Abstract
Context
Idiopathic male infertility is evident in half of infertile males. Vitamin D receptors are expressed throughout male reproductive tract, including spermatozoa, promoting motility. Epidemiological studies revealed the positive association between serum vitamin D and semen quality. However, there are no clinical studies examining the differential role of vitamin D in idiopathic male infertility.
Objectives
1) To investigate the association between vitamin D deficiency and idiopathic male infertility, and 2) To determine whether vitamin D deficient males would show restoration of semen quality parameters upon supplementation with vitamin D.
Design
This was a year-long case-control study from November 2015 to November 2016. A therapeutic intervention cohort for 2 months was also performed.
Subjects and Methods
117 Jordanian males were enrolled. Following a clinical evaluation by a urologist, baseline serum vitamin D and semen fluid analyses were collected. Participants were stratified into 3 groups: controls (n=30), idiopathic infertility (n=67), and secondary infertility (n=20). Idiopathic infertility patients with low vitamin D (n= 45) were supplemented with oral vitamin D, 5000 IU, once daily for two months. Thereafter, serum vitamin D and semen fluid analyses were reassessed (n= 34; 11 patients were lost to follow up).
Results
Vitamin D was significantly lower in patients with idiopathic infertility than in both controls and men with secondary infertility. Significant improvement of progressive and total sperm motility was observed after vitamin D treatment. Vitamin D correlated significantly with semen quality in the study population. However, no correlation was found between vitamin D and any of the semen quality parameters in the idiopathic infertility group.
Conclusions
Vitamin D supplementation improves sperm motility in idiopathic male infertility patients with low vitamin D. Larger and longer clinical trials are warranted to validate the use of vitamin D in these cases.
Keywords: vitamin D, male infertility, idiopathic
INTRODUCTION
Infertility is a multi-factorial problem, affecting approximately 15% of couples according to the World Health Organization in 2010 (1). Etiology of infertility is attributed to several factors, including ovulatory, utero-tubal, and semen migration factors. In almost 30% of couples, male reproductive abnormalities are the causative factors (2). Around 40% of all infertile couples exhibit a combination of factors, and about 15% of couples may not display any objective alteration aiding in a definitive diagnosis (3). Idiopathic male infertility, where no demonstrable cause of male infertility can be found, is characterized by history of infertility, unremarkable physical examination, and normal endocrine laboratory evaluation (4), and is evident in at least 44% of infertile men (3). According to the statement of the European Association of Urology in 2015 on the treatment of idiopathic male infertility (5): 1) empirical drugs, such as clomiphene citrate and tamoxifen, have no proven benefit (6), and 2) androgens, human chorionic gonadotrophin, bromocriptine, alpha-blockers, systemic corticosteroids, and magnesium supplementation are not effective in the treatment of idiopathic oligo-astheno-teratozo-ospermia syndrome. Thus, there is an urgent need for more studies to unravel the underlying pathogenic mechanisms, hence providing the proper foundation for new therapeutic targets, in the approach to this group of infertile males. Recent studies have addressed the correlation between male infertility and vitamin D (7-9). The physiological role of vitamin D in male reproduction is dependent on the efficiency of both its genomic (10), triggered by interaction with cytoplasmic receptors, and the rapid non-genomic effects via calcium and chloride channels on plasma membranes of reproductive organs and sperms (11, 12). Vitamin D also increases intracellular calcium concentrations within sperms enhancing their progressive motility (13). Preclinical studies illustrated the effect of vitamin D supplementation on male rats’ infertility, as Uhland et al. reported that vitamin D treatment restored fertility of vitamin D-deficient male rats (14). Furthermore, vitamin D-receptor knockout male mice were found to have decreased sperm concentration (15). Sood et al. had additionally reported that vitamin D3 injections successfully restored fertility in male and female rats previously fed on vitamin D-deficient diet (16). A positive correlation between 25-hydroxy vitamin D concentrations and sperm progressive motility was illustrated, as men with vitamin D deficiency showed a lower percentage of progressively motile and morphologically normal sperms (13). On the other hand, Hammoud et al. illustrated in 2012 the relationship between vitamin D and semen parameters as an inverse U-shaped association, with both low (<20 ng/mL) and high (>50 ng/mL) vitamin D levels corresponded to significantly lower quality of semen parameters compared to those associated with a normal range of vitamin D (17).
Previous clinical studies assessed the role of vitamin D in male fertility, by sampling the general population, and were not specific to any particular group of infertile patients. Furthermore, the effect of vitamin D supplementation on male infertility was tested on animals, but it has not been applied to humans thus far. Accordingly, we aimed in this study to: 1) investigate the association between vitamin D deficiency/insufficiency and idiopathic male-factor infertility, and 2) determine whether vitamin D deficient or insufficient males would show restoration of semen quality parameters upon supplementation with vitamin D.
SUBJECTS AND METHODS
The experimental design, therapeutic intervention, and clinical endpoints of this study were all approved by the Institutional Review Board (IRB) at Jordan University of Science and Technology. Patients attending the urology or assisted fertilization clinics at King Abdullah University Hospital (KAUH) were invited to the study and were formally consented upon enrolment. Participants were eligible for inclusion in the study if they were males of Arab descent, and aged 20 to 45 years old. Arab descent was set as an inclusion criterion to eliminate any confounding factors related to racial differences in the metabolism of vitamin D. Patients with chronic diseases known to interfere with vitamin D absorption, and men who receive vitamin D supplements regularly or have allergy towards vitamin D were excluded.
Baseline Evaluation
Consented participants were first interviewed by a trained research assistant for a thorough clinical evaluation. History included items like demographics (age, occupation), past medical, surgical, and medication history. The attending urologist at KAUH performed a thorough physical examination to report any testicular and/or post-testicular causes of male infertility. Thereafter, peripheral blood samples were obtained from all subjects for measurement of serum vitamin D levels (25(OH) vitamin D). An enzyme linked immunosorbent assay (ELISA) kit (Cobas®, ROCHE) was used, according to the manufacturer’s recommendations. Serum vitamin D levels were classified according to the Food and Nutrition Board criteria (18) as: vitamin D deficient (<12 ng/mL), vitamin D insufficient (12-20 ng/mL), or vitamin D sufficient (>20 ng/mL). Participants were then instructed to provide a semen sample by masturbating after at least 48 hours of sexual abstinence for a baseline semen fluid analysis (SFA).
Stratification of Subjects
Based on the baseline evaluation, participants were stratified into the following three experimental groups, according to a set of inclusion and exclusion criteria for each group:
Normal Controls (n = 30): healthy male volunteers, who showed normal semen quality in baseline SFA, according to the most recent WHO criteria in 2010.
Idiopathic Infertility Group (n = 67): males who were recently diagnosed with male-factor infertility of idiopathic origin, with normal follicular stimulating hormone and testosterone levels within the last 12 months, and who were found to have one or more abnormalities in the baseline SFA despite normal urological exam were included in this group. Based on the WHO criteria in 2010 (1, 19), abnormal semen quality was defined as: sperm concentration < 15 million/mL, total sperm motility < 40%, sperm progressive forward motility < 32%, or normal sperm morphology < 4%. Patients with confirmed diagnosis of germ cell aplasia, total or partly obstructive oligospermia, azoospermia, and those on drug therapy affecting fertile capacity (e.g. chemotherapy, potassium-sparing diuretics, calcium channel blockers), in addition to men who underwent vasectomy procedure were excluded from this group.
Secondary Infertility Group (n =20): males who were recently diagnosed with male-factor infertility of explained origin, namely varicoceles, and underwent a corrective surgery (varicocelectomy) at least 6 months prior to enrollment, however, showed normal semen quality in baseline SFA were included in this group. This group was studied as a second control group for idiopathic infertility cases besides the normal controls, to theoretically strengthen the possible evidence of a cause-effect relationship between idiopathic male infertility and serum vitamin D levels.
Vitamin D Intervention Cohort
Patients in the idiopathic infertility group who were found to have insufficient or deficient baseline vitamin D levels were subsequently treated with vitamin D3 tablets, 5,000 IU, once daily for 2 consecutive months, according to the recommended dosing regimen of the American Board of Family Medicine in 2014 (20). At the end of the intervention period, treated patients were recruited again for repetition of both SFA and serum vitamin D levels.
Statistical Analyses
Comparison of baseline vitamin D levels between the three experimental groups was analyzed using One-way Analysis of Variance (ANOVA) with Dunn’s Multiple Comparison post-hoc test. Nonparametric paired student t test with Wilcoxon matched-pairs signed rank test were used when comparing vitamin D levels and each of the semen quality parameters before and after treatment. Spearman Approximation test was used to examine the effectiveness of pairing of pre-post treatment readings. When testing for correlation between vitamin D levels and semen quality parameters, data were analyzed by Spearman nonparametric correlation test, using 95% confidence interval. Statistical significance was set at p <0.05. Data were analyzed using the GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
A total of 117 subjects participated in the study. Table 1 illustrates the general demographic and clinical characteristics of participants. According to the baseline evaluation, participants were assigned into the following experimental groups: 1- Controls (n= 30); 2- Idiopathic infertility patients (n= 67), who were then classified according to their baseline serum vitamin D results into: (a) low vitamin D (<20 ng/mL, n= 45) and (b) normal vitamin D (>20 ng/mL, n= 22). 3- Secondary infertility patients (n= 20). Idiopathic infertility patients with low baseline serum vitamin D (n= 45) constituted the intervention cohort in the study. Only 34 of those were successfully recruited again after completion of the treatment, as 11 patients were lost to follow up.
Table 1.
Description of demographic and clinical characteristics of study subjects. Values are expressed as means ± standard errors of the mean (SEM)
| Idiopathic | ||||
| Control | Secondary | |||
| Low D | Normal D | |||
| Sample size | n= 30 | n= 45 | n= 22 | n= 20 |
| Age (years) | 26.93 ± 1.277 | 31.93 ± 0.7838 | 28.75 ± 1.405 | |
| Income (<300, 300-600, 600-1000, >1000) | (27%, 53%, 13%, 7%) | (16%, 73%, 7%, 4%) | (32%, 50%, 9%, 9%) | (25%, 55%, 5%, 15%) |
| Smoking (never smoked, smoker, ex-smoker) | (53%, 40%, 7%) | (38%, 51%, 11%) | (41%, 45%, 14%) | (50%, 50%, 0%) |
| Baseline vitamin D level (ng/mL) | 21.27 ± 1.729 | 12.56 ± 0.7800 | 23.55 ± 1.119 | 25.91 ± 1.415 |
| Vitamin D level after treatment (ng/mL) | - | 38.45 ± 1.464 | - | - |
| Baseline sperm concentration (million/mL) | 61.77 ± 7.538 | 20.94 ± 4.270 | 15.58 ± 2.623 | 61.05 ± 8.103 |
| Sperm concentration after treatment (million/mL) | - | 25.15 ± 4.892 | - | - |
| Baseline progressive motility (%) | 48.37 ± 2.330 | 17.80 ± 1.936 | 15.43 ± 2.644 | 45.35 ± 3.089 |
| Progressive motility after treatment (%) | - | 27.94 ± 3.533 | - | - |
| Baseline total motility (%) | 53.50 ± 2.350 | 25.40 ± 2.153 | 21.74 ± 2.874 | 52.85 ± 2.924 |
| Total motility after treatment (%) | - | 35.76 ± 3.611 | - | - |
| Baseline Morphology (%) | 14.63 ± 1.031 | 6.556 ± 0.6494 | 7.091 ± 1.374 | 20.45 ± 2.083 |
| Morphology after treatment (%) | - | 8.176 ± 0.9417 | - | - |
Baseline serum vitamin D
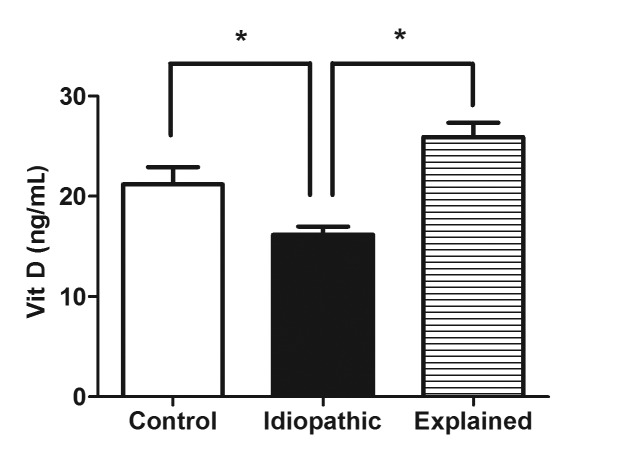
As illustrated in Figure 1, patients with idiopathic male infertility recorded significantly lower serum levels of vitamin D at baseline (mean= 15.89±0.8427), when compared to controls (mean= 21.27±1.729, p <0.0001), as well as patients with secondary male infertility (mean= 25.91±1.415, p <0.0001). Intriguingly, there was no significant difference in baseline vitamin D levels between the control and secondary infertility groups (p >0.05).
Figure 1.
Comparison between serum levels of vitamin D at baseline in the 3 experimental groups; control, idiopathic male infertility, and secondary male infertility. *, p< 0.05 versus idiopathic male infertility.
Analysis of correlation between vitamin D and semen quality parameters
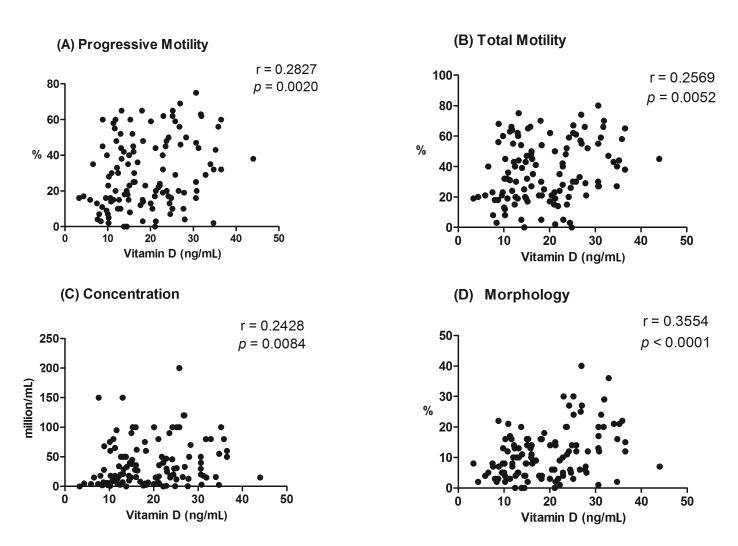
To identify the predictive value of serum vitamin D in male fertile capacity, we ran a set of correlation analyses between each of the semen quality parameters and baseline vitamin D levels in the whole study population (n= 117). All of the tested relationships showed a statistically significant correlation (Fig. 2). Correlation results included: sperm progressive motility (p= 0.0020, r= 0.2827, Fig. 2A), sperm total motility (p= 0.0052, r= 0.2569, Fig. 2B), sperm concentration (p= 0.0084, r= 0.2428, Fig. 2C), and sperm morphology (p= < 0.0001, r= 0.3554, Fig. 2D).
Figure 2.
Correlation between baseline serum levels of vitamin D and semen quality parameters; (A) progressive motility, (B) total motility, (C) concentration, and (D) morphology in all study population (n= 117). r represents Spearman correlation coefficient.
Changes in semen quality parameters after treatment in the intervention cohort
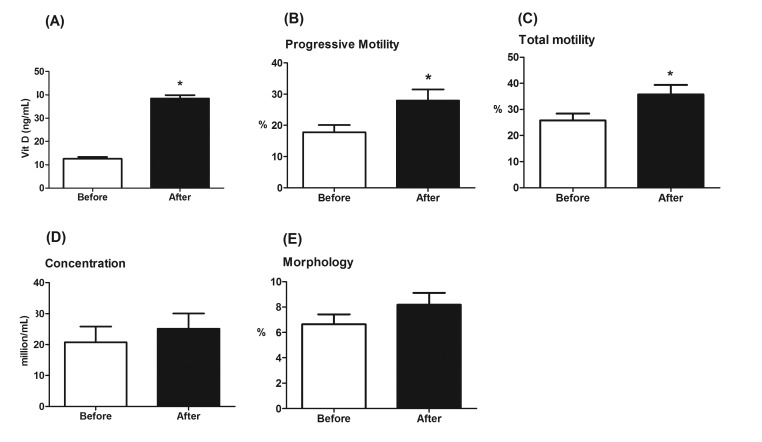
Patients in the idiopathic infertility group with low baseline vitamin D levels were treated with vitamin D3 tablets for 2 consecutive months. As shown in Figure 3A, post-intervention vitamin D levels (mean= 38.45±1.46 ng/mL) were significantly higher (p <0.001) than those before the treatment (mean= 12.56±0.78 ng/mL), indicating the adequacy of the treatment.
Figure 3.
(A) Comparison between serum levels of vitamin D in the intervention cohort of idiopathic infertility patients before and after vitamin D treatment. (B-E) Comparison between (B) progressive motility, (C) total motility, (D) sperm concentration, and (E) morphology of semen parameters in the intervention cohort of idiopathic infertility patients before and after vitamin D treatment. *, p<0.001 versus before treatment values.
Changes in the semen quality parameters between baseline and post-treatment SFAs were analyzed. Pairing of pre- and post-treatment values was significantly effective in all semen quality parameters. There was a statistically significant improvement in both sperm progressive forward motility (p= 0.0006, percent increase= 57%, Fig. 3B), and total sperm motility (p= 0.0006, percent increase= 38.66%, Fig. 3C) after vitamin D restoration. However, both sperm concentration (p= 0.1761, percent increase= 21.26%, Fig. 3D) and morphology (p= 0.079, percent increase= 23%, Fig. 3E) were not significantly improved after such therapeutic intervention.
Analysis of correlation between vitamin D and semen quality parameters in the intervention cohort
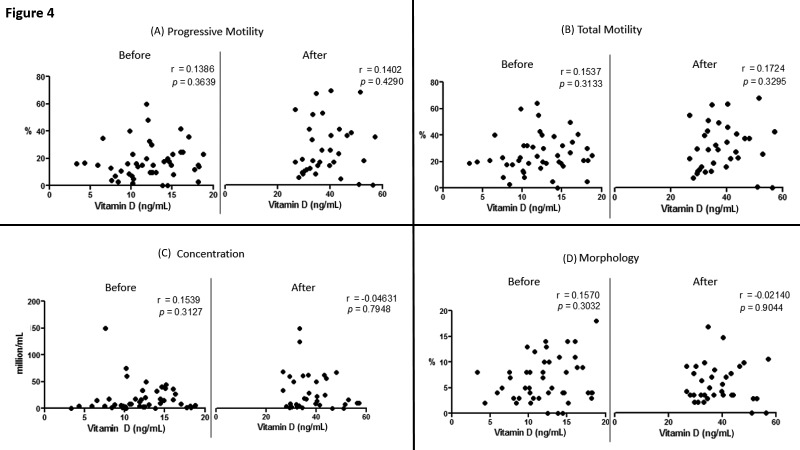
To better understand the type of relationship between vitamin D and idiopathic male infertility in particular, we ran a set of correlation analyses between each of the semen quality parameters and vitamin D levels before (n= 45) and after (n= 34) treatment. None of the tested relationships showed a statistically significant correlation, both before and after the therapeutic intervention (Fig. 4).
Figure 4.
Correlation between serum levels of vitamin D and semen quality parameters; (A) progressive motility, (B) total motility, (C) concentration, and (D) morphology BEFORE and AFTER treatment in the idiopathic infertility intervention cohort. r represents Spearman correlation coefficient.
DISCUSSION
To the best of our knowledge, this study is the first of its kind to demonstrate the effect of vitamin D supplementation on seminal fluid parameters in males who suffer from idiopathic infertility. The major highlights of this clinical trial are: (a) patients with idiopathic male infertility showed significantly lower serum levels of vitamin D at baseline, when compared to controls, as well as patients with secondary infertility; (b) the therapeutic intervention utilized in this study; oral vitamin D tablets, 5,000 IU, daily for two months, was sufficient to induce a statistically significant improvement in both sperm progressive forward motility and total motility; and (c) while baseline serum vitamin D significantly correlated with each of the semen quality parameters in the whole study population when results were pooled, the correlation ceased to exist upon selecting those with idiopathic male infertility alone.
Infertility harbours massive psychosocial burdens among young people and is one of the major stressors according to a recent study in 2016 (21). Male-factor infertility is incriminated in at least one third of infertility cases (22). Unfortunately, the most common type of male infertility is idiopathic (2), with no definitive etiology recognized. Such lack of understanding of the pathogenic mechanisms in these cases imperatively translates into debatable clinical practice with no clear-cut scientific evidence (6). Substantial in vitro (23), preclinical (14-16, 24, 25), and clinical evidence (7, 13, 17, 26) supports the association between vitamin D and male infertility. However, there are no clinical trials exploring the effect of vitamin D supplementation on idiopathic male infertility in particular; a fact that merits our study.
Our study revealed significantly lower levels of serum vitamin D in patients with idiopathic infertility than in both healthy fertile men and patients with secondary infertility. Such finding is partially congruent with what Blomberg et al. found in a large cross-sectional study in 2011, where men with vitamin D deficiency had a lower proportion of motile and morphologically normal spermatozoa compared to men with high vitamin D levels (13). Although this study utilized a good sample size to power the results, the sample was obtained from the general population, and it was not specific to a particular group of infertile patients. Accordingly, our study is the first clinical trial to compare serum vitamin D levels between idiopathic and secondary infertility patients against healthy fertile controls.
Furthermore, the significant correction of both progressive and total sperm motility observed in the intervention cohort after treatment is a novel finding in human male infertility, supporting the reported direct effects of activated vitamin D in vitro and in animal models. On the other hand, trends of improvement of both sperm concentration and morphology were not statistically significant in our study. This is in disagreement with a study that showed vitamin D-deficient and vitamin-D receptor knock-out mice recording significantly lower sperm numbers than controls (14, 25, 27). This conflict and the lack of an association between vitamin D status and human sperm production can be possibly interpreted by the long duration of human spermatogenesis (74 days) compared to rodents (9 – 11 days) (28). So, this duration that is required for generation of new sperms should be kept in mind if vitamin D supplementation is recommended in future treatment protocols of idiopathic male infertility. The positive correlation between pooled baseline vitamin D levels and each of the semen quality parameters is parallel to what was found in recent studies (13, 17). On the contrary, when we studied the correlation between serum vitamin D levels and semen parameters in the intervention cohort, we found no correlation between vitamin D levels and any of the semen quality parameters before and after intervention. This could indicate that male infertility is not a homogeneous entity. Thus, a well-guided stratification of infertile patients in future diagnosis, management, and follow-up plans is highly recommended.
Our study manifests several limitations. First, the sample size of our trial might not be sufficient. This shortage can be due to the study design itself, as we specified our study subjects according to strict inclusion and exclusion criteria. Furthermore, 11 patients were unfortunately lost to follow-up from the total intervention cohort after completion of treatment. Second, our clinical trial did not confirm the effect of vitamin D on fertility rates. This is seen as a limitation of the study because semen analysis itself is not an absolute indicator of fertility. However, following pregnancy rates and successful fertility outcomes needs a long-term cohort, which we are currently planning. Lastly, it was inevitable to enroll patients and study subject over a year-long period, spanning the four seasons. Unfortunately, we were unable to avoid the well-documented seasonal variations of vitamin D levels (29); a problem we would take into consideration in our subsequent long-term cohort.
In conclusion, the data presented in this study suggest a beneficial effect of vitamin D supplementation on semen quality parameters in a subset of idiopathic male infertility cases with low serum vitamin D levels, most notably on sperm progressive forward motility and total motility. Accordingly, standard treatment protocols of idiopathic male infertility patients should consider vitamin D supplements as an adjunct to other recommended therapeutic options.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
Acknowledgment
This work was supported by a grant from the Deanship of Research, Jordan University of Science and Technology (311/2015). The authors report no conflicts of interest. The authors wish to thank Dr. Firas Sahawneh (JUST) for his technical assistance in the urology clinic.
References
- 1.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–154. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Leaver RB. Male infertility: an overview of causes and treatment options. Br J Nurs. 2016;25(18):S35–S40. doi: 10.12968/bjon.2016.25.18.S35. [DOI] [PubMed] [Google Scholar]
- 3.Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am. 2014;41(1):195–204. doi: 10.1016/j.ucl.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Esteves SC. Novel concepts in male factor infertility: clinical and laboratory perspectives. J Assist Reprod Genet. 2016;33(10):1319–1335. doi: 10.1007/s10815-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jungwirth A. Guidelines on Male Infertility by the European Association of Urology; Update March 2014. 2015:1–42. [Google Scholar]
- 6.Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology. 2013;1(5):749–757. doi: 10.1111/j.2047-2927.2013.00107.x. [DOI] [PubMed] [Google Scholar]
- 7.Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, Ando S. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009;7:140–152. doi: 10.1186/1477-7827-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomberg Jensen M. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10(3):175–186. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- 9.Boisen IM, Bøllehuus Hansen L, Mortensen LJ, Lanske B, Juul A, Blomberg Jensen M. Possible influence of vitamin D on male reproduction. J Steroid Biochem Mol Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.023. pii: S0960-0760(16)30259-X. [DOI] [PubMed] [Google Scholar]
- 10.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3) Endocrinol Metab Clin North Am. 2010;39(2):255–269. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca(2+) store is involved in regulating sperm hyperactivated motility. Biol Reprod. 2001;65(5):1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- 12.Menegaz D, Barrientos-Duran A, Kline A, Silva FR, Norman AW, Mizwicki MT, Zanello LP. 1alpha,25(OH)2-Vitamin D3 stimulation of secretion via chloride channel activation in Sertoli cells. J Steroid Biochem Mol Biol. 2010;119(3-5):127–134. doi: 10.1016/j.jsbmb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Blomberg Jensen M, Gerner Lawaetz J, Andersson AM, Petersen JH, Nordkap L, Bang AK, Ekbom P, Joensen UN, Prætorius L, Lundstrøm P, Boujida VH, Lanske B, Juul A, Jørgensen N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26(6):1307–1317. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 14.Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992;122(6):1338–1344. doi: 10.1093/jn/122.6.1338. [DOI] [PubMed] [Google Scholar]
- 15.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sood S, Reghunandanan R, Reghunandanan V, Marya RK, Singh PI. Effect of vitamin D repletion on testicular function in vitamin D-deficient rats. Ann Nutr Metab. 1995;39(2):95–98. doi: 10.1159/000177848. [DOI] [PubMed] [Google Scholar]
- 17.Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14(6):855–859. doi: 10.1038/aja.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Food and Nutrition Board . Dietary Reference Intakes for Calcium and Vitamin D. In: Ross Catharine, Yaktine Ann L, Del Valle Heather B., editors. Washington (DC): National Academies Press (US); 2011. pp. 1–662. [PubMed] [Google Scholar]
- 19.Murray KS, James A, McGeady JB, Reed ML, Kuang WW, Nangia AK. The effect of the new 2010 World Health Organization criteria for semen analyses on male infertility. Fertil Steril. 2012;98(6):1428–1431. doi: 10.1016/j.fertnstert.2012.07.1130. [DOI] [PubMed] [Google Scholar]
- 20.Singh G, Bonham AJ. A predictive equation to guide vitamin D replacement dose in patients. J Am Board Fam Med. 2014;27(4):495–509. doi: 10.3122/jabfm.2014.04.130306. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Sharma PS, Narayan P, Binu VS, Dinesh N, Pai PJ. Prevalence and predictors of infertility-specific stress in women diagnosed with primary infertility: A clinic-based study. J Hum Reprod Sci. 2016;9(1):28–34. doi: 10.4103/0974-1208.178630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 23.Nangia AK, Hill O, Waterman MD, Schwender CE, Memoli V. Testicular maturation arrest to testis cancer: spectrum of expression of the vitamin D receptor and vitamin D treatment in vitro. J Urol. 2007;178(3 Pt 1):1092–1096. doi: 10.1016/j.juro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Hirai T, Tsujimura A, Ueda T, Fujita K, Matsuoka Y, Takao T, Miyagawa Y, Koike N, Okuyama A. Effect of 1,25-dihydroxyvitamin d on testicular morphology and gene expression in experimental cryptorchid mouse: testis specific cDNA microarray analysis and potential implication in male infertility. J Urol. 2009;181(3):1487–1492. doi: 10.1016/j.juro.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Kwiecinksi GG, Petrie GI, DeLuca HF. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol. 1989;256(4 Pt 1):E483–E487. doi: 10.1152/ajpendo.1989.256.4.E483. [DOI] [PubMed] [Google Scholar]
- 26.Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, Muciaccia B, Sartori L, Selice R. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab. 2011;96(4):E646–E652. doi: 10.1210/jc.2010-1628. [DOI] [PubMed] [Google Scholar]
- 27.Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr. 2001;131(6):1787–1791. doi: 10.1093/jn/131.6.1787. [DOI] [PubMed] [Google Scholar]
- 28.Zeng W, Avelar GF, Rathi R, Franca LR, Dobrinski I. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J Androl. 2006;27(4):527–533. doi: 10.2164/jandrol.05143. [DOI] [PubMed] [Google Scholar]
- 29.Klingberg E, Oleröd G, Konar J, Petzold M, Hammarsten O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine. 2015;49(3):800–808. doi: 10.1007/s12020-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]