Abstract
Context
Oral anti-diabetic drugs (OADs) are leading option for treatment of type 2 diabetes (T2D). However, availability of OADs are limited in the presence of renal impairment (RI).
Objective
In this study, we examined the efficacy of repaglinide, which is mainly metabolized and excreted via non-renal route, in patients with T2D and severe RI that consists mainly of chronic kidney disease (CKD) stage 4.
Design, Subjects and Methods
This was an open label, single arm, interventional study by repaglinide monotherapy. The primary efficacy end point was HbA1c change from baseline to week 12.
Results
Repaglinide treatment significantly reduced HbA1c levels from 7.7 ± 0.7% to 6.1 ± 0.3% (p<0.001) in 9 patients with severe RI (mean estimated glomerular filtration rate was 26.4 ± 7.5 mL/min/1.73m2). Focusing on 4 patients who received DPP-4 inhibitor monotherapy at enrolment, switching to repaglinide also significantly improved HbA1c levels. No hypoglycemic symptoms or severe hypoglycemia was reported in patients who completed the period of 12 weeks.
Conclusions
We demonstrated the efficacy of repaglinide in patients with T2D and severe RI. In case that DPP-4 inhibitors are not enough to achieve targeted range of glycemic control, repaglinide is another good candidate.
Keywords: repaglinide, type 2 diabetes, diabetic nephropathy, renal impairment
INTRODUCTION
Diabetes is known to be a major cause for renal impairment (RI) and subsequent end-stage kidney disease in the developed world (1). A profound problem may be lack of clear evidence that strict glycemic control could achieve better outcomes in this population, in contrast to patients without apparent diabetic complications including nephropathy. However, a recent large observational study demonstrated that both higher (≥ 8.0%) and lower (≤ 6.5%) levels of HbA1c were associated with higher mortality in patients with diabetes and RI (2). This finding suggests that appropriate glycemic control could prevent cardiovascular events and/or fatal infection (3, 4), although harmful hypoglycemia has to be avoided (1).
The presence of RI in patients with diabetes could change glucose and insulin metabolism together with alteration of drug kinetics, resulting in rather difficulties of glycemic control compared with those without RI. Of particular note is that RI leads to fragile condition prone to hypoglycemia because of impaired renal gluconeogenesis and insulin clearance. Indeed, it has been reported that the presence of RI is a significant risk factor for severe hypoglycemia (5). Although oral anti-diabetic drugs (OADs) are leading option for treatment of type 2 diabetes (T2D), the presence of RI confines availability of OADs. Especially, the accumulation of parent drugs or their active metabolites could lead to adverse events such as prolonged hypoglycemia (6, 7).
Although insulin therapy is preferred from the viewpoint of safety (8), sulfonylureas (SUs) are still one of options as anti-hyperglycemic treatment in some patients with visual and/or physical disabilities. With this difficult situation, the emergence of DPP-4 inhibitors has been to a boon to the patients with T2D and RI. Recent reports suggest the efficacy and safety of DPP-4 inhibitors in this population (9-12). However, in clinical practice, the anti-hyperglycemic action of DPP-4 inhibitors is not always enough in patients with poor glycemic control.
Repaglinide belongs to meglitinides which show rapid insulin secretagogue action. Compared with nateglinide and mitiglinide, repaglinide has unique pharmacokinetics. Orally-administered repaglinide is almost completely metabolized in the liver (13). Repaglinide metabolites, which have little glucose-lowering effect, are predominantly excreted through bile with less than 8% of administered dose being excreted into the urine (13). Therefore, repaglinide is theoretically thought to be a strong candidate as OADs in patients with T2D and RI. Contrary to our expectation, few reports are available focusing on effects of repaglinide on glycemic control under condition of RI, to date. In this study, we investigated the efficacy of repaglinide in patients with T2D and severe RI.
METHODS
This was a 12-week, open label, single arm, interventional study (Osaka Diabetes Mellitus and Kidney Diseases study-2: Diamond study-2). The participants were recruited from the inpatient and outpatient at Osaka City University Hospital between December 3, 2012 and July 17, 2014. The inclusion criteria were 1) T2D with ≥ 20 years of age, 2) HbA1c ≥ 6.5% and ≤ 10.0% or glycated albumin (GA) ≥ 18.0% and ≤ 30.0%, and 3) creatinine level of ≥ 1.5 mg/dL or estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 without dialysis. GA level was adopted as an inclusion criterion because HbA1c level is sometimes underestimated in this population as a result of renal anemia (14). The lower limit for GA level (18.0%) was estimated to be approximately equivalent to HbA1c of 6.5% in patients with RI. The main exclusion criteria were; impaired hepatic function (alanine transaminase [ALT] ≥ 100 IU/L or aspartate transaminase [AST] ≥ 100 IU/L); malignant tumors under medical treatment; untreated diabetic retinopathy.
After one to six weeks of washout period of any OADs or insulin therapy, eligible patients started to receive repaglinide monotherapy (0.25mg thrice a day) for 12 weeks. HbA1c, GA and fasting plasma glucose (FPG) levels were measured at screening, and at weeks 0, 4, 8, and 12.To achieve or maintain HbA1c < 6.2% or GA < 18.0%, dose adjustment was permitted with careful attention to hypoglycemia. The prespecified primary endpoint was the change from baseline to week 12 in HbA1c. Secondary endpoint included changes from baseline to week 12 in GA and FPG. In addition, we focused on 4 patients who received monotherapy with DPP-4 inhibitors at enrollment. We also evaluated the change from baseline to week 12 in HbA1c to compare the efficacy of repaglinide with that of DPP-4 inhibitor. Safety evaluations included adverse events (AEs), clinical laboratory tests, and hypoglycemic episodes.
This study adhered to the declaration of Helsinki. After explanation of the study objectives, all participants gave written informed consent. The study protocol was approved by the local ethics committee of Osaka City University Hospital (Registration No.2372). The clinical trial registration no. is UMIN-CTR (University Hospital Medical Information Network-Clinical Trials Registry) 000009166.
Data were expressed mean ± standard deviation (SD). Comparisons of blood parameters at baseline, after 4, 8, 12 weeks of therapy were performed with Dunnett test. Findings of p < 0.05 were considered significant.
RESULTS
Of 14 partients enrolled, 4 patients were drug-naïve, 3 patients were treated with insulin injections, 4 patients with DPP-4 inhibitors, 3 patients with combination of glimepiride and/or metformin and/or sitaglitpin. Nine patients completed the 12-week treatment period (Fig. 1). The reason for discontinuation in 3 participants was hyperglycemia due to lack of efficacy. One failed washout of OADs before starting repaglinide. Two others discontinued intervention within 10 days after repaglinide treatment. All of them started insulin injection. During the study period, dose escalation was performed in one patient not reaching target range of glycemic control. He received the increased dose of repaglinide (0.5 mg thrice a daily) at week 4 and maintained with the same dosage until the study end. One participant switched repaglinide to DPP-4 inhibitor (linagliptin) by attending physician’s decision at day 12 since he tended to show fasting hypoglycemia. The lowest blood glucose level was 55 mg/dL evaluated by finger-stick blood testing without apparent symptoms. Except glycemic control, one discontinued the intervention at week 9 because of acute exacerbation of renal failure, resulting in initiation of hemodialysis.
Figure 1.
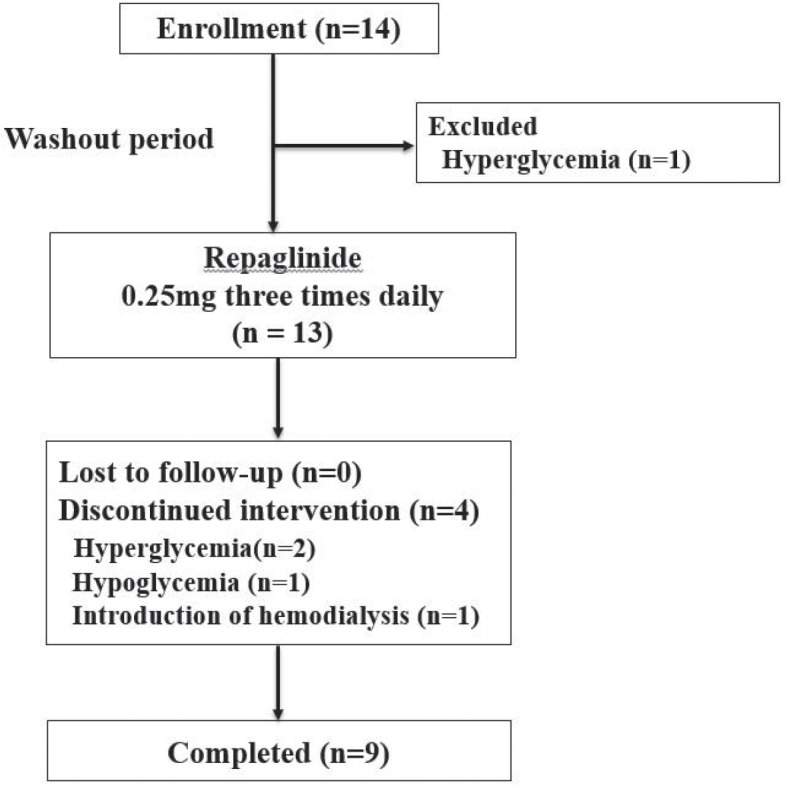
Flow chart for the study.
The baseline characteristics in 9 participants are shown in Table 1. They were mainly classified into chronic kidney disease (CKD) stage 4 (eGFR ranged from 14.9 to 37.4 mL/min/1.73m2). Mean HbA1c values during the study are presented in Figure 2. Twelve-week repaglinide treatment significantly improved HbA1c levels from 7.7 ± 0.7% to 6.1 ± 0.3% (p < 0.001). On the other hand, repaglinide tended to reduce FPG and GA levels at week 12 compared to those at baseline, although the differences were not statistically significant (Fig. 3, A and B).
Figure 2.
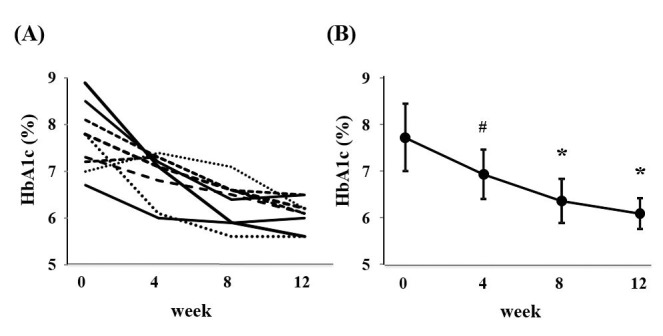
(A) Changes in HbA1c level in each participant during treatment. (B) Changes in mean HbA1c level during treatment. # P < 0.05 versus baseline. * P <0.001 versus baseline.
Figure 3.
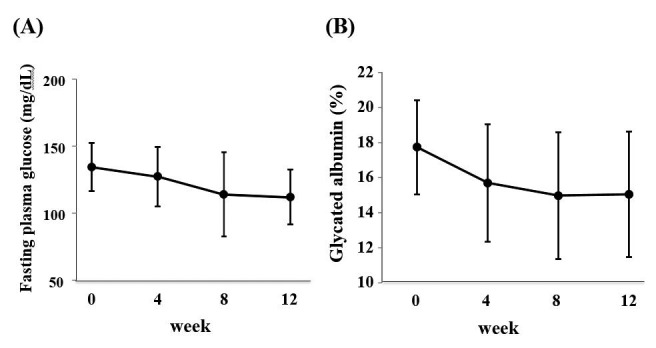
(A) Changes in mean fasting plasma glucose level. (B) Changes in mean GA level during treatment.
Table 1.
Baseline characteristics of patients with type 2 diabetes and renal impairment
| Sex (Male / Female) | 7/2 |
| Age (years) | 60.2 ± 10.7 |
| Body Mass Index (kg/m2) | 26.1 ± 4.9 |
| Systolic BP (mmHg) | 139 ± 19 |
| Diastolic BP (mmHg) | 69 ± 7 |
| Fasting Plasma Glucose (mg/dL) | 132 ± 13 |
| HbA1c (%) | 7.7 ± 0.7 |
| Glycated albumin (%) | 17.7 ± 2.7 |
| CPR (ng/mL) | 3.7 ± 1.1 |
| Blood Urea Nitrogen (mg/dL) | 28.9 ± 7.1 |
| Creatinine (mg/dL) | 2.2 ± 0.7 |
| eGFR (mL/min/1.73m2) | 26.4 ± 7.5 |
| Hemoglobin (g/dL) | 12.5 ± 2.0 |
| Serum albumin (g/dL) | 3.6 ± 0.6 |
| Urine protein (g/gCr) | 4.5 ± 3.0 |
| ACR (mg/gCr) | 3467 ± 2478 |
Data are means±standard deviation. BP, blood pressure; HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate; CPR, C-peptide immunoreactivity; ACR, albumin/creatinine ratio.
To explore whether switching from DPP-4 inhibitors to repaglinide could elicit better glycemic improvement, we focused on participants with DPP-4 inhibitor monotherapy at enrollment. Two participants were receiving sitagliptin (50mg per day) and two were receiving vildagliptin (50mg and 100mg per day). As shown in Figure 4, replacement of DPP-4 inhibitor monotherapy by repaglinide significantly reduced HbA1c levels (7.6 ± 0.8% at baseline vs. 6.3 ± 0.3% at week 12, p = 0.014), suggesting the superiority of repaglinide to DPP-4 inhibitors with regard to anti-hyperglycemic action.
Figure 4.
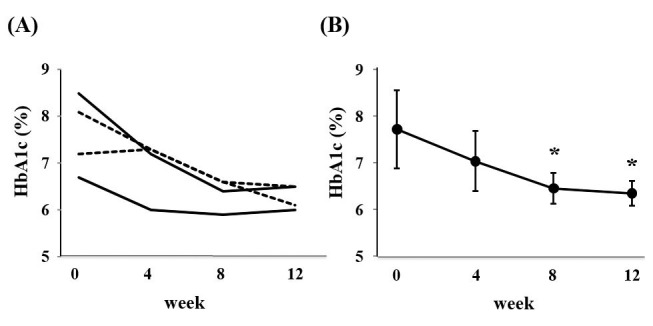
(A) Changes in HbA1c level in each participant who received monotherapy with DPP-4 inhibitor at enrollment during treatment. (B) Changes in mean HbA1c level in participants who received monotherapy with DPP-4 inhibitor at enrollment during treatment.
As described above, one participant discontinued repaglinide intervention since he tended to show fasting hypoglycemia without any hypoglycemic symptoms. Among the 9 completed participants, no hypoglycemic record or symptom was observed. Although one participant started hemodialysis, it was not considered treatment-related. Except for this case, apparent AE was not reported.
DISCUSSION
This is the first study to investigate the efficacy of repaglinide targeted for patients with T2D and severe RI (mainly CKD stage 4) to our knowledge. Repaglinide significantly reduced HbA1c level without hypoglycemia in this 12-week, open label, single arm, interventional study.
Although introduction of DPP-4 inhibitors has enabled glycemic control by OADs in patients with RI (9, 10), it is not rare that monotherapy of DPP-4 inhibitors cannot achieve target range of glycemia in clinical practice. Repaglinide, which has unique potential compared with other OADs including meglitinides, is one of good candidates as the next move in difficult glycemic control in this population.
Among meglitinides, repaglinide has a pharmacokinetic advantage under RI condition since its metabolites are mainly excreted via the bile (13). On the other hand, nateglinide is difficult to use because of accumulation of active metabolites which are excreted via renal route (15). Indeed, severe hypoglycemic coma due to nateglinide has been reported (16), resulting in contraindication for patients with T2D and RI in Japan. Another meglitinide, mitiglinide is available in Japan. Mitiglinide is metabolized in kidney and liver. Since predominant metabolites have little hypoglycemic action, it is plausible for anti-diabetic treatment in patients with RI. Abe et al. have already reported the efficacy and safety of mitiglinide in patients with T2D on hemodialysis (17, 18). However, mitiglinde has low penetration rate in the world and is not listed in a report of ‘Diabetic Kidney Disease’ from an ADA consensus conference (1).
Another unique characteristic of repaglinide may be powerful hypoglycemic action comparable to SUs. In previous clinical trials, repaglinide showed non-inferiority to glyburide (19) or superiority to glipizide (20) with regard to anti-hyperglycemic action. In addition to the efficacy, one report showed safety of repaglinide in patients with T2D and RI. Switching to repaglinide monotherapy in patients receiving various OADs and/or insulin at baseline resulted in no significant change of HbA1c level after a 3-month study period (21). Importantly, exchange for repaglinide significantly reduced incidence of hypoglycemia in patients with extreme renal impairment (20 ≤ creatinine clearance < 30) who received mainly SU monotherapy (80% of patients) (21). These studies suggest that repaglinide is equal to SUs with regard to glycemic improvement with less risk of hypoglycemia in patients with severe RI. In addition, direct comparison of anti-hyperglycemic action between meglitinides was also reported in 16-week, randomized trial (22). Repaglinide monotherapy was significantly more effective than nateglinide monotherapy in reducing HbA1c (22). Based on our findings, repaglinide seemed to be superior to DPP-4 inhibitors with regard to anti-hyperglycemic action (Fig. 4).
Although repaglinide clearly reduced HbA1c level in this study, we could not observe a significant reduction in FPG level. Since repaglinide is a short-acting insulin secretagogue, it can theoretically improve post-prandial hyperglycemia rather than fasting hyperglycemia. Moreover, we could not observe significant improvement of GA in this study. One possible explanation might be that participants in this study had relatively high proteinuria (4.5 ± 3.0 g/gCr), though they did not show apparent hypoalbuminemia (3.6 ± 0.6 g/dL). Previous report suggested that nephrotic range proteinuria decreases GA levels independent of the glycemic state, possibly through rapid albumin turnover (23). Reflecting this condition, mean GA level at baseline was relatively low (17.7 ± 2.7%) in this study.
There are critical limitations in this study. First, the sample size was very small. Second, this study was single-arm, short-duration interventional design. Third, the requirement of insulin therapy was not fully evaluated in patients with severe RI, although we tried measuring serum C-peptide immunoreactivity (CPR) to predict endogenous insulin secretion. However, it was very difficult to judge it using serum or urine CPR, because approximately 70% of plasma C-peptide is cleared in the kidney (24). Three participants with 2.2, 2.4, and 5.1ng/mL of CPR levels discontinued repaglinide monotherapy, resulting in insulin injections. On the other hand, CPR ranged from 2.1 to 5.6 ng/mL (3.7 ± 1.1 ng/mL) in 9 participants who successfully completed the study. Therefore, it is premature to conclude possible safety threshold for repaglinide monotherapy using CPR level.
In conclusion, we demonstrated the efficacy and tolerability of repaglinide monotherapy in patients with T2D and severe RI. In case that DPP-4 inhibitors are not enough to achieve targeted range of glycemic control, repaglinide is a good candidate before considering initiation of insulin therapy in patients with T2D and severe RI.
Conflict of interest
K. Mori received lecture fees from Sumitomo Dainippon Pharma Co., Ltd, Mitsubisi Tanabe Pharma Corporation, Daiichi Sankyo Co., Ltd, Sanofi, Nippon Boehringer Ingelheim Co., Ltd. M.E. received lecture fees from Sanofi, Nippon Boehringer Ingelheim Co., Ltd, Mitsubisi Tanabe Pharma Corporation, Daiichi Sankyo Co., Ltd, Takeda Pharmaceutical Company Limited, Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd, Ono Pharmaceutical Co., Ltd. T.S. received lecture fees from Bayer in Japan, Mochida Pharmaceutical Co., Ltd, Pfizer Inc., Takeda Pharmaceutical Company Limited. M.I. received lecture fees from Mitsubisi Tanabe Pharma Corporation, Daiichi Sankyo Co., Ltd., Astellas Pharma Inc, Asahi Kasei Pharm Corporation, Kyowa Hakko Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Teijin Pharma Limited, Takeda Pharmaceutical Company Limited, Eli Lilly Japan K.K., MSD K.K., Bayer in Japan, Ono Pharmaceutical Co., Ltd., K. Mori received unrestricted research grants from Sanofi and Eli Lilly Japan K.K. M.E. and M.I. received unrestricted research grants from Mitsubisi Tanabe Pharma Corporation, Daiichi Sankyo Co., Ltd., Astellas Pharma Inc, Asahi Kasei Pharm Corporation, Kyowa Hakko Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Teijin Pharma Limited, Takeda Pharmaceutical Company Limited. Ono Pharmaceutical Co., Ltd. T.S. received unrestricted research grants from Astellas Pharma Inc, Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited.
Acknowledgments
K. Mori contributed to the design of the study, the collection of data, and statistical analysis of data. K.Mori and M.E. wrote the manuscript. M.E. contributed to the design of the study. R.N., Y.Y., and H.U. contributed to the collection of data. K.Mot., T.M., T.S. and M.I. participated in the conduct and the interpretation of data.
All of the authors were fully responsible for all content and editorial decisions and have approved the final version. K. Mori is the guarantor of this work and had full access to all the data in the study and takes responsibility for the data.
References
- 1.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shurraw S, Hemmelgarn B, Lin M, Majumdar SR, Klarenbach S, Manns B, Bello A, James M, Turin TC, Tonelli M, Alberta Kidney Disease Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 3.Morioka T, Emoto M, Tabata T, Shoji T, Tahara H, Kishimoto H, Ishimura E, Nishizawa Y. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909–913. doi: 10.2337/diacare.24.5.909. [DOI] [PubMed] [Google Scholar]
- 4.Oomichi T, Emoto M, Tabata T, Morioka T, Tsujimoto Y, Tahara H, Shoji T, Nishizawa Y. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496–1500. doi: 10.2337/dc05-1887. [DOI] [PubMed] [Google Scholar]
- 5.Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, Fink JC. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12(1):57–69. doi: 10.2174/138920011794520053. [DOI] [PubMed] [Google Scholar]
- 7.Iglesias P, Heras M, Diez JJ. Diabetes mellitus and kidney disease in the elderly. Nefrologia. 2014;34(3):285–292. doi: 10.3265/Nefrologia.pre2014.Feb.12319. [DOI] [PubMed] [Google Scholar]
- 8).Urata H, Mori K, Emoto M, Yamazaki Y, Motoyama K, Morioka T, Fukumoto S, Koyama H, Shoji T, Ishimura E, Inaba M. Advantage of insulin glulisine over regular insulin in patients with type 2 diabetes and severe renal insufficiency. J Ren Nutr. 2015;25(2):129–134. doi: 10.1053/j.jrn.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Giorda CB, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine. 2014;46(3):406–419. doi: 10.1007/s12020-014-0179-0. [DOI] [PubMed] [Google Scholar]
- 10.Cheng D, Fei Y, Liu Y, Li J, Chen Y, Wang X, Wang N. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysis. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0111543. e111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada N, Mori K, Nakagawa C, Sawa J, Kumeda Y, Shoji T, Emoto M, Inaba M. Improved glycemic control with teneligliptin in patients with type 2 diabetes mellitus on hemodialysis: Evaluation by continuous glucose monitoring. J Diabetes Complications. 2015;29(8):1310–1313. doi: 10.1016/j.jdiacomp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Emoto M, Shoji T, Inaba M. Linagliptin monotherapy compared with voglibose monotherapy in patients with type 2 diabetes undergoing hemodialysis: a 12-week randomized trial. BMJ Open Diabetes Res Care. 2016;4(1) doi: 10.1136/bmjdrc-2016-000265. e000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marbury TC, Ruckle JL, Hatorp V, Andersen MP, Nielsen KK, Huang WC, Strange P. Pharmacokinetics of repaglinide in subjects with renal impairment. Clin Pharmacol Ther. 2000;67(1):7–15. doi: 10.1067/mcp.2000.103973. [DOI] [PubMed] [Google Scholar]
- 14.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y, Osaka CKDERG. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18(3):896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Shibahara N, Miyagawa K, Itahana R, Izumi M, Nakanishi T, Takamitsu Y. Pharmacokinetics of nateglinide and its metabolites in subjects with type 2 diabetes mellitus and renal failure. Clin Nephrol. 2003;60(2):90–95. doi: 10.5414/cnp60090. [DOI] [PubMed] [Google Scholar]
- 16.Nagai T, Imamura M, Iizuka K, Mori M. Hypoglycemia due to nateglinide administration in diabetic patient with chronic renal failure. Diabetes Res Clin Pract. 2003;59(3):191–194. doi: 10.1016/s0168-8227(02)00242-5. [DOI] [PubMed] [Google Scholar]
- 17.Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K. Efficacy and safety of mitiglinide in diabetic patients on maintenance hemodialysis. Endocr J. 2010;57(7):579–586. doi: 10.1507/endocrj.k09e-318. [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Okada K, Maruyama T, Maruyama N, Matsumoto K. Combination therapy with mitiglinide and voglibose improves glycemic control in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother. 2010;11(2):169–176. doi: 10.1517/14656560903530683. [DOI] [PubMed] [Google Scholar]
- 19.Marbury T, Huang WC, Strange P, Lebovitz H. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract. 1999;43(3):155–166. doi: 10.1016/s0168-8227(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 20.Madsbad S, Kilhovd B, Lager I, Mustajoki P, Dejgaard A, Scandinavian Repaglinide G Comparison between repaglinide and glipizide in Type 2 diabetes mellitus: a 1-year multicentre study. Diabet Med. 2001;18(5):395–401. doi: 10.1046/j.1464-5491.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 21.Hasslacher C, Multinational Repaglinide Renal Study G Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care. 2003;26(3):886–891. doi: 10.2337/diacare.26.3.886. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J, Hassman DR, Madder RD, Brazinsky SA, Farrell J, Khutoryansky N, Hale PM, Repaglinide Versus Nateglinide Comparison Study G Repaglinide versus nateglinide monotherapy: a randomized, multicenter study. Diabetes Care. 2004;27(6):1265–1270. doi: 10.2337/diacare.27.6.1265. [DOI] [PubMed] [Google Scholar]
- 23.Okada T, Nakao T, Matsumoto H, Nagaoka Y, Tomaru R, Iwasawa H, Wada T. Influence of proteinuria on glycated albumin values in diabetic patients with chronic kidney disease. Intern Med. 2011;50(1):23–29. doi: 10.2169/internalmedicine.50.4129. [DOI] [PubMed] [Google Scholar]
- 24.Iglesias P, Diez JJ. Insulin therapy in renal disease. Diabetes Obes Metab. 2008;10(10):811–823. doi: 10.1111/j.1463-1326.2007.00802.x. [DOI] [PubMed] [Google Scholar]


