Abstract
Context
Hepatitis C and diabetes represent important health problems globally. The new-onset diabetes after transplantation is a particular entity that appears due to the use of immunosuppression among transplanted patients.
Objective
We aim to describe the clinical and biological aspects of severe hyperglycemia in a kidney transplant recipient undergoing Interferon-free therapy for chronic hepatitis C, discussing the interference of different factors with the glucose metabolism.
Design
The occurrence of diabetes in a patient with history of renal transplantation and Interferon-free treated hepatitis C was studied from both clinical and paraclinical points of view.
Subjects and methods
When presenting to the hospital, extensive blood tests were performed on the patient, revealing significant hyperglycemia and an elevated level of blood tacrolimus. Creatinine clearance was calculated. ECG presented T-wave alterations. Intensive insulin protocol was applied, the case being managed in a multidisciplinary approach.
Results
Blood glucose and tacrolimus were slowly normalized, under therapy. The antiviral treatment was continued, with the achievement of sustained virologic response.
Conclusions
Diabetes mellitus can have many causes, hepatitis C and transplantation both having an impact on glucose metabolism. The association of the three entities should be carefully managed, due to its enhancing effect on morbidity and mortality.
Keywords: chronic hepatitis C, kidney transplant, diabetes mellitus, Paritaprevir, Ombitasvir, Dasabuvir
INTRODUCTION
Diabetes mellitus and hepatitis C are both growing worldwide epidemics. Approximately 180 million people are globally reported to be affected by the hepatitis C virus (HCV) infection, while 415 million individuals are currently estimated to have diabetes mellitus (1, 2). Apart from the fact that it leads to chronic hepatitis C, liver cirrhosis and hepatocellular carcinoma (HCC), HCV can interfere with glucose homeostasis, patients with chronic hepatitis C being at a higher risk of developing type 2 diabetes, by comparison with healthy individuals, as well as when compared with other causes of liver disease, such as the hepatitis B virus (3).
Furthermore, the HCV infection is associated with a significantly higher prevalence in individuals undergoing long-term hemodialysis and in kidney transplant recipients, leading to an increase in morbidity and mortality (4, 5).
A particular type of diabetes has been recently described, respectively the new-onset diabetes after transplantation (NODAT), with an occurrence rate between 2% and 53% of all transplanted patients (6). The first International Consensus Guidelines for the diagnosis and management of NODAT were published in 2003, and updated in 2014 (7).
The continuous efforts to improve the management of chronic hepatitis C resulted in the development of direct acting antivirals (DAAs), which have so far shown promising effects, increasing the rates of sustained virologic response and having a very good profile of safety and efficacy, even in transplanted patients. However, there are yet no guidelines regarding the use of these agents in kidney transplant recipients (8-11).
CASE PRESENTATION
A 42-year-old Caucasian man was admitted to our hospital, complaining of fatigue, blurred vision, trouble concentrating, headache, nausea, discrete abdominal pain and increased thirst, symptoms that had appeared during that same day and had become progressively aggravated. He had a history of chronic glomerulonephritis at the age of 12, which led to stage 5 chronic kidney disease (CKD) and hemodialysis by the age of 20, followed by his first kidney transplant from his mother. He was also diagnosed with chronic hepatitis C at the age of 38, for which he received interferon based therapy, with good outcome and undetectable viremia at the end of treatment. After chronic renal transplant rejection, he underwent a second kidney transplantation at the age of 40. The procedure was followed by viral reactivation, as the patient initially received immunosuppressive therapy with 10 mg of tacrolimus, 1.5 g of mofetil mycophenolate and 30 mg of prednisone daily, in the first 6 months after the transplantation – a period during which the blood tacrolimus level maintained between 10.3-12.2 ng/ mL. The dosage of the immunosuppressive drugs was progressively adjusted and lowered during the next year and a half, under the guidance of a nephrologist specialized in kidney transplant follow-up.
At the moment of the admission in our clinic, the patient was on his third week of receiving Interferon-free therapy with Ritonavir boosted Paritaprevir, Ombitasvir and Dasabuvir. The eligibility for the Interferon-free therapy was established based on Fibromax evaluation (resulting into a F3 degree of fibrosis), increased viral load (205000 copies/ mL) and a glomerular filtration rate (GFR) of 41 mL/ min/ 1.73 m2 (Stage 3 CKD).
Before starting antiviral therapy, the patient had been receiving daily immunosuppression with 2 mg of tacrolimus, 1 mg of mofetil mycophenolate and 5 mg of prednisone. At the initiation of antiviral treatment, the patient had been instructed to lower the dose of tacrolimus to 2 mg per week (as recommended by current guidelines), while maintaining the same doses of mofetil mycophenolate and prednisone. It is also worth mentioning from the patient’s history that, during the last year and a half and prior to the Interferon-free treatment initiation, the tacrolimus levels were maintained between a minimum of 4.3 and a maximum of 9 ng per mL. The patient had accidentally overlapped antiviral treatment and tacrolimus administration for two days at the beginning of therapy, afterwards continuing with the recommended 2 mg of tacrolimus weekly.
Physical examination at the moment of hospitalization revealed anxiety, confusion, dehydrated skin, sinus tachycardia and a blood pressure of 110/ 70 mmHg; there was a 3/6 systolic heart murmur, audible in the aortic area, but no further abnormalities were found. The neurologic examination was within normal limits.
The laboratory findings showed significant hyperglycemia (790 mg of glucose per dL), as well as a high level of blood tacrolimus (over 30 ng per mL). The previous tacrolimus determination had been made 20 days prior to this event and had revealed a value of 6 ng per mL.
The patient had no personal history of glucose metabolism impairment. None of the periodic blood tests following the transplantation showed any increase in the glycemic levels, that were always maintained between 75 and 90 mg per dL. However, the glycated hemoglobin (HbA1c) at admission had a value of 7.4%. There were no previous measurements of HbA1c as it was unnecessary, taking into consideration the fact that this was a non-diabetic patient.
The creatinine clearance was 38 mL/min. A urine stick evaluation was positive for ketones, as well as for glucose. It is worth mentioning that glycosuria was never present at the previous evaluations of the patient.
Although the patient had hepatitis C virus infection, the biological liver parameters were within normal ranges at the moment of the admission, as well as prior to this episode.
Intensive insulin protocol was applied, with a target glycemia of 150 mg/dL, and the administration of tacrolimus was discontinued. The patient was continuously and strictly monitored and the case was managed by a multidisciplinary team, consisting in an internist, a diabetologist and a nephrologist. Two days after admission the patient presented angina pectoris – like symptoms, with normal troponin levels and T-wave alterations on the ECG; echocardiography only revealed mild valvular abnormalities and was, otherwise, normal, while stress test for ischemia turned out to be positive, resulting in the association of nitrates to the therapeutic scheme.
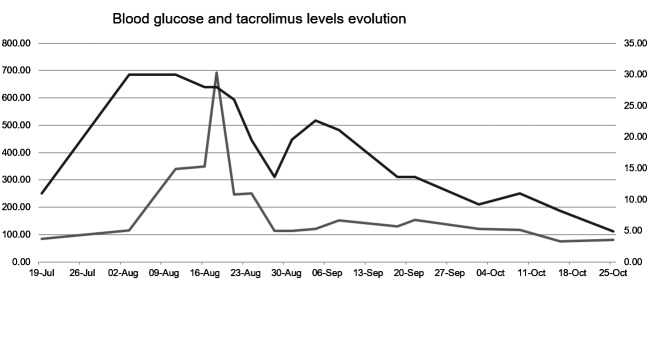
On follow-up, blood glucose and tacrolimus level had slowly decreased, reaching the normal values within several weeks (Fig. 1). The patient was discharged with the recommendation of a second-generation sulfonylurea (gliquidone), in order to maintain the blood sugar level under control.
Figure 1.
Blood glucose and tacrolimus level evolution under therapy: the glucose levels are in grey, while the tacrolimus levels are in black.
Furthermore, the antiviral therapy with DAAs was not interrupted and the patient achieved sustained virologic response (SVR), by maintaining the same therapeutic scheme, which resulted in undetectable viremia at 24 weeks after completion of antiviral therapy. There were no signs of impaired liver function before or during Interferon-free therapy.
DISCUSSION
In this paper, we reported a severe case of hyperglycemia in a kidney transplant recipient undergoing Interferon-free therapy for chronic hepatitis C, although the precise factor that caused the glucose metabolism impairment remains unclear in this situation, especially since the treatment of HCV infection with DAAs has been correlated with improved insulin sensitivity and satisfying glycemic control in patients already diagnosed with diabetes mellitus (12-14).
There is evidence that HCV induces glucose impairment both directly, via viral proteins, and, indirectly, by altering pro-inflammatory cytokines (15). It appears that the viral core proteins increase the degradation of insulin receptor substrate-1 (IRS-1), thus preventing its association with the insulin receptor (16-18). On the other hand, the indirect effect of the virus consists in the production of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) from sinusoidal liver cells. These cytokines interfere with insulin signaling pathways, leading to an enhancement in the gluconeogenesis process (19).
Not only the virus itself, but also interferon alpha therapy for chronic hepatitis C can play a key role in the pathogenesis of diabetes mellitus, due to its immunomodulatory effect (20). The mechanism by which interferon alpha induces type 1 diabetes may involve the activation of the JAK-STAT pathway, expressing interferon-stimulated genes, such as cytokines and adhesion molecules genes, thus triggering autoimmunity in genetically susceptible subjects (21, 22). In the presented case, the patient had previously received interferon alpha treatment at age 38 (with good response and undetectable viremia at the end of therapy). It is therefore possible that this substance may have produced changes at molecular levels, that only became evident 4 years later, in association with other factors, such as the long-term steroid intake (prednisone), which is known to determine an impairment in glucose transporter 2 (GLUT-2) expression. Not only do steroids exacerbate hyperglycemia in diabetic patients, but they also lead to the development of diabetes mellitus in healthy patients, with an incidence of 46% (23, 24).
It has also been demonstrated that the use of immunosuppressive agents, such as corticosteroids, calcineurin-inhibitors (for example, tacrolimus or cyclosporine), and sirolimus is related to new onset diabetes, in patients who underwent transplantation (25).
Tacrolimus is one of the most commonly used immunosuppressive drugs across the globe and it can have important side effects, such as nephrotoxicity, neurotoxicity, the aforementioned new onset diabetes after transplant, gastrointestinal and hepatic toxicity as well as microangiopathy, cardiomyopathy or post-transplant lymphoproliferative disease. Tacrolimus induced hepatic toxicity remains, however, an uncommon side effect and its mechanism is yet unclear (26). There is evidence found in rat models that high doses of tacrolimus may produce cholestasis, through the inhibition of glutathione biliary excretion (27).
In our patient’s case, there was only one significant side effect due to tacrolimus over dosage, despite the highly elevated levels of the drug – the acute episode of hyperglycemia and the onset of diabetes mellitus. The mechanisms involving tacrolimus in the development of diabetes are incompletely described; current data suggest that tacrolimus suppresses pancreatic insulin secretion (28).
New onset diabetes mainly occurs during the first 6 months after the transplant, because within this period the patients receive high doses of immunosuppressive agents (29). Besides the role of these drugs, there are several risk factors for post-transplant development of diabetes, including ethnicity, older age, familial history of diabetes, male sex of recipient, the presence of HLA phenotypes, donor-recipient mismatch, male sex of donor, personal history of acute rejection, autosomal dominant polycystic kidney disease, the type of immunosuppressive therapy chosen, obesity, pre-transplant hypertriglyceridemia, HCV infection, cytomegalovirus infection (30, 31).
Hyperparathyroidism, both primary and secondary, can determine glucose intolerance and insufficient insulin release, thus leading to diabetes. Literature data show that increased levels of intact parathormone are associated with new-onset diabetes after transplantation (32, 33).
The patient we chose to present already had HCV infection, as well as a personal history of acute rejection of the previous kidney transplant and a male donor, but he had no signs of hyperparathyroidism.
A particularity of the case consists in the elevation of glycated hemoglobin at admission, but with normal glycemic levels during the previous 2 years. As we already well know, the role of this parameter is to identify chronic hyperglycemia in the prior 2 or 3 months, being correlated with the risk of diabetic complications. Having less biological variability than blood glucose, the determination of glycated hemoglobin is recommended for diabetes diagnosis, as well as for risk stratification (34).
Higher levels of HbA1c may appear due to changes in the glycation rate, associated with aging and impaired renal function (35). Also, HbA1c can become elevated in cases of overt hypothyroidism, iron or vitamin B12 deficiency, alcoholism, hyperbilirubinemia, uremia, chronic use of aspirin or opiates (36). Furthermore, the use of corticosteroids, anti-seizure medication and human immunodeficiency virus (HIV) infection treatment, may lead to increased levels of glycated hemoglobin, often associated with autoimmunity (37, 39). In our patient’s case, the elevation of HbA1c can be, therefore, explained by the chronic use of prednisone, as well as by the renal function impairment.
Unlike the Interferon based regimens, the use of direct acting antivirals has been demonstrated to have minimal side effects in kidney transplant recipients (8-11). However, there is still little information concerning these patients, as no specific therapy protocol exists.
Paritaprevir is a non-structural protein 3/4A protease inhibitor. Being primarily metabolized by cytochrome P450 3A (CYP 3A), it requires co-administration with a CYP 3A inhibitor – ritonavir, which acts as a pharmacokinetic enhancer for paritaprevir. Ombitasvir inhibits NS5A, while dasabuvir acts on NS5B non-nucleoside polymerase (39). The pharmacologic interaction between tacrolimus and these direct acting antivirals may explain the high levels of tacrolinemia found in our patient, and implicitly, the occurrence of diabetes mellitus in this case, as there is an important action overlap between the tacrolimus and the antivirals. Tacrolimus is metabolized by CYP 3A and then it is transported by the transmembrane P-glycoprotein; the immunosuppressive drug also has a role in the inhibition of organic anion transporting polypeptide 1B1 (OATP1B1), which is known to be an uptake transporter in the liver. CYP 3A, the transmembrane P-glycoprotein, as well as OATP1B1 interfere with paritaprevir disposition. CYP 3A is also involved in the metabolism of dasabuvir (40). Therefore, the initiation of Interferon-free therapy in transplanted individuals should be associated with a decrease in the tacrolimus dose, as well as in the frequency of administration.
In conclusion, patients with chronic HCV infection are at an increased risk of developing diabetes mellitus, process that may occur by various mechanisms, implicating the virus both directly and indirectly. The association between the two entities is, however, a two-way one, as the diabetes also worsens hepatitis C outcome.
New-onset diabetes after transplantation is a severe complication that often appears due to the use of immunosuppressive agents, significantly increasing the morbidity and mortality. Therefore, another important association is the one between HCV infection and transplantation, both of the entities having an impact on glucose metabolism.
The particularity of our case consists in the diagnosis of severely unbalanced diabetes with recent onset in a patient with multiple risk factors (HCV chronic infection, impaired renal function, immunosuppressive therapy with corticosteroids and tacrolimus) in the setting of all oral antiviral treatment.
Conflict of interest
The authors declare that they do not have anything to disclose with respect to this manuscript.
Consent
Written informed consent was obtained from the patient prior to admission, for teaching/ scientific use of his medical data, provided his identity will be protected.
References
- 1.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global Epidemiology of Hepatitis C Virus Infection: An up-Date of the Distribution and Circulation of Hepatitis C Virus Genotypes. World J Gastroenterol. 2016;22(34):7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho N. Q&A: five questions on the 2015 IDF diabetes atlas. Diabetes Res Clin Pract. 2016;115:157–159. doi: 10.1016/j.diabres.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 3.White dL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49(5):831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi H, ElKhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baid-Agrawal S, Pascual M, Moradpour D, Frei U, Tolkoff-Rubin N. Hepatitis C virus infection in haemodialysis and kidney transplant patients. Rev Med Virol. 2008;18(2):97–115. doi: 10.1002/rmv.565. [DOI] [PubMed] [Google Scholar]
- 6.Pham PT, Pham PC, Lipshutz GS, Wilkinson AH. New onset diabetes mellitus after solid organ transplantation. Endocrinol Metab Clin North Am. 2007;36(4):873–890. doi: 10.1016/j.ecl.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Sharif A, Hecking M, de Vries APJ, Porrini E, Hornum M, Rasoul-Rockenschaub S, Krebs G, Berlakovich M, Kautzky-Willer A, Schernthaner G, Marchetti P, Pacini G, Ojo A, Takahara S, Larsen JL, Budde K, Eller K, Pascual J, Jardine A, Bakker SJL, Valderhaug TG, Jenssen TG, Cohney S, Säemann MD. Proceedings From an International Consensus Meeting on Posttransplantation Diabetes Mellitus: Recommendations and Future Directions. Am J Transplant. 2014;14(9):1992–2000. doi: 10.1111/ajt.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev dL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10:1238–1246. doi: 10.1111/j.1600-6143.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 9.Lubetzky M, Chun S, Joelson A, Coco M, Kamal L, Ajaimy M, Gaglio P, Akalin E, De Boccardo G. Safety and Efficacy of Treatment of Hepatitis C in Kidney Transplant Recipients With Directly Acting Antiviral Agents. Transplantation. 2017;101:1704–1710. doi: 10.1097/TP.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 10.Suna N, Etik DO, Ocal S, Selcuk H, Dagli U, Hilmioglu F, Boyacioglu S, Haberal M. Efficacy and Tolerability of Direct-Acting Antiviral Agents for Hepatitis C Virus Infection in Kidney Transplant Recipients. Transplantation. 2018;102:S909. [Google Scholar]
- 11.Sawinski D, Kaur N, Ajeti A, Trofe-Clark J, Lim M, Bleicher M, Goral S, Forde KA, Bloom RD. Successful Treatment of Hepatitis C in Renal Transplant Recipients With Direct-Acting Antiviral Agents. Am J Transplantation. 2016;16(5):1588–1595. doi: 10.1111/ajt.13620. [DOI] [PubMed] [Google Scholar]
- 12.Doyle MA, Cooper C. Successful Hepatitis C Antiviral Therapy Induces Remission of Type 2 Diabetes: A Case Report. Am J Case Rep. 2015;16:745–750. doi: 10.12659/AJCR.895064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahrani A, Bowler L, Singh P, Coates P. Resolution of diabetes in type 2 diabetic patient treated with IFN-alpha and ribavirin for hepatitis C. Eur J Gastroenterol Hepatol. 2006;18(3):291–293. doi: 10.1097/00042737-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AJ, Patel K, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Rustgi VK, Flisiak R, Pianko S, Diago M, Arora S, Foster GR, Torbenson M, Benhamou Y, Nelson DR, Sulkowski MS, Zeuzem S, Pulkstenis E, Subramanian GM, McHutchison JG for the ACHIEVE-1 and ACHIEVE-2/3 Study Teams Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61(1):128–134. doi: 10.1136/gut.2010.236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009;15(13):1537–1547. doi: 10.3748/wjg.15.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose SK, Ray R. Hepatitis C virus infection and insulin resistance. World J Diabetes. 2014;5(1):52–58. doi: 10.4239/wjd.v5.i1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, Baba S, Koga H, Kumashiro R, Ueno T, Ogata H, Yoshimura A, Sata M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. The Am J Pathology. 2004;165(5):1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55(4):529–535. doi: 10.1136/gut.2005.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonelli A, Ferrari SM, Giuggioli D, Di Domenicantonio A, Ruffilli I, Corrado A, Fabiani S, Marchi S, Ferri C, Ferrannini E, Fallahi P. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5(5):586–600. doi: 10.4239/wjd.v5.i5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabris P, Floreani A, Tositti G, Vergani D, De Lalla F, Betterle C. Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Aliment Pharmacol Ther. 2003;18(6):549–558. doi: 10.1046/j.1365-2036.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 21.Guo D, Dunbar JD, Yang CH, Pfeffer LM, Donner DB. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol. 1998;160(6):2742–2750. [PubMed] [Google Scholar]
- 22.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, Gadina M, O’Shea JJ, Biron CA. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297(5589):2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 23.Tamez Perez HE, Gómez de Ossio MD, Quintanilla Flores dL, Hernández Coria MI, Tamez Peña AL, Cuz Pérez GJ, Proskauer Peña SL. Glucose disturbances in non-diabetic patients receiving acute treatment with methylprednisolone pulses. Rev Assoc Med Bras. 2012;58(1):125–128. [PubMed] [Google Scholar]
- 24.Tamez-Pérez HE, Quintanilla-Flores dL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes. 2015;6(8):1073–1081. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesiraju S, Paritala P, Rao Ch UM, Sahariah S. New onset of diabetes after transplantation - an overview of epidemiology, mechanism of development and diagnosis. Transpl Immunol. 2014;30(1):52–58. doi: 10.1016/j.trim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Yadav DK, Gera DN, Gumber MR, Kute VB, Patel MP, Vanikar AV, Trivedi HL. Tacrolimus-induced severe cholestasis complicating renal transplantation. Ren Fail. 2013;35(5):735–737. doi: 10.3109/0886022X.2013.780621. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Campos S, Lopez-Acebo R, Gonzalez P, Culebras JM, Tuñon MJ, Gonzalez-Gallego J. Cholestasis and alterations of glutathione metabolism induced by tacrolimus (FK506) in the rat. Transplantation. 1998;66(1):84–88. doi: 10.1097/00007890-199807150-00013. [DOI] [PubMed] [Google Scholar]
- 28.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of Hepatitis C with Posttransplant Diabetes in Renal Transplant Patients on Tacrolimus. J Am Soc Nephrol. 2002;13(5):1374–1380. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 29.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3(5):590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 30.Pham P-TT, Pham P-MT, Pham SV, Pham P-AT, Pham P-CT. New onset diabetes after transplantation (NODAT): an overview. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2011;4:175–186. doi: 10.2147/DMSO.S19027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palepu S, Prasad GVR. New-onset diabetes mellitus after kidney transplantation: Current status and future directions. World J Diabetes. 2015;6(3):445–455. doi: 10.4239/wjd.v6.i3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivarsson KM, Clyne N, Almquist M, Akaberi S. Hyperparathyroidism and new onset diabetes after renal transplantation. Transplantation Proceedings. 2014;46(1):145–150. doi: 10.1016/j.transproceed.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 33.Sinangil A, Celik V, Barlas S, Koc Y, Basturk T, Sakaci T, Akin EB, Ecder T. The incidence of new onset diabetes after transplantation and related factors: Single center experience. Nefrologia. 2017;37(2):181–188. doi: 10.1016/j.nefro.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Bonora E, Tuomilehto J. The Pros and Cons of Diagnosing Diabetes With A1C. Diabetes Care. 2011;34(Suppl 2):S184–S190. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, Sullivan L, D’Agostino RB, Nathan DM. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care. 2008;31(10):1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MK, Kwon HS, Baek KH, Lee JH, Park WC, Sohn HS, Lee KW, Song KH. Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care. 2010;33(12):2546–2548. doi: 10.2337/dc10-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvaraj N, Bobby Z, Sathiyapriya V. Effect of lipid peroxides and antioxidants on glycation of hemoglobin: an in vitro study on human erythrocytes. Clin Chim Acta. 2006;366(1-2):190–195. doi: 10.1016/j.cca.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Lodh M, Mukhopadhyay R, Kumar B. A Case of Inappropriately High Glycated Hemoglobin. Ind J Clin Biochem. 2015;30(2):234–237. doi: 10.1007/s12291-014-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forsea AM, Mihai C, Predescu T, Tudose I, Margaritescu I, Giurcaneanu C. Polyglandular Autoimmune Syndrome Associated with Multiple Autoimmune Conditions and Atopic Dermatitis - An Unusual Manifestation of A Polyautoimmunity Phenotype. Acta Endocrinologica-Bucharest. 2017;13(1):106–110. doi: 10.4183/aeb.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badri P, Dutta S, Coakley E, Cohen D, Ding B, Podsadecki T, Bernstein B, Awni W, Menon R. Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT-450, ombitasvir, and dasabuvir. Am J Transplant. 2015;15(5):1313–1322. doi: 10.1111/ajt.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]