Abstract
The contamination of the widely used lifesaving anticoagulant drug heparin in 2007 has drawn renewed attention to the challenges that are associated with the characterization, quality control and standardization of complex biological medicines from natural sources. Heparin is a linear, highly sulfated polysaccharide consisting of alternating glucosamine and uronic acid monosaccharide residues. Heparin has been used successfully as an injectable antithrombotic medicine since the 1930s, and its isolation from animal sources (primarily porcine intestine) as well as its manufacturing processes have not changed substantially since its introduction. The 2007 heparin contamination crisis resulted in several deaths in the United States and hundreds of adverse reactions worldwide, revealing the vulnerability of a complex global supply chain to sophisticated adulteration. This Perspective discusses how the US Food and Drug Administration (FDA), the United States Pharmacopeial Convention (USP) and international stakeholders collaborated to redefine quality expectations for heparin, thus making an important natural product better controlled and less susceptible to economically motivated adulteration.
The anticoagulant heparin has been in clinical use since 1935 (refs. 1–4). Heparin is one of the oldest drugs still in clinical use, and in volume is second only to insulin as a natural product therapeutic agent. A monograph for heparin entered the USP in 1950, with tests for identity, strength, quality, purity and potency5. This pharmacopeia standard was one of several elements in the multi-tiered safety net that helped assure practitioners they were administering, and patients they were receiving, good quality, safe and effective heparin drug products. Gaps in this safety net appeared in late 2007 and early 2008 when epidemiologic6 and analytical investigations7 indicated that an adulterant in heparin products was associated with severe patient morbidity and mortality8. Although the details of this episode may never be fully known, it appears to have been an example of intentional adulteration for gain of profit. The adulterant was capable of meeting antiquated tests, including the USP clotting test for potency. An immediate recall of contaminated heparin was issued in United States, leading to acute shortages of this lifesaving drug in the US market.
The lack of specific tests for controlling the quality and purity of heparin products had failed to prevent contaminants from entering into the market place. In the wake of the heparin contamination crisis, a research team, including the Ronzoni Institute (Milan, Italy), the FDA, Rensselaer Polytechnic Institute (Troy, NY, USA) and the Massachusetts Institute of Technology (Cambridge, MA, USA), identified the contaminant as oversulfated chondroitin sulfate (OSCS)9,10. In addition, FDA reached out to the USP to initiate a rapid revision of the existing monograph. The USP responded by convening a panel of international experts on heparin to develop necessary compendial improvements. The initial changes, implemented in the United States after only three months, included the addition of critical identity tests, employing capillary electrophoresis and proton nuclear magnetic resonance (1H-NMR)11. These initial changes heralded a dramatic shift in our understanding of the role of identity testing for heparin products in danger of contamination or adulteration. In addition to testing for the identity of heparin, these tests introduce a requirement to detect the presence of a known adulterant and other impurities. Beyond the immediate measures taken, a comprehensive overhaul of quality expectations for heparin followed. This revision took into account the complexities and impact of manufacturing processes on heparin structure, and the realities of a global supply chain.
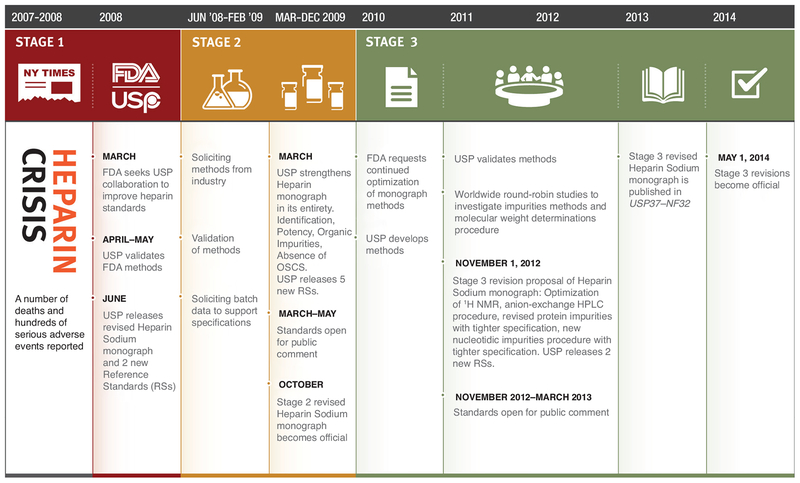
Over the years since the contamination crisis, the USP undertook (and has concluded) a three-stage set of revisions to the compendial quality requirements. The introduction of these additional changes aimed at striking a balance between two important goals: first, further strengthening the quality control of heparin; and second, at the same time, ensuring continuous availability of pharmaceutical heparin. The revisions were advanced in a data-driven manner (Fig. 1). This approach required the analysis of many batches of heparin by the FDA, USP, and heparin manufacturers. Beyond the initial addition of identity tests, the second stage of revisions shifted potency testing from a clotting test to measuring the antithrombin-mediated inhibition of factor Xa and factor IIa activities, two proteases of the blood coagulation cascade. This change increased the specificity for potency testing. Stage 2 revision also introduced additional tests and tightened existing limits for impurities12. At the same time, the spectroscopic and separations-based components of the identification test were further refined. Since 2009, additional focus has been placed on the following: first, the control of heparin raw materials, as reflected in the FDA-issued guidance13; second, the incentive to further tighten limits for additional process impurities, such as proteins and nucleic acids; and third, the control of heparin’s polydispersity by molecular size analysis14. This Perspective summarizes the scientific rationale behind a set of orthogonal tests and FDA’s actions to both enforce these tests and ensure the safety of heparin.
Figure 1.
Heparin crisis and resolution timeline. After the contamination crisis, the USP undertook and concluded a three-stage set of revisions to the Heparin Sodium monograph. Stage 1 involved the initial addition of identity tests and introduction of reference standards to prevent contaminated heparins from entering the US market. Stage 2 strengthened the entire monograph by including specific potency testing, additional tests and tightening existing limits for impurities. Stage 3 focused on further tightening limits for impurities and controlling heparin’s polydispersity by molecular weight analysis.
Structural heterogeneity and purity of heparin
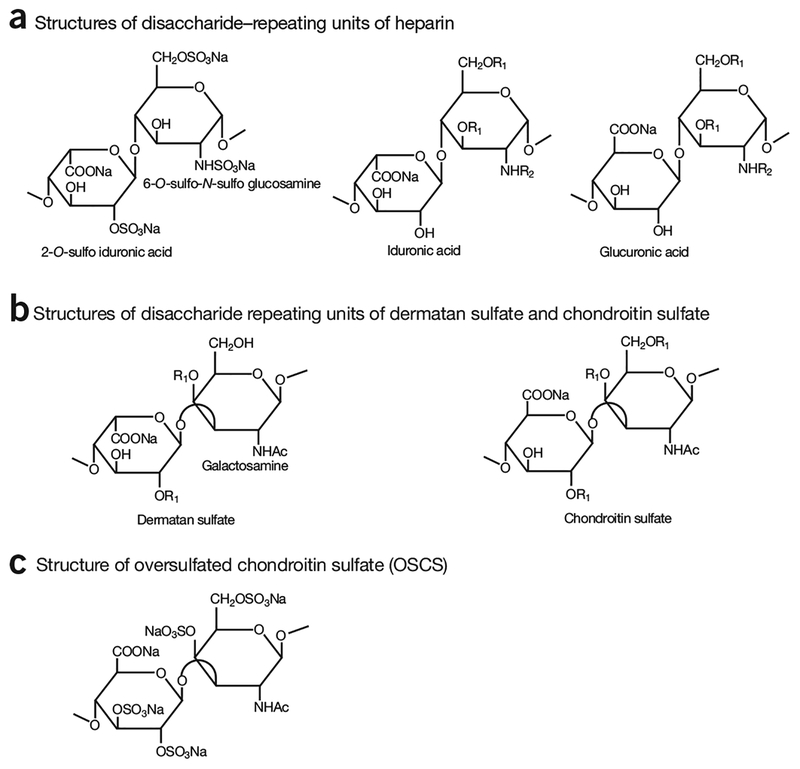
Although heparin has been used for 80 years, a simple set of standards for its identity and purity has not existed because heparin is a complex mixture of highly sulfated polysaccharides. Pharmaceutical grade heparin is a polydisperse glycosaminoglycan (GAG) composed of disaccharide repeating units of 1→4 linked uronic acid (α-l-iduronic acid or β-d-glucuronic acid) and α-d-glucosamine1–3,14,15. Each mono-saccharide unit can be differently sulfated, leading to a high degree of structural heterogeneity (Fig. 2)15. The structural heterogeneity of heparin is primarily attributed to heparin biosynthesis. Heparin is biosynthesized as a proteoglycan that consists of a core protein and heparin polysaccharide side chains. Heparin chain biosynthesis involves a series of glycosyltransferases, sulfotransferases and an epimerase. The modifications by these biosynthetic enzymes are only partially completed, resulting in a highly heterogeneous mixture of polysaccharides varying in sulfation pattern and saccharide chain length16. Heparin undergoes cleavage by heparanase, further increasing its structural heterogeneity17. Both the length of the chains and variation in sulfation patterns affect the anticoagulant activity of heparin18.
Figure 2.
Structures of disaccharide-repeating units of glycosaminoglycans. (a) Three different disaccharide-repeating units found in heparin. The disaccharide of -IdoA2S-GlcNS6S- is most abundantly present in heparin, where IdoA2S represents 2-O-sulfo-iduronic acid; and GlcNS6S represents 6-O-sulfo-N-sulfoglucosamine. (b) The disaccharide-repeating units of dermatan sulfate and chondroitin sulfate. The disaccharide-repeating units of dermatan and chondroitin sulfates contain galactosamine. Furthermore, the uronic acid and galactosamine residues are linked through α1→3 linkages. (c) The disaccharide-repeating unit of oversulfated glycosaminoglycans, oversulfated chondroitin sulfate (OSCS), an adulterant product of chemically modified chondroitin sulfates found in contaminated heparin products. R1 = −H or −SO3Na; R2 = −H, −Ac or −SO3Na.
Heparin approved in the United States is derived from porcine intestinal mucosa. The mucosa is extracted into an aqueous solution, captured from the extract by complex formation or binding to a resin, eluted with salt, precipitated with organic solvent, and finally purified by oxidation and further precipitations, filtration and a final drying step (Supplementary Note 1 and Supplementary Fig. 1). Other GAG impurities, such as chondroitin and dermatan sulfates, which have disaccharide-repeating units different from heparin (Fig. 2), can be found in pharmaceutical grade heparin. Improvements to manufacturing processes in recent years have reduced the levels of GAG impurities19. Certain manufacturing processes affect the structure of heparin. For example, oxidation by KMnO4 is used to remove the core protein from the heparin product. This reaction alters the reducing end of heparin through several chemical side-reactions, leading to unnatural saccharide residues20. Other structural changes in heparin (i.e., O-acetylation and desulfation) resulting from manufacturing processes have also been reported21,22.
Thus, the implementation of purity standards and tests to characterize heparin had to take into account three important issues: first, a molecular-level definition of heparin as a polydisperse product; second, a limit of detection of 0.1% for OSCS; and third, product variants arising from the manufacturing and purification steps employed.
Methods establishing heparin identification
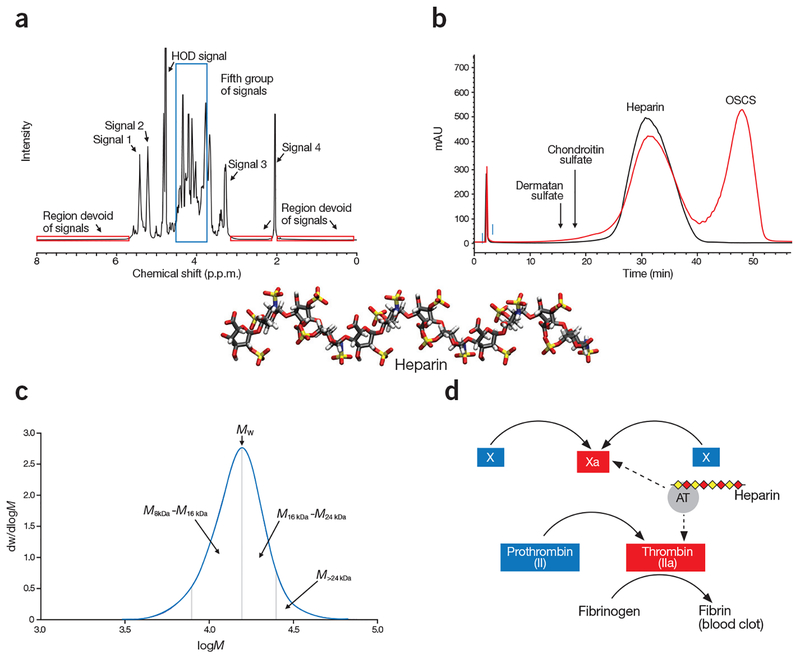
Orthogonal methods are used in the revised heparin monograph to determine heparin’s identity (Fig. 3). These four methods were designed to confirm the chemical structures of heparin products and to measure batch-to-batch or manufacturer-to-manufacturer variability. The tests should catch contaminants before they enter the supply chain, according to FDA’s guidelines13. The orthogonal tests are complementary, probing chemical fine structure, charge characteristics, molecular weight properties and anticoagulant activity, allowing comprehensive characterization of a complex macromolecule like heparin. 1H-NMR (nuclear magnetic resonance) is used to characterize the structures of monosaccharide building blocks and provides linkage information on heparin (Fig. 3a). Strong anion exchange–high performance liquid chromatography (SAX–HPLC) assesses the density of the sulfo groups present on heparin and serves as a tool to detect the presence of OSCS and other potential contaminants and impurities (Fig. 3b). The molecular weight distribution measurement is used to determine the polydispersity, an intrinsic property of GAGs (Fig. 3c). The anti-factor-Xa and anti-factor-IIa activity measurements are designed to determine the anticoagulant activity that is specifically attributed to the action of heparin (Fig. 3d).
Figure 3.
Orthogonal analysis of heparin. (a) Spectroscopic analysis shows a typical 1H-NMR spectrum of heparin. The specification for heparin included the identification of five groups of signals is: Signal 1 (5.42 p.p.m.) represents H1 of GlcNAc6S/GlcNS6S; Signal 2 (5.21 p.p.m.) represents H1 of IdoA2S; Signal 3 represents H2 of GlcNS (3.28 p.p.m.); and Signal 4 represents the methyl of GlcNAc (2.05 p.p.m.). The fifth group of signals (3.75–4.55 p.p.m.), corresponding to a number of heparin protons, are surrounded by a blue box. Signals 1, 2, and 4 are of comparable intensity. Signal 3 is substantially smaller than signal 2. The intensity of the fifth group of signals are 1–3 times higher than signal 2. In addition, the spectrum profile between 3.2 p.p.m. and 4.6 p.p.m., and between 4.9 p.p.m. and 5.7 p.p.m., closely matches the reference spectrum, both in intensity and signal position. The three red boxes surround regions of the spectrum where no signals should be observed. (b) Chromatographic mobility analysis showing compiled SAX–HPLC chromatograms of heparin standard (black tracing) and the mixture of HP and OSCS (red tracing). The eluted positions of other glycosaminoglycans, like dermatan sulfate and chondroitin sulfate, are indicated by arrows. (c) Molecular weight distribution analysis, shows a typical molecular weight distribution curve of heparin. In this particular determination, the weight average molecular weight (Mw) is 15,916; the proportion of material above 24 kDa (M>24 kDa) is 8.4% and the ratio of material 8–16 kDa to 16–24 kDa (M8–16 kDa/M16–24 kDa) is 1.46. dw/dlogM (y axis) represents the fraction of the material by weight. (d) Potency analysis of a partial blood coagulation cascade, demonstrating the mechanism of action of heparin. Heparin achieves its anticoagulant activity by binding to antithrombin (AT), and the heparin/AT complex inhibits the activities of factor Xa and factor IIa to prevent the formation of fibrin from fibrinogen, which is a key step in generating blood clots. The dashed arrows depict the action sites in the blood coagulation cascade by the complex of AT/heparin.
Given the structural complexity of heparin, comprehensive 1H-NMR analyses of the molecule can be performed only by highly trained NMR spectroscopists23–25. Concerns were raised over the reliability from the 1H-NMR analyses performed by relatively inexperienced technicians at heparin manufacturing facilities. In response to these concerns, the USP’s panel of experts designed a simplified version of 1H-NMR analysis. Instead of completely analyzing the entire spectrum, the operator is required to identify only five major groups of signals belonging to heparin (Fig. 3a). In addition, several designated regions from 0 to 10 p.p.m. of the 1H-NMR spectrum are also examined, as any signals found in these regions are likely to be associated with impurities in a heparin product. This simplified approach was then validated on 31 heparin samples26. Another critical application of 1H-NMR is to identify persulfonated polysaccharide-based adulterants, especially OSCS. The 1H-NMR spectrum of a heparin sample contaminated with OSCS displays a distinctive signal at around 2.15 p.p.m. (Supplementary Fig. 2), offering a reliable method to determine the OSCS contaminant in heparin products9. Appropriately validated 1H-NMR analysis using either a 300 MHz instrument or a 500 MHz instrument is capable of detecting the level of OSCS as low as 0.25% (w/w). The use of 500 MHz instrument, however, offers advantages over 300 MHz instrument: shortening analysis time and providing greater assurance of drug quality as the instrument has higher resolution and greater sensitivity. Additional descriptions about 1H-NMR analysis are presented in Supplementary Note 2.
As a negatively charged polysaccharide, heparin binds to an anion exchange chromatography column, and heparin’s elution correlates with its molecular size and the density of negative charges of its sulfo and carboxyl groups. After careful comparison of different chromatography methods, the USP panel chose SAX–HPLC as the method to characterize heparin products. Three factors influenced the USP panel’s choice: resolution, mobile phase suitability and the column matrix suitability. The mobile phase uses sodium perchlorate due to its transparency at 200 nm, the wavelength used to detect heparin eluted from the chromatographic column. The selection of the SAX column, tightly binding both heparin and OSCS, is especially important for detection of low levels of OSCS contaminant (Fig. 2c). An excellent separation of heparin and OSCS can be achieved by SAX–HPLC and method optimization allowed the detection of 0.1% OSCS in heparin (Fig. 3b)27. Capillary electrophoresis was initially used to detect OSCS in heparin11, but it was not used in the revised (stage 2 and stage 3) monograph, because SAX–HPLC analysis is more convenient in manufacturing quality control laboratories (Supplementary Note 3).
Measuring the molecular weight (MW) distribution ensures the consistency of heparin product28,29. Results from a coordinated study involving 21 laboratories from eight countries to measure 122 heparin samples show that the average MW of heparin lies in the range of 15–19 kDa30. In addition, the shape of the MW distribution curve is distinctive (Fig. 3c), with very high MW chains (>24 kDa) typically comprising <20% of a heparin sample, and shorter chains (8–16 kDa) having a larger area under the MW distribution curve than the longer heparin chains (16–24 kDa). Based on these findings, the USP panel set the upper and lower limits for the average MW, an upper limit for very high MW and a lower limit of 1.0 for the ratio of the populations of shorter and longer heparin chains ((8–16 kDa)/(16–24 kDa)) (Supplementary Note 4 and Supplementary Table 1).
Determination of heparin’s inhibition of factor Xa and factor IIa (or thrombin), known as anti-Xa and anti-IIa activities, improved the specificity of the potency test. This assay is based on heparin’s ability to potentiate antithrombin31, a coagulation inhibitor, to inactivate factor IIa and factor Xa (Fig. 3d). Utilization of purified reagents, including factor IIa, factor Xa, and specific polypeptide-based chromogenic substrates, in the assay allows the measurement of specific effects from heparin, avoiding the potential for interference from plasma proteins or the presence of OSCS (Supplementary Note 5 and Supplementary Table 2). Over the past 30 years, there has been a 10% drift in potency definition between USP unit and international unit (IU) due to the improved quality of reference materials and potency assay changes32. The employment of the new potency assay and new USP Heparin Sodium for Assays reference standard aligned both units, and brought minimum potency requirements in line with expectations for modern heparin products. Potency analysis of 90 heparin samples showed that narrowing the range of the anti-factor Xa to anti-factor IIa ratio, a characteristic potency indicator for heparin, to 0.9–1.1, minimizes the risk of adulteration (Supplementary Note 5 and Supplementary Fig. 3).
Tests for impurities
The new monograph tightens the allowable levels of organic solvents (introduced from manufacturing processes) and impurities, like chondroitin or dermatan sulfates, protein and nucleotidic impurities, in the heparin product. Improved analytical methods are introduced to improve impurity detection sensitivity.
HPLC-based methods are used for measuring the levels of galactosamine and nucleotides to monitor the level of chondroitin and dermatan sulfates and nucleotidic impurities, respectively (Supplementary Figs. 4 and 5). The protein impurity level is monitored by the Lowry method, to measure protein levels as low as 0.1% (w/w) (Supplementary Note 6).
Safeguarding heparin’s supply chain
The FDA’s responses to the 2008 heparin contaminant crisis were swift and comprehensive. Since the identification of OSCS as the contaminant in heparin7,9, the FDA has been maintaining an active research program for discovering new methods to monitor the level of OSCS or of various impurities in heparin products19,25,28,33–47. These extensive research activities provide a critical scientific foundation for the heparin monograph revisions from initial stages to the latest stage. Equipped with the most updated and advanced knowledge in heparin chemistry and analysis, the FDA research team advises the agency how to respond to any potential poor quality heparin products that may enter the supply chain. In addition, the FDA requires manufacturers to provide analytical data for every batch of drug product entering the US market. The FDA reviews these data before accepting shipments of the drug. Furthermore, the FDA has increased the number of random tests on those drugs considered to be at high risk for potential issues with drug quality, including heparin (http://www.fda.gov/drugs/scienceresearch/ucm407277.htm). Finally, the FDA in the new Office of Pharmaceutical Quality has established the Office of Process and Facilities with a mission to integrate review, inspection, surveillance and research across a product’s life cycle. For heparin, this integration will assure that quality heparin products are produced and monitored by the agency for as long as the drug is still used.
The heparin crisis in 2008 also triggered a series of policy changes at the FDA involving drug substances that are imported from other countries. Starting in November of 2008, FDA began to establish its offices in Asia and around the world. The FDA’s international offices increased collaboration with local regulatory agencies and established a better understanding of the foreign manufacturers. These efforts are particularly important to safeguard the supply chain of heparin as 50–70% of the heparin used in United States is imported from other countries.
Title VII of the Food and Drug Administration Safety and Innovation Act (FDASIA) gives the FDA wider authority to monitor drug supply chains. FDASIA Section VII enhances FDA’s functions in inspecting manufacture sites and production records of domestic and international suppliers, and authorizes FDA to detain or destroy those drug substances that are adulterated or counterfeit products. A new drug safety law, known as the Drug Supply Chain Security Act, became effective in January 2015, requiring an electronic identifier be present on the package of prescription drugs sold in the United States (http://www.fda.gov/Drugs/DrugSafety/DrugIntegrityandSupplyChainSecurity/DrugSupplyChainSecurityAct/default.htm). This system allows the agency to trace the drug supply chain and establishes the legitimacy of the drug product, facilitates any recalls of the product and strengthens supply chain oversight by manufacturers.
For US marketed heparin, the changes to the USP heparin sodium monograph in 2008, 2009 and 2014 enhanced the quality of this drug by improved detection of contamination, reducing impurity levels and establishing identity tests that are sensitive to the structure and composition of the drug19. Notable reduction in the impurity level of dermatan sulfate in heparin products was reported in those samples collected in 2009, after the revised heparin monograph was introduced19. In addition, the implementation of the new USP monograph assays led to the elimination of the spike in the number of reported adverse events observed in 2008 that were associated with the contamination of the heparin supply chain with OSCS.
As a product used globally, different regulatory agencies from around the world have their own jurisdiction to set the slightly different purity standards for heparin products. From 2008 to 2014, USP worked closely with European and Japanese Pharmacopeias to harmonize the Heparin Sodium monograph. This collaboration resulted in general harmonization of many monograph procedures, including 1H-NMR, chromatographic identity, potency assay and impurities methods. Although procedures are well harmonized, it should be noted that monograph acceptance criteria may vary slightly due to different local regulatory expectations. Despite those small differences, the rationales for the revised monographs, aimed at improving the purity to prevent the counterfeits from reentering the supply chain, are primarily based on the same principles.
Conclusions
The experience with heparin changed the way the FDA thought about complex drugs and drugs that were approved before modern testing paradigms were established (e.g., the International Conference on Harmonization). The FDA, proactively, in collaboration with USP and stakeholders, has initiated compendial improvements for safeguarding drug supply chains for many widely used pharmaceutical drugs (biologicals and small molecules) by updating the old specifications with emphasis on critical quality attributes like potency and impurities (see http://www.usp.org/usp-nf/development-process/monograph-modernization). The implementation of orthogonal USP tests for key intermediates, active pharmaceutical ingredients or drug products enables multi-layer confirmation of drug structural and functional properties along the supply chain. The FDA has established a Monograph Modernization Working Group that interfaces with the USP Monograph Modernization Program. The main task of this program is to update outdated test methods and improve the related critical quality attributes such as, identity, assay, potency, purity for many widely used drugs. In addition, this program provides updated public standards and materials useful for independent validation of analytical methodology pertinent to compendial requirements.
The morbidity and mortality arising from contaminated heparin from 2007 to 2008 was one of the most negative episodes of a toxic drug product reaching the market in the United States since the ‘Elixir of Sulfonilamide’ poisoning in the late 1930s, which led to the passage of the US Food, Drug and Cosmetic Act. Many might have argued that the safety nets in existence in 2008 arising in the 70 years since creation of that Act and its subsequent revisions should have prevented the event from occurring, but the event itself proved that this was not the case. Just as the Elixir of Sulfanilamide had a continuing impact on how the United States regulates the quality of medicines, the expectation is that the episode of contamination of heparin drug substance with OSCS will continue to have a profound influence on regulatory oversight. A particular example is the recently concluded Drug Quality and Security Act, the latter part of which speaks to good supply chain practices.
The new USP heparin monograph can now presumably protect against contamination with OSCS, provided Good Manufacturing Practices are followed and testing occurs when ingredients are taken out of quarantine and at batch release. Are there other contaminants that could ‘get past’ the tests of the new monograph? We do not think so, but even if that conviction is borne out, the overall challenge of a potency test, important for many natural source and recombination therapeutics, substantially broadens the risk, compared with unequivocal and specific analytical techniques for chemically synthesized medicines.
Taken together, the experience of heparin in the United States and elsewhere shows that ensuring the integrity of the supply chain of naturally sourced biologic drugs is fraught with challenges—to manufacturers, to regulatory agencies and to practitioners. The steps taken by US regulatory authorities and the US pharmacopeia following the heparin contamination events in 2007 and 2008 have put in place safeguards giving greater confidence that contaminants can be prevented from entering the marketplace in a similar manner.
Supplementary Material
ACKNOWLEDGMENTS
The authors appreciate the financial and logistical support provided by USP; the contributions of USP volunteers, the scientific community, and the collaborating laboratories; the public comments; and the collaboration of the FDA, including helpful review and discussion.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The ideas, findings and conclusions in this presentation have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Laremore TN, Zhang F, Dordick JS, Liu J & Linhardt RJ Recent progress and applications in glycosaminoglycan and heparin research. Curr. Opin. Chem. Biol 13, 633–640 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindahl U ‘Heparin’—from anticoagulant drug into the new biology. Glycoconj. J 17, 597–605 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Petitou M, Casu B & Lindahl U 1976–1983, a critical period in the history of heparin: the discovery of the antithrombin binding site. Biochimie 85, 83–89 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Barrowcliffe TW History of heparin. Handb. Exp. Pharmacol 2012, 3–22 (2012). [DOI] [PubMed] [Google Scholar]
- 5.United States Pharmacopeia. Official monographs: Heparin Sodium (US 14) (1950)
- 6.Blossom DB et al. Outbreak of adverse reactions associated with contaminated heparin. N. Engl. J. Med 359, 2674–2684 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishimoto TK et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med 358, 2457–2467 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon AW et al. Description of hypersensitivity adverse events following administration of heparin that was potentially contaminated with oversulfated chondroitin sulfate in early 2008. Pharmacoepidemiol. Drug Saf 19, 921–933 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Guerrini M et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol 26, 669–675 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Zhang Z & Linhardt RJ Lessons learned from the contamination of heparin. Nat. Prod. Rep 26, 313–321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.United States Pharmacopeia. Official monographs: Heparin Sodium (US 32–NF27) (2011).
- 12.United States Pharmacopeia Official monographs: Heparin Sodium (US 34–NF29) (2009).
- 13.FDA Guidance for Industry. Heparin for Drug and Medical Device Use: Monitoring Crude Heparin for Quality http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291390.pdf (June 2013).
- 14.Mulloy B Structure and physicochemical characterisation of heparin. Handb. Exp. Pharmacol 2012, 77–98 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Turnbull JE, Hopwood JJ & Gallagher JT A strategy for rapid sequencing of heparan sulfate and heparin saccharides. Proc. Natl. Acad. Sci. USA 96, 2698–2703 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J & Linhardt RJ Chemoenzymatic synthesis of heparan sulfate and heparin. Nat. Prod. Rep 31, 1676–1685 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogren S & Lindahl U Cleavage of macromolecular heparin by an enzyme from mouse mastocytoma. J. Biol. Chem 250, 2690–2697 (1975). [PubMed] [Google Scholar]
- 18.Raman K, Mencio C, Desai UR & Kuberan B Sulfation patterns determine cellular internalization of heparin-like polysaccharides. Mol. Pharm 10, 1442–1449 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keire DA et al. Characterization of currently marketed heparin products: key tests for quality assurance. Anal. Bioanal. Chem 399, 581–591 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Viskov C & Mourier P Process for oxidizing unfractionated heparins and detecting or absence of glycoserine in heparin and heparin products US patent 0,215,519 (2005).
- 21.Mourier PAJ, Guichard OY, Herman F & Viskov C Heparin sodium compliance to USP monograph: structural elucidation of an atypical 2.18 ppm NMR signal. J. Pharm. Biomed. Anal 67–68, 169–174 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Lee SE et al. NMR of heparin API: investigation of unidentified signals in the USP-specified range of 2.12–3.00 ppm. Anal. Bioanal. Chem 399, 651–662 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Guerrini M et al. Effects on molecular conformation and anticoagulant activities of 1,6-anhydrosugars at the reducing terminal of antithrombin-binding octasaccharides isolated from low-molecular-weight heparin enoxaparin. J. Med. Chem 53, 8030–8040 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Mazák K, Beecher CN, Kraszni M & Larive CK The interaction of enoxaparin and fondaparinux with calcium. Carbohydr. Res 384, 13–19 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Ye H et al. Characterization of currently marketed heparin products: key tests for LMWH quality assurance. J. Pharm. Biomed. Anal 85, 99–107 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z et al. Analysis of pharmaceutical heparins and potential contaminants using (1)H-NMR and PAGE. J. Pharm. Sci 98, 4017–4026 (2009). [DOI] [PubMed] [Google Scholar]
- 27.United States Pharmacopeia. Official monographs: Heparin Sodium (US 37–NF32) (Accessed January 8, 2014).
- 28.Sommers CD et al. Characterization of currently marketed heparin products: analysis of molecular weight and heparinase-I digest patterns. Anal. Bioanal. Chem 401, 2445–2454 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Neville GA, Mori F, Holme KR & Perlin AS Monitoring the purity of pharmaceutical heparin preparations by high-field 1H-nuclear magnetic resonance spectroscopy. J. Pharm. Sci 78, 101–104 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Mulloy B et al. USP compendial methods for analysis of heparin: chromatographic determination of molecular weight distributions for heparin sodium. Anal. Bioanal. Chem 406, 4815–4823 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Guerrini M et al. Antithrombin-binding octasaccharides and role of extensions of the active pentasaccharide sequence in the specificity and strength of interaction. Evidence for very high affinity induced by an unusual glucuronic acid residue. J. Biol. Chem 283, 26662–26675 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honchel R et al. A dose-response study in animals to evaluate the anticoagulant effect of the stage 2 unfractionated heparin USP monograph change. Regul. Toxicol. Pharmacol 60, 318–322 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Tami C et al. Inhibition of Taq polymerase as a method for screening heparin for oversulfated contaminants. Biomaterials 29, 4808–4814 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Spencer JA et al. Screening of heparin API by near infrared reflectance and Raman spectroscopy. J. Pharm. Sci 98, 3540–3547 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Trehy ML, Reepmeyer JC, Kolinski RE, Westenberger BJ & Buhse LF Analysis of heparin sodium by SAX/HPLC for contaminants and impurities. J. Pharm. Biomed. Anal 49, 670–673 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Keire DA et al. Analysis of crude heparin by 1H-NMR, capillary electrophoresis, and strong-anion-exchange-HPLC for contamination by over sulfated chondroitin sulfate. J. Pharm. Biomed. Anal 52, 921–926 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Keire DA, Mans DJ, Ye H, Kolinski RE & Buhse LF Assay of possible economically motivated additives or native impurities levels in heparin by 1H NMR, SAX-HPLC, and anticoagulation time approaches. J. Pharm. Biomed. Anal 52, 656–664 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Brustkern AM, Buhse LF, Nasr M, Al-Hakim A & Keire DA Characterization of currently marketed heparin products: reversed-phase ion-pairing liquid chromatography mass spectrometry of heparin digests. Anal. Chem 82, 9865–9870 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Zang Q et al. Combining (1)H NMR spectroscopy and chemometrics to identify heparin samples that may possess dermatan sulfate (DS) impurities or oversulfated chondroitin sulfate (OSCS) contaminants. J. Pharm. Biomed. Anal 54, 1020–1029 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Zang Q et al. Class modeling analysis of heparin 1H NMR spectral data using the soft independent modeling of class analogy and unequal class modeling techniques Anal. Chem 83, 1030–1039 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Sommers CD, Mans DJ, Mecker LC & Keire DA Sensitive detection of over-sulfated chondroitin sulfate in heparin sodium or crude heparin with a colorimetric microplate based assay. Anal. Chem 83, 3422–3430 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Zang Q et al. Identification of heparin samples that contain impurities or contaminants by chemometric pattern recognition analysis of proton NMR spectral data. Anal. Bioanal. Chem 401, 939–955 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Sommers CD & Keire DA Detection of possible economically motivated adulterants in heparin sodium and low molecular weight heparins with a colorimetric microplate based assay. Anal. Chem 83, 7102–7108 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Toby TK, Sommers CD & Keire DA Detection of native chondroitin sulfate impurities in heparin sodium with a colorimetric micro-plate based assay. Anal. Methods 4, 1488–1491 (2012). [Google Scholar]
- 45.Sommers CD, Montpas N, Adam A & Keire DA Characterization of currently marketed heparin products: adverse event relevant bioassays. J. Pharm. Biomed. Anal 67–68, 28–35 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Keire DA, Buhse LF & al-Hakim A Characterization of currently marketed heparin products: composition analysis by 2D-NMR. Anal. Methods 5, 2984–2994 (2013). [Google Scholar]
- 47.Nemes P, Hoover WJ & Keire DA High-throughput differentiation of heparin from other glycosaminoglycans by pyrolysis mass spectrometry. Anal. Chem 85, 7405–7412 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.