Abstract
Pollen is an important environmental cause of allergic asthma episodes. Prior work has established a proof of concept for assessing projected climate change impacts on future oak pollen exposure and associated health impacts. This paper uses additional monitor data and epidemiologic functions to extend prior analyses, reporting new estimates of the current and projected future health burden of oak, birch, and grass pollen across the contiguous United States. Our results suggest that tree pollen in the spring currently accounts for between 25,000 and 50,000 pollen‐related asthma emergency department (ED) visits annually (95% confidence interval: 14,000 to 100,000), roughly two thirds of which occur among people under age 18. Grass pollen in the summer season currently accounts for less than 10,000 cases annually (95% confidence interval: 4,000 to 16,000). Compared to a baseline with 21st century population growth but constant pollen, future temperature and precipitation show an increase in ED visits of 14% in 2090 for a higher greenhouse gas emissions scenario, but only 8% for a moderate emissions scenario, reflecting projected increases in pollen season length. Grass pollen, which is more sensitive to changes in climatic conditions, is a primary contributor to future ED visits, with the largest effects in the Northeast, Midwest, and Southern Great Plains regions. More complete assessment of the current and future health burden of pollen is limited by the availability of data on pollen types (e.g., ragweed), other health effects (e.g., other respiratory disease), and economic consequences (e.g., medication costs).
Keywords: aeroallergens, asthma
Key Points
We link pollen, climate, and epidemiological data to estimate the health burden of oak, birch, and grass pollen across the contiguous United States
We found that 35,000 to 60,000 asthma emergency department visits (two thirds of these among children) may be linked with pollen each year
We project that future climate changes could increase pollen‐related asthma emergency department visits by 14% in 2090
1. Introduction
Asthma is widespread in the United States, with a prevalence of approximately 25 million people, or 8% of the population, in 2015 (CDC, 2017). Nearly half of asthmatics in the United States reported having one or more asthma attacks in 2013, resulting in 1.6 million emergency department (ED) visits with asthma as the primary diagnosis. The economic burden of asthma is substantial, amounting to $56 billion in 2007 (CDC, 2011), including both direct costs (e.g., medication, healthcare utilization) and indirect costs (e.g., lost school and work days). The most common form of asthma is allergic asthma, associated with both indoor and outdoor environmental triggers such as pollen, dust mites, and mold (AAAAI, 2016). Understanding the contribution of environmental allergens such as pollen from trees, grasses, and weeds to the asthma burden can inform the design of approaches for preventing asthma exacerbation.
Several epidemiological studies over the last 10 to 15 years have examined the relationships between exposure to different pollen types on asthma‐related ED visits in various locations throughout the United States (e.g., Babin et al., 2007; Darrow et al., 2012), asthma hospitalizations in Canada (Dales et al., 2004, 2008), and allergic rhinitis exacerbation in New York City, as measured by sales of over‐the‐counter (OTC) anti‐allergic medications (Ito et al., 2015). Most studies examining the health effects of pollen exposure at the population level have focused on linkages between daily pollen concentrations and asthma ED visits, though the significance and magnitude of the associations vary by pollen type and between studies. Using epidemiologically derived health impact functions, Anenberg et al. (2017) developed a proof‐of‐concept method to estimate the burden of aeroallergens on asthma ED visits in the present day, finding that oak pollen could be associated with 21,000 (95% confidence interval: 10,000–35,200) asthma ED visits in the eastern United States in 2010. The total burden of aeroallergens on public health is likely to be substantially larger, since that study was limited to a single pollen type and excluded the Western United States. Despite the widespread influence of pollen on public health, the impacts of multiple pollen types on asthma exacerbation have not been quantified on a national scale.
The burden of allergenic pollen on public health is also expected to increase in the future, as population size increases and as projected climate change is expected to lengthen and intensify pollen seasons in some parts of the United States (Reid & Gamble, 2009; Sheffield, Weinberger, & Kinney, 2011) and elsewhere (Lake et al., 2016). Future changes in temperature and precipitation may alter the influence of aeroallergens on public health by affecting (1) the timing and length of the pollen season, (2) the total amount of pollen produced throughout the season (i.e., pollen season intensity), (3) the allergenicity of pollen grains, and (4) the geographic extent and distribution of pollen‐producing vegetation (Albertine et al., 2014; Beggs, 2004; Bielory et al., 2012; Cecchi et al., 2010; Fann et al., 2016). Though each plant species may respond differently to changes in meteorological conditions, warmer temperatures year‐round or in the months preceding the pollen season appear to be a key driver of increased pollen season length for multiple taxa (oak, birch, grass, and ragweed; Zhang et al., 2014, 2015). Previous studies have also linked temperature and precipitation in the months preceding the pollen season to increased pollen season intensity, typically calculated as the sum of daily pollen concentrations over an entire pollen season (Dahl et al., 2013; Frei, 1998; Gonzalez Minero et al., 1998; Hicks et al., 1994; Latorre, 1999; McLauchlan et al., 2011; Teranishi et al., 2000). These changes in temperature and precipitation patterns may also influence the geographic range of tree and other plant species that produce allergenic pollen, in turn changing the geographic range in which health impacts occur (Bellard et al., 2012; Woodall et al., 2009). Beyond temperature and precipitation, increased concentrations of ambient carbon dioxide (CO2) itself may influence pollen season intensity (e.g., Ziello et al., 2012).
Anenberg et al. (2017) used an ensemble of climate models and empirical relationships between oak pollen season length, temperature, and precipitation to estimate that, if unabated, climate change could lengthen the oak pollen season by 5% and 10% on average in 2050 and 2090 for the eastern United States, leading to a corresponding increase in asthma ED visits. Our literature review supports the expansion of that prior work both geographically and to additional pollen types. This paper extends the methods and results developed and reported by Anenberg et al. (2017) to include birch and grass pollen, in addition to oak pollen, and the entire continental United States. Both efforts were conducted as part of the U.S. Environmental Protection Agency's (U.S. EPA) ongoing Climate change Impacts and Risk Analysis project, specifically found in the Multi‐Model Framework for Quantitative Sectoral Impacts Analysis—A Technical Report for the Fourth National Climate Assessment (U.S. EPA, 2017a). The Climate Change Impacts and Risk Analysis project uses a consistent analytical framework of socioeconomic scenarios and climate projections to estimate and compare economic impacts on a range of sectors (e.g., health, infrastructure, water) under multiple climate scenarios. This work calculates health impacts using the U.S. EPA's environmental Benefits Mapping and Analysis Program—Community Edition (BenMAP‐CE; U.S. EPA, 2016), using a transparent and reproducible approach that can be used for future analyses of pollen health impacts.
2. Methods
As established in Anenberg et al. (2017), there are three main components to our approach: (1) estimate the impact of aeroallergen levels on key health endpoints under present‐day climatic conditions (baseline), using available monitor data, health impact functions from the epidemiological literature, and the BenMAP‐CE tool; (2) use projections of future climate and a reduced form climate/pollen relationship to project changes in aeroallergen season length, and by extension, estimated pollen exposure, in future years; (3) quantify health impacts of future aeroallergen exposures using the BenMAP‐CE tool. Completing these steps requires baseline pollen data—both average daily concentrations and current season length; climate projections; a method for projecting future pollen season length; a set of relevant pollen concentration‐response functions relating pollen to one or more health impacts, population projections, and baseline rates of health impact. A schematic diagram of the process applied for each of these steps is included in the supporting information.
2.1. Source of Pollen Data
Pollen data are available from the National Allergy Bureau (NAB, a network of daily pollen‐monitoring stations adhering to a common set of sampling and reporting standards). The NAB does not make historical daily pollen concentrations readily available; therefore, we relied on aggregated average pollen count and season length measures as reported for birch, oak, and grass pollen by Zhang et al. (2015) for 58 monitors across the United States and in Canada, near the U.S. border. These were supplemented by direct provision of data from three NAB monitor operators (Dayton, OH; Boise, ID; and Draper, UT), to fill gaps in spatial coverage across certain portions of the United States. All data apply to the period 1994–2010, but monitors differ on the number of years data are available for each pollen type. Most of the monitors we used have 10 years or more of data for the three pollen types we examine, but a few monitors have as few as 3 years of data for some pollen types.
2.2. Source of Climate Data
Climate projections used to support pollen projections were based on guidance from the USGCRP Scenarios and Interpretive Science Coordinating Group for use in the Fourth National Climate Assessment (USGCRP, 2017). Based on this USGCRP guidance, this analysis utilized Representative Concentration Pathway (RCP) 8.5 as a higher greenhouse gas emissions scenario and RCP4.5 as a lower scenario. These greenhouse gas emissions projections have been applied by over 20 climate modeling groups worldwide using more than 60 General Circulation Models (GCMs) as part of a coordinated climate model experiment called the fifth phase of the Coupled Model Intercomparison Project (Taylor et al., 2012).
From among these 60+ GCMs, we selected a subset of five that met criteria established in USEPA (2017a), including demonstrating independence and quality for U.S. projections, and ensuring a broad range of temperature and precipitation outcomes, as a group, over the continental United States. The full set of selection criteria and results are described more fully in USEPA (2017a) and the associated Technical Appendix. The five models applied here were from the Canadian Centre for Climate Modeling and Analysis (CanESM2, Von Salzen et al., 2013); the National Center for Atmospheric Research (CCSM4, Gent et al., 2011; Neale et al., 2013); the NASA Goddard Institute for Space Studies (GISS‐E2‐R, Schmidt et al., 2006); the Meteorological Office at the Hadley Centre (HadGEM2‐ES, Collins et al., 2011; Davies et al., 2005); and the Atmosphere and Ocean Research Institute, National Institute for Environmental Studies, and Japan Agency for Marine‐Earth Science and Technology (MIROC5, Watanabe et al., 2010).
This work also makes use of 20‐year climatic time periods, to ensure that results for any single year do not overrepresent or underrepresent the characteristics of a future period from any of the five models. For our baseline scenario, which is designed to reflect current climatic conditions, a 20‐year reference period of 1986–2005 is used. This reference period is roughly but not exactly consistent with the period for which we have measured pollen data (1994–2010). We considered an option to adjust the data within either the pollen or climatic reference period to align the temporal match, but rejected that approach as it would potentially introduce a new bias to the data.
The four projection eras are presented throughout using the central year in the 20‐year period: 2030 (2020–2039); 2050 (2040–2059); 2070 (2060–2079); and 2090 (2080–2099). Note that in subsequent steps, projected climatic conditions are used to estimate pollen concentrations over that full 20‐year period, and then pollen season length is averaged across the period and run as a single year's pollen season length in BenMAP‐CE. Because we have no information on the intensity of pollen levels, or potential changes in average daily pollen concentrations, we assume daily concentration is constant in all scenarios—total pollen exposure is therefore implicitly assumed to vary only with changes in season length.
Each combination of the two RCPs and five GCMs was downscaled from the native GCM spatial resolution to a 1/16° latitude and longitude scale (an approximately 6.25 km grid) over the contiguous United States using a data set that has been commissioned by the U.S. Bureau of Reclamation and Army Corps of Engineers and developed by the Scripps Institution of Oceanography with a number of collaborators. The data set is called LOCA (which stands for LOcalized Constructed Analogs), and features a statistical downscaling technique using a multiscale spatial matching scheme to pick appropriate analog days from observations. The LOCA data set provides daily projections through 2100 at a 1/16° resolution for three variables: daily maximum temperature (tmax), daily minimum temperature (tmin), and daily precipitation (see USEPA, 2017a, for more details).
2.3. Method to Project Pollen Season Length
Zhang et al. (2015) developed observation‐based statistical relationships between temperature and precipitation measures and season length for four pollen types measured at the genus level: oak (Quercus spp.), birch (Betula spp.), ragweed (Ambrosia spp.), and mugwort (Artemisia spp.), and for the grass pollen family (Poaceae), which is composed of multiple grass genera. Anenberg et al. (2017) used the simplified observation‐based regression model from that paper to calculate oak pollen season length, which is dependent on the temperature and precipitation in the same year. Our literature review concluded that health endpoint relationships were either not available or not reliable for ragweed and mugwort exposure—we therefore focused on projections for oak, birch, and grass only. The simplified observation‐based regression model to estimate season length of each aeroallergen is based on the equations listed below.
| (1a) |
| (1b) |
| (1c) |
Source: Zhang et al., 2015.
T and Pr represent temperature (in degrees Celsius) and precipitation (in monthly to seasonal total millimeter precipitation), respectively, in the same season. Subscripts in the aggregated climatic factors indicate the consecutive months in which the mean temperatures or total precipitations are calculated. For example, TJFMAM represents mean temperature in January, February, March, April, and May immediately preceding the season for which length is being calculated. The relationship to establish grass pollen season length uses Growing Degree Days (GDDs) in the simplified observation model. The calculation of GDD typically requires hourly temperature data, but we use a variation of the approach that relies on the average of daily minimum and maximum temperatures. This variation is not as accurate as using hourly temperature data, but results in daily temperature differences of less than 2 °F for the majority of days for which GDD is calculated by the two methods (Goff, 2012). This variation for calculating GDD is also known as the averaging method (Nugent, 2005) and has also been used in a recent study on grass pollen in Australia and New Zealand (Medek et al., 2015).
2.4. Application of Epidemiological Studies for Concentration Response Estimation
The primary health endpoint included by Anenberg et al. (2017) was asthma‐related ED visits; however, this is likely just one of the potential impacts of increased pollen exposures. For this work, we considered three additional health endpoints to estimate the health impacts of aeroallergen exposure: OTC anti‐allergy medication sales, rhinitis consultations/hospitalizations for asthma, and school absenteeism. For OTC anti‐allergy medication sales (Ito et al., 2015) and rhinitis consultations/hospitalizations for asthma (Dales et al., 2004, 2008; Villeneuve et al., 2006) associations have been established with changes in pollen. However, daily‐scale baseline sales or incidence data are not available for many areas and are not easily extrapolated to the majority of the United States. For school absenteeism, at least one study reported a relationship with asthma or allergic rhinitis (Nathan, 2007), and other studies (e.g., O'Connor et al., 2008) found links between outdoor air pollution exposure, asthma morbidity, and children's missed school days. However, we did not identify any studies documenting a relationship between school or work absenteeism and concentrations of specific pollen types. Due to these limitations, as well as limited availability of baseline incidence data for these health outcomes in BenMAP‐CE, we chose to only quantify the asthma ED visits endpoint in our analysis of climate‐induced health impacts of changes in aeroallergen season length.
For oak pollen, we use a pooled effect estimate based on the epidemiological studies described by Anenberg et al. (2017) as the source for the concentration‐response relationship. For each additional pollen type, we assessed the availability of epidemiological studies investigating the relationship between pollen levels and asthma ED visits in the United States and Canada. A summary of those results is provided below:
Grass pollen: We identified six studies—five from the United States, and one from Canada—examining the association between grass pollen and ED visits for asthma (Table S1). On the whole, these studies suggest a link between daily grass pollen concentrations and elevated rates of asthma ED visits, particularly among children. Specifically, out of these six studies, four (in Washington DC, Babin et al., 2007; Atlanta, GA, Darrow et al., 2012; the state of New Jersey, Gleason et al., 2014; and Montreal, QC, Heguy et al., 2008) documented statistically significant associations between grass pollen and asthma ED visits, while two studies (in Wake County, NC, Sun et al., 2016, and Cincinnati, OH, Zhong et al., 2006) documented null associations. While most pollen types are identified to the genus level, grass pollen is typically identified by pollen counters to the family level (a broader taxonomic classification) due to morphological similarities between different grass pollen genera. Thus, even more so than with pollen identified to the genus level, geographic heterogeneity in the association between grass pollen and health endpoints may be due in part to differences in the species of grass pollen present in different regions. Other factors contributing to heterogeneous results across studies may include differences in the statistical modeling approach, in the definition of the health endpoint, and in the age group(s) examined.
Birch: We identified two studies examining the association between birch pollen and asthma ED visits (Table S2). Ito et al. (2015) found that elevated concentrations of birch pollen are associated with an increase in the rate of asthma ED visits in New York City, while Darrow et al. (2012) did not find evidence of such an association in Atlanta. Notably, the association presented by Darrow et al. controls for the effects of oak pollen, another key allergen that is present at the same time of year as birch, while the association presented by Ito et al. does not. Thus, it is possible that the observed impact of birch pollen on asthma ED visits in New York City may be partially due to other tree pollen types that are present at the same time of year, including oak pollen. For this work, estimates for birch are based on Ito et al. (2015), and omit consideration of the result from Darrow et al. (2012).
We also considered the potential inclusion of other pollen types, namely, ragweed (Table S3) and mugwort. While there is some evidence of an association between ragweed pollen and an increased rate of asthma ED visits (Zhong et al., 2006), studies in our literature search did not account for the concurrent increase in rhinoviruses circulating in early fall, typically attributed to the resumption of the school year (Johnston et al., 2006). No U.S.‐based studies of a concentration‐response relationship were found for mugwort. Based on these limitations, we chose to quantify climate‐induced health impacts only for those species for which we had the highest confidence in the previously established associations with asthma ED visits: oak, birch, and grass pollen.
2.5. Other BenMAP Input Data
We used BenMAP‐CE to estimate the number of asthma ED visits using relevant epidemiological studies. For studies that specified a log‐linear risk model, we used equation (2) to estimate the number of pollen‐attributable ED Visits (y i) during a specific era i (i = 2010, 2030, 2050, 2070, 2090) for different population age groups a (a = 1–17 or 18–99) in each exposure analysis grid cell k (k = 1, … K, where K is the total number of cells in our study area). The exposure analysis is conducted using a 50‐km grid cell, consistent with Anenberg et al. (2017).
| (2) |
where β is a health effect coefficient relating a change in risk of asthma ED visits with a change in daily average exposure to a specified pollen type (oak, birch, or grass), y 0ka is the baseline rate of asthma ED visits in grid cell k for a given age group, ΔC ik is the change in annual mean pollen concentration in grid cell k between year i and the baseline, and P ika is the number of residents in air quality grid cell k in year i for age group a. This health impact function returns a count of the number of pollen‐related asthma ED visits occurring in each grid cell due to the seasonal pollen concentration in each era. BenMAP then spatially aggregates grid cell‐level results to the National Climate Assessment regions for each study year.
For the Ito et al. (2015) study of asthma ED visits and pollen exposures in New York City, the authors specified a log‐log risk model for exposures to oak and birch pollen. We used the following health impact function for this model:
| (3) |
where C ikb and C ikc are annual mean pollen concentrations in the baseline (b) and control (c) scenarios, respectively, for era i and grid cell k, and the other variables are defined as above.
For a given pollen type, if multiple health impact functions were identified for a specified age group, results were weighted and integrated using BenMAP‐CE's pooling function.
Data sources that were used as inputs to BenMAP‐CE are summarized below.
Population data at the census block level for our baseline year of 2010 is included in BenMAP‐CE. Disaggregated population projections were produced at the county level using EPA's Integrated Climate and Land Use Scenarios version 2 model (Bierwagen et al., 2010; USEPA 2017b). The spatial pattern of population change in Integrated Climate and Land Use Scenarios relies on assumptions regarding fertility, migration rate, and international immigration—these were parameterized using the storyline of Shared Socioeconomic Pathway 2, which suggests medium levels of fertility, mortality, and international immigration (O'Neill et al., 2014).
Baseline incidence rates for asthma ED visits at the county level for 2014 are included in BenMAP‐CE. These data are derived from the Health Care Utilization Project's Nationwide Emergency Department Sample database and State Emergency Department Database (for additional information on these baseline incidence rate data sources see USEPA, 2016). BenMAP‐CE includes in its default databases economic valuation estimates per asthma ED visit based on cost‐of‐illness studies published by Smith et al. (1997) and Stanford et al. (1999); these unit values allow the monetization of health impacts.
Forecast pollen exposures for the contiguous United States are derived by spatially interpolating forecast pollen concentrations from 61 NAB pollen monitor locations described above to a 50 km grid. A finer resolution would likely overstate precision, given the lack of spatially refined information on pollen exposure levels and concentration‐response relationships. The spatial interpolation uses BenMAP‐CE's Voronoi Neighbor Averaging approach (USEPA 2016). The estimated temporal distribution of pollen exposures in this work is slightly altered from that used by Anenberg et al. (2017). In this work we use the monitor‐specific start date reported in Zhang et al. (2015), which can vary between monitors by up to 75 days for oak and birch (between Tampa, FL, and two Massachusetts sites—see Table S2 in Zhang et al., 2015). Although the differences across monitors in some areas are slight, owing to high levels of spatial correlation in start dates by latitude, the resulting daily exposure surface using monitor‐specific start dates is a more realistic scenario of forecast pollen at spatially interpolated, nonmonitor portions of the geographic domain.
The value of an avoided ED visit is based on the same literature used in Anenberg et al. (2017). We assigned a monetary value to pollen‐related ED visits by applying the mean of two cost‐per‐visit estimates that are included in BenMAP‐CE's set of standard valuation functions (Smith et al., 1997; Stanford et al., 1999). Adjusted for inflation, the Smith and Stanford studies report a per‐visit cost in 2015 dollars of $532 and $447, with a mean of $490 per visit.
3. Results
Combining current pollen season monitor data with epidemiologic functions suggests that tree pollen in the spring (oak and birch) accounts for between 25,000 and 50,000 pollen‐related asthma ED visits annually in 2010 (95% confidence interval: 14,000 to 100,000), compared to summer season grass pollen's less than 10,000 cases (95% confidence interval: 4,000 to 16,000; Figure 1 and Table S4). Roughly two thirds of the tree pollen incidence is among the population under age 18. Baseline projections of asthma ED visits associated with currently measured levels of oak, birch, and grass pollen across the contiguous United States through the end of the century are based on anticipated population growth absent climate impacts on pollen season length. As illustrated in Figure 1, the baseline scenario shows nearly equal numbers of asthma ED visits associated with oak and birch pollen, with each growing from approximately 25,000 per year to over 30,000 per year by 2090 due to population growth alone. ED visits associated with grass pollen exposure, however, are much lower, less than 10,000 per year for the 2010 population and growing to just more than 10,000 per year in the 2090 era. Under the strong assumption that estimates are additive across tree pollen types, the total future asthma ED incidence attributable to a growing population's exposure to current levels of pollen increases from just under 60,000 asthma ED visits annually in 2010 to about 75,000 annually in 2090. As described below, however, there are reasons to suggest that results from the two tree genera may not be fully additive.
Figure 1.
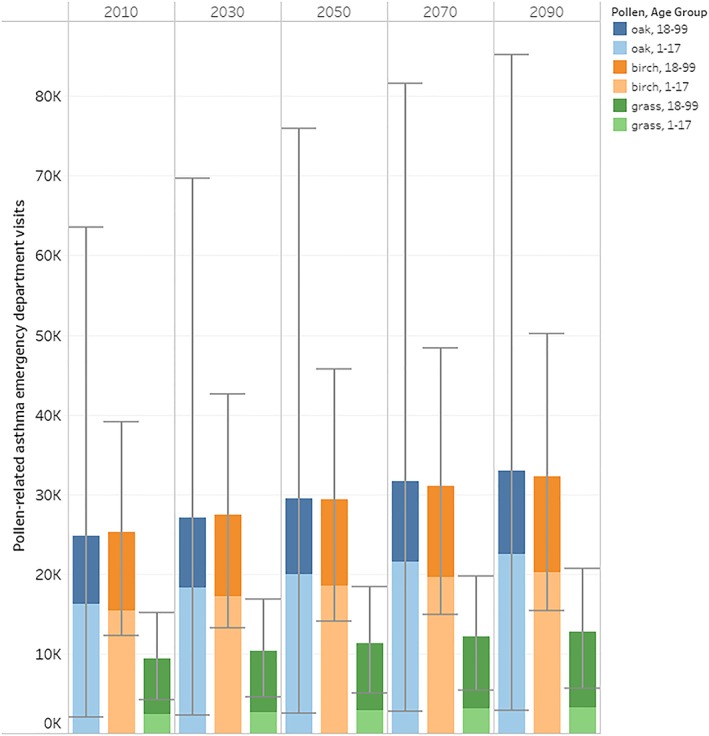
Baseline (1994–2010 pollen season with future population) annual pollen‐related asthma emergency department visits by year. Notes: Error bars represent the 95% confidence interval based on statistical uncertainty in the underlying concentration‐response effect estimate. The distribution of grass pollen incidence across age categories directly reflects the proportion by age in the overall population, because no age‐specific relative risk functions are available for this category. For oak and birch, estimated incidence is larger among children because the results reflect application of an age‐specific health impact function from the underlying literature that concludes children are more sensitive to oak and birch pollen exposure than adults.
The spatial distribution of baseline ED visits, monitors, and the outlines of the National Climate Assessment regions are shown in Figure 2 for oak pollen and in the supporting information for birch and grass pollen. As expected, counts of ED visits are spatially distributed roughly proportionately to population; interestingly, our results also suggest that the monitor network is appropriately distributed as most monitors track the areas with the largest ED visit counts attributed to pollen exposure. While our technique clearly reflects interpolation to grid cells away from monitors, much of the estimated exposure to all three pollen types, and the consequent health burden, is clustered in relatively close proximity to monitor sites.
Figure 2.
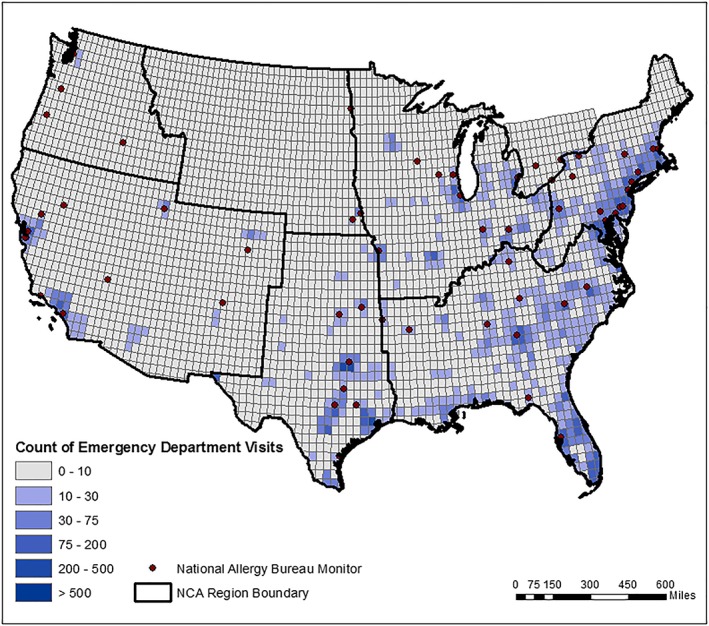
Asthma emergency department visits (all ages) in 2010 associated with 1994–2010 average oak pollen levels. Black outline indicates the U.S. Global Change Research Program NCA regions used for this study. Note: Estimates derived from National Allergy Bureau monitor data, with exposure extrapolation to unmonitored locations using BenMAP‐CE as described in the main text. Results for birch and grass pollen are provided in the supporting information. Estimates standardized for population exposure are also presented in supporting information. NCA = National Climate Assessment.
For both oak and birch, the baseline incidence occurs predominately among the 1–17 years age group, owing to the higher sensitivity of this group reflected in the relevant epidemiology (Ito et al., 2015). The relative risk function for grass, however, is not differentiated by age, and shows a distribution of effect proportional to age‐cohort population size, yielding larger incidence for adults than children.
Confidence intervals in Figure 1 illustrate differences in statistical uncertainty of the underlying epidemiological functions and suggest that the results for oak are more uncertain than those for birch and grass. Such comparisons across pollen types are generally not valid, however, as the confidence intervals necessarily omit other sources of unquantified uncertainty. For example, as noted above, the estimates for birch are based on Ito et al. (2015), and omit consideration of the result from Darrow et al. (2012), which shows a lack of an association between birch pollen and asthma ED visits in a function where oak pollen levels are controlled. The true associations for both birch and oak may lie between those documented by Ito et al. (2015) and Darrow et al. (2012). The results further suggest that ideally a multiaeroallergen approach to tree pollen sensitivity could be applied to more robustly estimate incidence attributable to this exposure. The timing of grass pollen release, which occurs predominantly in summer when fewer other allergenic taxa are present, provides a stronger assurance that the effects measures for tree and grass pollen exposure are independent and therefore more reliably additive.
The projections of future changes in oak, birch, and grass pollen season lengths reflect the higher sensitivity of grass pollen to changes in temperature over time (and to a lesser extent, the effect of precipitation, a factor which Zhang et al., 2015, conclude has a statistically significant effect only for the oak pollen projection and acts to reduce pollen concentration). The two panels of Figure 3 present pollen season length projection results for birch and grass—oak projections are predominantly unchanged from those provided by Anenberg et al. (2017), with the exception of the two additional monitors added for this work, and are therefore provided in the supporting information. The y axis of Figure 3 is a normalized pollen season length scalar, with 1.0 equal to the current pollen season length at that monitor. Results for each of the 61 monitor sites are individually presented for each of the eight forecast year/RCP combinations, and the monitors are arrayed on the x axis in order of decreasing latitude. In general, the results in Figure 3a for birch show a relatively modest sensitivity to future climate, with the largest increase of approximately 15% in the highest latitude monitor location, for the 2090 projection under RCP 8.5. Increases in birch pollen season length are expected, as projections are based solely on temperature estimates, which is generally projected to increase over time, at a faster rate under RCP 8.5 than for RCP 4.5. Nonetheless, Figure 3a indicates that pollen concentrations are projected to slightly decrease at two monitor locations, for RCP 4.5 in the 2030 timeframe, consistent with the forecast of a slight temperature decrease at those locations across all five GCMs used in this work.
Figure 3.
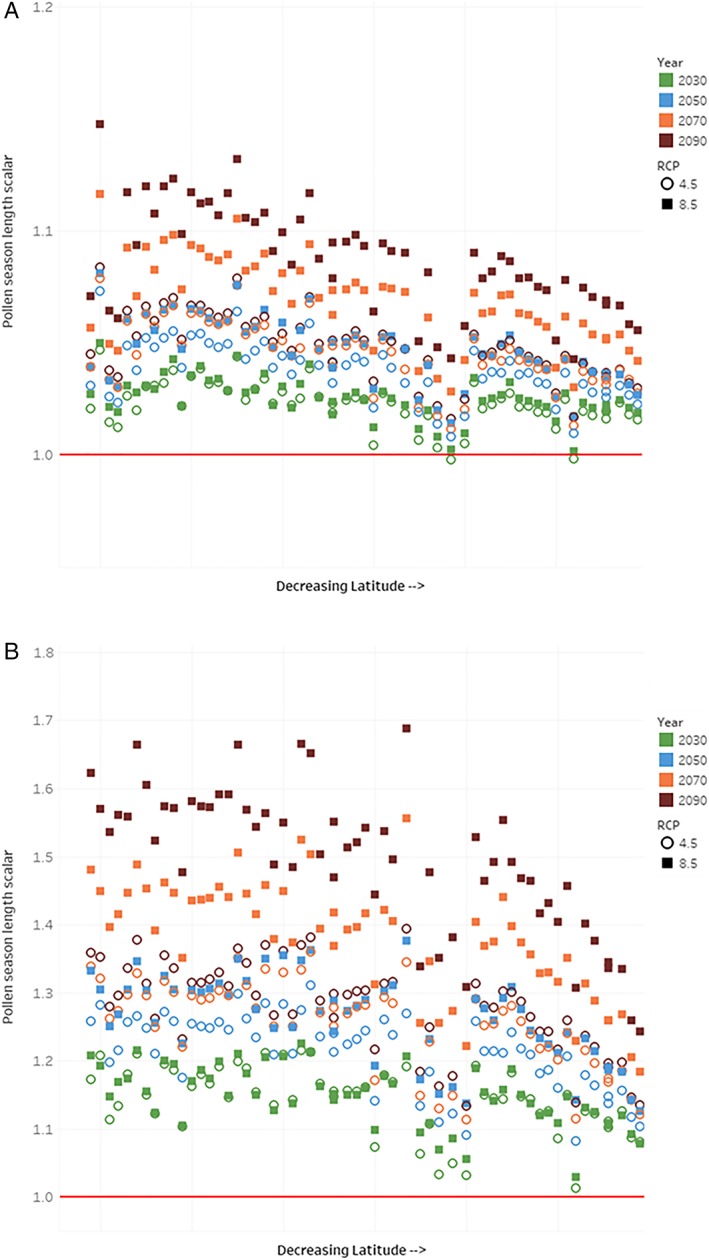
Estimates of change in pollen season length by monitor. (a) Birch. (b) Grass. Note: Pollen monitors along x axis are arranged by decreasing latitude, indicating effects on season length are more pronounced in the north (left side of graphic). Estimates averaged across five General Circulation Models, for each era and RCP assessed. Y axis is pollen season length scalar, with baseline (current climate) season length equal to 1. RCP = Representative Concentration Pathway.
The results for grass, in Figure 3b, show a much higher sensitivity to projected temperature (or, according to the function used, to GDD changes). At some sites, grass pollen is projected to increase 60% to 70% from current baseline conditions. In addition, the grass pollen results are very different across the two climate scenarios. Sensitivity of grass pollen season length scalars to temperature, via the GDD calculation, yields an almost 50% difference in the change in pollen season length when comparing RCP 8.5 to RCP 4.5 across monitor/projection year combinations. For example, several of the high and midlatitude sites show increases in season length of between 60% and 70% for the RCP 8.5 scenario in 2090, but the same monitors show a 30% to 40% increase in season length for RCP 4.5 in that year.
We estimated changes in asthma ED visits associated with spatially interpolated monitor‐level pollen projections to the full contiguous U.S. geographic domain. Figure 4 presents estimates of the average annual change in asthma ED visits from baseline—that is, the incremental increase in asthma ED visits attributable to projected changes in relevant climatic indicators—for the three pollen types (oak, grass, and birch), for six regions, four time periods, and two RCPs. The patterns that emerge reflect important spatial differences in pollen source species distribution, changes in the spatial pattern of temperature and precipitation change, and changes in the projected population exposed to pollen across regions. The largest counts of incremental increases in asthma ED visits are found in the Midwest and Northeast regions, with the Southeast and Southern Great Plains regions also showing increased health burden. While grass pollen exposure accounts for the largest change in the number of ED visits overall compared to the two tree genera, estimates associated with birch pollen exposure are of comparable magnitude to grass in the Midwest and Northeast. Increases in ED visits associated with birch pollen are somewhat lower than increases due to grass pollen in the Southeast and are a small portion of the cases in the Southern Great Plains and the western regions, where grass pollen is a more potent health threat. Increases in ED visits are lowest in the Northern Great Plains, owing to smaller exposed populations, lower pollen concentrations in the baseline time period, and hence lower projected future pollen concentrations.
Figure 4.
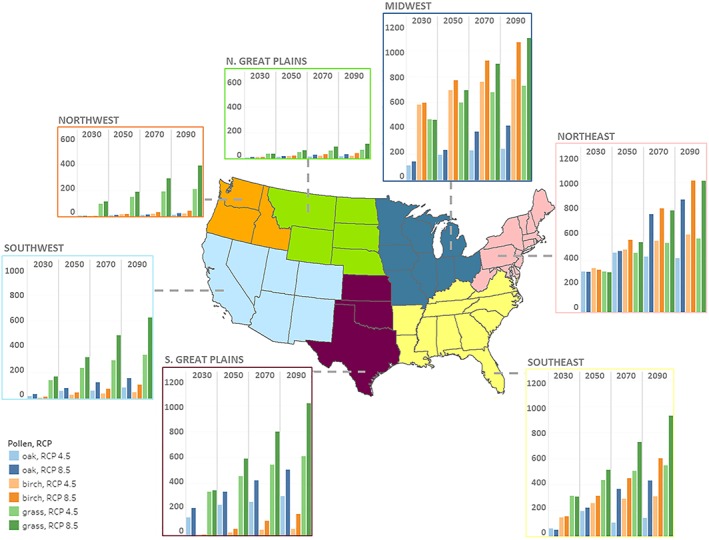
Annual regional pollen‐related asthma emergency department visits (all ages)—change from baseline for each RCP scenario, averaged across results from five General Circulation Models. Note: Estimates reflect application of pollen season length projections that incorporate the effect of projected climate change from each of the five General Circulation Models; estimated future population exposure spatially extrapolated across the United States; and application of the health impact function described in the main text. Estimates also incorporate projections of future population and population distribution as described in the main text. Vertical axes are presented at the same scale. RCP = Representative Concentration Pathway.
The spatial patterns in Figure 4 also reflect differences in the sensitivity of exposed populations to different types of pollen exposure. As noted above with respect to Figures 3a and 3b, grass pollen is much more sensitive to changes in climatic factors, but the health effects literature shows a stronger per unit response to birch pollen concentrations than for grass, which explains the rough comparability of results for birch and grass in the Northeast and Midwest. The spatial distribution of ED visits also reflects nonlinearity in the concentration‐response function for birch pollen exposure, where the functional form from Ito et al. (2015) reflects an association with percentage changes in tree pollen exposure rather than absolute increments. Detailed results by pollen type, age group, year, RCP, and region are included in the Tables S5 to S7.
The overall results, summing across all three pollen types, indicate excess incidence of allergic asthma ED visits attributed to changes in climate increasing over time in the RCP 8.5 scenario, from roughly an additional 3,700 cases per year in 2030 to over 10,000 additional cases per year in 2090 (i.e., about 6% over the baseline, increasing to 14% in 2090)—see Table 1 for details. Excess incidence attributed to changes in climate under RCP 4.5 are comparable to those under RCP 8.5 in 2030, but level off in most regions after 2050, as temperature changes are more moderate under this lower emissions scenario in the second half of the century. This generalization is perhaps most noticeable in the Northeast region. Here the overall number of ED visits grows faster than in other regions under RCP 8.5 but this increase is significantly moderated under RCP 4.5. It is also of interest that in the Northeast and Southeast, impacts from exposure to oak pollen actually decrease from 2050 to 2070 and from 2070 to 2090, while the same is not true for grass and birch. The differing effect for oak across scenarios and regions is attributable to balance of temperature and precipitation as a factor in pollen season length for the oak genera—that is, in these regions and time periods, somewhat flatter trajectories of temperature are also accompanied by slight increases in precipitation, which negatively affects oak pollen.
Table 1.
Change in Annual Regional and National Oak, Birch, and Grass Pollen‐Related Asthma Emergency Department Visits for Rach RCP Scenario, Averaged Across all Five General Circulation Models
| Projection era and climate scenario | Northeast | Southeast | Midwest | Northern Great Plains | Southern Great Plains | Northwest | Southwest | National total |
|---|---|---|---|---|---|---|---|---|
| 2030 | ||||||||
| RCP 4.5 | ||||||||
| 2.5th percentile | 301 | 194 | 500 | 23 | 144 | 48 | 52 | 1,262 |
| Mean | 974 | 523 | 1,170 | 59 | 466 | 113 | 166 | 3,472 |
| 97.5th percentile | 1,838 | 960 | 1,981 | 107 | 893 | 197 | 310 | 6,287 |
| RCP 8.5 | ||||||||
| 2.5th percentile | 284 | 100 | 506 | 24 | 183 | 56 | 70 | 1,223 |
| Mean | 952 | 516 | 1,210 | 63 | 566 | 131 | 221 | 3,659 |
| 97.5th percentile | 1,819 | 976 | 2,081 | 116 | 1,116 | 225 | 424 | 6,756 |
| 2050 | ||||||||
| RCP 4.5 | ||||||||
| 2.5th percentile | 477 | 313 | 615 | 33 | 236 | 78 | 121 | 1,874 |
| Mean | 1,406 | 887 | 1,492 | 86 | 716 | 180 | 324 | 5,091 |
| 97.5th percentile | 2,636 | 1,670 | 2,586 | 157 | 1,382 | 306 | 615 | 9,352 |
| RCP 8.5 | ||||||||
| 2.5th percentile | 547 | 288 | 697 | 42 | 325 | 98 | 167 | 2,165 |
| Mean | 1,574 | 1,049 | 1,701 | 109 | 981 | 226 | 449 | 6,090 |
| 97.5th percentile | 2,926 | 1,987 | 2,952 | 200 | 1,874 | 389 | 860 | 11,186 |
| 2070 | ||||||||
| RCP 4.5 | ||||||||
| 2.5th percentile | 506 | 285 | 684 | 39 | 294 | 99 | 151 | 2,058 |
| Mean | 1,520 | 903 | 1,671 | 101 | 849 | 229 | 393 | 5,666 |
| 97.5th percentile | 2,815 | 1,618 | 2,919 | 184 | 1,599 | 391 | 734 | 10,260 |
| RCP 8.5 | ||||||||
| 2.5th percentile | 799 | 572 | 870 | 59 | 459 | 150 | 257 | 3,166 |
| Mean | 2,346 | 1,543 | 2,201 | 156 | 1,340 | 345 | 690 | 8,621 |
| 97.5th percentile | 4,372 | 2,865 | 3,924 | 288 | 2,529 | 586 | 1,328 | 15,892 |
| 2090 | ||||||||
| RCP 4.5 | ||||||||
| 2.5th percentile | 574 | 232 | 712 | 43 | 331 | 109 | 169 | 2,168 |
| Mean | 1,590 | 999 | 1,753 | 111 | 964 | 252 | 470 | 6,138 |
| 97.5th percentile | 2,910 | 1,845 | 3,059 | 202 | 1,825 | 431 | 904 | 11,177 |
| RCP 8.5 | ||||||||
| 2.5th percentile | 1,013 | 710 | 1,029 | 74 | 591 | 201 | 335 | 3,953 |
| Mean | 2,904 | 1,964 | 2,587 | 192 | 1,691 | 466 | 893 | 10,696 |
| 97.5th percentile | 5,351 | 3,607 | 4,608 | 351 | 3,166 | 798 | 1,711 | 19,592 |
Note. Estimates reflect application of pollen season length projections that incorporate the effect of projected climate change from each of the five General Circulation Models; estimated future population exposure spatially extrapolated across the United States; and application of the health impact function described in the main text. Estimates also incorporate projections of future population and population distribution as described in the main text. RCP = Representative Concentration Pathway.
After assigning a monetary value of $490 (2015$) per pollen‐related ED visit as indicated in the methods section above, we estimate undiscounted results for each future year and an overall net present value estimated by applying a 3% discount rate per year, and linearly interpolating between analysis years. The results indicate an undiscounted sum of incidence associated with changes in climate through 2090 of $280 million under RCP 8.5, but $190 million under RCP 4.5. After discounting, the net present value of these damages is $75 million under RCP8.5, and $58 million under RCP4.5. The annual undiscounted results for the value of excess burden attributed to climate change in 2050 are comparable for the two RCPs—$2.5 million for RCP 4.5 and $3.0 million for RCP 8.5—but by 2090 the results reflect differing century‐end temperature trajectories—growing to $3.0 million for RCP 4.5 and $5.2 million for RCP 8.5.
4. Discussion
This study builds on and addresses several limitations of prior work conducted by Anenberg et al. (2017). First, this work extends the method developed in Anenberg et al. (2017) for the Northeast, Southeast, and Midwest to the full continental United States. In doing so, it also clarifies that those three regions examined in the prior work are the regions with the largest current and future health burden from aeroallergens. Second, this study expands estimates of recent and future impacts of changes in seasonality and concentration of oak pollen to also include birch and grass pollen. Here we only examine effects of climate change on season length. In addition to season length, climate change is also expected to change the amount of pollen produced by plants (Damialis et al., 2007; Frei, 1998; Frei & Leuschner, 2000; Jager et al., 2009; Levetin, 1998; Rasmussen, 2002; Spieksma et al., 1995, 2003; Teranishi et al., 2000; Ziello et al., 2012), the allergenicity of that pollen (Ahlholm et al., 1998; Cecchi et al., 2010), and the geographic range of plant species (Bellard et al., 2012; Tang et al., 2012; Woodall et al., 2009). Despite improvements in geographic coverage and pollen types, the focus on asthma‐related ED visits as the sole measure of health endpoints remains an important limitation. Existing epidemiologic literature in Canada, the Netherlands, and elsewhere suggests that aeroallergen exposure may also be linked to cardiovascular disease, allergen sensitization, and allergic rhinitis (Brunekreef et al., 2000; Meng et al., 2016; Weichenthal et al., 2016) as well as lost school or work days and lower overall productivity. Therefore, we likely underestimate both the full impact of climate change on pollen and the full impact of increased exposure to aeroallergens on human health.
Asthma is a major chronic disease of childhood, with nearly 6.8 million children affected in the United States (Bloom et al., 2013). A recent review confirmed the strong association between aeroallergens and pediatric asthma (Pollock et al., 2017). Throughout our recent and future projections of the health burden expected from increased tree pollen, roughly two thirds of asthma‐related ED visits occur in children under age 18. Children make up a smaller percentage of the total number of people projected to visit the ED for grass pollen, based on their proportion of the population, but a significant increase in the absolute number of children experiencing this health outcome is expected. In 2090 under RCP8.5, nearly 5,000 additional children are projected to visit the ED each year for asthma related to oak, birch, or grass pollen (see supporting information for tables with age‐specific results). Though the number of cases for oak and birch may not be additive due to correlation in the timing of pollen season (Ito et al., 2015), this number also does not include other pollen types (e.g., other tree species and ragweed) or the potential for pollen seasons to lengthen enough to overlap, creating multiple stressors for people allergic to both pollen types. This increase in the number of children visiting EDs for pollen induced asthma by 2090 could be reduced by more than 40% under the lower emissions scenario (RCP4.5).
Though the current number of ED visits associated with grass pollen is low compared to tree pollens, primarily because there is a relatively low level of sensitivity of those exposed to effects as severe as an ED visit, exposure to grass allergens accounts for the largest change in the number of ED visits in all regions as a result of climate change. The combination of large increase in season length coupled with the high prevalence of grass pollen allergy (20% of the general population and 40% of atopic individuals are allergic, though perhaps few currently experience effects severe enough to trigger an ED visit; Andersson & Lidholm, 2003) result in significant increases in ED visits, particularly in Western states. The high sensitivity of grass pollen to changes in temperature over time modeled in this study, relative to oak and birch pollen season length, may be further aggravated by increases of up to ~200% in grass pollen exposure predicted under higher levels of CO2 (Albertine et al., 2014).
As noted above, our results are likely underestimates of the full health impact of aeroallergens. While it is difficult to quantify the degree of underestimation, one broad measure of impact—the use of OTC medications in response to tree pollen concentrations—provides some indication. An association between pollen exposure and purchase of OTC medications is quantified in Ito et al. (2015) and Sheffield, Weinberger, Ito, et al. (2011). These studies found a cumulative impact of 7‐day lagged peak exposures on OTC allergy medication sales of 141% (Sheffield, Weinberger, Ito, et al., 2011), or a cumulative rate ratio of up to 2.0 (Ito et al., 2015). While there are difficulties in directly linking these effects measures to our analysis of asthma ED visits, and in understanding whether the daily pattern of OTC medication purchases represents a true incremental increase or merely the timing of the first seasonal purchase of these medications (see Sheffield, Weinberger, Ito, et al., 2011, for a discussion of this effect), the large overall size of the OTC allergy medication market nationally suggests even a small incremental effect could amount to a significant additional economic effect. Recent survey data indicate that allergy medication sales amount to over $6.8 billion annually and more than 735 million medication units sold in 2016 (Johnsen, 2017), an increase from comparable estimates of $6.2 billion in 2015 from the same source (Johnsen, 2016).
A second category of economic impact, school and work absence days, has not been directly linked to pollen exposures but can be linked to the increased incidence of asthma ED visits. Nathan (2007) analyzed the burden of allergic rhinitis in the United States for 2006, including physical, nonphysical, social, and economic consequences. According to this source, allergic rhinitis from all causes (including pollen, mold, animal dander, or dust) results in 3.5 million lost workdays and 2 million lost schooldays annually; Nathan (2007) further reports that 38% of those with allergic rhinitis have asthma, and 78% of those with asthma have allergic rhinitis. The value of these losses is informed by the BenMAP‐CE program, which provides an estimate of a lost work day at the median wage rate of $106 (2015$), and an estimate of a lost school day of about $75 based on the probability that, if a school child stays home from school, a parent will have to stay home from work to care for the child, combined with the value of the parent's lost productivity (USEPA 2016). This suggests that the annual current burden of lost work and schooldays from allergic rhinitis is more than $0.5 billion per year, not including lost educational development for affected children. While not all of this annual damage estimate is attributable to pollen exposure, a portion of these lost work and school days are nonetheless very likely associated with the asthma ED visits that are the focus of this analysis. At this stage, however, we can conclude that the overall burden of allergic rhinitis suggests the economic effect of climate‐induced changes in pollen alone is much larger than we can currently reliably quantify.
This analysis is subject to a number of uncertainties, only a subset of which we were able to quantify. The estimated number of pollen‐attributable asthma ED visits incorporate error bars representing the 95% confidence interval (Figure 1); the numerical values for which are available in Tables S5–S7 and in summary form in Table 1. Because these intervals were calculated by sampling from the standard error reported in each epidemiological study, they reflect only the statistical error reported. Other “upstream” uncertainties—including the accuracy of monitored recent‐year pollen levels, our approach for projecting population, the technique for interpolating pollen levels, the simulated future temperature and precipitation levels, and estimated change in pollen season length—were not accounted for. In addition, a key assumption that underlies our method is the reliance on pollen season length as the key metric of the effect of climate change on pollen exposure. While we assume that the average daily pollen concentration remains the same, which is reasonable based on current information, further research is needed to better understand the effect of climate change on the full temporal distribution of daily pollen concentrations during the lengthened season. A second key assumption is that, when we apply the future season‐length models, we assume that in each location, the set of species that contributed to the baseline pollen data are going to be the same in the future. At this point, tree and grass species prevalence modeling, which would also need to be coupled with species‐specific pollen production data, is not available to support a species‐level simulation, but there is some evidence that climate change could alter the geographic range of tree species in the future (e.g., Bellard et al., 2012; Tang et al., 2012; Woodall et al., 2009).
We also elected to construct our 2010 baseline era estimates using slightly inconsistent periods for pollen data (1994–2010), baseline climate (1986–2010), and population assumption (2010). This is primarily the consequence of inconsistent data availability, and also reflects our choice to not introduce new uncertainties associated with a potential temporal correction of the data. Our choices in the construction of the base period estimate likely means that our characterization of the baseline health burden somewhat underestimates current burdens, because the pollen concentration temporal trend within the reference period is upward, and we effectively attribute concentrations from 1994 to 2010 to a year (2010) at the end of that period. Finally, our results are limited by the lack of primary epidemiologic information on the pollen concentration‐response function for many regions of the United States. To the extent that the response varies over space, this limitation adds to the uncertainty of our analysis. We are unable to characterize the level of uncertainty contributed by each of these inputs.
5. Conclusions
This work provides an enhanced understanding of the attribution of asthma ED visits to two tree pollen types (oak and birch) and grass pollen, both currently and in the future under two climate scenarios. The roughly 60,000 cases attributed to these three pollen types in 2010 represents about 4% of the approximately 1.6 million asthma‐related emergency room visits. In addition, other pollen types omitted from this analysis due to data or methodological limitations, including many other tree species (in spring), ragweed (in late summer/early fall), and other flowering plants (season‐wide), mean the full impact of aeroallergens on asthma ED visits is much higher.
Our results suggest that the future grass pollen season will be a more important component of the overall aeroallergen impact than it is currently, largely because of the estimated high sensitivity of grass pollen to temperature changes. The impact on grass pollen will be particularly large in the Southeast, Southern Great Plains, Southwest, and Northwest, where tree pollen levels are generally lower.
Continued research to develop a more complete assessment of the health burden of pollen, currently and for the future under climate change, is needed. Key limitations in the literature related to the epidemiology of some pollen types (e.g., ragweed) might be overcome with more refined epidemiological attribution techniques. The expansion of geographic and pollen type scope that is the main focus of this paper could continue to be augmented by future studies, on more individual pollen types, in more locations. Research investigating the effects of pollen on other health endpoints and other socioeconomic consequences (e.g., spending on OTC drugs), and better characterization of the disproportionate impacts experienced by vulnerable populations, is also important. In addition, as observations of both pollen exposures and health outcomes accumulate over time, there will be an increasing opportunity to analyze climate change attributable changes in the observed timing and magnitude of pollen exposures and associated health effects. Scientific advancements building on this work to quantify projected changes to the burden of disease are also crucial to improve the effectiveness of adaptation actions. Finally, we suggest that a multitaxa approach to quantifying health effects from pollen would be a fruitful direction for research, in light of the temporal overlap between oak, birch, and other tree pollen taxa in the spring.
Supporting information
Supporting Information S1
Acknowledgments
Opinions expressed in this article are those of the authors and do not necessarily represent those of their employers or affiliated institutions, including the U.S. EPA. Weinberger's contribution to this publication was as a paid consultant and was not part of her Brown University duties or responsibilities. The authors are grateful to Lewis Ziska for constructive comments; to The Allergy Group for pollen monitor data from Boise, Idaho; to Duane Harris for pollen monitor data from Draper, Utah; to Natalie Weiss and Alisa White for excellent research assistance; and to Stefani Penn for assistance with portions of the literature review and discussion sections. This research was funded by the U.S. Environmental Protection Agency under contract EP‐D‐14‐031. The authors declare that they have no competing financial interests. The data used are listed in the references and supporting information.
Neumann, J. E. , Anenberg, S. C. , Weinberger, K. R. , Amend, M. , Gulati, S. , Crimmins, A. , et al. (2019). Estimates of present and future asthma emergency department visits associated with exposure to oak, birch, and grass pollen in the United States. GeoHealth, 3, 11–27. 10.1029/2018GH000153
References
- Ahlholm, J. U. , Helander, M. L. , & Savolainen, J. (1998). Genetic and environmental factors affecting the allergenicity of birch (Betula pubescens ssp. Czerepanovii [Orl.] hamet‐ahti) pollen. Clinical and Experimental Allergy, 28, 1384–1388. [DOI] [PubMed] [Google Scholar]
- Albertine, J. M. , Manning, W. J. , DaCosta, M. , Stinson, K. A. , Muilenberg, M. L. , & Rogers, C. A. (2014). Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PLoS One, 9, e111712 10.1371/journal.pone.0111712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Allergy, Asthma and Immunology (AAAAI) (2016). Allergic asthma. [Available at https://www.aaaai.org/conditionsand-treatments/conditions-dictionary/allergic-asthma, accessed July 6 2016.]
- Andersson, K. , & Lidholm, J. (2003). Characteristics and immunobiology of grass pollen allergens. International Archives of Allergy and Immunology, 130(2), 87–107. [DOI] [PubMed] [Google Scholar]
- Anenberg, S. C. , Weinberger, K. R. , Roman, H. , Neumann, J. E. , Crimmins, A. , Fann, N. , Martinich, J. , & Kinney, P. L. (2017). Impacts of oak pollen on allergic asthma in the United States and potential influence of future climate change. GeoHealth, 1, 80–92. 10.1002/2017GH000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin, S. M. , Burkom, H. S. , Holtry, R. S. , Tabernero, N. R. , Stokes, L. D. , Davies‐Cole, J. O. , DeHaan, K. , & Lee, D. H. (2007). Pediatric patient asthma‐related emergency department visits and admissions in Washington, DC, from 2001–2004, and associations with air quality, socio‐economic status and age group. Environmental Health, 6, 9 10.1186/1476-069X-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs, P. J. (2004). Impacts of climate change on aeroallergens: Past and future. Clinical and Experimental Allergy, 34, 1507–1513. 10.1111/j.1365-2222.2004.02061.x [DOI] [PubMed] [Google Scholar]
- Bellard, C. , Bertelsmeier, C. , Leadley, P. , Thuiller, W. , & Courchamp, F. (2012). Impacts of climate change on the future of biodiversity. Ecology Letters, 15, 365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielory, L. , Lyons, K. , & Goldberg, R. (2012). Climate change and allergic disease. Current Allergy and Asthma Reports, 12, 485–494. 10.1007/s11882-012-0314-z [DOI] [PubMed] [Google Scholar]
- Bierwagen, B. , Theobald, D. M. , Pyke, C. R. , Choate, A. , Groth, A. P. , Thomas, J. V. , & Morefield, P. (2010). National housing and impervious surface scenarios for integrated climate impact assessments. Proceedings of the National Academy of Sciences of the United States of America, 107, 20,887–20,892. See http://www.epa.gov/iclus for more information, 10.1073/pnas.1002096107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, B. , Jones, L. I. , & Freeman, G. (2013). Summary health statistics for U.S. children: National health interview survey, 2012. Vital and Health Statistics, 258, 1–81. [PubMed] [Google Scholar]
- Brunekreef, B. , Hoek, G. , Fischer, P. , & Spieksma, F. T. M. (2000). Relation between airborne pollen concentrations and daily cardiovascular and respirator‐disease mortality. Lancet, 355(9214), 1517–1518. 10.1016/S0140-6736(00)02168-1 [DOI] [PubMed] [Google Scholar]
- Cecchi, L. , D'Amato, G. , Ayres, J. G. , Galan, C. , Forastiere, F. , Forsberg, B. , Gerritsen, J. , Nunes, C. , Behrendt, H. , Akdis, C. , Dahl, R. , & Annesi‐Maesano, I. (2010). Projections of the effects of climate change on allergic asthma: The contribution of aerobiology. Allergy, 65(9), 1073–1081. 10.1111/j.1398-9995.2010.02423.x [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2011). Vital signs, May 2011. Retrieved from http://www.cdc.gov/vitalsigns/asthma/, accessed July 6 2016.
- Centers for Disease Control and Prevention (CDC) (2017). Most recent asthma data. Retrieved from https://www.cdc.gov/asthma/most_recent_data.htm, accessed January 24, 2018.
- Collins, W. J. , Bellouin, N. , Doutriaux‐Boucher, M. , Gedney, N. , Halloran, P. , Hinton, T. , Hughes, J. , Jones, C. D. , Joshi, M. , Liddicoat, S. , Martin, G. , O'Connor, F. , Rae, J. , Senior, C. , Sitch, S. , Totterdell, I. , Wiltshire, A. , & Woodward, S. (2011). Development and evaluation of an Earth system model–HadGEM2. Geoscience Model Development, 4, 1051–1075. 10.5194/gmd-4-1051-2011 [DOI] [Google Scholar]
- Dahl, A. , Galán, C. , Hajkova, L. , Pauling, A. , Sikoparija, B. , Smith, M. , & Vokou, D. (2013). The onset, course and intensity of the pollen season In Sofiev M. & Bergmann K. C. (Eds.), Allergenic pollen: A review of the production, release, distribution and health impacts (pp. 29–70). Dordrecht, Netherlands: Springer; 10.1007/978-94-007-4881-1_3 [DOI] [Google Scholar]
- Dales, R. E. , Cakmak, S. , Judek, S. , & Coates, F. (2008). Tree pollen and hospitalization for asthma in urban Canada. International Archives of Allergy and Immunology, 146(3), 241–247. 10.1159/000116360 [DOI] [PubMed] [Google Scholar]
- Dales, R. E. , Cakmak, S. , Judek, S. , Dann, T. , Coates, F. , Brook, J. R. , & Burnett, R. T. (2004). Influence of outdoor aeroallergens on hospitalization for asthma in Canada. Journal of Allergy and Clinical Immunology, 113(2), 303–306. 10.1016/j.jaci.2003.11.016 [DOI] [PubMed] [Google Scholar]
- Damialis, A. , Halley, J. M. , Gioulekas, D. , & Vokou, D. (2007). Long‐term trends in atmospheric pollen levels in the city of Thessaloniki, Greece. Atmospheric Environment, 41, 7011–7021. 10.1016/j.atmosenv.2007.05.009 [DOI] [Google Scholar]
- Darrow, L. A. , Hess, J. , Rogers, C. A. , Tobert, P. E. , Klein, M. , & Sarnat, S. E. (2012). Ambient pollen concentrations and emergency department visits for asthma and wheeze. Journal of Allergy and Clinical Immunology, 130, 630–638.e4. 10.1016/j.jaci.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, T. , Cullen, M. J. P. , Malcolm, A. J. , Mawson, M. H. , Staniforth, A. , White, A. A. , & Wood, N. (2005). A new dynamical core for the Met Office's global and regional modelling of the atmosphere. Quarterly Journal of the Royal Meteorological Society, 131, 1759–1782. 10.1256/qj.04.101 [DOI] [Google Scholar]
- Fann, N. , Brennan, T. , Dolwick, P. , Gamble, J. L. , Ilacqua, V. , Kolb, L. , Nolte, C. G. , Spero, T. L. , & Ziska, L. (2016). Ch. 3: Air quality impacts In The impacts of climate change on human health in the United States: A scientific assessment (pp. 69–98). Washington, DC: U.S. Global Change Research Program; 10.7930/J0GQ6VP6 [DOI] [Google Scholar]
- Frei, T. (1998). The effects of climate change in Switzerland 1969–1996 on airborne pollen quantities from hazel, birch and grass. Grana, 37, 172–179. 10.1080/00173139809362662 [DOI] [Google Scholar]
- Frei, T. , & Leuschner, R. M. (2000). A change from grass pollen induced allergy to tree pollen induced allergy: 30 years of pollen observation in Switzerland. Aerobiologia, 16, 407–416. 10.1023/A:1026532307090 [DOI] [Google Scholar]
- Gent, P. R. , Danabasoglu, G. , Donner, L. J. , Holland, M. M. , Hunke, E. , Jayne, S. , Lawrence, D. , Neale, R. B. , Rasch, P. J. , Vertenstein, M. , & Worley, P. H. (2011). The community climate system model version 4. Journal of Climate, 24, 4973–4991. 10.1175/2011JCLI4083.1 [DOI] [Google Scholar]
- Gleason, J. A. , Bielory, L. , & Fagliano, J. A. (2014). Associations between ozone, PM2.5, and four pollen types on emergency department pediatric asthma events during the warm season in New Jersey: A case‐crossover study. Environmental Research, 132, 421–429. 10.1016/j.envres.2014.03.035 [DOI] [PubMed] [Google Scholar]
- Goff, J. M. (2012). A value‐added approach in degree day calculation. Burlington VT: National Weather Service. [Google Scholar]
- Gonzalez Minero, F. J. , Candau, P. , Tomas, C. , & Morales, J. (1998). Airborne grass (Poaceae) pollen in southern Spain. Results of a 10‐year study (1987–96). Allergy, 53, 266–274. 10.1111/j.1398-9995.1998.tb03886.x [DOI] [PubMed] [Google Scholar]
- Heguy, L. , Garneau, M. , Goldberg, M. S. , Raphoz, M. , Guay, F. , & Valois, M.‐F. (2008). Associations between grass and weed pollen and emergency department visits for asthma among children in Montreal. Environmental Research, 106(2), 203–211. 10.1016/j.envres.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Hicks, S. , Helander, M. , & Heino, S. (1994). Birch pollen production, transport and deposition for the period 1984–1993 at Kevo, northernmost Finland. Aerobiologia, 10, 183–191. 10.1007/BF02459234 [DOI] [Google Scholar]
- Ito, K. , Weinberger, K. R. , Robinson, G. S. , Sheffield, P. E. , Lall, R. , Mathes, R. , Ross, Z. , Kinney, P. L. , & Matte, T. D. (2015). The associations between daily spring pollen counts, over‐the‐counter allergy medication sales, and asthma emergency department visits syndrome in New York City, 2002–2012. Environmental Health, 14, 71 10.1186/s12940-015-0057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager, S. , Nilsson, S. , Berggren, B. , Pessi, A.‐M. , Helander, M. , & Ramfjord, H. (2009). Trends of some airborne tree pollen in the Nordic countries and Austria, 1980–1993: A comparison between Stockholm, Trondheim, Turku and Vienna. Grana, 35, 171–178. [Google Scholar]
- Johnsen, M . (2016). Flonase continues market strength. Drugstore News. August 2016: 64.
- Johnsen, M . (2017). Record‐breaking heat, precipitation make allergy medications more important. Drugstore News. March 2017: 16.
- Johnston, N. W. , Johnston, S. L. , Norman, G. R. , Dai, J. , & Sears, M. R. (2006). The September epidemic of asthma hospitalization: School children as disease vectors. Journal of Allergy and Clinical Immunology, 117, 557–562. 10.1016/j.jaci.2005.11.034 [DOI] [PubMed] [Google Scholar]
- Lake, I. R. , Jones, N. R. , Agnew, M. , Goodess, C. M. , Giorgi, F. , Hamaoui‐Laguel, L. , Semenov, M. A. , Solomon, F. , Storkey, J. , Vautard, R. , & Epstein, M. M. (2016). Climate change and future pollen allergy in Europe. Environmental Health Perspectives, 125, 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre, F. (1999). Differences between airborne pollen and flowering phenology of urban trees with reference to production, dispersal and interannual climate variability. Aerobiologia, 15, 131–141. 10.1023/A:1007523316024 [DOI] [Google Scholar]
- Levetin, E. (1998). A long‐term study of winter and early spring tree pollen in the Tulsa, Oklahoma atmosphere. Aerobiologia, 14, 21–28. 10.1007/BF02694591 [DOI] [Google Scholar]
- McLauchlan, K. K. , Barnes, C. S. , & Craine, J. M. (2011). Interannual variability of pollen productivity and transport in mid‐North America from 1997 to 2009. Aerobiologia, 27, 181–189. 10.1007/s10453-010-9186-7 [DOI] [Google Scholar]
- Medek, D. E. , Beggs, P. J. , Erbas, B. , Jaggard, A. K. , Campbell, B. C. , Vicendese, D. , Johnston, F. H. , Godwin, I. , Huete, A. R. , Green, B. J. , Burton, P. K. , Bowman, D. M. J. S. , Newnham, R. M. , Katelaris, C. H. , Haberle, S. G. , Newbigin, E. , & Davies, J. M. (2015). Regional and seasonal variation in airborne grass pollen levels between cities of Australia and New Zealand. Aerobiologia, 32(2), 289–302. 10.1007/s10453-015-9399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, Q. , Nagarajan, S. , Son, Y. , Koutsoupias, P. , & Bielory, L. (2016). Asthma, oculonasal symptoms, and skin test sensitivity across National Health and Nutrition Examination Surveys. Annals of Allergy, Asthma & Immunology, 116, 118–125. 10.1016/j.anai.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Nathan, R. A. (2007). The burden of allergic rhinitis. Allergy and Asthma Proceedings, 28, 3–9. [DOI] [PubMed] [Google Scholar]
- Neale, R. B. , Richter, J. , Park, S. , Lauritzen, P. H. , Vavrus, S. J. , Rasch, P. , & Zhang, M. (2013). The mean climate of the community atmosphere model (CAM4) in forced SST and fully coupled experiments. Journal of Climate, 26, 5150–5168. 10.1175/JCLI-D-12-00236.1 [DOI] [Google Scholar]
- Nugent, J. (2005). Calculating growing degree days. Michigan State University. Retrieved https://www.canr.msu.edu/uploads/files/Research_Center/NW_Mich_Hort/General/CalculatingGrowingDegreeDays.pdf
- O'Connor, G. T. , Neas, L. , Vaughn, B. , Kattan, M. , Mitchell, H. , Crain, E. F. , Evans, R. , Gruchalla, R. , Morgan, W. , Stout, J. , Adams, G. K. , & Lippmann, M. (2008). Acute respiratory health effects of air pollution on children with asthma in US inner cities. The Journal of Allergy and Clinical Immunology, 121(5), 1133–1139.e1. 10.1016/j.jaci.2008.02.020 [DOI] [PubMed] [Google Scholar]
- O'Neill, B. C. , Kriegler, E. , Riahi, K. , Ebi, K. L. , Hallegatte, S. , Carter, T. R. , Mathur, R. , & van Vuuren, D. P. (2014). A new scenario framework for climate change research: The concept of shared socioeconomic pathways. Climatic Change, 122, 387–400. 10.1007/s10584-013-0905-2 [DOI] [Google Scholar]
- Pollock, J. , Lu, S. , & Gimbel, R. W. (2017). Outdoor environment and pediatric asthma: An update on the evidence from North America. Canadian Respiratory Journal, 2017, 1–16. 10.1155/2017/8921917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, A. (2002). The effects of climate change on the birch pollen season in Denmark. Aerobiologia, 18, 253–265. 10.1023/A:1021321615254 [DOI] [Google Scholar]
- Reid, C. E. , & Gamble, J. L. (2009). Aeroallergens, allergic disease, and climate change: Impacts and adaptation. EcoHealth, 6, 458–470. 10.1007/s10393-009-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, G. A. , Ruedy, R. , Hansen, J. E. , Aleinnov, I. , Bell, N. , Bauer, M. , Bauer, S. , Cairns, B. , Canuto, V. , Cheng, Y. , & Del Genio, A. (2006). Present‐day atmospheric simulations using GISS ModelE: Comparison to in situ, satellite, and reanalysis data. Journal of Climate, 19, 153–192. 10.1175/JCLI3612.1 [DOI] [Google Scholar]
- Sheffield, P. E. , Weinberger, K. R. , Ito, K. , Matte, T. D. , Mathes, R. W. , Robinson, G. S. , & Kinney, P. L. (2011). The association of tree pollen concentration peaks and allergy medication sales in New York City: 2003–2008. ISRN Allergy, 2011, 1–7. 10.5402/2011/537194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield, P. E. , Weinberger, K. R. , & Kinney, P. L. (2011). Climate change, aeroallergens and pediatric allergic disease. Mount Sinai Journal of Medicine, 78, 78–84. 10.1002/msj.20232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. H. , Malone, D. C. , Lawson, K. A. , Okamoto, L. J. , Battista, C. , & Saunders, W. B. (1997). A national estimate of the economic costs of asthma. American Journal of Respiratory and Critical Care Medicine, 156(3), 787–793. 10.1164/ajrccm.156.3.9611072 [DOI] [PubMed] [Google Scholar]
- Spieksma, F. T. M. , Corden, J. M. , Detandt, M. , Millington, W. M. , Nikkels, H. , Nolard, N. , Schoenmakers, C. H. H. , Wachter, R. , de Weger, L. A. , Willems, R. , & Emberlin, J. (2003). Quantitative trends in annual totals of five common airborne pollen types (Betula, Quercus, Poaceae, Urtica, and Artemisia) at five pollen‐monitoring stations in western Europe. Aerobiologia, 19(3/4), 171–184. 10.1023/B:AERO.0000006528.37447.15 [DOI] [Google Scholar]
- Spieksma, F. T. M. , Emberlin, J. C. , Hjelmroos, M. , Jager, S. , & Leuschner, R. M. (1995). Atmospheric birch (Betula) pollen in Europe: Trends and fluctuations in annual quantities and the starting dates of the seasons. Grana, 34, 51–57. 10.1080/00173139509429033 [DOI] [Google Scholar]
- Stanford, R. , Mclaughlin, T. , & Okamoto, L. J. (1999). The cost of asthma in the emergency department and hospital. American Journal of Respiratory and Critical Care Medicine, 160(1), 211–215. 10.1164/ajrccm.160.1.9811040 [DOI] [PubMed] [Google Scholar]
- Sun, X. , Waller, A. , Yeatts, K. B. , & Thie, L. (2016). Pollen concentration and asthma exacerbations in Wake County, North Carolina, 2006–2012. Science of the Total Environment, 544, 185–191. 10.1016/j.scitotenv.2015.11.100 [DOI] [PubMed] [Google Scholar]
- Tang, G. , Beckage, B. , & Smith, B. (2012). The potential transient dynamics of forests in New England under historical and projected future climate change. Climatic Change, 114, 357–377. 10.1007/s10584-012-0404-x [DOI] [Google Scholar]
- Taylor, K. E. , Stouffer, R. J. , & Meehl, G. A. (2012). An overview of CMIP5 and the experiment design. Bulletin of the American Meteorological Society, 93, 485–498. 10.1175/BAMS-D-11-00094.1 [DOI] [Google Scholar]
- Teranishi, H. , Kenda, Y. , Katoh, T. , Kasuya, M. , Oura, E. , & Taira, H. (2000). Possible role of climate change in the pollen scatter of Japanese cedar Cryptomeria japonica in Japan. Climate Research, 14, 65–70. 10.3354/cr014065 [DOI] [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) (2016). Environmental benefits mapping and analysis program—Community edition (BenMAP‐CE). Research Triangle Park, NC. Retrieved from http://www.epa.gov/air/benmap, Accessed October 27, 2016.
- U.S. Environmental Protection Agency (USEPA) (2017a). Multi‐model framework for quantitative sectoral impacts analysis: A technical report for the fourth national climate assessment. EPA 430‐R‐17‐001.
- U.S. Environmental Protection Agency (USEPA) (2017b). Updates to the demographic and spatial allocation models to produce integrated climate and land use scenarios (Iclus) (Version 2). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R‐16/366F. Available online at https://cfpub.epa.gov/ncea/iclus/recordisplay.cfm?deid=322479 [Google Scholar]
- USGCRP (2017). In Wuebbles D. J., Fahey D. W., Hibbard K. A., Dokken D. J., Stewart B. C., & Maycock T. K. (Eds.), Climate science special report: Fourth national climate assessment (Vol. I, p. 470). Washington, DC, USA: U.S. Global Change Research Program; 10.7930/J0J964J6 [DOI] [Google Scholar]
- Villeneuve, P. J. , Doiron, M. S. , Stieb, D. , Dales, R. , Burnett, R. T. , & Dugandzic, R. (2006). Is outdoor air pollution associated with physician visits for allergic rhinitis among the elderly in Toronto, Canada? Allergy, 61, 750–758. 10.1111/j.1398-9995.2006.01070.x [DOI] [PubMed] [Google Scholar]
- Von Salzen, K. , Scinocca, J. F. , McFarlane, N. A. , Li, J. , Cole, J. N. , Plummer, D. , Verseghy, D. , Reader, M. C. , Ma, X. , Lazare, M. , & Solheim, L. (2013). The Canadian fourth generation atmospheric global climate model (CanAM4). Part I: representation of physical processes. Atmosphere‐Ocean, 51, 104–125. 10.1080/07055900.2012.755610 [DOI] [Google Scholar]
- Watanabe, M. , Suzuki, T. , O'ishi, R. , Komuro, Y. , Watanabe, S. , Emori, S. , Takemura, T. , Chikira, M. , Ogura, T. , Sekiguchi, M. , & Takata, K. (2010). Improved climate simulation by MIROC5: Mean states, variability, and climate sensitivity. Journal of Climate, 23, 6312–6335. 10.1175/2010JCLI3679.1 [DOI] [Google Scholar]
- Weichenthal, S. , Lavigne, E. , Villenveuve, P. J. , & Reeves, F. (2016). Airborne pollen concentrations and emergency room visits for myocardial infarction: A multicity case‐crossover study in Ontario, Canada. American Journal of Epidemiology, 183(7), 613–621. 10.1093/aje/kwv252 [DOI] [PubMed] [Google Scholar]
- Woodall, C. W. , Oswalt, C. M. , Westfall, J. A. , Perry, C. H. , Nelson, M. D. , & Finley, A. O. (2009). An indicator of tree migration in forests of the eastern United States. Forest Ecology and Management, 256, 1434–1444. [Google Scholar]
- Zhang, Y. , Bielory, L. , & Georgopoulos, P. (2014). Climate change effect on Betula (birch) and Quercus (oak) pollen seasons in US. International Journal of Biometeorology, 58(5), 909–919. 10.1007/s00484-013-0674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Bielory, L. , Mi, Z. , Cai, T. , Robock, A. , & Georgopoulos, P. (2015). Allergenic pollen season variations in the past two decades under changing climate in the United States. Global Change Biology, 21, 1581–1589. 10.1111/gcb.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, W. , Levin, L. , Reponen, T. , Hershey, G. K. , Adhikari, A. , Shukla, R. , & LeMasters, G. (2006). Analysis of short‐term influences of ambient aeroallergens on pediatric asthma hospital visits. Science of the Total Environment, 370(2‐3), 330–336. 10.1016/j.scitotenv.2006.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziello, C. , Sparks, T. H. , Estrella, N. , Belmonte, J. , Bergmann, K. C. , Bucher, E. , Brighetti, M. A. , Damialis, A. , Detandt, M. , Galán, C. , Gehrig, R. , Grewling, L. , Gutiérrez Bustillo, A. M. , Hallsdóttir, M. , Kockhans‐Bieda, M. C. , de Linares, C. , Myszkowska, D. , Pàldy, A. , Sánchez, A. , Smith, M. , Thibaudon, M. , Travaglini, A. , Uruska, A. , Valencia‐Barrera, R. M. , Vokou, D. , Wachter, R. , de Weger, L. A. , & Menzel, A. (2012). Changes to airborne pollen counts across Europe. PLoS One, 7(4), e34076 10.1371/journal.pone.0034076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


