Abstract
Context
Regular training program is recognized to play a key role in chronic low-grade inflammation in obese patients.
Objective
The aim of this study was to evaluate the effects of a continued aerobic training on serum Interleukine-10 (IL-10) as anti-inflammatory cytokine in obese women.
Subjects and Methods
Thirty non-trained obese women aged 30-40 years with BMI 30-36 (kg/m2) participated in the study and divided into exercise (n=15) and control groups. Exercise subjects completed a 6 weeks aerobic training at 60-75% of heart rate max and continued to 12 weeks. Anthropometrical markers and fasting blood samples were collected of all subjects at pre, mid (6 weeks) and post training (12 weeks) for measuring serum IL-10. Data were analyzed using repeated measures ANOVA. Significance was accepted at P<0.05.
Results
At baseline (pre training), there were no differences in the age, body weight and other anthropometrical indexes also in serum IL-10 between the two groups (p>0.05). Six and 12 weeks of aerobic training resulted in a significant decrease in body weight and other anthropometrical indexes and a significant increase in IL-10 compared to baseline (p<0.05). No significant difference was observed in serum IL-10 between 6 and 12 weeks aerobic training (p=0.361). There were no changes in all variables in the Control group (p>0.05).
Conclusion
Based on this data we concluded that anti-inflammatory effects of long term aerobic training can be attributed in part to IL-10 but further studies over other markers of inflammatory profile are necessary in order to sustain the anti-inflammatory effect of aerobic training.
Keywords: Aerobic training, Obesity, Inflammation, Anthropometry.
INTRODUCTION
Lipid and hormonal disorders, changes in glucose homeostasis, and inflammatory profile imbalance are the most important complications of obesity and body fat increase. These complications predispose to many chronic diseases such as cardiovascular and respiratory diseases, kidney disease, cancer, and type-2 diabetes (1).
In addition to being the largest source of body triglyceride, adipose tissue, as an endocrine organ, discharges a lot of peptide or protein mediators which have a major role in the metabolic mechanisms and fat or glucose homeostasis (2). This tissue together with some other body tissues discharges peptide mediators such as adipokines and or inflammatory and anti-inflammatory cytokine such as leptin, adiponectin, resistin, C-reactive protein (CRP), and many interleukins (3). The literature supports these cytokines’ disorder in some chronic diseases, particularly diseases such as cardiovascular diseases, type-2 diabetes, or metabolic syndrome which are consequences of overweight and obesity (4). Lower levels of anti-inflammatory cytokines such as interleukin 10 (IL-10) in obese populations or related diseases compared to people with normal or healthy weight have been reported by many recent studies (5).
IL-10 has an important role in regulating the response of the immune system and reducing inflammation. This anti-inflammatory cytokine reduces inflammation through some different mechanisms such as inhibiting the synthesis of pro-inflammatory cytokines such as interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) through the suppression of p65 NF-kappaB (NF-kB) in macrophages (6). These anti-inflammatory cytokines are also crucial in negative regulation of reactive oxygen species, nitrogen mediators, and immune system tolerance (7). In this regard, the suppression of proliferation and cytotoxic T-cell responses are of high importance (8). There are substantial evidences on the relationship between IL-10 and obesity (9), metabolic syndrome, and cardiovascular disease (10). In adults, the levels of IL-10 decrease in the presence of obesity (11), cardiovascular disease (12), and type-2 diabetes (13).
Recently, several studies have suggested solutions to reduce or improve levels of these cytokines in the so-called populations. Since obese people have higher levels of body fat compared to those with a normal weight, it seems that weight loss or low body fat percentage are of particular importance in improving these cytokines’ profile in this population. Considering pharmacological or non-pharmacological interventions, the role of exercise or physical activity, as a non-pharmacological intervention for maintaining or improving levels of cytokines in obese or sick populations, has attracted the attention of many researchers in the field of health sciences (14). However, depending on the level of preparation, type of training program, and the type of study population, contradictory findings have been observed regarding the inflammatory profile response to exercise. Some studies have introduced body weight loss as the necessary condition for reducing the inflammatory cytokines or increasing the anti-inflammatory cytokines (15). Some studies also have reported the lack of changes in cytokines in the presence of weight loss (16) or significant improvement of cytokines in the absence of weight loss (17).
The findings of a recent study showed that improvement of inflammatory profile in response to aerobic training programs appears through a significant increase in anti-inflammatory cytokines such as IL-10, but not through changes in inflammatory cytokines such as CRP (18). In the mentioned study, 8 weeks of aerobic training led to a significant increase in IL-10 without any changes in CRP. However, in another study, a period of high intensity interval training (HIIT) did not result in any significant changes in IL-10 and the inflammatory cytokines TNF-α and interleukin-6 (IL-6) in obese men (19). Hence, due to lack of sufficient evidence on the response of IL-10 exercise intervention as well as conflicts regarding the effects of weight change or body fat percentage on the pattern of changes in inflammation profile markers, the present study aimed to determine the effect of 12 weeks of aerobic exercise on IL-10, weight, body fat percentage, and other markers of obesity in overweight inactive women.
MATERIAL AND METHODS
Subjects
Thirty non-trained age-matched (30-40 year of old) obese women (BMI; 30-36 kg/m2) participated in the study and divided into exercise (n=15) and control (n=15) groups. Ethics approval was given by the Ethics Committee of Islamic Azad University, Iran. The experimental protocol and potential risks of the study were explained to each subject both verbally and in writing before their informed consent was obtained for participation.
Inclusion and exclusion criteria
Obesity (30 kg/m2 ≤ BMI ≤ 36 kg/m2) is the main criterion to participate in the study. All subjects were inactive, non-smoker and non-alcoholics. Participants were included if they had not been involved in regular physical activity/diet in the previous 6 months. None of the subjects used drugs or therapies for obesity, and none had a past history of disease or injury that would prevent daily exercise. We also excluded people who had any self reported physician diagnosed chronic disease (arthritis, stroke, diabetes, hypertension, cancer, heart attack, chronic cough, or bronchitis).
Anthropometric measures
Anthropometric measurements were taken of all study participants before breakfast, with the subject wearing light clothing without shoes in the physiology laboratory. Weight was measured to the nearest 100g using digital scales. Standing height was measured to the nearest 0.1 cm with the use of a wall-mounted stadiometer. BMI was calculated by dividing body mass (kg) by height in squared metres (m2). Abdominal circumference and hip circumference were measured in the most condensed part using a non-elastic cloth meter. Percentage of body fat was estimated by bioelectrical impedance method (Omron Body Fat Analyzer, Finland). All of these measurements were conducted by the same researcher. Each of these measurements was conducted two times and the average was reported. Anthropometrical indexes were measured before and after exercise program.
Training protocol and Biochemical Analysis
As mentioned above, subjects were randomly divided in exercise and control groups. Control subjects did not participate in the training program and were instructed to maintain their habitual activities. Aerobic intervention lasted 12 weeks. As exercise subjects were completed with a 6 weeks supervised aerobic training, 3 times per week at 60-75 % of heart rate max (HRmax) (The first two weeks at 60-65 % of HRmax, third and fourth week at 65-70% of HRmax, fifth and sixth week at 70-75 % of HRmax) (20). After that, the subject continued aerobic exercise for 6 weeks until the twelfth week at 70-75 % of HRmax. Each session started by 10 min of warm up, 30-40 min of aerobic exercise and 5–10 min of cool down activity. Aerobic exercises included running on flat surface with no slope. The intensity of the activity of any person was controlled using the Polar heart rate tester (made in the US).
Blood samples were taken before and 48 hours after last exercise session. So, all subjects were asked to attend Hematology Lab after an overnight fast. Blood samples were obtained between 8:00 and 9:00 a.m. after 10 to 12 hours overnight fast, and then centrifuged for separate serum. Serum was used to measuring IL-10 by ELISA method (Enzyme-linked Immunosorbent Assay for quantitative detection of human IL-10, Austria). The inter- and intra-assay coefficients of variance were 3.2 and 5.6% pg/mL respectively.
Data analysis
After calculation of the mean and the standard deviation, the statistical analysis was conducted using the SPSS software version 15.0. Shapiro-Wilk test was used to determine the normal status of the data. A repeated measure analysis of variance (ANOVA) was used to evaluate time-course change in variables for serum IL-10 and anthropometrical indexes and Bonferroni post hoc test used to determine significant values between specific means. Then independent sample T-test was used to compare each variable between 2 groups at each stage. A criterion alpha level of P ≤ 0.05 was used for all statistical comparisons.
RESULTS
As mentioned above, this study aimed to determine the anti-inflammatory effect of aerobic training with emphasis on serum IL-10 in non-trained obese women.
Pre, mid (6 weeks) and post (12 weeks) training anthropometrical indexes of two groups are shown in Table 1. Based on independent T test, there were no statistically significant differences between the exercise and control groups with regard to all anthropometrical indexes at baseline (P>0.05, Table 1).
Table 1.
Pre, mid and post-training of anthropometrical variables of the subjects
| Variables | Pre-training | Mid-training | Post-training | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | Sig | Exercise | Control | Sig | Exercise | Control | Sig | |
| Weight (kg) | 83.3 ± 6.87 | 83.47 ± 5.40 | 0.43 | 82.18 ± 6.55 | 83.6 ± 5.82 | 0.023 | 80.08 ± 6.41 | 83.31 ± 5.37 | 0.014 |
| AC (cm) | 112 ± 5.24 | 113 ± 3.97 | 0.61 | 110 ± 5.26 | 114 ± 4.37 | 0.019 | 106 ± 5.66 | 113 ± 4.25 | 0.028 |
| HC (cm) | 115 ± 5.73 | 117 ± 3.42 | 0.32 | 113 ± 5.44 | 116 ± 3.85 | 0.032 | 109 ± 5.41 | 116 ± 3.81 | 0.033 |
| BMI (kg/m2) | 32.03 ± 1.26 | 32.07 ± 1.33 | 0.51 | 31.60 ± 1.18 | 32.12 ± 1.53 | 0.029 | 30.8 ± 1.19 | 32.01 ± 1.30 | 0.022 |
| Body fat (%) | 45.04 ± 1.96 | 45.39 ± 1.64 | 0.64 | 43.28 ± 1.92 | 45.04 ± 2.01 | 0.009 | 40.66 ± 1.42 | 45.08 ± 2.37 | 0.011 |
| Visceral fat | 8.6 ± 0.91 | 8.47 ± 0.74 | 0.36 | 8.2 ± 0.86 | 8.47 ± 0.91 | 0.010 | 7.27 ± 0.45 | 8.27 ± 0.88 | 0.015 |
AC, abdominal circumference; HC, hip circumference; BMI: body mass index.
Based on data of repeated measure analysis, significant changes were observed in body weight (p = 0.031), abdominal circumference (p = 0.018), hip circumference (p = 0.028), BMI (p = 0.019), body fat percentage (p = 0.011) and visceral fat (p = 0.026) between measuring stages.
In addition, data by Bonferroni test showed a significant decrease in each anthropometrical marker after 6 or 12 weeks aerobic training compared with pre-training (p < 0.05), but no significant difference was observed between mid and post-training (p > 0.05).
As mentioned above, independent T test was used to compare each variable between 2 groups in each sampling stage. Based on data, all anthropometrical markers in exercise subjects were significantly lower than those of control group in mid-training as well as post-training (p < 0.05, Table 1, Figs 1-4).
Figure 1.
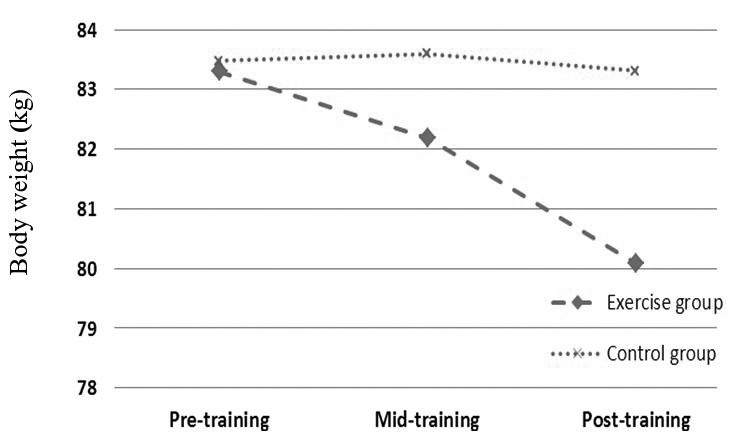
Effects profile of aerobic training on body weight in obese females.
Figure 4.
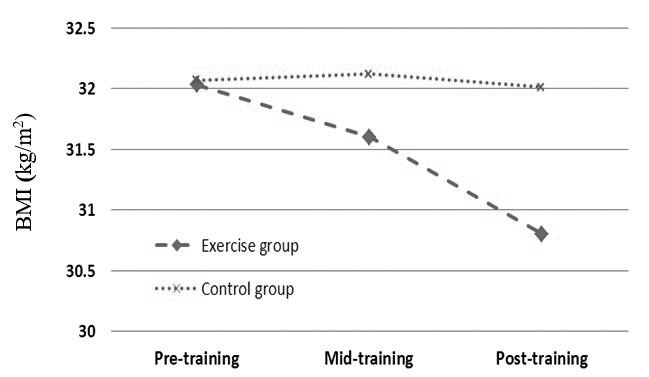
Effects profile of aerobic training on BMI in obese females.
Figure 2.
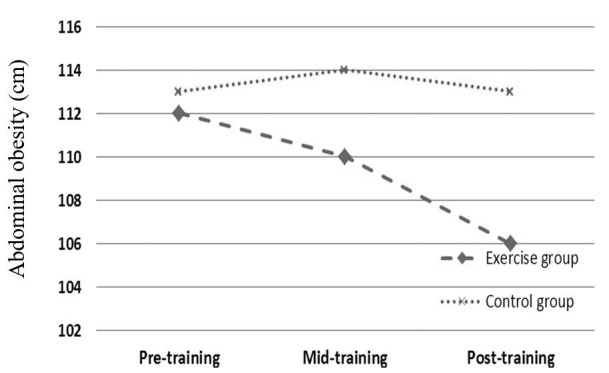
Effects profile of aerobic training on abdominal circumference in obese females.
Figure 3.
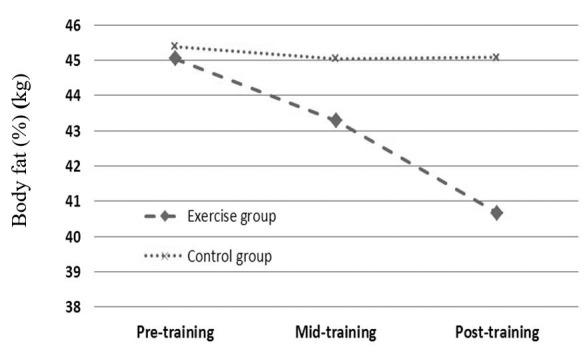
Effects profile of aerobic training on body fat in obese females.
The effect of 6 and 12 weeks aerobic training on serum IL-10 was the main aim of the present study. Table 2 presents data of serum IL-10 at pre, mid and post training of studied groups. At baseline, there was no difference in serum IL-10 between the two groups (see Table 1).
Table 2.
Pre, mid and post training of serum IL-10 of the subjects
| Variables | Pre-training | Mid-training | Post-training | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | Sig | Exercise | Control | Sig | Exercise | Control | Sig | |
| IL-10 (pg/mL) | 14.79 ± 3.04 | 17.83 ± 2.27 | 0.046 | 17.36 ± 2.72 | 14.5 ± 2.44 | 0.028 | 17.79 ± 3 | 15.03 ± 2.24 | 0.025 |
Based on data of repeated measure analysis, significant changes were observed in serum IL-10 between measuring stages (p = 0.023). On the other hand, data by Bonferroni test showed a significant decrease in serum IL-10 after 6 (p = 0.001) or 12 (p = 0.002) weeks aerobic training compared with pre-training. But no significant difference was observed between mid and post-training (p = 0.361).
Based on independent T analysis, serum IL-10 level in exercise subjects were significantly higher than those of control group in mid-training (p = 0.014) as well post-training (p = 0.023) (Table 2, Fig. 5).
Figure 5.
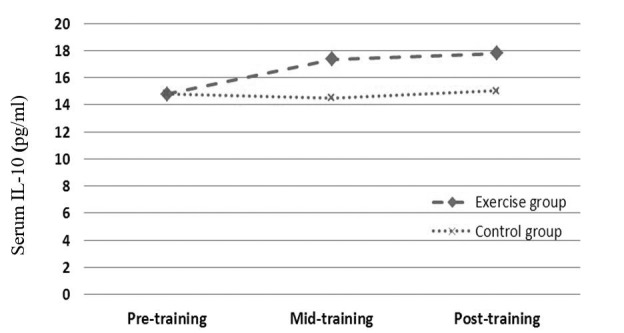
Effects profile of aerobic training on serum IL-10 in obese females.
DISCUSSION
The findings confirm the anti-inflammatory effects of 6 and 12 weeks of aerobic training on adult obese women. The serum levels of IL-10 in response to 6 weeks of aerobic training significantly increased. In other words, 6 weeks of aerobic training, three times a week, significantly increased the serum levels of IL-10 as an anti-inflammatory cytokine in obese adult women. Despite little evidence on response or compatibility of IL-10 to exercise training, some studies have attributed a decrease in inflammation through training programs to the increase of this anti-inflammatory cytokine rather than the inflammatory cytokines’ response (18). Similarly, in another study, long-term endurance trainings caused a significant increase in IL-10 along with decreased levels of leptin in mice (14). In a recent study, despite the lack of changes in inflammatory and anti-inflammatory cytokines such as CRP and adiponectin in response to 6-month aerobic exercise in type-2 diabetics with overweight, a significant increase was observed in serum levels of IL-10 with the ratio of IL-10 to interleukin-18 (IL-18); it was associated with reduced levels of glucose and insulin resistance in these patients (21). In contrast to these findings, in another study, lack of significant changes in IL-10 and some pro-inflammatory cytokines such as TNF-α and IL-6 after a period of HIIT has been reported in obese men (19).
A significant increase in IL-10 was observed in the study while the training program was followed by losing weight and body fat percentage among women. It should be noted that levels of IL-10 decrease in the presence of obesity (10). Hence, increasing this inflammatory cytokine followed by the training program can be attributed to weight loss or reduction of body fat levels. Although some studies have reported an increase in this anti-inflammatory cytokine following an intense interval training session (22), this increase can be accounted as an unstable response to an exercise session, not compatibility. In contrast, in another study, a resistance training session did not lead to a change in serum levels of IL-10, immediate and 60-minute recovery in women with metabolic syndrome (23). Laboratory studies have revealed that the increase in IL-10 by anti-inflammatory properties is probably due to anti-inflammatory effects on the vascular system through the inhibition of leukocyte-endothelial cell interactions and inhibition of the production of pro-inflammatory cytokines and chemokines by macrophages and lymphocytes (24).
Recently, it has been specified that anti-inflammatory effects of adiponectin by IL-10 are to some extent a strong anti-inflammatory cytokine mediator (10). However, the main mechanisms of the association between abdominal obesity and IL-10 are not fully known yet. It has been reported that macrophages are the main source of proteins derived by adipose tissue. However, some other studies consider IL-10 levels in obese people, revealing that the increase in the number of macrophages in visceral adipose tissue of obese individuals is the main source of secretion of this inflammatory cytokine (25). In some other studies, the increase in visceral adipose tissue is the main source of IL-10 production in obese individuals (10). Nevertheless, it is hypothesized that obese patients have higher circulating IL-10 than obese healthy individuals. In this context, there is considerable evidence that the increase in IL-10 in obese patients occurs to inhibit the production of pro-inflammatory cytokines (26). On the other hand, some studies refer to the fact that obese children have lower levels of IL-10 (26). The researchers attribute the increase in IL-10 in obese people to a compensatory response of the body to inhibit the production of pro-inflammatory cytokines (26). On the other hand, the increase in the secretion of compensation IL-10 in obese children reduces its discharge capacity in adulthood, promoting the risk of developing type-2 diabetes and cardiovascular diseases (27).
Despite these pieces of evidence, some other studies confirm the close relationship between lower levels of IL-10 or less secretion of it and clinical diseases and disorders (28, 29). Hence, more studies are required to be conducted on the mechanisms responsible for the link between obesity or body fat levels with IL-10 and other inflammatory or anti-inflammatory mediators. However, laboratory studies have revealed that about three-quarters of the difference in the production of IL-10 in humans depend on the hereditary and genetic features (30). Thus, attributing the increase in serum levels of IL-10 on the obese women to weight loss or decline in body fat levels is somewhat controversial. In obese women in this study, continuation of aerobic training program for 12 weeks was followed by ascending increase in IL-10 compared to baseline levels, however no significant difference was observed in IL-10 levels between 6 and 12 weeks of the training program. However, the rate of weight loss or decline in body fat percentage was perceptible between weeks 6 and 12 of training. In other words, despite the significant difference in weight and body fat percentage between weeks 6 and 12 of aerobic training, the serum levels of IL-10 did not significantly change between these two training conditions. In this regard, some studies have reported a significant improvement of cytokines in the absence of weight loss (17).
Hence, it is hypothesized that increased IL-10 serum levels in obese women can be mainly attributed to compatibility of long-term continuous aerobic exercises rather than weight loss or decline in body fat levels, which requires conduction of further researches. Some studies have indicated that improvement in metabolic parameters and inflammatory marker following training programs becomes more visible when the training program is associated with at least 10% reduction in body weight (31). It should be noted that in this study, despite a significant reduction in the weight and body fat percentage of the subjects following each training period, the numerical value of this reduction was less than 10 percent compared to baseline levels.
Some studies have attributed improvements in inflammatory mediators to the increase in cardiovascular fitness in response to the training program. In a recent study, 6 months of aerobic exercise reduced IL-6 and increased IL-10 along with improving VO2max. And researchers, assuming the significant relationship between IL-10 changes and changes in VO2max in response to the training program, have attributed changes of IL-10 to improvement of cardiovascular fitness of the subjects (32). In this regard, literature review shows that baseline levels of inflammation can be a decisive factor of an individual’s relative fitness (33). It should be noted that some studies on diabetic (34) and metabolic syndrome (35) patients have approved an inverse relationship between cardiovascular fitness and inflammation independent of body fat levels. At the end, it should be also noted that small sample size (n=15) is a limitation of the present study which cannot allow generalization of the results on the target population. In addition, lack of measuring other inflammatory or anti-inflammatory cytokines such as adiponectin and TNF-α in response to the training program is another limitation of the current study.
In conclusion, regular aerobic training can be considered a none-pharmacologic treatment and an anti-inflammatory strategy with an emphasis on IL-10. This anti-inflammatory property may be attributed to weight loss and improvement of the body composition in response to the aerobic training program. However, examination of other indicative markers of inflammatory profile is required to make a more comprehensive conclusion. Hence, future studies should focus on markers of inflammatory profile.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgment
The authors thank all the participants and Islamic Azad University for their financial support and cooperation in implementing this project.
References
- 1.Prado WL, Lofrano-Prado MC, Oyama LM, Cardel M, Gomes PP, Andrade ML, Freitas CR, Balagopal P, Hill JO. Effect of a 12-Week Low vs. High Intensity Aerobic Exercise Training on Appetite-Regulating Hormones in Obese Adolescents: A Randomized Exercise Intervention Study. Pediatr Exerc Sci. 2015;27(4):510–517. doi: 10.1123/pes.2015-0018. [DOI] [PubMed] [Google Scholar]
- 2.Parvaresh Rizi E, Teo Y, Leow MK, Yin Hao Khoo E, Rou Yeo C, Chan E, Song T, Anand Sadananthan S, Sendhil Velan S, Gluckman PD, Seng Lee Y, Seng Chong Y, Shyong Tai E, Toh SA, Meng Khoo C. Ethnic differences in the role of adipocytokines linking abdominal adiposity and insulin sensitivity among Asians. J Clin Endocrinol Metab. 2015;100(11):4249–56. doi: 10.1210/jc.2015-2639. [DOI] [PubMed] [Google Scholar]
- 3.Maltais ML, Perreault K, Courchesne-Loyer A, Lagacé JC, Barsalani R, Dionne IJ. Effect of Resistance Training and Various Sources of Protein Supplementation on Body Fat Mass and Metabolic Profile in Sarcopenic Overweight Older Adult Men: A Pilot Study. Int J Sport Nutr Exerc Metab. 2016;26(1):71–77. doi: 10.1123/ijsnem.2015-0160. [DOI] [PubMed] [Google Scholar]
- 4.Vinagre I, Sánchez-Quesada JL, Sánchez-Hernández J, Santos D, Ordoñez-Llanos J, De Leiva A, Pérez A. Inflammatory biomarkers in type 2 diabetic patients: effect of glycemic control and impact of LDL subfraction phenotype. Cardiovasc Diabetol. 2014;13:34. doi: 10.1186/1475-2840-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonia LC, Yoaly AL, Enrique EL, Guadalupe TP, Antonio GC, Galileo E, Javier VM. Reduced Systemic Levels of IL-10 Are Associated with the Severity of Obstructive Sleep Apnea and Insulin Resistance in Morbidly Obese Humans. Mediators of Inflammation. 2015;2015:1–9. doi: 10.1155/2015/493409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahim SS, Khan N, Boddupalli CS, Hasnain SE, Mukhopadhyay S. Interleukin-10 (IL-10) mediated suppression of IL-12 production in RAW 264.7 cells also involves c-rel transcription factor. Immunology. 2005;114(3):313–321. doi: 10.1111/j.1365-2567.2005.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasu C, Gorla SR, Prabhakar BS, Holterman MJ. Targeted engagement of CTLA-4 prevents autoimmune thyroiditis. Int Immunol. 2003;15(5):641–654. doi: 10.1093/intimm/dxg061. [DOI] [PubMed] [Google Scholar]
- 8.Couper KN, Blount DG. Riley EM: IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 9.Chang JS, Chang CC, Chien E, Lin SS, Cheng-Shiuan T, Bai CH, Chao KC. Association between interleukin 1β and interleukin 10 concentrations: a cross-sectional study in young adolescents in Taiwan. MC Pediatr. 2013;13:123. doi: 10.1186/1471-2431-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Ward J, Dauch JR, Tanzi RE, Cheng HT. Cytokine-mediated inflammation mediates painful neuropathy from metabolic syndrome. PLoS One. 2018;13(2):e0192333. doi: 10.1371/journal.pone.0192333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Daghri NM, Al-Ajlan AS, Alfawaz H, Yakout SM, Aljohani N, Kumar S, Alokail MS. Serum cytokine, chemokine and hormone levels in Saudi adults with pre-diabetes: a one-year prospective study. Int J Clin Exp Pathol. 2015;8(9):11587–93. [PMC free article] [PubMed] [Google Scholar]
- 12.Bagchi AK, Akolkar G, Mandal S, Ayyappan P, Yang X, Singal PK. Toll-like receptor 2 dominance over Toll-like receptor 4 in stressful conditions for its detrimental role in the heart. Am J Physiol Heart Circ Physiol. 2017;1312(6):1238–1247. doi: 10.1152/ajpheart.00800.2016. [DOI] [PubMed] [Google Scholar]
- 13.Letícia da Silva Pereira B, Regina Polina E, Crispim D, Cesar Sbruzzi R, Henrique Canani L, Gonçalves Dos Santos K. Interleukin-10 -1082A>G (rs1800896) polymorphism is associated with diabetic retinopathy in type 2 diabetes. Diabetes Res Clin Pract. 2018 doi: 10.1016/j.diabres.2018.01.023. S0168-8227(17)31815-6. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins NT, Padilla J, Arce-Esquivel AA, Bayless DS, Martin JS, Leidy HJ, Booth FW, Rector RS, Laughlin MH. Effects of endurance exercise training, metformin, and their combination on adipose tissue leptin and IL-10 secretion in OLETF rats. J Appl Physiol (1985) 2012;113(12):1873–1883. doi: 10.1152/japplphysiol.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2008;19(6):371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 16.de Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Bellioo D, Conde R. Effects of a low-fat versus a low-carbohydrate diet on adipocytokines in obese adults. Horm Res. 2007;67(6):296–300. doi: 10.1159/000099329. [DOI] [PubMed] [Google Scholar]
- 17.Kadoglou NP, Perrea D, Iliadis F, Angelopoulou N, Liapis C, Alevizos M. Exercise Reduces Resistin and Inflammatory Cytokines in Patients With Type 2 Diabetes. Diabetes Care. 2007;30(3):719–719. doi: 10.2337/dc06-1149. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro F, Alves AJ, Teixeira M, Miranda F, Azevedo C, Duarte JA, Oliveira J. Exercise training increases interleukin-10 after an acute myocardial infarction: a randomised clinical trial. Int J Sports Med. 2012;33(3):192–798. doi: 10.1055/s-0031-1297959. [DOI] [PubMed] [Google Scholar]
- 19.Leggate M, Carter WG, Evans MJ, Vennard RA, Sribala-Sundaram S, Nimmo MA. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males.J Appl Physiol (1985) 2012;112(8):1353–1360. doi: 10.1152/japplphysiol.01080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eizadi M, Karimy M, Kohandel M, Doaly H. Effect of aerobic exercise on serum leptin response and insulin resistance of patients with type 2 diabetes. J Qazvin Univ Med Sci. 2013;16(4):33–39. [Google Scholar]
- 21.Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, Alevizos M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14(6):837–843. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 22.Zwetsloot KA, John CS, Lawrence MM, Battista RA, Shanely RA. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J Inflamm Res. 2014;7:9–17. doi: 10.2147/JIR.S54721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira GB1, Tibana RA, Navalta J, Sousa NM, Córdova C, Souza VC, Nóbrega OT, Prestes J, Perez SE. Acute effects of resistance training on cytokines and osteoprotegerin in women with metabolic syndrome. Clin Physiol Funct Imaging. 2013;33(2):122–130. doi: 10.1111/cpf.12004. [DOI] [PubMed] [Google Scholar]
- 24.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88(9):877–887. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- 25.Manica-Cattani MF, Bittencourt L, Rocha MI, Algarve TD, Bodanese LC, Rech R, Machado MM, Santos GF, Gottlieb MG, Schwanke CH, Piccoli JE, Duarte MF, Cruz IB. Association between interleukin-1 beta polymorphism (+3953) and obesity. Mol Cell Endocrinol. 2010;314(1):84–89. doi: 10.1016/j.mce.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, Giugliano D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88(3):1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 27.Calcaterra V, De Amici M, Klersy C, Torre C, Brizzi V, Scaglia F, Albanesi M, Albertini R, Allais B, Larizza D. Adiponectin, IL-10 and metabolic syndrome in obese children and adolescents. Acta Biomed. 2009;80(2):117–123. [PubMed] [Google Scholar]
- 28.Fang L, Ellims AH, Beale AL, Taylor AJ, Murphy A, Dart AM. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res. 2017;9(11):5063–5073. [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena M, Srivastava N, Banerjee M. Cytokine gene variants as predictors of type 2 diabetes mellitus. Curr Diabetes Rev. 2018;14(3):307–319. doi: 10.2174/1573399813666170112145429. [DOI] [PubMed] [Google Scholar]
- 30.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, Vandenbroucke JP. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349(9046):170–713. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 31.Johnson WD, Brashear MM, Gupta AK, Rood JC, Ryan DH. Incremental weight loss improves cardiometabolic risk in extremely obese adults. Am J Med. 2011;124(10):931–938. doi: 10.1016/j.amjmed.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Babbitt DM, Diaz KM, Feairheller DL, Sturgeon KM, Perkins AM, Veerabhadrappa P, Williamson ST, Kretzschmar J, Ling C, Lee H, Grimm H, Thakkar SR, Crabbe DL, Kashem MA, Brown MD. Endothelial activation microparticles and inflammation status improve with exercise training in african americans. Int J Hypertens. 2013;2013:538017. doi: 10.1155/2013/538017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Julia W, Karen C, Javier R, Ascension M. Role of physical activity on immune function Physical activity, exercise and low-grade systemic inflammation. Proceedings of the Nutrition Society. 2010;69:400–406. doi: 10.1017/S0029665110001928. [DOI] [PubMed] [Google Scholar]
- 34.McGavock JM, Mandic S, Vonder Muhll I. Low cardiorespiratory fitness is associated with elevated C-reactive protein levels in women with type 2 diabetes. Diabetes Care. 2004;27(2):320–325. doi: 10.2337/diacare.27.2.320. [DOI] [PubMed] [Google Scholar]
- 35.Aronson D, Sheikh-Ahmad M, Avizohar O, Kerner A, Sella R, Bartha P, Markiewicz W, Levy Y, Brook GJ. C-reactive protein is inversely related to physical fitness in middle-aged subjects. Atherosclerosis. 2004;176(1):173–179. doi: 10.1016/j.atherosclerosis.2004.04.025. [DOI] [PubMed] [Google Scholar]


