to look separately into the 1-year dynamic of prolactin levels for each antipsychotic, separately in females and males.
METHODS
Data analysed in our paper are drawn from European First Episode Schizophrenia Trial study and represent a secondary analysis of data about Romanian patients (N=113).
The rationale for EUFEST study has been described elsewhere (38). Basically, the main aim of EUFEST study was to compare atypical antipsychotics (amisulpride 200–800 mg/day, olanzapine 5–20 mg/day, quetiapine 200–750 mg/day, ziprasidone 40–160 mg/day) with low doses of typical neuroleptics (haloperidol 1–4 mg/day) in terms of effectiveness.
The trial took place at 50 centres in Europe and Israel.
The patients included were 18– 40 years of age and met DSM- IV criteria for schizophrenia, schizophreniform, or schizoaffective disorder confirmed by the Mini International Neuropsychiatric Interview Plus; at the first episode of psychosis and with less than 2 years between the onset of psychosis and enrolment. In this article we used the term ‘schizophrenia’ for diagnostics of schizophrenia, schizophreniform and schizoaffective disorder.
Previous use of antipsychotic drugs was less than 2 weeks during the preceding year and less than 6 weeks lifetime.
The trial complied with the Declaration of Helsinki and was approved by the Ethics Committees of the participating centres.
Baseline data were obtained between 4 weeks before and 1 week after randomization on demographics, diagnoses, current medication, psychopathology (Positive and Negative Syndrome Scale - PANSS), severity of illness, overall psychosocial functioning (global assessment of functioning scale - GAF), extrapyramidal symptoms, depression, neurocognitive performance) etc. All evaluations have been made at Baseline, 1, 2, 3, 6, 9, and 12 months. Prolactin levels were obtained at baseline, 6 and 12 months and measured in mcg/L.
A total number of 113 patients took part. From this we have eliminated the patients treated with typical antipsychotics (haloperidol, N=18), therefore remaining 95 people to be studied.
Because the Study Protocol stated that: “Previous use of antipsychotic drugs was less than 2 weeks during the preceding year and less than 6 weeks lifetime” we decided to look separately at the two groups: antipsychotic naïve, N=7 (5.4%) and antipsychotic non-naïve N=88 (94.6%). However, due to the small number of patients in the antipsychotic naïve group (7) we decided to analyse only antipsychotic non-naïve group. From the entire group of 88 patients, data for prolactin levels were available for only 73 patients: Olanzapine (N=22), Quetiapine (N=12), Amisulpride (N=19), Ziprasidone (N=20).
Because the purpose of our study was to analyse the differences between prolactin blood levels in schizophrenic patients treated with AAs on long run, we looked only into the group of patients (N=73) who finished the entire study (completers) (i.e. one year) for whom all the prolactin data on the full 1-year length were available.
At the beginning of the analysis we first analysed if there are significant differences between completers and non-completers, searching for putative differences: socio economic status, clinical data and prolactin blood levels or treatment allocation, which may have represented a potential source of bias for the generalizability of data regarding prolactin level.
Afterwards, we compared the four AAs to see if the allocation of patients at the baseline to the four treatment arms is uniform regarding: socio economic status, clinical status and prolactin levels.
We compared the dynamic (baseline, 6 months, 12 months) of prolactin level for each antipsychotic separately for males and for females.
Effect size (r) was determined for each pair of groups with the formula r= Z/√N (where N= is the total number of participants in a pair of groups) and we used Cohen’s effect size estimates (0.2 = “small”; 0.5 = “medium” and 0.8 = “large” effect size).
Statistical analysis
Descriptive methods have been used. The Shapiro– Wilk and Kolmogorov–Smirnov tests were used to assess whether the data were normally distributed. Because the data analysed (age, education, PANSS, and plasma PRL level) were not normally distributed, the data between the four AAs were evaluated by non-parametric tests (Kruskal–Wallis test for detecting putative differences between antipsychotics, followed by Mann-Whitney test for comparing each antipsychotic between each other). Differences among the level of prolactin at different times compared to baseline were tested by Wilcoxon signed rank test.
Therefore, the data will be presented by the median and interval instead of mean.
RESULTS
A number of 73 patients (83.9%) is representing the completers group. The non-completers group is comprised of 14 people (16.1%).
Mean dose before drug discontinuation (in mg.): Haloperidol=3 (+/1.2), Amisulpride=450.8 (+/-171.9), Olanzapine=12.6 (+/-4.7), Quetiapine=498.6 (+/-201.4), Ziprasidone=107.2 (+/-35).
Demographical data, socio-economic, clinical status, medication allocation and values of prolactin at baseline are presented for the two groups in Table 1.
Table 1.
Analysis of completers versus non-completers in EUFEST Romanian patient group
| Completers N=73 |
Non-completers N=14 |
p-value | |
| Women N (%) | 41 (87.2) | 6 (42.8) | .394 |
| Age (interval) | 26(18.29-39.92) | 28.60 (19-18.2) | .661 |
| Education | 12. (1-19) | 11 (7-13) | .009* |
| Olanzapine | 22 | 4 | .638 |
| Quetiapine | 12 | 4 | .516 |
| Amisulpride | 19 | 4 | .200 |
| Ziprasidone | 20 | 2 | .420 |
| PANSS | 85.50 (60-187) | 94.50 (60-118) | .328 |
| Prolactin Baseline males (mcg/L) | 23.58 (5.1-174.53) | 41.1 (14.15-75.47) | .117 |
| Prolactin Baseline females (mcg/L) | 70.75 (7.1-314.5) | 69.1 (40.5-88) | .640 |
There are no differences between completers and non-completers regarding age, sex, clinical status as evaluated by PANSS and baseline prolactin blood level. The only difference between the completers and non-completers is the fact that completers tend to have a better education compared to non-completers. However, this does not seem to represent a confounding factor since it could not bias the non-completers to different prolactin levels secondary to treatment. The groups are the same in terms of baseline prolactin levels and allocation to different AAs, therefore we do not expect the non-completers to have a different prolactin level, had they finished the entire study.
Data about socio economic status, clinical status and baseline prolactin blood level for the four AAs are presented in Table 2.
Table 2.
Comparison between antipsychotics in completers at baseline
| Olanzapine N=22 |
Quetiapine N=12 |
Amisulpride N=19 |
Ziprasidone N=20 |
p-value | |
| Age (years) (range) | 25.16 (18.95-35.27) | 23.67 (18.35-36.23) | 25.04 (18.29-39.92) | 28.51 (18.7-39.81) | .441 |
| Women N (%) | 12 (54.5) | 6 (50) | 9 (47.4) | 14 (70) | .402 |
| Education (years) | 12.5 (1-19) | 12 (10-17) | 12 (8-17) | 12 (6-17) | .990 |
| PANSS (range) | 85.5 (61-113) | 92 (76-187) | 89 (61-116) | 83.5 (60-125) | .851 |
| Prolactin baseline (mcg/L) males | 29 (9-89.62) | 12.58 (5.1- 68.3) | 30 (6-122.64) | 13.08 (9-174.53 | .864 |
| Prolactin baseline (mcg/L) females | 58.96 (24.5-200 | 58.96 (24.5-200) | 127.5 (7.1-268.87) | 73.11 (23.58-314.5) | .341 |
There are no statistically significant differences between the four groups.
The results of prolactin blood levels (with evaluation at baseline, 6 and 12 months) of the male patients (entire group) treated with the four AAs are presented in Table 3 and Graph 1 and for the females patients in Table 4 and Graph 2.
Graph 1.
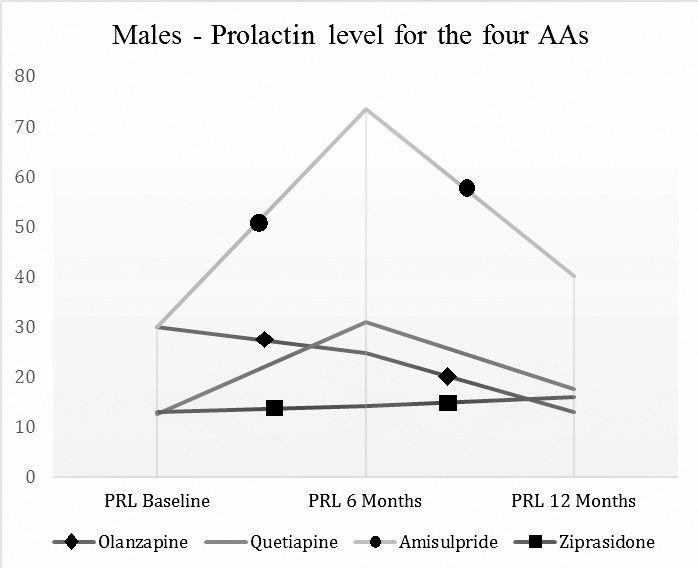
Prolactin levels by visits by antipsychotics (Males).
Graph 2.
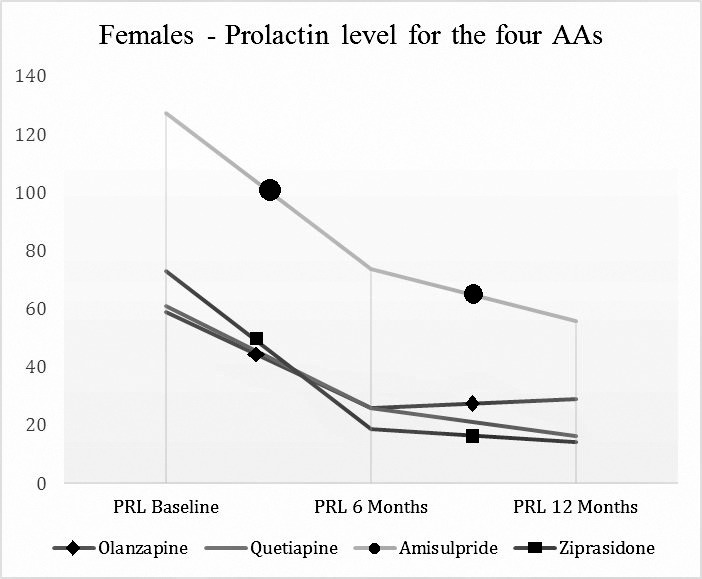
Prolactin levels by visits by antipsychotics (Females).
Table 3.
Prolactin level results for EUFEST- male patients
| AA (number of patients analysed per visits) | Prolactin Baseline (mcg/L) | Prolactin 6 Months (mcg/L) | Prolactin 12 Months (mcg/L) |
| Olanzapine (10; 8; 8) | 29 (9-89.62) | 24.84 (4.72-37.74) | 13.08 (4.72-105.5) |
| Quetiapine (6; 4; 4) | 12.58 (5.1-68.3) | 31 (16-66.6) | 17.5 (7.1-36.1) |
| Amisulpride (9;7; 8) | 30 (6-122.64) | 73.5 (19-87) | 40.14 (15.7-95) |
| Ziprasidone (6; 4; 3) | 13.08 (9-174.53) | 14.28 (13.9-37.74) | 16 (14.15-18.87) |
Table 4.
Prolactin level results for EUFEST- female patients
| AA (number of patients analysed per visits) | Prolactin Baseline (mcg/L) | Prolactin 6 Months (mcg/L) | Prolactin 12 Months (mcg/L) |
| Olanzapine (10; 10; 11) | 58.96 (24.5-200) | 25.94 (5-109) | 28.9 (5.6-51.4) |
| Quetiapine (6; 4; 4) | 60.93 (20.8-179.25) | 25.95 (10.6-28.3) | 16.43 (10.7-18.87) |
| Amisulpride (9; 8; 6) | 127.5 (7.1-268.87) | 73.81 (9.43-235.85) | 55.8 (13.2-212.26) |
| Ziprasidone (14; 8; 10) | 73.11 (23.58-314.5) | 18.75 (9.43-87) | 14.15 (0-121.70) |
Results for males patients: Neither of the four antipsychotics have been associated with a statistically significant (Wilcoxon signed rank test) increase of prolactin level in the entire study (we compared prolactin level at 6 months compared with the level at baseline, prolactin level at 12 months with the one at 6 months and prolactin level at 12 months with the one at baseline): Olanzapine (z=-1.4, p=.889, z=-734, p=.463, z=-280, p=.779), Quetiapine (z=-.-730, p=.465, z=-1.604, p=.109, z=-.365, p=.715), Amisulpride (z=-2.366, p=.0.18, z=-676, p=.499, z=-1.521, p=.128), Ziprasidone (z=-.365 p=.715, z=-.447 p=.655, z=0 p=1).
Comparing (Mann Whitney test) the four AAs one by one we obtained for the group of males (we present only the statistically significant ones) the next results:
Amisulpride vs. Olanzapine: the blood level of prolactin is statistically significantly increased by Amisulpride compared to Olanzapine at 6 months (Z=-2.493, p=.013), with a rather small effect size (r=0.38), but not at 12 months (Z=-1.629, p=.103).
Amisulpride vs. Ziprasidone the blood level of prolactin is statistically significant increased by Amisulpride compared to Ziprasidone at 6 months (Z=-2.273, p=.023), with a rather small effect size (r=0.36), but there is only a trend to significance at 12 months (Z=-1.739 p=.082).
Results for females patients: Olanzapine: there is a statistically significant decrease of prolactin blood level between 12 months and baseline (Z=-2.310, p=.021).
The changes of prolactin blood level induced by Quetiapine and Amisulpride are non-significantly statistic either at 6 or 12 months (data not presented, available on request).
For Ziprasidone is a statistically significant decrease in prolactin blood levels at 6 months (Z=-2.521. p=.012). The level of blood prolactin at 12 months is statistically significant lower compared to baseline (Z=-2.810 p=.005).
Comparing (Mann Whitney test) the four AAs one by one we obtained for the group of females (we present only the statistically significant ones) the next results: Amisulpride vs. Ziprasidone: there is an increase in prolactin blood level at 12 months in the group treated with amisulpride compared to the group treated with ziprasidone (Z=-2.065, p=.039), with a rather small effect size (r=0.33).
DISCUSSION
To the best of our knowledge this is the first study of this type ever done in Romania. In practice we find a significant number of patients with psychiatric disorders, most of them in need for long-term treatment. Hyperprolactinemia has multiple negative consequences, especially when it lasts for a long time. Thus, it is important for our practice to understand long-term dynamics of prolactin level in patients treated with neuroleptics. Therefore, information derived from our analysis are important, although the follow-up is limited to one year.
Literature data report the rapid increase of prolactin levels at 72 hours after the administration of antipsychotics and normalization of levels in 2-3 weeks after drug discontinuation (39).
An important aspect of our study is that most schizophrenic patients (N=88 (94.6%)) were not neuroleptic native. This could be an explanation for the fact that we report higher values of prolactinemia at baseline both in men and in women comparatively with normal values (<20 mcg/L in men and <25 mcg/L in women) (40). On the other hand, we cannot speak of a chronic exposure to high prolactinemia since every patient included in the study did not cumulate a period longer than 6 weeks of treatment with antipsychotics.
Another important aspect is the fact that patients were included in their first episode of psychosis, therefore there are no cases of chronic patients. In the case of chronic schizophrenia, both the disease and the prolonged exposure to antipsychotics are important factors related to prolactin data obtained from these patients. Moreover, some data show that patients at a first episode of schizophrenia have higher percentages of hyperprolactinemia compared with general population (16).
Results of our study have shown that there are small differences between patients of male and female gender related to long-term evolution of prolactin level during treatment with one of the four atypical antipsychotics.
Thus, in the case of male patients, none of the four antipsychotics has been associated with statistically significant modifications of prolactin level during the entire study. We can state that in the case of men, the four AAs are neutral as for prolactin level modification in long-term. The results of our analysis show in this case that long-term administration of any of the four AAs is safe regarding the risk of inducing hyperprolactinemia, and, implicitly, consequences of hyperprolactinemia in long-term in patients of male gender.
Comparing antipsychotics, we have seen that Amisulpride is associated with a statistically significant increase in prolactin level at 6 months compared with Olanzapine and Ziprasidone. However, analysis at 12 months shows that these differences cease to be statistically significant, which could suggest that the administration of any of the four AAs in long term in male patients with first episode schizophrenia is safe as per inducing hyperprolactinemia.
In the case of female gender, the situation is more complex. Literature data suggest that women are more sensitive to increased prolactin level (36). Treatment with Olanzapine and Ziprasidone has been associated with a decrease of prolactin level in one year compared with baseline. Quetiapine and Amisulpride have been neutral from this point of view. Analysing the four AAs relations, we could see that Amisulpride has determined statistically significant increases at the end of the study compared with Ziprasidone.
The results of this study are largely optimistic, as for all male patients the four AAs have been proven neutral as per prolactin level at the end of 12 months of treatment. Also, in the case of male patients, Amisulpride has been associated with the highest increase in prolactin levels compared with Olanzapine and Ziprasidone, but this only at 6 months not at 12 months, which suggests a process of adaptation in organisms of men being treated in long-term with these AAs.
In the case of female patients, Olanzapine and Ziprasidone have been associated with a significant decrease of prolactine levels in 12 months treatment, while Amisulpride and Quetiapine have proven neutral regarding prolactin levels. Comparing the 4 AAs, Amisulpride at the end of the study has been significantly associated with higher prolactin levels than in the case of Ziprasidone.
However, the number of patients in each individual group of AAs potentially limit the generalizability of our data. Even though, the results are in agreement with the data from other international similar studies.
In conclusion, all these data suggest that, in total, both in the case of men and women, the administration of these four AAs is not associated with the increase of prolactin levels, moreover, in the case of women, there is a reduction of prolactin values at administration of Olanzapine and Ziprasidone. These results are optimistic, suggesting that long term administration of these antipsychotics is safe as per prolactin level.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Torniainen M, Mittendorfer-Rutz E, Tanskanen A, Björkenstam C, Suvisaari J, Alexanderson K, Tiihonen J. Antipsychotic treatment and mortality in schizophrenia. Schizophrenia Bulletin. 2015;41(3):656–663. doi: 10.1093/schbul/sbu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. www.nice.org.uk/guidance/cg185- Last accessed June 2017.
- 3.Citrome L, Kalsekar I, Guo Z, Laubmeier K, Hebden T. Diagnoses associated with use of atypical antipsychotics in a commercial health plan: A claims database analysis. Clinical Therapeutics. 2013;35:1867–1875. doi: 10.1016/j.clinthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4. http://www.nytimes.com/2012/09/25/health/a-call-for-caution-in-the-use-of-antipsychotic-drugs.html.
- 5.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer HY. An atypical compound by any other name is still A. Psychopharmacology. 2000;148(1):16–19. doi: 10.1007/s002130050018. [DOI] [PubMed] [Google Scholar]
- 7.Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498–511. doi: 10.7326/0003-4819-157-7-201210020-00525. [DOI] [PubMed] [Google Scholar]
- 8.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 9.Classification Research System 1997. Oslo: WHO Collaborating Centre for Drug Statistics Methodology, Report No. 5;
- 10.Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second- generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- 11.Orsolini L, Tomasetti C, Valchera A, Vecchiotti R, Matarazzo I, Vellante F, Iasevoli F, Buonaguro EF, Fornaro M, Fiengo ALC, Martinotti G, Mazza M, Perna G, Carano A, De Bartolomeis A, Di Giannantonio M, De Berardis D. An update of safety of clinically used atypical antipsychotics. Expert Opinion on Drug Safety. 2016;15(10):1329–1347. doi: 10.1080/14740338.2016.1201475. [DOI] [PubMed] [Google Scholar]
- 12.Matei VP, Mihailescu A, Paraschiv G, Al-Bataineh R, Purnichi T. Weight Gain and Antipsychotics. Data from Eufest Study. Acta Endocrinologica-Bucharest. 2016;12(2):177–184. doi: 10.4183/aeb.2016.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byerly M, Suppes T, Tran QV, Baker RA. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: recent developments and current perspectives. J Clin Psychopharmacol. 2007;27(63):639–661. doi: 10.1097/jcp.0b013e31815ac4e5. [DOI] [PubMed] [Google Scholar]
- 14.Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129–134. doi: 10.1192/bjp.bp.106.023671. [DOI] [PubMed] [Google Scholar]
- 15.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominantly premenopausal women. Cancer Res. 2006;66:2476–2482. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- 16.Riecher-Rössler A, Rybakowski JK, Pflueger MO, Beyrau R, Kahn RS, Malik P, Fleischhacker WW, EUFEST Study Group Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychological Medicine. 2013;43:2571–2582. doi: 10.1017/S0033291713000226. [DOI] [PubMed] [Google Scholar]
- 17.Wesselmann U, Windgassen K. Galactorrhea: subjective response by schizophrenic patients. Acta Psychiatr. Scand. 1995;91(3):152–155. doi: 10.1111/j.1600-0447.1995.tb09758.x. [DOI] [PubMed] [Google Scholar]
- 18.Kleine B, Rossmanith WG. Hormones and the Endocrine System Textbook of Endocrinology. 2015:79–83. [Google Scholar]
- 19.Engler H, Doenlen R, Riether C, Engler A, Niemi MB, Besedovsky HO, del Rey A, Pacheco-López G, Feldon J, Schedlowski M. Time-dependent alterations of peripheral immune parameters after nigrostriatal dopamine depletion in a rat model of Parkinson’s disease. Brain Behav. Immun. 2009;23:518–526. doi: 10.1016/j.bbi.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Oride A, Kanasaki H, Purwana IN, Miyazaki K. Possible involvement of mitogen-activated protein kinase phosphatase-1 (MKP-1) in thyrotropin-releasing hormone (TRH)-induced prolactin gene expression. Biochem. Biophys. Res. Commun. 2009;382:663–667. doi: 10.1016/j.bbrc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 21.Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Anifandis G, Messinis IE. Effect of ghrelin and thyrotropin-releasing hormone on prolactin secretion in normal women. Horm. Metab. Res. 2010;42:204–208. doi: 10.1055/s-0029-1241197. [DOI] [PubMed] [Google Scholar]
- 22.Melmed S, Polonsky K, Reed Larsen P, Kronenberg H. Saunders, Philadelphia, PA.: 2008. Williams Textbook of Endocrinology- Neuroendocrinology; pp. 85–295. [Google Scholar]
- 23.Jørgensen HS. Studies on the neuroendocrine role of serotonin. Dan Med Bull. 2007;54:266–288. [PubMed] [Google Scholar]
- 24.Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. 1999;35(Suppl):S67–73. doi: 10.1016/s0920-9964(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 25.Tschoner A, Engl J, Rettenbacher MA, Kaser S, Ott HW, Fleischhacker WW, Patsch JR, Ebenbichler CF. Is second generation antipsychotic-induced hyperprolactinemia due to biologically active prolactin or to biologically inactive macroprolactin? Results from a prospective study. J Clin Psychiatry. 2009;70(2):293–294. doi: 10.4088/jcp.08l04509. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, Saito M, Someya T. Differences in plasma pro- lactin levels in patients with schizophrenia treated on monotherapy with five second-generation antipsychotics. Schizophr Res. 2013;145(1-3):116–119. doi: 10.1016/j.schres.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Naber D, Fischer H, Ackenheil M. Effect of long-term neuroleptic treatment on dopamine tuberoinfundibular system: development of tolerance? Commun Psychopharmacol. 1979;3:59–65. [PubMed] [Google Scholar]
- 28.Migliardi G, Spina E, D’Arrigo C, Gagliano A, Germanò E, Siracusano R, Diaz FJ, de Leon J. Short- and long-term effects on prolactin of risperidone and olanzapine treatments in children and adolescents. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1496–1501. doi: 10.1016/j.pnpbp.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, Arnold LE, Martin A, Katsovich L, Posey DJ, Shah B, Vitiello B. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–550. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 30.Eberhard J, Lindström E, Holstad M, Levander S. Prolactin level during 5 years of risperidone treatment in patients with psychotic disorders. Acta Psychiatr Scand. 2007;115:268–276. doi: 10.1111/j.1600-0447.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- 31.Paparrigopoulos T, Liappas J, Tzavellas E, Mourikis I, Soldatos C. Amisulpride- induced hyperprolactinemia is reversible following discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):92–96. doi: 10.1016/j.pnpbp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Ahn YM, Lee KY, Kim CE, Kang DY, Seok JH, Shin YM, Chung IW, Jun TY, Chang JS, Kim YS. The acute and long-term effectiveness of amisulpride in patients with schizophrenia: results of a 12-month open-label prospective follow-up study. Hum Psychopharmacol. 2011;26(8):568–577. doi: 10.1002/hup.1246. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, Josiassen RC. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. 2004;65(11):1491–1498. doi: 10.4088/jcp.v65n1108. [DOI] [PubMed] [Google Scholar]
- 34.Crespo-Facorro B, Ortiz-Garcia de la Foz V, Suarez-Pinilla P, Valdizan EM, Pérez-Iglesias R, Amado-Señaris JA, Teresa Garcia-Unzueta M, Labad J, Correll C, Ayesa-Arriola R. Effects of aripiprazole, quetiapine and ziprasidone on plasma prolactin levels in individuals with first episode nonaffective psychosis: Analysis of a randomized open-label 1 year study. doi: 10.1016/j.schres.2017.01.046. Sch. 2017 Feb 17. pii: S0920-9964(17)30059-2. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Park TW, Yang JC, Huang GB, Kim MG, Lee KH, Chung YC. Efficacy and safety of ziprasidone in the treatment of first-episode psychosis: an 8-week, open-label, multicenter trial. Int Clin Psychopharmacol. 2012;27(4):184–190. doi: 10.1097/YIC.0b013e3283528d22. [DOI] [PubMed] [Google Scholar]
- 36.Smith S, Wheeler MJ, Murray R, O’Keane V. The effect of antipsychotic– induced hyperprolactinemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol. 2002;22(2):109–114. doi: 10.1097/00004714-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Peuskens J, Pani L, Detraux J, De Hert M. The Effects of Novel and Newly Approved Antipsychotics on Serum Prolactin Levels: A Comprehensive Review. CNS Drugs. 2014;28(5):421–453. doi: 10.1007/s40263-014-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleischhacker WW, Keet IP, Kahn RS, EUFEST Steering Committee The European First Episode Schizophrenia Trial (EUFEST): Rationale and design of the trial. Schizophrenia Res. 2005;78:147–156. doi: 10.1016/j.schres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Marken PA, Haykal RF, Fisher JN. Management of psychotrop induced hyperprolactinemia. Clin Pharm. 1992;11:851–856. [PubMed] [Google Scholar]
- 40.Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and Prolactinomas. Endocrinol Metab Clin North Am. 2008;37(1):67–99. doi: 10.1016/j.ecl.2007.10.013. [DOI] [PubMed] [Google Scholar]


