Abstract
IMPORTANCE
The complement factor H R1210C rare variant confers the strongest genetic risk for age-related macular degeneration and earlier age at onset; however, its associated phenotype has not been well characterized.
OBJECTIVE
To describe specific fundus features of a white population with the R1210C rare variant.
DESIGN, SETTING, AND PARTICIPANTS
Fundus features specific for diagnosis and disease staging were retrospectively characterized by systematic review of all available fundus images for each patient, including color photography, fluorescein angiography, fundus autofluorescence, and optical coherence tomography, at a tertiary ophthalmologic referral center. For this retrospective observational study conducted from 2012 to 2014, enrolled patients with the variant and their family members without the variant were identified from the Age-Related Macular Degeneration Study for a family-based study arm. For patients with the variant but without a family member enrolled in the study, age-matched comparison individuals without the variant were selected randomly from the database.
MAIN OUTCOMES AND MEASURES
The presence of drusen in the macula (macular drusen score) and estimated number (total macular drusen score) were assessed. The presence of drusen in the extramacular regions (extramacular drusen score), pigmentary abnormalities, and disease staging were also evaluated. Binary logistic regression models were used to evaluate the association between rare variant status and ocular phenotypes.
RESULTS
Images from a total of 143 patients (283 eyes), including 62 patients with the rare variant, were analyzed. Drusen score covariates were associated with the R1210C rare variant. A larger proportion of patients carrying the variant had the highest level of macular and total macular drusen scores compared with those without the variant (57.9% vs 16.7% and 52.9% vs 14.2%, respectively; P for trend < .001 for both scores). Patients carrying the rare variant had a much greater likelihood of having advanced disease (odds ratio, 7.0; 95% CI, 3.1-16.2; P < .001). A higher prevalence of geographic atrophy was observed among patients carrying the variant (odds ratio, 13.7; 95% CI, 5.0-37.7; P < .001).
CONCLUSIONS AND RELEVANCE
The typical phenotype of the complement factor H R1210C rare variant is associated with extensive drusen accumulation in the macula and throughout the fundus, as well as with a high risk for having advanced disease. Better characterization of genetic profiles in age-related macular degeneration may be important for screening and future therapeutic strategies for this vision-threatening condition.
Age-related macular degeneration (AMD) is a complex disorder triggered by a wide range of environmental and genetic risk factors.1-3 Numerous common variants are known markers of AMD including several complement pathway genes: complement factor H (CFH),4-8 complement factor I,9 complement component 2, complement factor B,7,10 and complement 3.11
We previously reported the association of a rare CFH variant with AMD, R1210C, which is the strongest genetic risk factor to date, with an odds ratio (OR) of 20.12,13 The R1210C variant is also known to be associated with inherited forms of atypical hemolytic uremic syndrome and primary glomerulonephritis.14-21 In addition to linking 2 clinically unrelated conditions, such as AMD and atypical hemolytic uremic syndrome, the R1210C finding suggests that compromised function of the factor H protein is involved in AMD pathogenesis as a causal factor and not merely as an associated factor.12
In that initial report, the R1210C rare variant was associated with an earlier age at diagnosis of advanced AMD.12 This variant is also significantly associated with progression from early or intermediate AMD to advanced stages in a multigene prediction model.22,23 However, the fundus phenotype typically related to the variant is still to be determined. This knowledge is needed to better understand the manifestations of this rare CFH mutation and to help detect and characterize this phenotype in clinical practice. Identification of such high-risk individuals will be important for screening, potential new therapeutic strategies, and personalized medicine. Therefore, the objective of this study, conducted from 2012 to 2014, was to determine specific fundus features of a white population carrying the CFH R1210C rare variant.
Methods
Study Population
All participants were previously enrolled in our ongoing genetic and epidemiologic studies of AMD, which included standardized protocols for assessment of demographic, behavioral, and ocular variables of patients without AMD, those with all stages of AMD, and relatives.7-9,11-13,24 In a family-based arm of the study, individuals with the CFH R1210C rare variant and their family members without the variant were included. Family members without the R1210C variant were selected as a comparison group. Patients with the R1210C variant who did not have family members in the AMD Study were randomly matched to patients from the database without the variant according to age, comprising the nonfamily-based arm of the study. These individuals were matched using a random number generator based on age at most recent AMD grade in a ratio of 2 individuals for each person with the variant. The mean age at most recent AMD grade was 74.6 years in the individuals without the variant and 73.8 years in those with the variant. Exclusion criteria included any previous or concomitant ophthalmological condition that could confound the interpretation of AMD features on multimodal fundus imaging, inadequate image quality, or unavailability of fundus images. This research adhered to the tenets of the Declaration of Helsinki and was performed under approved institutional review board protocol from the New England Eye Center and Ophthalmic Epidemiology and Genetics Service, Tufts University School of Medicine. Signed informed consent was obtained for all patients.
Genotypic Characterization
The rare CFH R1210C variant and other AMD-related genotypes for common and rare variants were determined by genotyping and gene-sequencing platforms, as previously described.7,9,11-13,25,26
Phenotypic Characterization
Ocular records and all fundus images available for each participant (with and without the R1210C variant) were systematically reviewed to characterize specific fundus features relevant for the diagnosis and staging of AMD. Imaging modalities included color photography, fluorescein angiography, fundus autofluorescence imaging, and spectral-domain optical coherence tomography. When more than 1 follow-up visit was available for a given participant, all visits were taken into consideration to allow complete characterization of the most advanced manifestation for each specific fundus feature.
For the purpose of this study, the fundus was divided into 2 areas of interest: the macular area (defined as a circular area of 3 mm or 2 disc diameters in radius, centered at the fovea) and the extramacular regions, including temporal to the macula, nasal to the optic disc (defined as a semicircular area of 3 mm or 2 disc diameters in radius, nasal to the optic disc), along the temporal vascular arcade, and the superotemporal, inferotemporal, superonasal, and inferonasal fundus quadrants (defined as the retina beyond the retinal vascular arcades extending to or beyond the equator). Color fundus photographs and fluorescein angiography were obtained in up to 7 standard fields based on the modified Airlie House classification, which was adopted by the Early Treatment Diabetic Retinopathy Study and previously described elsewhere.27 Briefly, images were obtained with 30° fundus cameras. Standard field 1 was centered on the optic disc, standard field 2 was centered on the macula, standard field 3 was temporal to the macula, and standard fields 4 to 7 were tangential to horizontal lines passing through the upper and lower poles of the disc and to a vertical line passing through its center.27 The macular area was assessed on fields 2 and 3, the peripapillary area nasal to the optic disc was assessed on field 1, and the 4 fundus quadrants (superotemporal, inferotemporal, superonasal, and inferonasal) were assessed on fields 4 to 7.
The phenotypic features characterized in the fundus areas of interest were the presence of soft, hard, and cuticular drusen and pigmentary abnormalities classified as either hyperpigmentation or hypopigmentation (relative loss of pigment in a circumscribed area of the fundus with indistinct borders and only partial visualization of underlying choroidal vessels, thus non-geographic atrophy type of hypopigmentation).28-32 In the macular area, AMD staging also included the presence of advanced AMD with geographic atrophy (GA) or choroidal neovascularization (CNV). The AMD grading was based on the Clinical Age-Related Maculopathy Staging (CARMS) system and noted for all enrolled eyes.5,24 The CARMS system has a 5-step scale as follows: grade 1 = no drusen; grade 2a = several small drusen and no retinal pigment epithelium (RPE) abnormalities; grade 2b = RPE abnormalities but no drusen; grade 2c = both small drusen and RPE abnormalities; grade 3a = several intermediate and large soft drusen; grade 3b = drusenoid RPE detachment; grade 4 = central or noncentral GA; grade 5a = RPE detachment secondary to CNV; and grade 5b = disciform scar secondary to CNV.4,24
The characteristics of drusen in the macular area were further evaluated using the Early Treatment Diabetic Retinopathy Study grid described in detail elsewhere.27 Briefly, this grid has 3 concentric circles of 0.5 mm, 1.5 mm, and 3.0 mm in radius that are divided into 4 quadrants (superior, temporal, inferior, and nasal). It is centered at the fovea and divides the macular area into 9 concentric subfields. A 10th subfield of interest was the extramacular area temporal to the Early Treatment Diabetic Retinopathy Study grid (defined as a semicircular area of 1.5 mm or 1 disc diameter in radius extending temporal to the grid along the horizontal meridian). The grid was properly placed on slides or on digital images according to the image format available for analysis. The specific features characterized in each of the 10 macular subfields were predominant drusen type (hard, soft, or cuticular); largest drusen size (small, intermediate, or large); and intermediate or large drusen count.27 We determined a range of intermediate or large drusen present in each of the 10 macular subfields defined as no drusen, 1 to 5, 6 to 10, 11 to 20, and more than 20 drusen.
All phenotypic characterization was performed masked for the rare variant status. The CARMS grade was assessed by 2 masked observers (D.F. and J.M.S.). The rate of agreement was 94.4%, and the discrepant grades were solved by opened adjudication. The other clinical features were graded by a single observer (D.F.).
Drusen Scores
Drusen scores were created to facilitate clinical comparisons and statistical analyses. Three scores were calculated: macular drusen score, total macular drusen score, and extramacular drusen score. The macular drusen score (range, 0-10) was calculated by assessing the presence of drusen in each of the 10 macular subfields. The total macular drusen score (range, 0-40) was defined as the range of intermediate and large drusen counted in each macular subfield. Each of the 10 macular subfields was weighted as follows: no drusen = 0; 1-5 drusen = 1; 6-10 drusen = 2; 11-20 drusen = 3; and more than 20 drusen = 4. The extramacular drusen score (range, 0-7) comprised the presence of drusen in each of the 7 extramacular regions (temporal to the macula; nasal to the optic disc; along the temporal vascular arcades; and the superotemporal, inferotemporal, superonasal, and inferonasal fundus quadrants).
Statistical Analysis
Demographic, behavioral, and ocular phenotype variables were analyzed for patients with and without the CFH R1210C rare variant. Patients were additionally classified by whether they were a member of an enrolled family (family-based arm) or an individual participant (nonfamily-based arm). Demographic and behavioral factors were assessed as follows: age at recent CARMS grade (<70, 70-79.9, and ≥80 years), sex, body mass index (calculated as weight in kilograms divided by height in meters squared) classification (<25, 25-29, and ≥30), and smoking status (never or ever). Among patients with advanced disease in the AMD Study, age at diagnosis was compared between patients with and without the variant.
Ocular phenotype variables included the macular drusen score, total macular drusen score, extramacular drusen score, macular pigmentary abnormalities, and most recent CARMS grade. Demographic and behavioral variables were analyzed with the individual participant as the unit of analysis. Analyses pertaining to ocular phenotype variables were based on the individual eye. Patients were additionally classified into AMD groups defined by CARMS grade both in the worse eye and in each eye for analyses of patients vs individual eyes, respectively. These groups were classified as follows: no AMD = grade 1; early/intermediate AMD = grades 2 and 3; and advanced AMD = grades 4 and 5. Covariates were assessed using the Pearson or Mantel-Haenszel χ2 test. Ocular phenotype covariates were also assessed for trend using the clustered Wilcoxon rank sum test that incorporates the effect of clustering between individual eyes.33
Binary logistic regression models were used to evaluate the multivariate associations between the CFH R1210C rare variant and ocular phenotype. Multivariate associations were adjusted for the participant’s sex and age at the most recent grade. All regression modeling used generalized estimating equations to account for the effect of clustering in the assessment of individual eyes. For categorical variables with more than 2 levels, separate logistic regression models were used to compare each level with the reference group for each outcome characteristic. This method was used because multinomial logistic regression modeling is not available in generalized estimating equations. Odds ratios and 95% CIs were calculated for each nonreference category of each outcome characteristic vs the reference category.
Eyes that were assigned a CARMS grade indicating another maculopathy or a disease that was not AMD (grade 6) were excluded from all statistical analyses. Patients without adequate photographs displaying the 7 fields of the fundus were excluded from analyses related to extramacular drusen scores. All statistical analyses were performed using SAS version 9.3 (SAS Institute).
Results
The sample size enrolled for analysis comprised 143 patients (283 eyes), among which 62 patients carried the CFH R1210C rare variant. Demographic, behavioral, and ocular characteristics of the total study population, as well as for the family-based and nonfamily-based arms, are reported by R1210C rare variant status (eTable 1 in the Supplement). Each group was predominately female (62.9% and 64.2%, respectively). Body mass index and smoking status were not associated with rare variant status (P = .49 and P = .80, respectively). In the total AMD Study database, age at diagnosis was younger among 30 advanced cases carrying the rare variant compared with 1627 advanced cases who did not (64.3 vs 69.6 years; P = .003). The comparatively smaller sample and age matching of nonfamily comparison individuals without the variant precluded the analysis of age at diagnosis in the study population. A higher proportion of patients with the variant in the family-based arm was classified as having advanced AMD compared with those without the variant (74.2% vs 15.8%; P = .001).
In the Supplement, eTable 2 displays the fundus phenotypes and ocular characteristics of the 3 study groups analyzed (family-based arm, nonfamily-based arm, and total study population), defined by CFH R1210C rare variant status. We found an association between the presence of the rare variant and all fundus phenotype characteristics, with significance retained in both the family-based and nonfamily-based arms of the analysis. Patients with the variant had the highest levels of macular drusen and total macular drusen scores compared with those without the variant: 57.9% vs 16.7% and 52.9% vs 14.2%, respectively. Noteworthy trends were observed for both scores (P for trend < .001). A higher prevalence of macular pigmentary abnormalities was seen among patients carrying the rare variant (79.3% vs 54.9%; P = .005).
Advanced disease was also more likely in the analysis of individual eyes among patients with the variant: 62.8% of patients with the variant were classified as having advanced disease compared with 43.2% without the variant (P for trend = .01).Patients carrying the variant tended to have higher CARMS grades. These patients had a higher proportion of GA compared with those without the rare variant (46.4% vs 17.9%; P for trend < .001). Similar results for GA were reported for the family- and nonfamily-based arms of the study (P for trend < .001 and P for trend = .006, respectively). The family-based arm also exhibited a difference in CNV (CARMS grade 5) based on variant status (58.9% vs 5.7%; P for trend = .009).
The multivariate associations between CFH R1210C rare variant status and the prevalence of phenotypic characteristics by fundus area, adjusting for age at last known CARMS grade and sex, are displayed in the Table. The R1210C rare variant was associated with all macular phenotype variables examined including drusen scores, pigmentary abnormalities, and CARMS grade. The presence of advanced AMD was 7 times more likely among patients carrying the variant (OR, 7.0; 95% CI, 3.1-16.2; P < .001). The highest odds of advanced disease were seen specifically among patients with GA (OR, 13.7; 95% CI, 5.0-37.7; P < .001). The strongest effects of the R1210C rare variant were observed in relation to the presence of drusen, particularly in the highest classification for the macular drusen score, total macular drusen score, and extramacular drusen score. For example, carrying the R1210C variant was associated with 13-fold higher odds of having drusen in 8 to 10 of the macular subfields vs no subfields compared with the population without the variant (OR, 13.4; 95% CI, 6.7-26.7; P < .001). Similar associations were seen for the total macular and extramacular drusen scores (ORs, 17.1 and 3.5, respectively; P < .001). Macular pigmentary abnormalities were also related to rare variant status when comparing patients with the variant with those without (OR, 3.1; 95% CI, 1.8-5.3; P < .001). Noteworthy trends were observed for higher macular (P for trend = .02) and total macular drusen scores (P for trend = .02), more advanced AMD group (P for trend = .02), and higher CARMS grade (P for trend < .001) comparing those with the variant with those without.
Table.
Multivariate Associations Between the CFH R1210C Rare Variant and Prevalence of Phenotypic Characteristics
| Characteristic | Odds Ratio (95% CI) | P Valuea | P Value for Trendb |
|---|---|---|---|
| No. of patients (eyes) | 143 (283) | ||
| Macular drusen scorec | |||
| 0 | 1 [Reference] | .02 | |
| 1-3 | 2.6 (1.2-5.5) | .01 | |
| 4-7 | 2.6 (1.0-6.9) | .047 | |
| 8-10 | 13.4 (6.7-26.7) | <.001 | |
| Total macular drusen scored | |||
| 0 | 1 [Reference] | .02 | |
| 1-5 | 1.5 (0.7-3.1) | .27 | |
| 6-10 | 4.7 (2.0-11.5) | <.001 | |
| >10 | 17.1 (7.7-38.0) | <.001 | |
| Macular pigment abnormality | |||
| No | 1 [Reference] | .08 | |
| Yes | 3.1 (1.8-5.3) | <.001 | |
| Extramacular drusen scoree | |||
| 0 | 1 [Reference] | .16 | |
| 1-3 | 0.8 (0.3-1.9) | .60 | |
| 4-7 | 3.5 (1.7-7.1) | <.001 | |
| AMD groupf | |||
| No AMD | 1 [Reference] | .02 | |
| Early/intermediate AMD | 5.8 (2.4-14.1) | <.001 | |
| Advanced AMD | 7.0 (3.1-16.2) | <.001 | |
| CARMS grade | |||
| 1 | 1 [Reference] | ||
| 2 | 6.9 (2.0-23.4) | .002 | |
| 3 | 7.6 (2.6-21.9) | <.001 | |
| 4 | 13.7 (5.0-37.7) | <.001 | <.001 |
| 5 | 5.2 (2.2-12.4) | <.001 | .11 |
Abbreviations: AMD, age-related macular degeneration; CARMS, Clinical Age-Related Maculopathy Staging; CFH, complement factor H.
Calculated using generalized estimating equation, comparing each covariate level with the reference level after adjusting for age and sex.
Calculated using a clustered Wilcoxon rank sum test with stratification by age/sex groups. Pvalue for trend was assessed separately for CARMS grades 1-4 (geographic atrophy as the advanced stage) and 1, 2, 3, and 5 (neovascular disease as the advanced stage).33
Presence of drusen in any of the 10 macular subfields (0-10).
Total macular drusen, with each subfield weighted by the total number of drusen present (0-40).
Presence of drusen in any of the 7 extramacular fields (0-7).
Age-related macular degeneration groups classified using the CARMS system: no AMD (grade 1); early/intermediate AMD (grades 2 and 3); and advanced AMD (grades 4 and 5).24
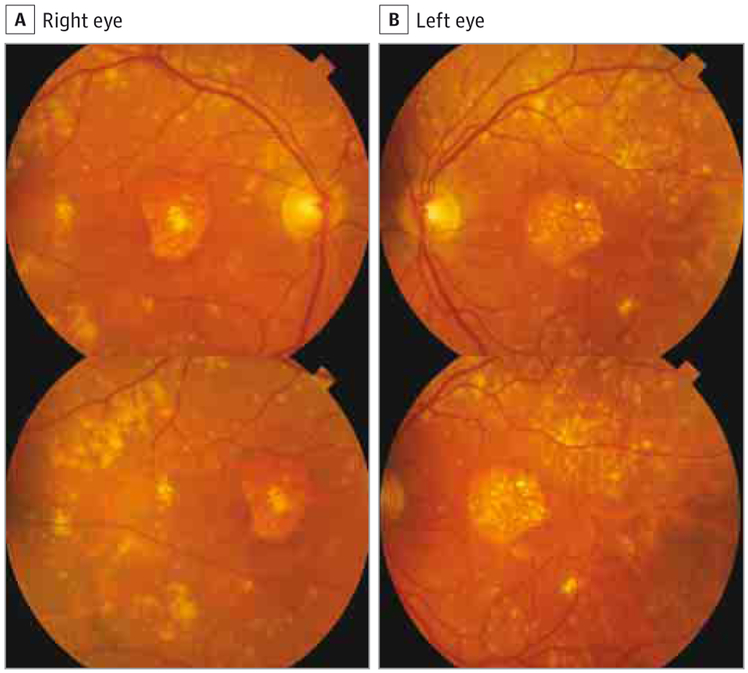
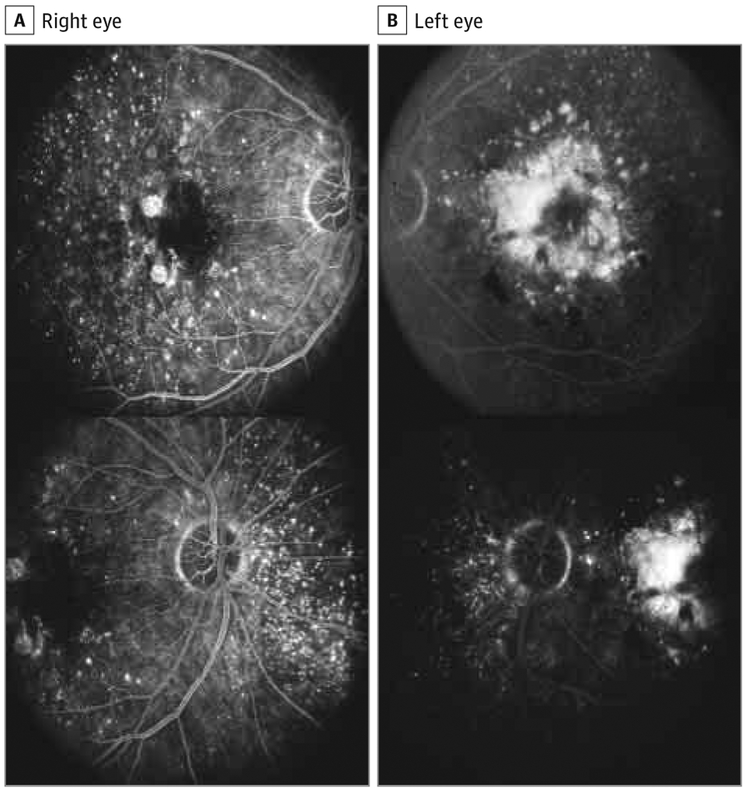
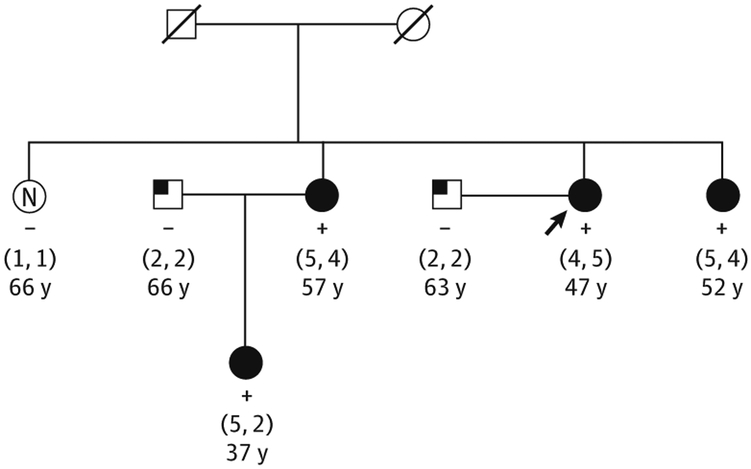
Inpatients with the rare variant, drusen deposition was typically characterized by voluminous accumulation of confluent soft drusen in the entire macula, sometimes associated with drusenoid retinal pigment epithelial detachment (Figure 1). Extensive extramacular drusen deposition throughout the fundus was also observed in association with the variant (Figure 2 and Figure 3). A representative pedigree shown in Figure 4 depicts 4 siblings among whom 3 carry the rare variant. Each sibling with advanced AMD has GA in 1 eye and CNV in the other. The one unaffected sibling does not have the variant. The macular phenotype of the proband at age 52 years is shown in Figure 3; numerous drusen are present throughout the macula and nasal to the optic disc.
Figure 1. Voluminous Soft Drusen Accumulation in the Macula Associated With the Complement Factor H (CFH) R1210C Rare Variant.
Color photographs of a patient with the CFH R1210C mutation show voluminous soft drusen accumulation, large drusenoid retinal pigment epithelium detachments, and marked retinal pigment epithelium abnormalities.
Figure 2. Extensive Drusen Accumulation Throughout Retinal Vascular Arcades Associated With the Complement Factor H (CFH) R1210C Rare Variant.
Color fundus photographs of a patient with the CFH R1210C rare variant and geographic atrophy in the right (A) and left (B) eyes are shown. Large confluent soft drusen are seen in the macular area and extending throughout the topography of retinal vascular arcades and beyond.
Figure 3. Extensive Drusen Accumulation Temporal to the Macula and Nasal to the Optic Disc Associated With the Complement Factor H (CFH) R1210C Rare Variant.
Fluorescein angiography of a 52-year-old woman with the CFH R1210C rare variant presenting with an advanced stage of age-related macular degeneration in both eyes. A, The right eye has the dry form of age-related macular degeneration, with foci of geographic atrophy (Clinical Age-Related Maculopathy Staging [CARMS] system grade 4). B, The left eye has neovascular age-related macular degeneration with a disciform scar (CARMS grade 5). A large number of drusen of different sizes are evident throughout the macular area, temporal to the macula and nasal to the optic disc.
Figure 4. Representative Pedigree Showing Segregation of the Complement Factor H R1210C Rare Variant With Age-Related Macular Degeneration.
The arrow indicates the proband (images in Figure 3). The numbers within parentheses indicate the age-related macular degeneration grades (right eye, left eye); numbers below grades, age at diagnosis (affected patients) or age at last known grade (unaffected patients). The filled circles indicate Clinical Age-Related Maculopathy Staging (CARMS) grades 4 and 5; the partially filled squares, CARMS grade 2; the open circle with an N, unaffected (CARMS grade 1); +, positive R1210C carrier status; and −, negative R1210C carrier status.
Discussion
To our knowledge, this is the first systematic evaluation and demonstration that the R1210C rare variant is strongly associated with soft drusen accumulation throughout the macula and ocular fundus, as well as with advanced AMD, compared with individuals not carrying the variant. Although the R1210C rare variant is known to confer the strongest genetic risk for AMD,12 its typical phenotypic presentation had not been well characterized prior to this study. Therefore, we investigated a relatively large population with the rare variant and identified the typical clinical presentations.
The complement cascade is a key component of the innate immunological system with several fundamental roles including elimination of microorganisms and damaged cells, formation of activation products that initiate inflammation, enhancement of adaptive immune reactions, and clearance of immune complexes. The complement cascade consists of 3 different pathways that ultimately converge: the classic, lectin, and alternative pathways. Factor H is a potent inhibitor of both the classic and alternative complement pathways, and CFH gene mutations have been associated with a broad spectrum of pathological conditions including membranoproliferative glomerulonephritis, atypical hemolytic uremic syndrome, and AMD.34,35 Histopathological studies on drusen in membranoproliferative glomerulonephritis type II showed deposits in the Bruch membrane that are similar to glomerular dense deposits in the kidney.36-40 There are notable histological similarities between the choriocapillaris-Bruch membrane–RPE complex and the capillary–glomerular basal membrane–glomerular epithelium interface.36 Previous genetic studies also suggested association of CFH mutations with basal laminar drusen, which have distinct clinical manifestation and natural course in comparison with typical AMD.30,41
Limitations of this study included the retrospective nature and the study design being dependent on the completeness of the medical records and multimodal imaging. Assessments performed by a single grader for some features were also a limitation with potential impact on the interpretation of the results. For the nonfamily-based arm of the study, comparison patients without the variant were selected randomly from the AMD Study database, which includes a large proportion of advanced cases. This selection of comparison individuals could potentially underestimate the differences between patients with and without the variant in regards to AMD grade in the nonfamily-based arm. The strength of this study was that many years of cumulative data were accessible through our AMD Study to substantiate the phenotypic characterization we describe, although CFH R1210C is a rare variant in the population. Rare variants have been shown to be highly associated with an increased risk for AMD in case-control association studies12,13,42-44 and in models predicting progression to advanced stages over time,22,23 and they can segregate in families, as described in this study, and in families with other rare CFH variants.45,46
Conclusions
The phenotypic presentation associated with CFH R1210C described in this study will facilitate its clinical recognition in ophthalmological practice and supports the potential benefit of genetic screening in selected patients with AMD. A better characterization of genetic profiles in AMD may have an important role in developing more efficacious therapeutic strategies for this vision-threatening condition.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants RO1-EY11309 and RO1-EY022445 from the National Institutes of Health; the Massachusetts Lions Eye Research Fund Inc, New Bedford; unrestricted grants from Research to Prevent Blindness Inc, New York, New York; Foundation Fighting Blindness, Columbia, Maryland; the American Macular Degeneration Foundation, Northampton, Massachusetts; and the Age-Related Macular Degeneration Research Fund, Ophthalmic Epidemiology and Genetics Service, Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ferrara is an employee of Genentech Inc and has equity/options from Roche. No other disclosures were reported.
Contributor Information
Daniela Ferrara, New England Eye Center, Tufts University School of Medicine, Boston, Massachusetts.
Johanna M. Seddon, New England Eye Center, Tufts University School of Medicine, Boston, Massachusetts; Ophthalmic Epidemiology and Genetics Service, Department of Ophthalmology, Tufts University School of Medicine, Boston, Massachusetts.
REFERENCES
- 1.Sobrin L, Seddon JM. Nature and nurture: genes and environment predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritsche LG, Chen W, Schu M, et al. ; AMD Gene Consortium. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45(4):433–439, e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. [DOI] [PubMed] [Google Scholar]
- 4.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308(5720):419–421. [DOI] [PubMed] [Google Scholar]
- 6.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet. 2006;38(9):1055–1059. [DOI] [PubMed] [Google Scholar]
- 8.Seddon JM, Reynolds R, Rosner B. Peripheral retinal drusen and reticular pigment: association with CFHY402H and CFHrs1410996 genotypes in family and twin studies. Invest Ophthalmol Vis Sci. 2009;50(2):586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerness JA, Maller JB, Neale BM, Reynolds RC, Daly MJ, Seddon JM. Variation near complement factor I is associated with risk of advanced AMD. Eur J Hum Genet. 2009;17(1):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold B, Merriam JE, Zernant J, et al. ; AMD Genetics Clinical Study Group. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39(10):1200–1201. [DOI] [PubMed] [Google Scholar]
- 12.Raychaudhuri S, Iartchouk O, Chin K, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011; 43(12):1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seddon JM, Yu Y, Miller EC, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45(11):1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Barricarte R, Pianetti G, Gautard R, et al. ; European Working Party on the Genetics of HUS. The complement factor H R1210C mutation is associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2008;19(3):639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann HP, Salzmann M, Bohnert-Iwan B, et al. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J Med Genet. 2003;40 (9):676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Corral P, Pérez-Caballero D, Huarte O, et al. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am J Hum Genet. 2002;71(6):1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caprioli J, Bettinaglio P, Zipfel PF, et al. ; Italian Registry of Familial and Recurrent HUS/TTP. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol. 2001;12(2):297–30. [DOI] [PubMed] [Google Scholar]
- 18.Servais A, Frémeaux-Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44(3):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira VP, Herbert AP, Cortés C, et al. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol. 2009;182 (11):7009–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Józsi M, Heinen S, Hartmann A, et al. Factor H and atypical hemolytic uremic syndrome: mutations in the C-terminus cause structural changes and defective recognition functions. J Am Soc Nephrol. 2006;17(1):170–177. [DOI] [PubMed] [Google Scholar]
- 21.Manuelian T, Hellwage J, Meri S, et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003; 111(8):1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon JM, Reynolds R, Yu Y, Rosner B. Three new genetic loci (R1210C in CFH, variants in COL8A1 and RAD51B) are independently related to progression to advanced macular degeneration. PLoS One. 2014;9(1):e87047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seddon JM, Silver RE, Kwong M, Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates [published online February 5, 2015]. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.14-15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology. 2006;113(2):260–266. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y,Bhangale TR, Fagerness J, et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet. 2011;20(18):3699–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A. 2010;107(16):7395–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786–806. [PubMed] [Google Scholar]
- 28.Bird AC, Bressler NM, Bressler SB, et al. ; The International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367–374. [DOI] [PubMed] [Google Scholar]
- 29.Boon CJ, van de Ven JP, Hoyng CB, den Hollander AI, Klevering BJ. Cuticular drusen: stars in the sky. Prog Retin Eye Res. 2013;37:90–113. [DOI] [PubMed] [Google Scholar]
- 30.Gass JD, Jallow S, Davis B Adult vitelliform macular detachment occurring in patients with basal laminar drusen. Am J Ophthalmol. 1985;99(4): 445–459. [DOI] [PubMed] [Google Scholar]
- 31.Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117(9):1775–1781. [DOI] [PubMed] [Google Scholar]
- 32.Kanagasingam Y, Bhuiyan A, Abràmoff MD, Smith RT, Goldschmidt L, Wong TY. Progress on retinal image analysis for age related macular degeneration. Prog Retin Eye Res. 2014;38:20–42. [DOI] [PubMed] [Google Scholar]
- 33.Rosner B, Glynn RJ, Lee ML. Extension of the rank sum test for clustered data: two-group comparisons with group membership defined at the subunit level. Biometrics. 2006;62(4):1251–1259. [DOI] [PubMed] [Google Scholar]
- 34.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61(2):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol. 2013; 33(6):508–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16(5):1392–1403. [DOI] [PubMed] [Google Scholar]
- 37.Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond). 2001;15(Pt 3):390–395. [DOI] [PubMed] [Google Scholar]
- 38.Bresin E, Rurali E, Caprioli J, et al. ; European Working Party on Complement Genetics in Renal Diseases. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24(3):475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Quintrec M, Zuber J, Moulin B, et al. Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant. 2013;13(3):663–675. [DOI] [PubMed] [Google Scholar]
- 40.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boon CJ, van de Kar NC, Klevering BJ, et al. The spectrum of phenotypes caused by variants in the CFH gene. Mol Immunol. 2009;46(8-9):1573–1594. [DOI] [PubMed] [Google Scholar]
- 42.Helgason H, Sulem P, Duvvari MR, et al. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nat Genet. 2013;45(11):1371–1374. [DOI] [PubMed] [Google Scholar]
- 43.van de Ven JP, Nilsson SC, Tan PL, et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat Genet. 2013;45(7):813–817. [DOI] [PubMed] [Google Scholar]
- 44.Zhan X, Larson DE, Wang C, et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nat Genet. 2013;45(11):1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Y, Triebwasser MP, Wong EK, et al. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014;23(19):5283–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sobrin L, Maller JB, Neale BM, et al. Genetic profile for five common variants associated with age-related macular degeneration in densely affected families: a novel analytic approach. Eur J Hum Genet. 2010;18(4):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.