Abstract
Plants of Eruca sativa Mill. (Brassicaceae) from desert and Mediterranean populations in Israel differ in flower color and size. In the desert habitat, the population has higher abundance of flowers with cream color and longer petals, whereas in the Mediterranean habitat, the population has higher abundance of flowers with yellow and shorter petals. Choice experiments with honey bee foragers (Apis mellifera Linn., Apidae, Hymenoptera), the main pollinator in the natural habitat in Israel, confirmed that they are more attracted to the yellow flower morph than to the cream one. A proboscis extension response test indicated that honey bees are able to discriminate between flower scents of the desert and Mediterranean populations. Considering the advantage of plants of the yellow morph in attracting pollinators, we further tested in a common garden experiment whether these possess higher fitness than plants of the desert population. Indeed, a significant association was found between flower color and fruit set, and seed mass. In general, our results provide evidence for ecotypic differentiation between populations imposed by pollinators. The advantage of the yellow color morph in attracting pollinators may explain its dominance among plants of the Mediterranean population. We discuss why the cream color morph may be dominant in the desert habitat, considering the possibility of different pollinators, tradeoffs between traits, or pleiotropy.
Keywords: fitness, flower morph, foraging experiment, proboscis extension response test
In plants that rely on insect pollination, preference and visitation behavior of pollinators is affected by the rewards that the flowers offer, and on floral display features such as their shape, size, color, and scent. It is generally thought that the ability to attract a broad range of potential pollinators provides an advantage over species-specific advertisement (Galen 1999, Glover 2007). Based on this hypothesis it is also expected that species that are dependent on generalist pollinators will possess higher intraspecific variation in floral characteristics as compared to species that depend on specialization (Herrera et al. 2008).
Natural phenotypic variation in floral traits in several Brassicaceae species has been used to understand the preference of generalist pollinators to different shapes, sizes, colors, UV patterning, and odors (Kay 1976; Stanton 1987a,b; Conner and Rush 1996; Lee and Snow 1998; Irwin and Strauss 2005; Majetic et al. 2009; Gomez and Perfectti 2010; Norton et al. 2015; Brock et al. 2016). Providing evidence for the adaptive value of the traits in an ecological context, only few of these studies have coupled trait variation with differences in plant reproduction success (Stanton 1987b, Irwin and Strauss 2005, Majetic et al. 2009, Norton et al. 2015). Based on the pollination syndrome concept, phenotypic differentiation in floral attraction traits should be the outcome of selection by pollinators (Raguso 2009, Parachnowitsch and Kessler 2010). But, such variation cannot always be associated with adaptive differentiation. For example, a study on seven populations of Brassica cretica in Crete showed that polymorphism in flower color is not associated with adaptive differentiation among the geographically isolated populations, but the result of genetic drift (Edh et al. 2007).
Eruca sativa (Brassicaceae) is a self-incompatible winter annual species pollinated by insects. We previously described phenotypic variation among natural populations of E. sativa along a climatic gradient in Israel (Barazani et al. 2012, Westberg et al. 2013, Ogran et al. 2016). Qualitative assessment over a few years of observations suggested that the abundance of flowers with yellow petals is higher in populations originating from Mediterranean habitats than in these from the desert, where we observed high abundance of flowers with cream petals (Supp. Fig. S1). We, therefore, hypothesized that in Mediterranean populations the yellow color morph confers a greater advantage than the cream morph. Based on this hypothesis, we investigated the relationship between variation in floral traits and preference of honey bees (Apis mellifera), one of the most common pollinators in the Mediterranean habitats of Israel (as in many other natural ecosystems; Hung et al. 2018). We tested the prediction that such floral color variation is associated with differences in the attraction of honey bees. But considering the myopic vision of bees, which constrains their ability to discriminate colors from a long-distance (Chittka and Raine 2006, Dotterl and Vereecken 2010), we tested also whether floral attraction may be associated with olfactory cues. Our choice experiments were designed to test initial preference of honey bees to focal floral traits, excluding the effect of potential differences between morphs in floral traits associated with gains and costs (e.g., reward). Upon finding that honey bees preferred the yellow morph in binary choice experiments, we tested whether the yellow morph was also preferred when rare and surrounded by many cream morph flowers, as would be typical in the desert habitat. Finally, we tested whether plants of the yellow petal morph had higher fitness than plants of the cream petal morph in a common garden experiment in Mediterranean climate.
Materials and Methods
Plant Material and Growth Conditions
Seeds of two populations, representing desert (32°04′49″N, 35° 29′ 46″E, ≤200 mm annual rainfall) and Mediterranean (32°46′39″N, 35°39′29″E, ≥430 mm annual rainfall) habitats were used for the experiments below. To reduce maternal effects, seeds for each population were generated following one generation of random mating by bumble bees in their respective common garden (Barazani et al. 2012). Seeds were germinated on moistened Whatman No. 1 filter paper in 9-cm Petri dishes in a growth chamber at 25°C with a 8:16 (L:D) h photoperiod. Seedlings (4-d-old) were transferred to germination trays and placed in a climate controlled greenhouse with 8:16 (L:D) h photoperiod and 22/16°C day/night temperatures. Two weeks later, plants were transferred to 1-liter pots and grown in climate controlled greenhouse until flowering for foraging behavior experiments, or in an open net-house for assessment of plant performance (below). All experiments were conducted in a Mediterranean climate.
Honey Bee Foraging Behavior Experiments
Three foraging experiments were conducted to assess the preference of honey bees to different flower morphs of E. sativa. Experiments were conducted in a screened enclosure (950 × 800 × 400 cm). A colony (six-frame nucleus) was placed in the enclosure 3–4 d prior to the behavioral tests; during this time older foragers tend to be replaced by younger, inexperienced bees.
In the first experiment, two plants from each of the two populations were randomly selected. We cut inflorescence stalks, ca. 11 cm in length, each with the four youngest flowers, and placed them in 20 ml glass flasks containing tap water. Two stalks for each population were placed in alternating order on a rotary green round plate (41 cm in diameter), with a distance of 16 cm between the stalks. The plate rotated slowly (2 rpm) throughout the experiment to prevent location bias. The honey bee hive was at a distance of 3 m from the plate. As a bee approached the flowers, her choice was recorded, and the bee was caught and removed, to exclude the possibility of dance recruitment. The stalks were then replaced by others, representing a total of four other different plants (herein referred to as trial). The experiment included a total of 86 trials, i.e., a total of 172 plants for each population.
Another similar experiment (experiment II) was performed, in which plants of each of the two populations were selected on the basis of their visible flower color. Accordingly, each of a total of 72 trials included four flower stalks, two with visible cream color and two of yellow color, of the desert and Mediterranean populations, respectively. The experiments were performed in 17 continuous days between 09:00 to 16:00 hours.
Based on the results of the two foraging experiments, showing initial preference of honey bees to yellow color morph, we designed a third experiment in which we tested whether yellow color would possess an ecological advantage also in the desert population, where this morph is less abundant (Supp. Fig. S1). In this experiment, 10 inflorescence stalks from three plants with flowers with low saturation values (i.e., cream color, below), each including four flowers, were set in 20 ml flasks and placed at equal distances at the edge of the green rotating plate, herein referred as periphery flowers (PF). An additional flask was placed in the center of the plate, in which we replaced stalks between trials in alternating order, a stalk of a plant with flowers of high saturation value with one of a low saturation value; we will herein refer to these as target central flowers (CF). Significant differences were found in the average saturation values (as described below) between cream PF (mean + SE: 0.154 ± 0.004), and the yellow CF (0.274 ± 0.008) (one-way analysis of variance [ANOVA], F2,171 = 206.65, P < 0.001). Each trial ended when a single honey bee foraged on a CF, or after 1 h, if she did not visit the target flower. The honey bee was then caught and removed to exclude the possibility of dance recruitment. In each trial, we monitored the number of PF that were visited before visiting the CF and the time it took to reach it. The experiment was performed in eight continuous days between 09:00 to 16:00 hours and included a total of 98 trials, 49 for each CF of the yellow and cream color.
Due to the limited forage available in the enclosure in all three experiments, only few foragers were active, and there was never more than one bee visiting the flowers at any one time. Before each trial, one flower of each plant that was included in the three sets of experiments was cut and the scanned image was used for measurement of petal size and its saturation value, as described below; the petal’s UV reflectance was also measured in the flowers of the second and third experiments.
Proboscis Extension Response Conditioning Experiment
The proboscis extension response (PER) experiment was set as previously described (Shafir et al. 1999, Afik et al. 2006, Matsumoto et al. 2012) to test the ability of bees to discriminate between odors released from flowers (ODF) of plants of the Mediterranean and desert populations (conditioned stimuli). Inflorescence stalks with four to six flowers (but always an equal number between the two populations during each trial) were cut from four random plants of each of the two populations, and the tip of each stalk was placed in a 0.5-ml Eppendorf tube containing water. Four tubes containing the flowers of four plants of one population were placed in a 150-ml Erlenmeyer flask. Another flask contained the flowers of four plants of the other population. An air pump blew air at 826 ml/min via silicon tubes through the flask, and ODF was delivered to the honey bees, avoiding any visual cue of the flowers. In the first experiment, bees had to discriminate between a conditioned stimulus (CS) of 5-s exposure to the ODF of one of the two populations versus to clean air. The floral stimuli (CS+) were associated with a positive unconditioned stimulus (US+, 40% sucrose solution), whereas the air stimulus (CS−) was associated with a negative unconditioned stimulus (US−, 2M NaCl solution). The US were delivered by a Gilmont microsyringe following the 5-s exposure to the CS. Bees imbibed the sucrose solution (0.4 microliters), but the salt solution was only touched to the antennae, without allowing the bees to imbibe it. This experiment was repeated twice, with 24 bees each time, for a total of 48 bees. Each bee was tested in 12 trials, six with each of the two conditioned stimuli, CS+ and CS− (A and B, respectively), in pseudorandom order (ABBABAABABBA). During each trial, we noted whether the bee extended its proboscis after onset of CS, but prior to delivery of US.
In a second experiment, using a new set of bees, we tested whether honey bees could discriminate between floral odors of the two populations. The set up was the same as the first experiment but here every bee was exposed to the ODF of both populations, one as CS+ and one as CS−. The experiment was repeated six times, with 24 bees each time, for a total of 144 bees. In each replicate, for 12 bees the ODF of the desert population was CS+ and the ODF of the Mediterranean population was the CS−, and for the other 12 bees the ODF of the Mediterranean population was CS+ and the ODF of the desert population was the CS−.
In a third experiment, as an assessment of whether bees can simply discriminate between two groups of flowers from the same population due to plant individual differences, and not due to differences between the populations, we tested whether honey bees could discriminate between two groups of ODFs from the same population. The set up was as in the other experiments. Half the bees were exposed to two groups of ODFs of the desert population, one group as CS+ and the other as CS−. The other half of the bees were similarly exposed to two groups of ODFs of the Mediterranean population, one as CS+ and the other as CS−. The experiment was repeated six times, with 24 bees each time, for a total of 144 bees. Of a total of 336 bees tested in the three experiments, 13 escaped or died during the experiments and their partial data were excluded from analyses.
Flower Morphology
One petal of each cut flower (above) was scanned with a digital scanner; the scanned images were used to measure petal dimension (its length and width) with Digital Earth Watch (DEW, http://www.globalsystemsscience.org/software). We also wrote a MatLab (MathWorks, Natick, MA) program, using the image analysis toolbox, to analyze the scanned petal images in HIS (hue, intensity, saturation) color space. Hue is a color attribute (in arbitrary HIS color space), saturation represents how strong that color is (with higher values indicating a stronger color, so yellow was more saturated than cream), and intensity represents how light is the color (with higher values meaning a lighter color, so cream was lighter than yellow). Based on the comparison between qualitative assessment of flower color (using Munsell’s charts, below) and quantitative (Matlab) evaluations (data not shown), we used the saturation values for assessment of yellow to cream flower color. In addition, we used an AVASpec-2048 fiber-optic spectrometer (Avantes Starline, the Netherlands) to measure reflectance (200 to 1,100 nm) from petals of additional freshly picked flowers. The saturation value and petal size were used to associate flower traits to honey bee foraging preference (above); the assessment of flower color with Munsell charts was used as a convenient method to evaluate flower color in the common garden experiment (below).
Evaluation of Plant Performance
The experiment was conducted in an open net-house (to allow visitation by pollinators) at the Agricultural Research Organization experimental field site (32° 46′ 39″ N, 35° 39′ 28″ E, ca. 50 m above sea level) between January and March, with average max/min temperatures of 20/14°C. Plant performance was evaluated from rosette vegetative stage until the reproductive stage and fruit maturation. The experiment included 32 plants of each population arranged in a randomized order. We first recorded the flowering date, and with the use of the Munsell charts, flower color of each individual plant was categorized to one of five color groups on a scale of 1 to 5, in a range of cream to yellow petals, respectively.
At the date of flowering, 16 plants of each of the desert and Mediterranean populations were harvested and the dry plant biomass was determined 2 d after drying at 70°C. The remaining plants continued to grow and after flowering ended (3 mo after germination) irrigation was stopped and the number of fruits per plant was determined. Fruits were harvested after full maturation, and seeds mass and number of seeds per fruit were evaluated to represent female reproductive success.
Data Analysis
Data were analyzed with JMP software v. 12.0.1 (SAS Institute, Cary, NC). Analyses of variance (ANOVA) were used to test phenotypic differences between plants of the two populations, and χ2 goodness of fit test was used to examine the preference of honey bees between the flower morphs. Linear regression analysis was used to assess the relations between morphological and plant performance traits. Post hoc ANOVA and Student’s t-tests (with degrees of freedom estimated without assuming equal variances) were used to assess the differences between data sets, and multivariate analysis and pairwise Pearson’s correlations were used to evaluate the association between phenotypic and fitness traits. Olfactory discrimination in PER experiments was assessed by calculating for each bee a discrimination index (deltaPE), consisting of the difference between the sum of responses during the last three trials to the CS+ and to the CS−, when learning curves reach asymptotic values, e.g., Shafir and Yehonatan 2014. A Wilcoxon signed rank test was applied to compare the deltaPE to a hypothesized value of zero.
Results
Preference of Honey Bees to Plants of the Two Populations of E. sativa: Foraging Experiment I
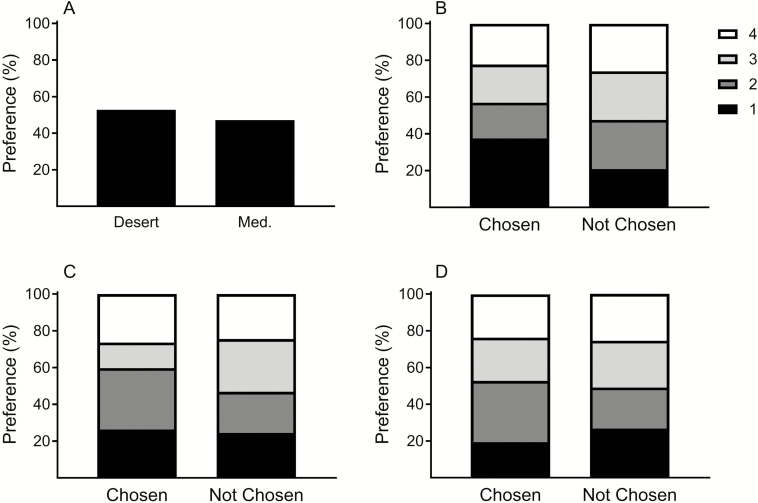
In the first experiment, bees foraged on flowers in 72 out of 86 trials. In 34 of the 72 trials, the initial choice was a flower of the Mediterranean population, and in 38 trials the initial foraging choice was a flower of the desert population (Fig. 1). Thus, no differences were found in the preference to one population over the other (χ2 test, P > 0.05).
Fig. 1.
Choice of honey bees to flowers of the two population of E. sativa (A), and based on the level of saturation (B), petal length (C), and width (D). The results describe the percentage of visits out of a total number of trials (n = 72) in which the bee visited flowers ranked at a decreasing order of high (1) to low (4) saturation value or petal size.
Petals of the desert population were longer than those of the Mediterranean population (P < 0.001), but those of the Mediterranean population were wider (P = 0.01; Table 1). Petals of the Mediterranean population had slightly greater mean saturation value (more yellow; 0.236) than those of the desert population (0.234), but the differences were not statistically significant (Table 1). Linear regression analyses showed significant correlation between petal length and width (R2 = 0.53, F1,286 = 138.22, P < 0.001), but not between flower color and its size, in neither petal length (R2 = 0.01, F1,286 = 2.92, P = 0.08) nor width (R2 < 0.01, F1,286 = 0.04, P = 0.38).
Table 1.
Floral attraction traits (mean ± SE) in populations of E. sativa, and the results of Student’s t-tests for differences between populations
| Color | Petal length (cm) | Petal width (cm) | |
|---|---|---|---|
| Desert | 0.234 ± 0.002 | 1.44 ± 0.012 | 0.85 ± 0.009 |
| Mediterranean | 0.236 ± 0.003 | 1.38 ± 0.013 | 0.88 ± 0.009 |
| t | 0.61 | 3.69 | 2.68 |
| df | 269.9 | 284.2 | 285.9 |
| P | 0.545 | <0.001 | 0.01 |
The petal scanned image was used to measure its saturation value and size.
Since plants of the two populations were randomly selected, regardless of visible or measured flower color, and variation existed in color across flowers within a population (Supp. Fig. S1B), we further analyzed the association between morphological features of the flowers and preference of honey bees in each of the trials, i.e., the preference to petal color or size. In each of 72 trials, a bee had a choice between flowers from four different plants, and we had a measurement of saturation, petal length, and width from a flower from each of these plants. We, therefore, ranked the flowers in each trial separately for each of the three measures and compared the rank distributions of the chosen and non-chosen flowers (Fig. 1). The flower with the highest saturation value was chosen in 37.5% of trials, which is a significantly greater percentage than the null hypothesis of 25% (Likelihood ratio = 5.49, n = 72, P = 0.0192). Overall, bees tended to choose flowers with higher saturation values ( = 7.74, n = 288, P = 0.052) and longer petals ( = 8.02, n = 288, P = 0.046), but regardless of petal width ( = 3.9, n = 288, P = 0.272).
Preference of Honey Bees to Cream and Yellow Flower Morphs of E. sativa: Foraging Experiment II
To determine whether bees preferred yellow flower plants over cream ones, another experiment was conducted, this time plants of the two populations were selected on the basis of their visible color (i.e., yellow and cream color; Supp. Fig. S1). The mean saturation value of plants of visible yellow color flowers was twice as high as that of cream color (P < 0.0001), and flowers of cream color possessed higher UV reflectance (209.4% reflectance) than the yellow color morph (155.0% reflectance; P < 0.0001) (Table 2). Significant differences (P < 0.0001) between flowers of cream and yellow color were also observed in petal length (1.54 and 1.34 cm, respectively) and width (0.87 and 0.81 cm, respectively). Pairwise correlations revealed significant negative or positive correlations between pairs of morphological traits, except between UV reflectance and petal width (Supp. Table 1).
Table 2.
Floral attraction traits (mean ± SE) in plants of a cream and yellow flower morph of E. sativa used in bee foraging experiment II, and the results of Student’s t-tests for differences between populations
| Morph | Color | Petal length (cm) | Petal width (cm) | UV reflectance |
|---|---|---|---|---|
| Cream | 0.150 ± 0.002 | 1.54 ± 0.016 | 0.87 ± 0.009 | 209.4 ± 9.50 |
| Yellow | 0.297 ± 0.004 | 1.34 ± 0.012 | 0.81 ± 0.009 | 155.0 ± 8.76 |
| t | 34.8 | 9.87 | 4.93 | 4.21 |
| df | 169.7 | 208.3 | 221.5 | 218.3 |
| P | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
The petal scanned image was used to measure its saturation value and size; UV reflectance was measured with the AVASpec-2048 fiber-optic spectrophotometer.
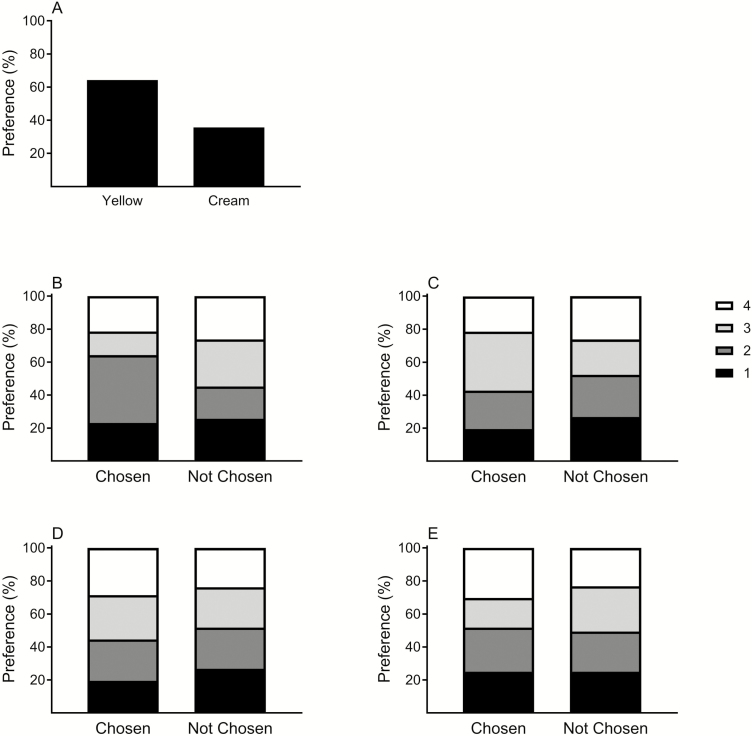
Honey bees foraged on flowers in 56 out of the total 72 trials. The percent of trials in which honey bees foraged on flowers of the yellow morph (64.3%) was significantly higher than those in which they foraged on flowers of the cream morph ( = 4.64, P = 0.0313) (Fig. 2A). The results indicated that bees tended to visit flowers with high saturation value ( = 11.28, n = 224, P = 0.0103) but regardless of UV reflectance ( = 4.56, n = 224, P = 0.2067), petal length ( = 1.37, n = 224, P = 0.7132) and width ( = 2.56, n = 224, P = 0.4655) (Fig. 2B–E).
Fig. 2.
Choice of honey bees in experiment II to flowers of E. sativa with visible yellow and cream color (A), and based on their level of saturation (B), UV reflectance (C), petal length (D), and width (E). The results describe the % number of visits out of a total number of trials (n = 56) in which the bee visited flowers ranked at a decreasing order from high (1) to low (4) values.
Preference of Honey Bees to Plants of Yellow or Cream Visible Color When Surrounded by Cream Color Plants: Foraging Experiment III
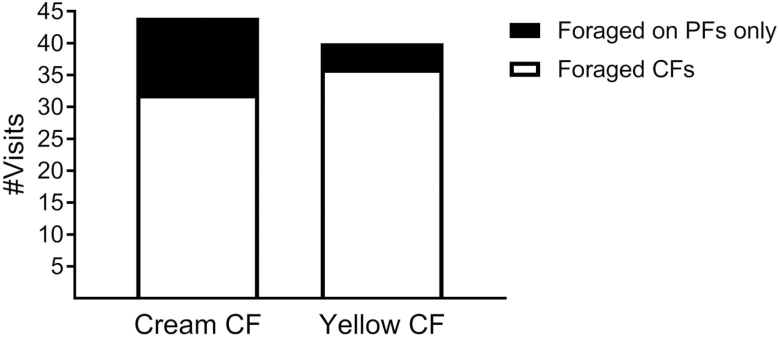
The third experiment aimed to test whether the yellow flower morph still maintains its advantage when surrounded by many cream flower morphs, typical of the desert habitat. The results showed that honey bees foraged on flowers (either PF or CF) in 84 of the total 98 trials. In 16 of the 84 trials, they visited only the PF stalks; 12 of these when the CF was of the cream morph, and only four when the CF was of the yellow morph ( = 4.19, P < 0.05; Fig. 3). Bees were more likely to visit the CF after visiting PF when the CF was of the yellow morph (36 out of 40 trials) rather than the cream morph (32 out of 44 trials) ( = 98.27, P < 0.001) (Fig. 3 and Table 3). In addition, bees visited a higher number of PF before foraging on the CF when the CF was of cream color in comparison to when it was yellow (means of 9.6 and 5.4, respectively; one-way ANOVA, F1,66 = 4.14, P < 0.05) (Table 3). Furthermore, it took bees twice as long until they first visited a cream CF (mean of 18 min) than a yellow CF (mean of 10 min) (ANOVA, F1,66 = 6.31, P ≤ 0.01).
Fig. 3.
Choice of honey bees in experiment III to CF stalks of E. sativa with visible yellow or cream color surrounded with PF of cream color. The results present the number of visitation on PF only in comparison to trials in which bees visited CFs.
Table 3.
Preference of honey bees to flowers of yellow or cream morphs (foraged CFs) in intraspecific competitive interactions
| N | Foraged CFs | No. of PF | Time (min) | Saturationa | ||
|---|---|---|---|---|---|---|
| No. | % | |||||
| Yellow CF | 40 | 36 | 90.0a | 5.4 ± 1.1a | 9.9 ± 1.6a | 0.274 ± 0.008a |
| Cream CF | 44 | 32 | 72.7b | 9.6 ± 1.8b | 18.4 ± 3.1b | 0.145 ± 0.006b |
The results present the total number of trials (N) with yellow or cream morph as CF, the number and percent of trials a bee foraged on a CF of each color, number of PF visited before visiting the CF, time till visiting the CF, and the mean flower saturation value. Also presented are SE. Different letters represent statistically different values within each column (detailed statistics are presented in the text).
aThe mean value was calculated for a total 40 yellow or 44 cream CFs separately.
PER Conditioning Experiment
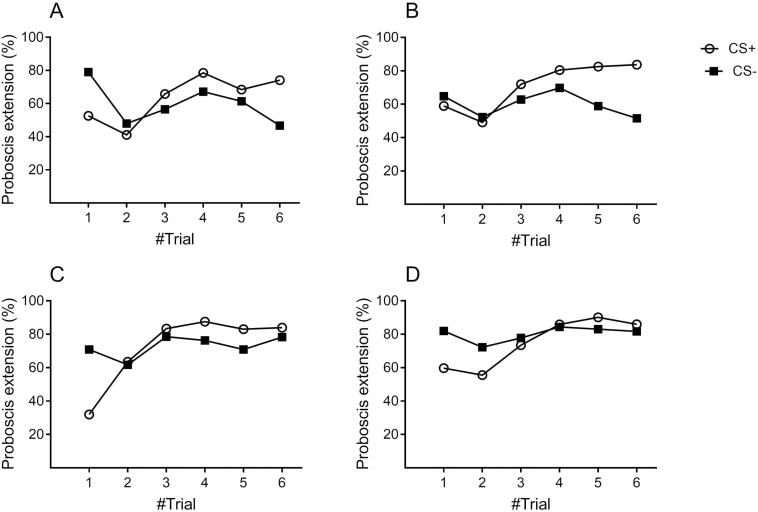
The high percentage of PER of honey bees to exposure to either ODF of the desert or Mediterranean populations (CS+), and not to exposure to clean air (CS−), shows that bees could easily detect the ODF of both populations (Supp. Fig. S2). The results also showed a learning curve to the CS, i.e., increase in PER associated with positive reward. In the follow-up experiments, we tested PER after alternative exposure to ODFs of the two populations, one as CS+ and the other as CS−. Percent PER was greater for ODF of the desert population associated with US+ than for that of the Mediterranean with US− (Wilcoxon signed ranked test: W = 460.5, df = 66, P = 0.0019) (Fig. 4A). Similarly, percent PER was greater for ODF of the Mediterranean population associated with US+ than for that of the desert population with US− (W = 789.5, df = 68, P < 0.0001) (Fig. 4B). Discrimination between ODFs of plant groups from a single population was more difficult; bees were still able to discriminate between two plant groups from the desert population (W = 415, df = 68, P = 0.0027, Fig. 4C), but were unable to discriminate between two plant groups from the Mediterranean population (W = 223, df = 70, P = 0.0996, Fig. 4D).
Fig. 4.
The percent of honey bees that extended their proboscis in a PER conditioning experiment, in response to exposure to odors of flowers (ODF) of the desert and Mediterranean populations that were positively (CS+) or negatively (CS−) associated with sucrose or NaCl solutions, respectively: (A) CS+ and CS− are ODF of the desert and Mediterranean populations, respectively; (B) CS+ and CS− are ODF of the Mediterranean and desert populations, respectively; (C) CS+ and CS− are ODF of two different flowers of the desert population. (D) CS+ and CS− are ODF of two different flowers of the Mediterranean population.
Evaluation of Plant Performance and Fitness in Populations of E. sativa
Plants of the desert population flowered 6.5 d earlier than plants of the Mediterranean population (Student’s t-test, P < 0.001), consequently, plants of the latter gained more vegetative biomass in comparison to plants of the desert population (5.1 and 3.8 g DW, respectively), but this difference was not statistically significant (Table 4). Significant differences between plants of the two populations were found in the average values of visible petal color, as assessed by Munsell color charts (Table 4, P < 0.001). No differences were found between plants of the desert and Mediterranean populations in life time number of fruits, the average total seed weight per plant and the average number of seeds per fruit (Table 4).
Table 4.
Phenotypic evaluation (mean ± SE) of plants of the desert and Mediterranean populations, and post hoc Student’s t-test (df = 30) comparisons between populations
| Flowering (d from sowing) | Biomass (g DW) | Petal colora | No. of fruit set | Total seeds (mg) | No. of seeds/fruit | |
|---|---|---|---|---|---|---|
| Desert | 57.4 ± 0.8 | 3.8 ± 0.6 | 2.1 ± 0.2 | 65.6 ± 6.6 | 696.5 ± 61.7 | 9.9 ± 0.7 |
| Mediterranean | 63.9 ± 0.8 | 5.1 ± 0.9 | 3.4 ± 0.2 | 87.5 ± 7.2 | 673.5 ± 61.9 | 8.3 ± 0.5 |
| t | 3.73 | 1.22 | 1.22 | 1.75 | −0.26 | −1.89 |
| P | ≤0.001 | 0.23 | ≤0.0001 | 0.09 | 0.79 | 0.07 |
aPetal color was determined by visible observation using Munsell color chart.
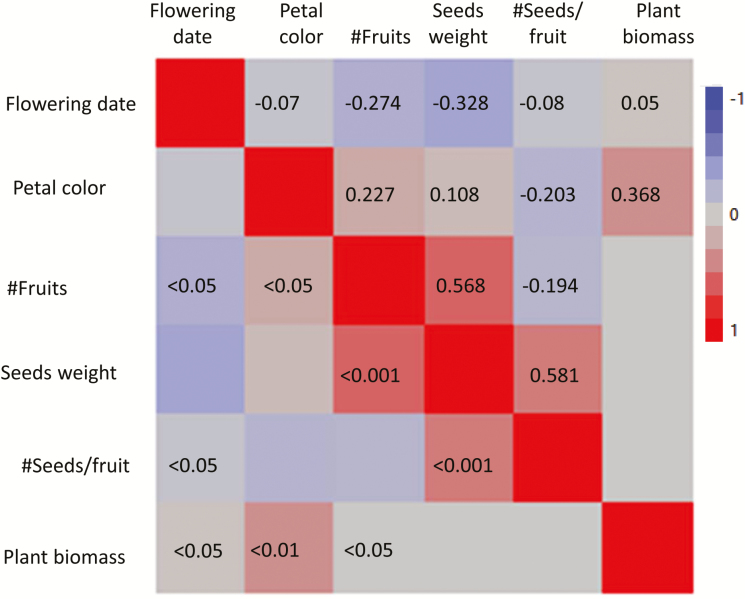
Multivariate analysis and pairwise correlations indicated a significant and relatively high positive correlation between the number of fruits and the total seed mass per plant (n = 33, R = 0.568, P < 0.001) and between the number of seeds per fruit and seed mass (n = 33, R = 0.581, P < 0.001) (Fig. 5). A significant positive correlation was also found between petal color (the more yellow the higher the color value) and plant biomass (n = 32, R = 0.368 P < 0.01), and between petal color and the number of fruits per plant (n = 33, R2 = 0.227, P < 0.05).
Fig. 5.
A color map of pairwise correlations between phenotypic traits: Values above diagonal present the R values, P values are given below diagonal only for significant correlations.
Discussion
Based on the differences between the two habitats of E. sativa (Supp. Fig. S1), and variation that exists in flower color and corolla size (Table 1 and Supp. Fig. S1), we here tested the hypothesis that in the heterogeneous Mediterranean plant community, plants of the yellow color morph possess an ecological advantage over those of cream color in the attraction of pollinators. Indeed, in a choice experiment with inexperienced honey bees, which included random selection of flower stalks of each of the tested populations (foraging experiment I), significant effect of the petal saturation value was found for foraging preference, but not for petal size (width and length) (Fig. 1). Similarly, no association was found in Raphanus raphanistrum (Brassicaceae) between flower size and pollination behavior of small bees (Conner and Rush 1996), and in R. sativus, strong association was found between preference of honey bees and flower color (Stanton 1987a). Note that in our foraging preference experiment, honey bees did not choose flowers of plants of the Mediterranean population over the desert ones (Fig. 1A). Considering the variation in saturation values among plants of the desert population (Supp. Fig. S1B) and that plants were randomly selected, these results were not surprising. The results of foraging experiments II and III, in which flowers were selected based on their visible color and saturation value, further supported the advantage of the yellow flower morph in the attraction of honey bees (Figs. 2 and 3; Tables 2 and 3). In addition, the third foraging experiment showed that a single honey bee spent on average significantly less time and visited fewer PF when the target flower (CF stalk) was a yellow morph, rather than a cream morph (Table 3; Fig. 3). We found significant negative correlation between saturation and UV reflectance values (Supp. Table 1), and accordingly, a marginally significant correlation between preference of honey bees and the flowers UV reflectance (Fig. 2C). Thus, bees generally preferred yellow morphs of low UV reflectance, showing that visitation behavior of honey bees can be related to conspecific variation in flower color. Floral spectral characteristics may be influenced by ambient conditions and vary between habitats that vary greatly in climate and altitude (Nordstrom et al. 2017). Our experiments were conducted under conditions resembling more the Mediterranean than the desert habitat; future work will need to address potential habitat effects on floral spectral characteristics of E. sativa.
When considering the low resolution of the bee eye, minor differences in flower color do not always result in complete constancy or discrimination, especially against a green background (Chittka et al. 2001, Spaethe et al. 2001), as might be the case for variation in colors in E. sativa (Supp. Fig. S1A). But, bees are able to distinguish small quantitative variations in flower scent (Wright et al. 2005, Wright and Schiestl 2009), and utilize olfactory cues emitted from flowers for long-distance attraction, especially when foraging in environments of dense vegetation (Dotterl and Vereecken 2010). Accordingly, we further hypothesized that preference of honey bees to the yellow morph in the foraging choice experiments, might be due to association between color and olfactory cues. The PER experimental system was therefore used to neutralize the visual cues, and test whether honey bees differentiate between odors emitted from flowers of the two investigated populations. The PER results indicated that honey bees significantly discriminated between odors of flowers of the two populations (Fig. 4A and B). The pattern of lack of discrimination in the early conditioning trials, and increasing discrimination in later trials, is typical for discrimination between odors that are similar one to the other; bees tend to generalize initially between the odors until they learn to discern the differences between them (Paldi et al. 2003). By the last trial, bees discriminated better between floral odors of the two populations than between two groups of plants from within either population (Fig. 4). This supports the hypothesis that variation between populations is greater than within populations. Such a pattern has also been recently found in Lithophragma (Saxifragaceae) (Friberg et al. 2019). In fact, bees were unable to discriminate between two groups of plants in the Mediterranean population (Fig. 4D). However, they were able to statistically significantly discriminate between two groups of plants in the desert population (Fig. 4C). This may possibly reflect greater variability in floral bouquet in the desert than in the Mediterranean population, which can be coupled with variation in flower color between plants of the two populations (above). Our results thus provide evidence that honey bees are able to discriminate between scents of flowers of the two populations of E. sativa. Similarly, in Hesperis matronalis (Brassicaceae), association was found between intraspecific variation in flower color and polymorphism in volatile compounds, which was shown to affect pollinator’s visitation behavior (Majetic et al. 2007).
A significant association that was found between petal color and plant biomass, and between petal color and the number of fruit set (Fig. 5) supports our hypothesis that the yellow petal morph possesses higher reproductive fitness in a Mediterranean habitat. However, no differences were found between plants of the two investigated populations in total fruit and seed production, and in the number of seeds per fruit (Table 4). Plants of the desert population flowered significantly earlier (6.5 d) and produced 1.3-fold less vegetative biomass than those of the Mediterranean population (Table 4). It is, therefore, possible that under the current net-house favorable experimental conditions of continuous irrigation, flowers of the desert population gained reproductive success by an early and prolonged flowering period. Earlier flowering in plants of the desert population is consistent with the hypothesis of the adaptive value of early reproduction in annual species of arid habitats of the southeastern Mediterranean (Aronson et al. 1992, Nevo et al. 2012).
Based on the results of the third foraging experiment (Table 3) it is expected that if pollinators can indeed impose selection, the yellow color morph should be more abundant at the desert site. Thus, the high abundance of plants of cream color petals in the desert habitat (Supp. Fig. 1) might have other adaptive value. Geographical polymorphism in flower color may result through indirect selection due to pleiotropy, where abiotic factors pose selection on traits that confer advantages in different environments, and which are correlated with floral morphology. Such a process is attributed to the floral color polymorphism among populations of Lysimachia arvensis (Primulaceae) from western Europe and Macaronesia (Arista et al. 2013). Similarly, differences in floral traits between desert and Mediterranean populations of E. sativa may be due pleiotropy.
However, the relative weak selection imposed by pollinators on L. arvensis may be due to it being easily self-pollinated (Arista et al. 2013). We expect selection by pollinators to be stronger in a self-incompatible species such as E. sativa, but there may be other biotic selection factors involved. Pollinators can impose directional selection on flowering time, as was shown to be the case in Arabidopsis lyrata (Sandring and Agren 2009). In addition, floral attraction traits (i.e., color and scent) are not always a target for selection by pollinators (e.g., Edh et al. 2007). For example, in Raphanus sativus (Brassicaceae) variation in flower color was attributed to opposing selective pressures of pollinators and herbivores (Strauss et al. 2004), and a relationship was also found between polymorphism in flower color of Petunia hybrida (Solanaceae) and variation in defense against herbivores (Johnson et al. 2008). We previously reported differences between populations of E. sativa in induced defense mechanisms against generalist herbivores, which suggest different adaptations to generalist and specialist herbivores in the Mediterranean and desert populations, respectively (Ogran et al. 2016). Thus, the association between defense mechanisms and flower color may suggest an adaptive tradeoff between the traits in plants of the desert population. In contrast, a literature survey conducted by Parachnowitsch and Kessler (2010) indicated that pollinators exert stronger selection on flower traits than herbivores. Thus, according to the accepted pollination syndrome paradigm, it is also possible that the potential advantage of the two morphs of E. sativa in the attraction of different pollinators may explain their dominance at the two sites.
Supplementary Material
Acknowledgments
This project was funded by the Chief Scientist, Ministry of Agriculture and Rural Development (research grant 261-0924) and the Israel Academy of Sciences (research grant 2037/17).
References Cited
- Afik O., Dag A., and Shafir S.. . 2006. The effect of avocado (Persea americana) nectar composition on its attractiveness to honey bees (Apis mellifera). Apidologie 37: 317–325. [Google Scholar]
- Arista M., Talavera M., Berjano R., and Ortiz P. L.. . 2013. Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. J. Ecol. 101: 1613–1622. [Google Scholar]
- Aronson J., Kigel J., Shmida A., and Klein J.. 1992. Adaptive phenology of desert and Mediterranean populations of annual plants grown with and without water stress. Oecologia. 89: 17–26. [DOI] [PubMed] [Google Scholar]
- Barazani O., Quaye M., Ohali S., Barzilai M., and Kigel J.. . 2012. Photo-thermal regulation of seed germination in natural populations of Eruca sativa Miller (Brassicaceae). J. Arid Environ. 85: 93–96. [Google Scholar]
- Brock M. T., Lucas L. K., Anderson N. A., Rubin M. J., Markelz R. J., Covington M. F., Devisetty U. K., Chapple C., Maloof J. N., and Weinig C.. 2016. Genetic architecture, biochemical underpinnings and ecological impact of floral UV patterning. Mol. Ecol. 25: 1122–1140. [DOI] [PubMed] [Google Scholar]
- Chittka L., and Raine N. E.. . 2006. Recognition of flowers by pollinators. Curr. Opin. Plant Biol. 9: 428–435. [DOI] [PubMed] [Google Scholar]
- Chittka L., Spaethe J., Schmidt A., and Hickelsberger A.. . 2001. Adaptation, constraint, and chance in the evolution of flower color and pollinator color vision, pp. 106–126. InChittka L. and Thomsons J. D. (eds.), Cognitive ecology of pollination animal behavior and floral evolution. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Conner J. K., and Rush S.. . 1996. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia. 105: 509–516. [DOI] [PubMed] [Google Scholar]
- Dotterl S. and Vereecken N. J.. . 2010. The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Can. J. Zool. 88: 668–697. [Google Scholar]
- Edh K., Widén B., and Ceplitis A.. 2007. Nuclear and chloroplast microsatellites reveal extreme population differentiation and limited gene flow in the Aegean endemic Brassica cretica (Brassicaceae). Mol. Ecol. 16: 4972–4983. [DOI] [PubMed] [Google Scholar]
- Friberg M., Schwind C., Guimarães P. R., Raguso R. A., and Thompson J. N.. . 2019. Extreme diversification of floral volatiles within and among species of Lithophragma (Saxifragaceae). Proc. Natl. Acad. Sci. U.S.A. 116: 4406–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C. 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience, 49: 631–640. [Google Scholar]
- Glover B. J. 2007. Understanding flowers and flowering, an integrated approach. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Gomez J. M., and Perfectti F.. . 2010. Evolution of complex traits: the case of Erysimum corolla shape. Int. J. Plant Sci. 171: 987–998. [Google Scholar]
- Herrera J., Arista M., and Ortiz P. L.. 2008. Perianth organization and intra-specific floral variability. Plant Biol. (Stuttg). 10: 704–710. [DOI] [PubMed] [Google Scholar]
- Hung K. L. J., Kingston J. M., Albrecht M., Holway D. A., and Kohn J. R.. . 2018. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc B Biol Sci. 285: 20172140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R. E., and Strauss S. Y.. . 2005. Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. Am. Nat. 165: 225–237. [DOI] [PubMed] [Google Scholar]
- Johnson E. T., Berhow M. A., and Dowd P. F.. 2008. Colored and white sectors from star-patterned petunia flowers display differential resistance to corn earworm and cabbage looper larvae. J. Chem. Ecol. 34: 757–765. [DOI] [PubMed] [Google Scholar]
- Kay Q. O. N. 1976. Preferential pollination of yellow-flowered morphs of Raphanus raphanistrum by Pieris and Eristalis spp. Nature. 261: 230–232. [Google Scholar]
- Lee T., and Snow A.. . 1998. Pollinator preferences and the persistence of crop genes in wild radish populations (Raphanus raphanistrum, Brassicaceae). Am. J. Bot. 85: 333. [PubMed] [Google Scholar]
- Majetic C. J., Raguso R. A., Tonsor S. J., and Ashman T. L.. 2007. Flower color-flower scent associations in polymorphic Hesperis matronalis (Brassicaceae). Phytochem. 68: 865–874. [DOI] [PubMed] [Google Scholar]
- Majetic C. J., Raguso R. A., and Ashman T. L.. . 2009. The sweet smell of success: floral scent affects pollinator attraction and seed fitness in Hesperis matronalis. Funct. Ecol. 23: 480–487. [Google Scholar]
- Matsumoto Y., Menzel R., Sandoz J. C., and Giurfa M.. 2012. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J. Neurosci. Methods. 211: 159–167. [DOI] [PubMed] [Google Scholar]
- Nevo E., Fu Y. B., Pavlicek T., Khalifa S., Tavasi M., and Beiles A.. . 2012. Evolution of wild cereals during 28 years of global warming in Israel. Proc. Natl. Acad. Sci. U.S.A. 109: 3412–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom K., Dahlbom J., Pragadheesh V. S., Ghosh S., Olsson A., Dyakova O., Suresh S. K., and Olsson S. B.. . 2017. In situ modeling of multimodal floral cues attracting wild pollinators across environments. Proc. Natl. Acad. Sci. U.S.A. 114: 13218–13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton N. A., Fernando M. T., Herlihy C. R., and Busch J. W.. 2015. Reproductive character displacement shapes a spatially structured petal color polymorphism in Leavenworthia stylosa. Evolution. 69: 1191–1207. [DOI] [PubMed] [Google Scholar]
- Ogran A., Landau N., Hanin N., Levy M., Gafni Y., and Barazani O.. 2016. Intraspecific variation in defense against a generalist lepidopteran herbivore in populations of Eruca sativa (Mill.). Ecol. Evol. 6: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paldi N., Zilber S., and Shafir S.. 2003. Associative olfactory learning of honeybees to differential rewards in multiple contexts–effect of odor component and mixture similarity. J. Chem. Ecol. 29: 2515–2538. [DOI] [PubMed] [Google Scholar]
- Parachnowitsch A. L., and Kessler A.. . 2010. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytol. 188: 393–402. [DOI] [PubMed] [Google Scholar]
- Raguso R. A. 2009. Floral scent in a whole-plant context: moving beyond pollinator attraction. Funct. Ecol. 23: 837–840. [Google Scholar]
- Sandring S., and Agren J.. . 2009. Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution. 63: 1292–1300. [DOI] [PubMed] [Google Scholar]
- Shafir S., and Yehonatan L.. . 2014. Comparative evaluations of reward dimensions in honey bees: evidence from two-alternative forced choice proboscis-extension conditioning. Anim. Cogn. 17: 633–644. [DOI] [PubMed] [Google Scholar]
- Shafir S., Wiegmann D. D., Smith B. H., and Real L. A.. 1999. Risk-sensitive foraging: choice behaviour of honeybees in response to variability in volume of reward. Anim. Behav. 57: 1055–1061. [DOI] [PubMed] [Google Scholar]
- Spaethe J., Tautz J., and Chittka L.. . 2001. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl. Acad. Sci. U.S.A. 98: 3898–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. L. 1987a. Reproductive-biology of petal color variants in wild populations of Raphanus- sativus .1. Pollinator response to color morphs. Am. J. Bot. 74: 178–187. [Google Scholar]
- Stanton M. L. 1987b. Reproductive-biology of petal color variants in wild populations of Raphanus sativus .2. Factors limiting seed production. Am. J. Bot. 74: 188–196. [Google Scholar]
- Strauss S. Y., Irwin R. E., and Lambrix V. M.. . 2004. Optimal defence theory and flower petal colour predict variation in the secondary chemistry of wild radish. J. Ecol. 92: 132–141. [Google Scholar]
- Westberg E., Ohali S., Shevelevich A., Fine P., and Barazani O.. 2013. Environmental effects on molecular and phenotypic variation in populations of Eruca sativa across a steep climatic gradient. Ecol. Evol. 3: 2471–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. A., and Schiestl F. P.. . 2009. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 23: 841–851. [Google Scholar]
- Wright G. A., Lutmerding A., Dudareva N., and Smith B. H.. 2005. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honeybees (Apis mellifera). J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 191: 105–114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.