Abstract
Purpose of review:
It has long been considered that tolerance in a transplant recipient is a binary all-or-none state: either the graft is accepted without immunosuppression identifying the recipient as tolerant, or the recipient rejects the graft and is not tolerant. This tolerance paradigm, however, does not accurately reflect data emerging from animal models and patients and requires revision.
Recent Findings:
It is becoming appreciated that there may be different gradations in the quality of tolerance based on underlying cellular mechanisms of immunological tolerance, and that individuals may enhance their tolerance by strengthening or combining different cellular mechanisms. Furthermore, evidence suggests that even if tolerance is lost, the loss may be only temporary, and in some circumstances tolerance can be restored.
Summary:
Shifting our focus from an all-or-nothing tolerance paradigm to one with many shades may help us better understand how tolerance operates, and how this state may be tracked and enhanced for better patient outcomes.
Keywords: Tolerance, Transplantation, Robustness, Erosion
Introduction
Tolerance is a polysemic term: it can refer to alterations in immune cells so that they that fail to enact a rejection response —be it to self, tumors, allergens, or transplanted organs/tissues. In addition, tolerance can be used to describe a transplant recipient whose allograft remains functional following withdrawal of pharmacological immunosuppression, irrespective of underlying immunological mechanisms. Achieving tolerance in transplanted patients has been a goal of the transplant field for many years. Conventional immunosuppression has allowed organs to be transplanted in patients that are genetically distinct from their donor, but these treatments must be taken for a patient’s lifetime to prevent graft rejection and have many undesirable side effects. As a result, non-adherence to immunosuppression regimens is one of the major risk factors for graft rejection and loss [1]. Transplantation might be more widely used to benefit a greater number of patients if transplant tolerance could be routinely induced, following a short-term treatment that would permanently prevent the immune system from rejecting the organ, while maintaining immune competence to other antigens. Tolerance, induced in animal models and identified in rare patients who have ceased immunosuppression without rejecting their allografts, as well as in patients in whom tolerance has been prospectively induced, offers a starting point to discover what may be necessary to make inducible and stably maintained tolerance a feasible goal for all patients. Increasingly, evidence from tolerance studies in animals and humans is revealing that tolerance is not an all-or-none state, and that even if tolerance is robustly induced initially, it may not be sustained long-term. Strategies to induce more robust tolerance may arise from achieving multiple redundant cellular mechanisms of tolerance to make transplant tolerance less metastable. Furthermore, if tolerance is not a fixed state, but may fluctuate in response to environmental cues, new strategies to monitor the state of tolerance and the cellular mechanisms that maintain it, as well as approaches to boost them, will need to be developed to ensure the preservation of the transplanted organ for the life of the tolerant patient.
In this review, we discuss a new conceptual model of tolerance that is graded, rather than binary. This model arises from data in animal systems and in patients that revealed that some states of tolerance are more resilient than others, and that tolerance can be eroded over time. Moreover, if tolerance is broken after it has been stably induced, it may spontaneously return, albeit not always at the same level as before its transient abrogation. This graded model of tolerance supports the hypothesis that multiple cellular mechanisms are necessary for sustaining a robust state of tolerance, and that each of these may be susceptible to its own type of reversal signals. In support of this new graded model of tolerance, we have summarized results showing different mechanisms of cellular tolerance, as well as highlighted studies in which tolerance is likely to be robust, requiring disruption of multiple underlying cellular tolerance pathways to be broken.
Animal Models that Reveal the Robustness and Resilience of Tolerance
Our group has shown that tolerance induced with a short-term treatment of anti-CD154 (anti-CD40L) and donor splenocyte transfusion (DST) is donor-specific and robust. Importantly its maintenance phase is resistant to many inflammatory insults, including half-lethal doses of systemic Toll-like receptor (TLR) agonists (Alegre et al. unpublished and [2]). In addition, increasing the frequency of alloreactive T cells by injecting 105 alloreactive TCR-transgenic T cells, depleting regulatory T cells (Tregs), or blocking PD-1/PD-L1 interactions, either as single or pairwise interventions, all failed to precipitate transplant rejection in stably tolerant hosts [3•]. Only the combination of all three of these interventions was able to break tolerance [3•], underscoring the redundant/additive effects of controlling alloreactive T cell numbers, and regulating their function in both cell-extrinsic and cell-intrinsic manners. However, transferring greater numbers of alloreactive naïve T cells (>2×106 cells) was ultimately sufficient to break stable tolerance. This high-dose transfer resulted in a modest increase in the numbers of endogenous alloreactive T cells producing IFNγ in the graft, which was sufficient to overwhelm the tolerance mechanisms in the host and trigger graft rejection [3•]. Another intervention that has led to cardiac allograft rejection in costimulation-blockade-mediated tolerance in mice is lymphodepletion resulting from antibody-mediated T cell depletion or irradiation during the maintenance period of tolerance [4]. Indeed, the residual effector T cells not only expanded faster than the remaining regulatory cells but they also took on a memory phenotype and were resistant to Treg suppression [4]. Finally, established transplantation tolerance could also be abrogated with the administration of agonistic anti-CD40 mAb [2], as could tolerance to a malignancy in a model of acute myeloid leukemia [5]. Agonistic anti-CD40 likely provides surrogate help to alloreactive CD8+ T cells, as well as activates dendritic cells that then present donor-derived antigens indirectly to CD4+ T cells [6].
Infection with Listeria monocytogenes has also been shown to be able to break established anti-CD154/DST-induced transplantation tolerance [7]. Listeria infection, but not LCMV or S. aureus infections, has been the only acute infection identified to date that can not only prevent the induction of tolerance when it occurs at the time of transplantation, but also break tolerance when experienced later, during the maintenance phase [7–10]. Listeria infection results in a systemic increase in IL-6 and type I interferon (IFNβ) that act to suppress Treg function and promote alloreactive T cell proliferation and IFNγ production, respectively [7]. Listeria infection in stably tolerant recipients can cause the complete cessation of cardiac allograft heartbeat and loss of tolerance in a subset of animals, but it can also induce a rejection crisis, defined by a slowing heartbeat that does not progress to complete heartbeat cessation in another subset of mice. Recovery from Listeria-induced rejection crisis was marked by an erosion in tolerance, which could then be completely abrogated by antibodies that disrupt the PD-1/PD-L1 inhibitory checkpoint pathway [11••]. Thus eroded tolerance after infection required PD-1/PD-L1 interactions, whereas in uninfected tolerant mice the combination of simultaneous PD-1/PD-L1 blockade, adoptive transfer of alloreactive T cells, and Treg depletion [3•] was necessary to precipitate the rejection of the stably accepted allograft.
Abrogation of tolerance, defined as the complete loss of cardiac allograft function, is not an invariably permanent state. In tolerant mice that fully rejected their allografts following Listeria infection, tolerance was spontaneously restored as early as one week after the rejection event, as evidenced by the ability of these mice to accept secondary donor-matched allografts without the need for additional immunosuppression [12••]. While this spontaneous return of tolerance permitted the acceptance of the second graft, the new tolerant state was not as robust as before the infection. Post-infection mice were prevented from accepting second donor-matched heart grafts if Tregs were depleted, but not when the second hearts were transplanted into non-infected tolerant mice [12••]. The erosion of tolerance following Listeria infection is likely due to an increase in alloreactive T cells and/or reversal of their cell-intrinsic tolerance mechanisms following the Listeria infection, resulting in a greater dependence on Tregs for tolerance maintenance.
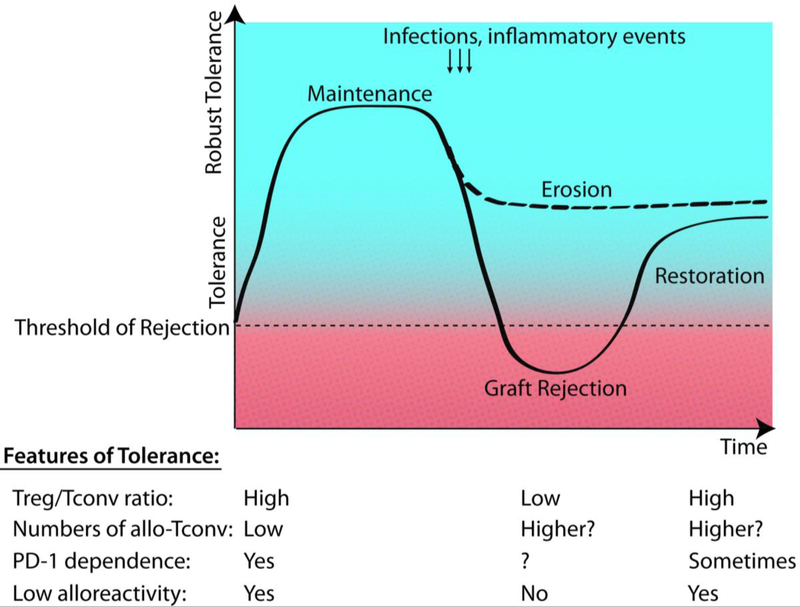
A list of interventions that have been used to disrupt tolerance, their effects, and impact on graft outcomes is summarized in Table 1. These data illustrate that anti-CD154+DST treatment induces a robust tolerance, because it takes multiple simultaneous disruptions of different cellular/molecular tolerance mechanisms to break it. We posit that if some of these cellular/molecular tolerance mechanisms are only transiently disrupted, and revert back to their original tolerant state, tolerance can be spontaneously restored. However, if not all mechanisms are restored, the resulting tolerance might not be as robust as it once was (Figure 1). Thus, depending on the cellular mechanisms of maintenance of tolerance elicited by specific therapeutic regimens, and the types of inflammatory pathways triggered by particular infections, as well as the duration of their impact, one could theorize that certain infections might have no significant effect on tolerance while others might erode tolerance, or precipitate a reversible or irreversible loss of tolerance.
Table 1:
Multiple mechanisms of tolerance need to be compromised to result in cardiac graft rejection in anti-CD154+DST-induced robust tolerance
| Intervention during the maintenance phase of tolerance | ↑Cell #a | Treg brake removedb | PD-1 brake removedc | IFNγd | ↑Cell # +IFNγe | Rej? f | Loss of tolerance scoreg | Ref |
|---|---|---|---|---|---|---|---|---|
| Listeria | + | + | − | + | + | ▯ | 4 | [7] |
| 105 TCR75 | + | − | − | − | − | − | 1 | [3•] |
| >2×106 TCR75 | + | − | − | + | + | ▯ | 3 | [3•] |
| Lymphodepletion | + | + | − | + | + | ▯ | 4 | [4] |
| αCD25 | − | +/− | − | −? | −? | − | 0.5 | [3•, 59] |
| αPD-L1 | − | − | + | −? | −? | − | 1 | [3•] |
| αCD25+105 TCR75 | + | +/− | − | −? | −? | − | 1.5 | [3•] |
| αPD-L1+105 TCR75 | + | − | + | −? | −? | − | 2 | [3•] |
| αCD25+αPD-L1 | − | +/− | + | −? | −? | − | 1.5 | [3•] |
| αCD25+αPD-L1 +105 TCR75 | + | +/− | + | +?h | +?h | ▯ | 4.5 | [3•] |
| αPD-L1 post-Listeria | + | − | + | +?h | +?h | ▯ | 4 | [11••] |
| αCD25 post-Listeria 2nd ❤ | + | + | − | +?h | +?h | ▯ | 4 | [12••] |
| αCD25 no Listeria 2nd ❤ | − | + | − | +/−? | −? | − | 1.5 | [12••] |
Increased cell number; “+” given for known increase in alloreactive T cells that occurs post-Lm and for increasing the numbers of alloreactive cells with adoptive transfer. “-” no known increase in cells, or not experimentally determined (grey)
Treg brake removed; “+” given for known reduction in Treg percentages in the graft or for αCD25 given prior to a 2nd donor-matched graft. “+/−” given for αCD25 treatment with a pre-existing graft because depletion of Tregs is less efficient in tissues “–” given for no known change in Treg percentages, or not experimentally determined (grey)
PD-1 brake removed; “+” given for αPD-L1 “–” given for no αPD-L1 given and high PD-1 expression intact, but some values were not experimentally determined (grey)
IFNγ production “+” given for Lm, HD TCR75 transfer, and lymphodepletion based on experimental data. “+/−” given for post-Lm day 14 (having recently expressed IFNγ and potentially poised to do so again with inflammation) or with 2nd donor-matched transplant as a source of inflammation (combined two (+/−) values (each 0.5) = 1 = +). “-” given for no known change in IFNγ; grey values not experimentally determined
If a given treatment resulted in an increase in allospecific T cells and IFNγ, the same value was given in this column as what was in the IFNγ column to give additional weight to what is likely causing rejection (an increased number of IFNγ-producing T cells)
“▯”=rejection in the majority of recipients, “-”= no rejection
The sum of each value across a row was used to determine the loss of tolerance score with each “+” = 1, “+/−” = 0.5 and “-” = 0. The rejection column was not used.
“+” values were given based on the hypothesis that having increased alloreactive Tconv cells with an additional brake removed would result in the induction of IFNγ-producing T cells that mediate rejection. This hypothesis needs to be experimentally verified.
Figure 1: Threshold model of tolerance and mechanisms involved.
Tolerance is not an all-or-none state but rather exists as a gradient of robust tolerance resulting from the number of combined mechanisms of T cell tolerance. If multiple mechanisms are simultaneously compromised this may result in dipping below the threshold necessary to prevent rejection (solid line). If individual mechanisms are disrupted, this may result in an erosion of tolerance (dashed line). Tolerance mechanisms may later recover resulting in a restoration of tolerance. Whether all tolerance mechanisms are fully restored or not will determine whether the restored tolerance state is as robust as the initial tolerance, or is potentially eroded. The example shown above lists different tolerance mechanisms examined and how their strength changes during a Listeria-triggered loss of tolerance and return of tolerance. Graft-specific Tregs are expanded or induced following tolerance induction, but these cells can be overwhelmed transiently by alloreactive conventional T cells (allo-Tconv) and Listeria-specific T cells in the graft. Tolerant mice maintain low numbers of allo-Tconv, similar to naïve mice, but these cells expand and stay elevated post-Listeria infection. Many tolerant T cells express the negative regulator PD-11, and in certain circumstances, such as post-infection, eroded tolerance becomes susceptible to PD-1/PD-L1 blockade. Naïve T cells have the potential for high alloreactivity but tolerance induction can result in their inhibition of cytokine production either extrinsically or intrinsically. Alloreactivity transiently increases during infection-mediated rejection, but approaches baseline levels during the restoration of tolerance.
Transplantation tolerance is robust not only when induced with anti-CD154+DST, but also when induced with non-depleting anti-CD4 and anti-CD8 [13–15]. The latter is also resistant to breaking by individual interventions, such as increasing the numbers of naïve alloreactive T cells, or allowing T cells to homeostatically proliferate, or providing additional alloantigen and ischemia-reperfusion inflammation via giving a new transplant, or depleting Tregs [13–15]. Additionally, Schroeder et al., showed that established tolerance induced with anti-CD4 alone was resistant to breakage with FTY720, an intervention that is capable of preventing the induction of tolerance [16]. This observation suggests that recirculation of new immune cells to the graft is not necessary for maintenance of tolerance, but is necessary for its induction.
Transient cyclosporine treatment induced a robust tolerance in a MHC-I-mismatch transplant model in swine that was resistant to the removal of regulatory cells via a leukapheresis protocol, and to retransplantation [17]. Only the combination of both procedures precipitated rejection [17]. Robust tolerance was also induced in a combined bone marrow/costimulation blockade chimerism model, in which treatment with exogenous IL-2 or depletion of CD25+ cells did not break tolerance to the bone marrow transplant [18]. In contrast, while Treg depletion or anti-CTLA-4 treatment did not break established tolerance induced by anti-CD3 mAb in an islet transplantation model [19], anti-PD-L1 alone could precipitate rejection [20], suggesting tolerance in this model may be less robust. PD-1/PD-L1 blockade was also sufficient to break tolerance of cardiac allografts induced with CTLA-4-Ig [21], and of long-term single-mismatched skin grafts in synchimeric mice induced with anti-CD154 and CTLA-4-Ig [22]. Similarly, spontaneous kidney transplantation tolerance that occurs in select strain combinations in mice may be less robust, as it was susceptible to Treg depletion alone [23]. Collectively these observations are consistent with our overall model of tolerance existing in different states of robustness and resilience.
The return of tolerance after its transient abrogation following infection of tolerant mice is not unique to this animal model. In a non-human primate model of combined bone marrow/kidney transplantation, a similar transient breaking of established tolerance was reported with high dose IL-2 administration that resulted in the expansion of alloreactive effector T cells, primarily memory T cells [24•]. While the IL-2 also expanded Tregs, this was not sufficient to prevent the increase in IFNγ production by conventional T cells, which precipitated acute cellular rejection. Strikingly, after IL-2 was withdrawn, graft function recovered [24•]. In contrast, when bone marrow transplantation was combined with costimulation blockade in mice, providing exogenous IL-2 did not break tolerance to the bone marrow transplant [18], suggesting a more robust state of tolerance. In a study by de Vries et al., costimulation-blockade-induced tolerance was broken following mast cell degranulation that resulted in Treg egress from the graft and impaired Treg expression of suppressor molecules such as IL-10, TGFβ and granzyme B [2]. Though not formally tested, a return of tolerance in this setting is likely, as the changes in Tregs were only transient [2].
Transplantation Tolerance in Patients—Deliberately Induced or Spontaneous Acquired
In a few patients, renal transplantation tolerance has been prospectively induced successfully by promoting donor hematopoietic cell engraftment through combined bone marrow/kidney transplantation [25–27]. This mixed bone-marrow chimerism approach harnesses mechanisms of central tolerance, primarily through the elimination of donor-reactive T cell clones. Indeed, evidence of deletion has been identified in one group of patients [28]. Other mechanisms of cellular tolerance besides T cell deletion may also play a role in maintaining allograft tolerance in these patients, as animal models of mixed chimerism had suggested [18, 29]. Relevant to the stability and robustness of transplantation tolerance, several of these patients have been followed long-term. Two of eight tolerized patients were diagnosed at 5 and 7 years post-transplantation with chronic rejection, and 1 patient experienced acute cellular rejection just 3 weeks after acute pyelonephritis [30]. These data suggest that even chimerism-induced tolerance may not always be permanent, and may be susceptible to breaking, perhaps by select infections that trigger the right inflammatory pathways.
In other patients, transplantation tolerance has emerged spontaneously after the withdrawal of immunosuppression. In rare, operationally tolerant kidney transplant patients (estimated to occur at a frequency of less than 5 out of 10,000 transplants) [31], a B cell signature has been identified in peripheral blood [32, 33], as well as evidence of a signature of reduced T follicular helper cells [34] and increased memory Tregs [35]. Although this B cell signature has been attributed to tolerance, recent findings suggest that it may instead be representative of a signature of an absence of immunosuppression, arising from the comparison of tolerant individuals with stable patients on immunosuppression [36–38]. In spontaneously tolerant liver transplant recipients, an intragraft iron metabolism signature was predictive of tolerance [39]. Interestingly, in both groups of patients, prior acute rejection episodes did not preclude these patients from later developing tolerance [40•, 41••], suggesting that tolerance can still be induced or restored following rejection. In addition, in a cohort of kidney transplant patients that were followed long-term post-immunosuppression withdrawal, there was a higher incidence of infections that preceded a loss of tolerance than in those that retained functional grafts [41••]. These data suggest that tolerance in certain individuals may not be robust enough to withstand some infections and may not stably persist for the life of the patient. Successful weaning of conventional immunosuppression in liver transplant recipients has been correlated with the time elapsed since transplantation, recipient male gender and age at the time of transplantation. Indeed, in patients at >10 years post-transplantation, successful weaning was observed in 79.2% (n=19 of 24) of patients. Determining whether liver tolerance is robust and persistent in patients will require long-term follow up, as rejection has been reported in rare tolerant patients after many years of drug discontinuation [42, 43].
Cellular Mechanisms of Tolerance—Their Potential Reversibility
Individual immune cell subsets may undergo transient or permanent reversal of tolerance under certain circumstances. Loss of regulatory T cell suppressive function, levels of intrinsic T/B cell anergy/hyporesponsiveness/exhaustion and/or expansion of alloreactive T and B cells during inflammatory insults may individually and additively contribute to a loss of tolerance.
T cells
Chronic inflammation including inflammatory cytokines such as IL-6 and TNF, have been shown to promote the instability of the Treg lineage and impair Treg function [44], or diminish the susceptibility of conventional T cells to Treg suppression [45, 46]. PD-1/PD-L1 blockade can result in an increase in effector functions including increased production of IFNγ [19, 22]. Homeostatic proliferation has been shown to drive self-tolerant CD8+ T cells temporarily out of a cell-intrinsic dysfunctional state [47, 48•], but if these cells are left to quiesce, they can revert to their previous tolerant state as they appear to have an epigenetic imprinting of their hyporesponsiveness [48•].
B cells
If CD4 T cell tolerance is broken, it may allow these cells to provide additional help to B cells. Increasing the multi-valency of B cell antigens and providing B cells with additional CD4 help allowed self-tolerant B cells to reverse their anergic state [49]. If this occurred in a transplant setting, it might result in alloantibody production and chronic graft rejection.
NK cells
NK cells can become tolerant/anergic in the long-term presence of MHC I-deficient cells because of chronic stimulation via activating receptors, unopposed by the inhibitory signals normally induced by self-MHC engagement. NK cell anergy could be overridden with high levels of IL-12, IL-18 or a mutant IL-2 “superkine” to provide control of MHC-I-deficient tumors [50].
Dendritic cells
Dendritic cell maturation has been shown to be impaired upon immunization with a self-antigen in a Treg-, IL-10R-dependent manner [51, 52]. Maturation markers were restored however, in the presence of pro-inflammatory TLR3 signals [51], suggesting this type of DC tolerance could be overridden during an infection if necessary. Other TLR ligands such as CpG or co-administration of viruses and dendritic cells have helped override established T cell tolerance to tumors [53, 54].
Conclusion
In this review, we have proposed a new working model explaining outcomes of transplantation tolerance versus rejection, one that is based on a gradient rather than a binary state (Figure 1). Robust tolerance has multiple fail-safes to prevent rejection from occurring. Only when several mechanisms are simultaneously disrupted does rejection ensue. The notion that transplantation tolerance can exist in different states of robustness is reminiscent of the recent findings of graded mechanisms of tolerance to tissue-restricted self-antigens. Legoux et al. reported that ubiquitously expressed self-antigens induced T cell deletion, and that tissue-restricted self-antigens expressed in the lung and intestine enhanced thymically-derived FoxP3+ Tregs, while pancreatic-restricted self-antigens were ignored by T cells [55•]. Importantly, regulatory tolerance exhibited limited durability and could be reversed with repeated antigen rechallenge while deletional tolerance was more robust. Likewise, Malhotra and colleagues reported that self-peptide expression patterns in the thymus determined the mechanisms of self-tolerance, with peptides presented uniformly inducing clonal T cell deletion, those with limited thymic expression inducing partial clonal deletion, impaired effector T cell but enhanced Treg potentiation, and those excluded from the thymus having no impact the T cell repertoire [56]. The existence of graded states of tolerance has profound implications for the search of tolerance biomarkers, and for the design of therapeutic approaches targeting multiple cellular tolerance mechanisms to preserve allografts for the life of the transplant recipient.
The concept that tolerance can be overridden transiently during inflammatory events, but then resurfaces when the inflammation resolves (Figure 1), may have wide-reaching clinical implications. It may help explain why certain transplant patients can be successfully weaned of immunosuppression revealing a state of operational tolerance despite having experienced prior acute rejection events [40•, 41••]. Moreover, several autoimmune diseases are known to undergo phases of relapse and stages of remission. It is possible that disease relapse is triggered by pro-inflammatory events that overwhelm an already suboptimal self-tolerant state in individuals genetically predisposed to autoimmunity. With the quiescence of inflammation, regulation may dominate again to explain disease remission. In a similar fashion, initial immune-dependent regression of tumors can be followed by tumor recurrence. Tumor elimination by anti-tumor T cells may be aided by bystander inflammation, while tumor recurrence may be facilitated by activated Tregs [57]. In fact, Listeria monocytogenes is currently being used in clinical trials to improve anti-tumor immunity [58] suggesting that similar mechanisms of tolerance in the context of cancer as in the transplant model may be overcome by infection. A better understanding of the loss and spontaneous restoration of antigen-specific tolerance may have wide clinical applicability for therapeutic approaches to transplantation, autoimmunity and cancer.
Acknowledgements:
M.L.M. was funded by American Heart Association predoctoral fellowships (13PRE14550022 and 15PRE22180007), a Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), and a Howard Hughes Medical Institute Med-into-Grad Program training grant (56006772). The work was also supported by National Institutes of Health P01AI-97113 to A.S.C. and M.-L.A.
Footnotes
Human and Animal Rights: All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References:
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. (2015) Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 15:2921–2930. doi: 10.1111/ajt.13347 [DOI] [PubMed] [Google Scholar]
- 2.de Vries VC, Wasiuk A, Bennett KA, et al. (2009) Mast cell degranulation breaks peripheral tolerance. Am J Transplant 9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.•.Miller ML, Daniels MD, Wang T, et al. (2016) Tracking of TCR-Tg T cells reveals multiple mechanisms maintain cardiac transplant tolerance in mice. Am J Transplant. doi: 10.1111/ajt.13814 Study showing that robust tolerance is maintained by multiple additive/redundant cellular tolerance mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iida S, Suzuki T, Tanabe K, et al. (2013) Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection. Am J Transplant 13:2268–2279. doi: 10.1111/ajt.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Chen X, Liu X, et al. (2013) CD40 ligation reverses T cell tolerance in acute myeloid leukemia. Journal of Clinical Investigation 123:1999–2010. doi: 10.1172/JCI63980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali JM, Negus MC, Conlon TM, et al. (2016) Diversity of the CD4 T Cell Alloresponse: The Short and the Long of It. Cell Rep 14:1232–1245. doi: 10.1016/j.celrep.2015.12.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Ahmed EB, Chen L, et al. (2010) Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant 10:1524–1533. doi: 10.1111/j.1600-6143.2010.03066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, Chen L, Ahmed EM, et al. (2008) Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol 180:5991–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed EB, Wang T, Daniels M, et al. (2011) IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant 11:936–946. doi: 10.1111/j.1600-6143.2011.03476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welsh RM, Markees TG, Woda BA, et al. (2000) Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol 74:2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.••.Young JS, Daniels MD, Miller ML, et al. (2017) Erosion of Transplantation Tolerance After Infection. Am J Transplant 17:81–90. doi: 10.1111/ajt.13910 A study showing that a severe infection can erode transplantation tolerance long-term even in animals in which it does not cause graft loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.••.Miller ML, Daniels MD, Wang T, et al. (2015) Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun 6:7566. doi: 10.1038/ncomms8566 A study showing that the memory of tolerance can dominate over the memory of transplant rejection triggered by an infection, albeit the restored tolerance is eroded when compared to the robust tolerance prior to infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin S, Cobbold SP, Pope H, et al. (1993) “Infectious” transplantation tolerance. Science 259:974–977. [DOI] [PubMed] [Google Scholar]
- 14.Kendal AR, Chen Y, Regateiro FS, et al. (2011) Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med 208:2043–2053. doi: 10.1084/jem.20110767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graca L, Thompson S, Lin C-Y, et al. (2002) Both CD4+CD25+ and CD4+CD25− Regulatory Cells Mediate Dominant Transplantation Tolerance. J Immunol 168:5558–5565. doi: 10.4049/jimmunol.168.11.5558 [DOI] [PubMed] [Google Scholar]
- 16.Schroeder G, Risch K, Kotsch K, et al. (2004) FTY720 prevents anti-CD4 mAb-induced tolerance but cannot reverse established tolerance in a rat kidney transplantation model. Am J Transplant 4:863–871. doi: 10.1111/j.1600-6143.2004.00442.x [DOI] [PubMed] [Google Scholar]
- 17.Scalea JR, Okumi M, Villani V, et al. (2014) Abrogation of Renal Allograft Tolerance in MGH Miniature Swine: The Role of Intra-Graft and Peripheral Factors in Long-Term Tolerance. American Journal of Transplantation 14:2001–2010. doi: 10.1111/ajt.12816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigenzahn S, Blaha P, Koporc Z, et al. (2005) The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant 5:1237–47. [DOI] [PubMed] [Google Scholar]
- 19.Besançon A, Baas M, Goncalves T, et al. (2017) The Induction and Maintenance of Transplant Tolerance Engages Both Regulatory and Anergic CD4(+) T cells. Front Immunol 8:218. doi: 10.3389/fimmu.2017.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baas M, Besançon A, Goncalves T, et al. (2016) TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife. doi: 10.7554/eLife.08133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Albin MJ, Yuan X, et al. (2007) PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. Journal of Immunology 179:5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koehn BH, Ford ML, Ferrer IR, et al. (2008) PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol 181:5313–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyajima M, Chase CM, Alessandrini A, et al. (2011) Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol 178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.•.Yamada Y, Nadazdin O, Boskovic S, et al. (2015) Repeated Injections of IL-2 Break Renal Allograft Tolerance Induced via Mixed Hematopoietic Chimerism in Monkeys. American Journal of Transplantation 15:3055–3066. doi: 10.1111/ajt.13382 The authors provide evidence of breaking of transplantation tolerance in non-human primates, and of its spontaneous restoration following cessation of IL-2 administration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leventhal J, Abecassis M, Miller J, et al. (2012) Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 4:124ra28. doi: 10.1126/scitranslmed.3003509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai T, Cosimi AB, Spitzer TR, et al. (2008) HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 358:353–361. doi: 10.1056/NEJMoa071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. (2008) Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 358:362–368. doi: 10.1056/NEJMoa074191 [DOI] [PubMed] [Google Scholar]
- 28.Morris H, DeWolf S, Robins H, et al. (2015) Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med 7:272ra10. doi: 10.1126/scitranslmed.3010760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haspot F, Fehr T, Gibbons C, et al. (2008) Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood 112:2149–2155. doi: 10.1182/blood-2007-12-127449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Sachs DH, Sprangers B, et al. (2014) Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 14:1599–1611. doi: 10.1111/ajt.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massart A, Pallier A, Pascual J, et al. (2016) The DESCARTES-Nantes survey of kidney transplant recipients displaying clinical operational tolerance identifies 35 new tolerant patients and 34 almost tolerant patients. Nephrol Dial Transplant 31:1002–1013. doi: 10.1093/ndt/gfv437 [DOI] [PubMed] [Google Scholar]
- 32.Sagoo P, Perucha E, Sawitzki B, et al. (2010) Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120:1848–1861. doi: 10.1172/JCI39922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newell KA, Asare A, Kirk AD, et al. (2010) Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120:1836–1847. doi: 10.1172/JCI39933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chenouard A, Chesneau M, Bui Nguyen L, et al. (2016) Renal Operational Tolerance Is Associated With a Defect of Blood Tfh Cells That Exhibit Impaired B Cell Help. Am J Transplant. doi: 10.1111/ajt.14142 [DOI] [PubMed] [Google Scholar]
- 35.Braza F, Dugast E, Panov I, et al. (2015) Central Role of CD45RA- Foxp3hi Memory Regulatory T Cells in Clinical Kidney Transplantation Tolerance. J Am Soc Nephrol 26:1795–1805. doi: 10.1681/ASN.2014050480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asare A, Kanaparthi S, Lim N, et al. (2017) B Cell Receptor Genes Associated With Tolerance Identify a Cohort of Immunosuppressed Patients With Improved Renal Allograft Graft Function. Am J Transplant. doi: 10.1111/ajt.14283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, et al. (2016) Biomarkers of Tolerance in Kidney Transplantation: Are We Predicting Tolerance or Response to Immunosuppressive Treatment? Am J Transplant 16:3443–3457. doi: 10.1111/ajt.13932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bottomley MJ, Chen M, Fuggle S, et al. (2017) Application of Operational Tolerance Signatures Are Limited by Variability and Type of Immunosuppression in Renal Transplant Recipients: A Cross-Sectional Study. Transplant Direct 3:e125. doi: 10.1097/TXD.0000000000000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohne F, Martínez-Llordella M, Lozano J-J, et al. (2012) Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest 122:368–382. doi: 10.1172/JCI59411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.•.Benítez C, Londoño M-C, Miquel R, et al. (2013) Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 58:1824–1835. doi: 10.1002/hep.26426 In one of the first studies to prospectively wean patients from immunosuppression, the authors find that episodes of acute rejection do not preclude tolerance from later developing. [DOI] [PubMed] [Google Scholar]
- 41.••.Brouard S, Pallier A, Renaudin K, et al. (2012) The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant 12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x This study provides evidence that spontaneous tolerance in human patients is not always permanent. [DOI] [PubMed] [Google Scholar]
- 42.Tryphonopoulos P, Ruiz P, Weppler D, et al. (2010) Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation 90:1556–1561. doi: 10.1097/TP.0b013e3182003db7 [DOI] [PubMed] [Google Scholar]
- 43.Mazariegos GV, Sindhi R, Thomson AW, Marcos A (2007) Clinical tolerance following liver transplantation: long term results and future prospects. Transpl Immunol 17:114–119. doi: 10.1016/j.trim.2006.09.033 [DOI] [PubMed] [Google Scholar]
- 44.Barbi J, Pardoll D, Pan F (2014) Treg functional stability and its responsiveness to the microenvironment. Immunol Rev 259:115–139. doi: 10.1111/imr.12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasare C, Medzhitov R (2003) Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033–6. [DOI] [PubMed] [Google Scholar]
- 46.Nish SA, Schenten D, Wunderlich FT, et al. (2014) T cell-intrinsic role of IL-6 signaling in primary and memory responses. Elife 3:e01949. doi: 10.7554/eLife.01949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown IE, Blank C, Kline J, et al. (2006) Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol 177:4521–9. [DOI] [PubMed] [Google Scholar]
- 48.•.Schietinger A, Delrow JJ, Basom RS, et al. (2012) Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335:723–727. doi: 10.1126/science.1214277 In this work, the authors identify an epigenetically-programmed T cell-intrinsic tolerant state, that can be reversed and later spontaneously restored. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chackerian B, Durfee MR, Schiller JT (2008) Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol 180:5816–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ardolino M, Azimi CS, Iannello A, et al. (2014) Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest 124:4781–4794. doi: 10.1172/JCI74337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farkas AM, Finn OJ (2014) Novel mechanisms underlying the immediate and transient global tolerization of splenic dendritic cells after vaccination with a self-antigen. J Immunol 192:658–665. doi: 10.4049/jimmunol.1301904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farkas AM, Marvel DM, Finn OJ (2013) Antigen choice determines vaccine-induced generation of immunogenic versus tolerogenic DC that are marked by differential expression of pancreatic enzymes. J Immunol 190:3319–3327. doi: 10.4049/jimmunol.1203321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Huang C-T, Huang X, Pardoll DM (2004) Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol 5:508–515. doi: 10.1038/ni1059 [DOI] [PubMed] [Google Scholar]
- 54.Horkheimer I, Quigley M, Zhu J, et al. (2009) Induction of type I IFN is required for overcoming tumor-specific T-cell tolerance after stem cell transplantation. Blood 113:5330–5339. doi: 10.1182/blood-2008-05-155150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. •.Legoux FP, Lim J-B, Cauley AW, et al. (2015) CD4(+) T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity 43:896–908. doi: 10.1016/j.immuni.2015.10.011. Demonstration that self-tolerance is also functionally graded in its robustness based on the mechanism of tolerance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra D, Linehan JL, Dileepan T, et al. (2016) Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol 17:187–195. doi: 10.1038/ni.3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goding SR, Wilson KA, Xie Y, et al. (2013) Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol 190:4899–4909. doi: 10.4049/jimmunol.1300271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood LM, Paterson Y (2014) Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol 4:51. doi: 10.3389/fcimb.2014.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang X, Sun W, Guo D, et al. (2011) Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+CD25+ regulatory T cells. Surgery 149:336–346. doi: 10.1016/j.surg.2010.08.012 [DOI] [PubMed] [Google Scholar]