Abstract
Swiss needle cast (SNC), caused by Nothophaeocryptopus gaeumannii, is an important foliage disease of Douglas-fir (Pseudotsuga menziesii) forests of the Pacific Northwest. The fungus lives endophytically within the foliage, until forming reproductive structures (pseudothecia) that plug stomates and cause carbon starvation. When pseudothecia appear on one- and two-year-old foliage, significant needle abscission can occur, which reduces productivity of the tree. While there is considerable evidence of SNC disease in coastal Douglas-fir plantations, the severity of SNC in mature and old-growth forests is poorly understood. We compared tree crowns of mature and old-growth conifer forests and nearby young forests at three locations in the Oregon Coast Range and four locations in the western Cascade Range of Oregon. We assessed disease severity for N. gaeumannii on two-year-old foliage, incidence by presence of N. gaeumannii on all foliage, foliage retention for the first four years, and foliar nitrogen of one-year-old foliage. We also compared leaf wetness at three heights in one mature and one young tree at five of the seven sites. Disease severity was greater in young forests than mature forests at all sites except for high elevation Cascade Range areas. Incidence of disease was highest for two-year-old needles in young trees and 3–5 year-old needles in mature trees, except for one coastal site. Retention of 1–4 year-old needle cohorts differed between young and mature trees, and mature trees had much larger complements of > four-year-old needles. Total foliar nitrogen (TN) concentration did not differ in needles of young and mature trees, but at some locations total N differed between canopy positions. Leaf wetness differences were not consistent between young and mature tree crowns. However, at one study site in the core epidemic area, the younger stand had longer periods of wetness in the upper crowns than a nearby old stand. Leaf wetness and foliar N were hypothesized to play a role in SNC disease severity, but they do not explain differences in adjacent young and mature trees. Although the fungus is present in old and young trees, the likelihood of disease expression and lower foliage retention appears to be greater in younger plantation trees than mature and older trees in western Oregon Douglas-fir forests.
1. Introduction
Swiss needle cast (SNC), caused by Nothophaeocryptopus gaeumannii (T. Rohde) Videira et al. (2017) (Ascomycete: Mycosphaerellaceae), is an important foliage disease of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) plantations in the coastal region of the Pacific Northwest (Hansen et al. 2000; Shaw et al. 2011). Nothophaeocryptopus gaeumannii is a common native fungus that occurs everywhere Douglas-fir grows. The fungus does not spread per se, but intensifies when conditions allow. Aerial detection surveys across coastal Oregon and Washington have shown the disease symptoms to be intensifying, with 1996 aerial survey in Oregon detecting 53,050 ha of forest land having disease symptoms, increasing to 238,705 ha in 2015 (Ritóková et al. 2016). Rather than directly attacking host cells, N. gaeumannii is an endophyte that causes disease by carbon starvation when the reproductive structures (pseudothecia) plug the stomates and inhibit carbon uptake and transpiration (Manter et al. 2000). Disease impact in forest plantations is associated with loss of foliage. Foliage retention of less than three years results in reduced tree volume growth < 25%, and foliage retention of two years is associated with a volume loss >25% (Maguire et al. 2002, 2011). Mortality is rare but stands with õne year or less of foliage retention are associated with the most severe disease impacts observed (Maguire et al. 2011).
Foliage retention is a common metric for assessing disease severity in young Douglas-fir plantations (Maguire et al. 2011). Foliage retention is known to vary with site productivity and elevation, the lowest productivity and highest elevation sites have the greatest foliage retention (Ewers and Schmid 1981, Reich et al. 1995). The overall effect of SNC is to lower foliage retention in forest plantations across this gradient (Shaw et al. 2014), but the influence of SNC on foliage retention in older stands is not known.
Disease severity, assessed on two-year-old needles, varies with canopy position in younger forests, with severity greatest in the upper crown (Hansen et al. 2000; Manter et al. 2003; Shaw et al. 2014). This is unusual because foliage disease severity is typically greatest in the most humid portion of the crown, which for conifers is typically the lower and inner crown. Therefore, the assumption has been that leaf wetness is not a limiting factor within the epidemic area (~ within 50 km of the coast). However, leaf wetness and humidity are necessary for spore dispersal, germination on the leaf, and growth of the hyphae into stomates (Manter et al. 2005).
Epidemiology of SNC has focused on winter temperature and leaf wetness during spore dispersal from May through August (Manter et al. 2005). Subsequent models using foliage retention found that needle survival was positively related to minimum winter temperature (December-February) and spring (March-May) precipitation (Zhao et al. 2012). Dendrochronological analysis has shown that mature forests in the coastal mountains are susceptible to growth reduction by SNC, specifically associated with warmer spring and summer temperature (Black et al. 2010). Forests further from the coast in the Cascade Mountains also have been reported with SNC, particularly the low elevation foothills (Ritóková et al. 2016), and it has been shown that disease severity decreases with increasing elevation. Lee et al. (2013, 2017) demonstrated that current- and previous-year’s winter and summer temperatures and summer precipitation were strongly correlated with SNC impacts on radial stem growth in mature and old-growth forests of western Oregon. In addition, the relative importance of these climate variables varied by elevation and distance from the coast (Lee et al. 2013, 2016, 2017).
Anecdotal observations suggest that SNC is more severe in young trees than in mature trees, however, this has not been measured quantitatively. SNC has been well studied in young-growth stands but there is still uncertainty regarding what controls disease severity. Data on SNC severity in mature and old-growth forests are rare and there is little understanding of SNC disease epidemiology in older forests. We hypothesized that the vertical and horizontal complexity of older stands would lead to differentiation of microclimate within the vertical profile of older tree crowns and that would lead to less uniform infection of the crown by N. gaeumannii. We predicted that this could result in less severe SNC disease in old forests.
Two factors thought to be influential in fungal disease epidemiology in conifer forests are leaf wetness during spore dispersal (Capitano 1999) and nitrogen content of the leaf (El-Hajj et al. 2004). These factors are expected to be different in crowns of young versus older trees because of differences in tree morphology, needle age composition and microclimate within the tree. Given that, we hypothesized that wetter needles and greater nitrogen content would be associated with greater disease severity.
We investigated SNC disease patterns in tree crowns of mature and old-growth forests and nearby young forests at three locations in the Oregon Coast Range and four locations in the western Cascade Range. We compared needle samples from young and old trees at each site to determine if SNC disease severity differed between tree age. We also compared infection incidence in different needle age classes, foliage retention for first 4 years, and foliage nitrogen patterns and leaf wetness (May – August) to determine if these variables differed between tree age classes. We expected that (1) Severity of SNC would be greater in younger than adjacent older trees; (2) Incidence of N. gaeumannii would vary by tree age, needle age, and canopy position; (3) Foliage retention would be greatest in older trees; (4) Total nitrogen would be greater in foliage with higher SNC severity; and (5) Leaf wetness would be higher where SNC was more severe.
2. Methods
2.1. Study sites
Foliage samples were collected in 2016 and again in 2017 at seven sites in western Oregon, including five sites at long-term ecological monitoring plots established by the Environmental Protection Agency (hereafter EPA; Beedlow et al. 2013, Lee et al. 2016) and two sites in the Siuslaw National Forest (Fig. 1). Four sites were located on the west slope of the Cascade Range (Moose Mountain, Fall Creek, Toad Creek, and Soapgrass Mountain), and three sites were in the Coast Range (Cascade Head, Woods Creek, and Klickitat Mountain). Forests at each site included a mixture of old stands of Douglas-fir that were 114–470 years old and young stands of Douglas-fir that were 20–30 years old. The old forests were unmanaged stands that regenerated after fires, whereas the young stands were plantations, growing on areas that had been clear-cut and replanted. Elevation ranged from 140 m at the lowest plot in the Coast Range to 1200 m at the highest plot in the Cascade Range. Precipitation varied from 1300–2700 mm (Table 1). Associated tree species included western hemlock (Tsuga heterophylla) and western red cedar (Thuja plicata), as well as Pacific silver fir (Abies amabilis) and noble fir (Abies procera) at higher elevations in the Cascades (Table 2).
Figure 1.
Northwestern Oregon study area showing the seven study sites and major cities. The Oregon Coast Range adjacent to the coast, and the Cascade Range to the east.
Table 1.
Location, tree age, elevation, and climate variables of the seven research sites. Cascade Head, Woods Creek, and Klickitat Mountain are in the Oregon Coast Range, and Moose Mountain, Falls Creek, Soapgrass Mountain, and Toad Creek are in the western Cascade Range.
| Site | Stand | Latutude | Longitude | Elev (m) | Tree age (year) | Annual mean temperature6 (°C) | Annual precipitation6 (mm) | Dec-Feb average temperature6 (°C) |
May-Aug precipitation6 (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Cascade Head | Mature | 45°02′26.82″ | 123°55′08.13″ | 147 | ~1501 | 10.5 | 2517 | 5.9 | 301 |
| Young | 45°02′14.75″ | 123°51′06.65″ | 171 | ~302 | 10.1 | 2760 | 5.4 | 310 | |
| Klickitat Mountain | Mature | 44°14′38.03″ | 123°56′16.52″ | 383 | ~1301 | 10.8 | 2198 | 5.9 | 247 |
| Young | 44°14′10.89″ | 123°56′33.86″ | 610 | ~153 | 10.4 | 2236 | 5.6 | 252 | |
| Woods Creek | Mature | 44°32′00.67″ | 123°32′59.30″ | 523 | ~1501 | 10.6 | 2353 | 4.7 | 183 |
| Young | 44°32′12.13″ | 123°33′21.58″ | 496 | ~104 | 10.7 | 2253 | 4.9 | 181 | |
| Moose Mountain | Mature | 44°24′52.92″ | 122°23′39.48″ | 664 | ~1201 | 9.7 | 1786 | 2.7 | 274 |
| Young | 44°24′40.95″ | 122°23′52.26″ | 679 | ~205 | 9.7 | 1868 | 2.8 | 273 | |
| Falls Creek | Mature | 44°23′44.24″ | 122°22′25.47″ | 556 | ~1301 | 10.1 | 1922 | 2.9 | 276 |
| Young | 44°23′42.38″ | 122°22′35.08″ | 562 | ~302 | 10 | 1908 | 2.8 | 274 | |
| Soapgrass Mountain | Mature | 44°20′52.67″ | 122°17′30.45″ | 1169 | ~4701 | 7.9 | 2541 | 1.8 | 372 |
| Young | 44°20′42.66″ | 122°17′38.10″ | 1193 | ~302 | 8.2 | 2489 | 2.1 | 360 | |
| Toad Creek | Mature | 44°25′32.86″ | 122°01′57.65″ | 1210 | ~2001 | 7.4 | 2279 | 0.9 | 286 |
| Young | 44°25′32.98″ | 122°02′19.68″ | 1193 | ~302 | 7.4 | 2280 | 0.9 | 293 |
Tree age of mature trees were determined by coring trees using increment borer in a previous study (Lee et al. 2016), except for Klickitat Mountain. Mature trees in Klickitat Mountain were newly estimated by coring trees using increment borer in this study.
Young trees in Cascade Head, Falls Creek, Soapgrass Mountain, and Toad Creek were planted in the early 1990’s.
Young trees in Klickitat Mountain were cored by increment borer and estimated the tree age is about 15 years old.
Young trees in Woods Creek were planted in 2005.
Young trees in Moose Mountain were replanted within the next year after a clear cut in 1997–1998.
Climate data were collected from PRISM at Oregon State University (http://www.prism.oregonstate.edu/explorer/, accessed 11 December 2018) by providing study site coordinates. Using 30 year average climate database and 800m special resolution.
Table 2.
Stand structure, density, diameter, basal area, and tree height attributes of the seven research sites where we conducted studies of SNC severity in western Oregon, 2016–2017.
| Trees per plot2 | Trees per Ha | Average DBH (cm) | Basal Area (m2/Ha) | Average Tree Height (m) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Plot | Douglas-fir | Other Trees | Douglas-fir | Other Trees | Douglas-fir | Other Trees | Douglas-fir | Other Trees | Douglas-fir | Other Trees | |
| CH1 | Mature | 3 | 4 | 133 | 160 | 112.9 | 43.6 | 119.5 | 34.4 | 54.7 | 29.5 | |
| Young | 17 | 0 | 680 | 0 | 28.3 | NA3 | 42.9 | NA | 20.0 | NA | ||
| KT1 | Mature | 2 | 1 | 93 | 27 | 158.4 | 43.7 | 176.3 | 6.0 | 62.3 | 28.0 | |
| Young | 3 | 0 | 120 | 0 | 23.8 | NA | 5.4 | NA | 14.6 | NA | ||
| WC1 | Mature | 2 | 8 | 93 | 307 | 107.0 | 21.2 | 68.3 | 11.0 | 57.7 | NA | |
| Young | 9 | 1 | 360 | 40 | 10.3 | 2.9 | 3.0 | 0.0 | 6.0 | 4.0 | ||
| MM1 | Mature | 4 | 6 | 173 | 253 | 85.1 | 15.7 | 93.7 | 5.6 | 53.5 | 12.0 | |
| Young | 15 | 19 | 600 | 760 | 9.8 | 8.7 | 4.6 | 4.5 | 8.1 | 7.7 | ||
| FC1 | Mature | 4 | 10 | 173 | 400 | 78.6 | 9.4 | 83.6 | 2.2 | 66.0 | 6.4 | |
| Young | 6 | 36 | 240 | 1440 | 24.4 | 4.0 | 11.2 | 1.8 | 10.6 | 5.9 | ||
| SG1 | Mature | 2 | 5 | 67 | 213 | 156.4 | 42.6 | 133.2 | 30.8 | 54.1 | 21.4 | |
| Young | 10 | 8 | 400 | 320 | 15.5 | 14.9 | 7.5 | 5.5 | 9.4 | 8.8 | ||
| TC1 | Mature | 4 | 13 | 173 | 533 | 81.4 | 18.3 | 88.4 | 13.8 | 45.7 | 11.5 | |
| Young | 4 | 32 | 160 | 1280 | 9.7 | 8.5 | 1.2 | 7.2 | 7.8 | 7.1 | ||
The abbreviation of study sites is Cascade Head (CH), Woods Creek (WC), and Klickitat Mountain (KT), Moose Mountain (MM), Falls Creek (FC), Soapgrass Mountain (SG), and Toad Creek (TC).
For each of mature stands, we investigated three 8.9m radius plots centered with our sample tree, and averaged all 3 plots data to represent mature stand. For each of young stands, because the trees are closed to EPA weather station and the young trees grow evenly in stand, so we only investigated one 8.9m radius plot centered with EPA weather station. Dead trees and saplings were not included.
“NA” means no data or not sufficient data to present.
Three sampled trees for the mature stands are spaced at least 10m apart from each other (mostly >20m), and the three sampled trees for the young stands were located in proximity to a weather station at different orientations and were spaced at least 3 m apart from each other (mostly >5m).
Two weather stations were previously installed at each of the five mature stands managed by EPA (Environmental Protection Agency, Western Ecology Division) (Cascade Head, Moose Mountain, Falls Creek, Soapgrass Mountain and Toad Creek), one at the base and another at the top of a dominant tree which had been rigged for climbing. At these mature stands, branch samples were collected from the instrumented tree and two nearby dominant or co-dominant trees which were previously rigged for climbing (5 sites × 3 mature trees). In each adjacent young stand, the EPA installed a single weather station that was placed 2 m above ground and three trees were selected for sampling near the weather station (5 sites × 3 young trees). Branch samples from mature and young trees were collected from upper, middle and lower crown. Leaf wetness sensors were connected to the datalogger of the weather station and placed along the vertical gradient of one study tree at the upper, middle and lower parts of the canopy at each old and young EPA site. Five old trees and five young trees had leaf wetness sensors.
In each of the two sites that were not instrumented with weather stations (Klickitat Mountain and Woods Creek), we selected sample trees that had well-developed crowns and were easily accessible without placing leaf wetness sensors in canopies (2 sites × 3 trees = 6 old trees and 6 young trees). Branch samples from mature and young trees were collected from the upper, middle and lower parts of the crown.
2.2. Field Sampling
We collected 1–3 branches from three canopy positions (lower, middle, and upper crown) in each tree (total of 21 mature trees and 21 young trees). Samples were collected on the south side of the tree in late May through early June in both 2016 and 2017, after bud-break and before new branchlets were elongated. At least one branch >1 meter in length was selected to ensure sufficient needle material for measurements and foliage nutrient analysis. Several shorter branches were chosen if there were no branches greater than 1 meter in length. Branches were transported to the lab and stored in a 5°C cold room. Foliage retention was determined by estimating the number of years (annual cohorts) of foliage present on 1–4 year-old branches (Maguire et al. 2011). We rated the needle amount present within each age class along the branch on a scale from 0 to 1, With a 1 indicating all needles were still present. We combined the four age classes for analysis with needle retention possible from 0 to 4.
2.3. Lab Analysis
For each canopy height position of 21 mature trees and 21 young trees, 50 individual needles were randomly selected from each cohort of all foliar age classes. Needles were taped on an index card and stored at −20 °C. The SNC incidence is defined as the percentage of the 50 needles with pseudothecia present. All needle ages were examined for SNC incidence under a microscope for presence or absence of pseudothecia occluding the stomates. The SNC disease severity index was calculated based on the two-year old needles as the product of the incidence and the pseudothecia density. Pseudothecia density was determined by selecting the first 10 two-year-old needles with pseudothecia present and then counting the % of stomates occluded in three regions (base, middle, and tip) of the needle. In each region evenly divided along length of needle, we picked a random starting point from the needle base and examined 100 stomates from the starting point to determine the number that were occluded by pseudothecia. Pseudothecial occlusion in the three regions was then averaged for each needle and then averaged for 10 needles per canopy position per tree.
Foliar nitrogen was determined on dried and ground foliar material using dry combustion in a FlashEA 1112 NC Analyzer (Thermo Fisher Scientific Inc. USA). Only one-year-old needles were collected for foliage nitrogen measurements. After transporting branches to the lab, we randomly selected ~ 200 needles from each canopy position of each sample tree, dried them for 48 hours in a drying oven at 40 °C, and ground them with a ball grinder and stored in clean vials. We then placed 3–5 mg of the powder into a tin capsule and used FlashEA1112 to measure total nitrogen concentration (%).
2.4. Leaf Wetness Data Collection
We estimated leaf wetness duration during May-August as the ratio of total wet hours in each month / total hours in each month. Only May-August leaf wetness data were examined because that is the primary period of spore dispersal and leaf colonization (Michaels and Chastagner 1984). Leaf wetness data were collected every 5 minutes with a PHYTOS31 sensor (Decagon Devices, Inc. USA) then averaged and recorded as hourly data in mV with a CR1000 datalogger (Campbell Scientific Inc. USA). Based on the excitation voltage for our datalogger (2.5V), we assumed that leaf surfaces were wet when resistance values were > 280mV (The manual of leaf wetness sensor is available at http://library.metergroup.com/Manuals/20434_PHYTOS31_Manual_Web.pdf accessed 14 March 2019). In addition, as a quality control of the sensors, we also compared the leaf wetness data with rainfall and humidity sensor data that were collected on site, and 280mV was the mean value from all sensors when no rainfall was present, and the needle surface was dry.
2.5. Statistical Analysis
Multivariate analysis of variance (MANOVA) was used to test for the main effects of canopy position (upper, middle, and lower), tree age (mature or young), sites, and years (2016 and 2017), and their interactions on the SNC severity index, foliage retention, and total foliar nitrogen at the 0.05 level of significance. Canopy position and year were treated as within-subject factors in the MANOVA whereas tree age and sites were between-subject factors where the subject was an individual tree. The SNC severity index data from Soapgrass Mountain and Toad Creek were excluded from the analysis because almost all SNC values from those sites were zeros (Fig. 2 & 3). In the preliminary results (Table 3), there were interactions involving sites, so we also ran the MANOVA on individual sites, to test for differences in SNC severity index between canopy position, year, and tree age (Table 4). When one or more main effects but not their interactions were statistically significant, we conducted a Bonferroni mean separation test to infer which treatment means were different. MANOVA tests were performed using R (v. 3.4.3, R Core Team 2017) and package car (Fox and Weisberg 2011), dplyr (Wickham et al. 2017), emmeans (Lenth 2018), ggplot2 (Wickham 2009), and nlme (Pinheiro et al. 2017).
Figure 2.
Incidence of Nothophaeocryptopus gaeumannii pseudothecia along needle age at three canopy positions and seven sites in western Oregon in 2016–2017. Panels are in sets of upper, middle and lower canopy positions, for mature and young stands. Needle age was determined by counting the number of internodes on twigs from the current year needles. All classes of needle age are included in the figure.
Figure 3.
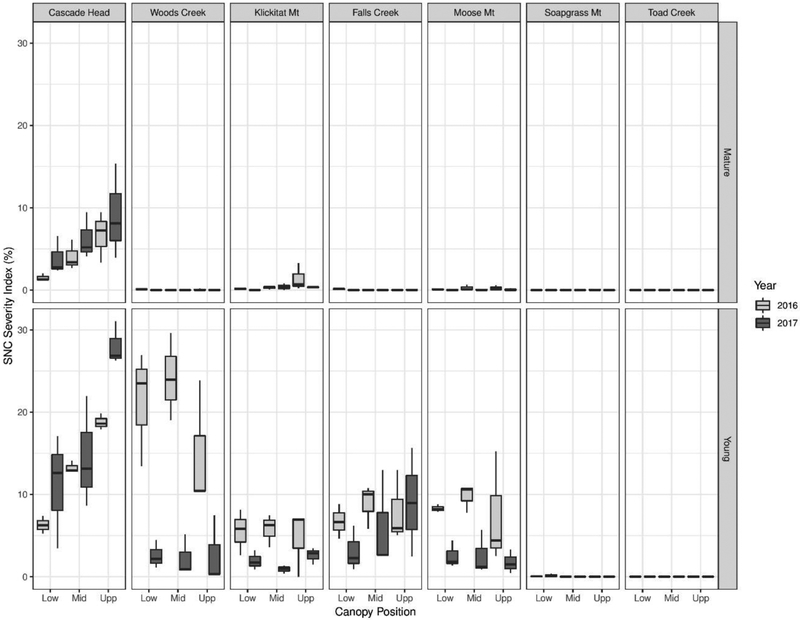
Swiss needle cast disease severity index for mature and young trees at three canopy positions (x-axis) across seven sites in western Oregon in 2016–2017. Only two-year-old needles were used for SNC disease severity index. The whiskers represented the range of mean values.
Table 3.
Results of preliminary MANOVA tests for the main effects of tree age, site, year and canopy position and their interactions on SNC severity index, foliage retention, and foliage nitrogen (TN) in study areas in western Oregon.
| SNC Severity Index (5 sites2) | Foliage retention (7 sites3) | Foliage TN (7 sites3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | ||||
| (Intercept) | 295.9 | < 0.001 | *** | 4197.1 | < 0.001 | *** | 3705.1 | < 0.001 | *** |
| Tree age | 172.6 | < 0.001 | *** | 5.1 | 0.033 | * | 0.0 | 0.865 | |
| Site | 28.9 | < 0.001 | *** | 21.4 | < 0.001 | *** | 7.1 | 0.001 | ** |
| Tree age × Site | 6.7 | 0.002 | ** | 2 | 0.098 | . | 0.4 | 0.794 | |
| Year | 12.1 | 0.003 | ** | 0.1 | 0.740 | 74.0 | < 0.001 | *** | |
| Tree age × Year | 17.1 | 0.001 | *** | 0 | 0.886 | 2.2 | 0.156 | ||
| Site × Year | 10.9 | < 0.001 | *** | 0.6 | 0.740 | 3.6 | 0.024 | * | |
| Tree age × Site × Year | 7.7 | 0.001 | *** | 2.5 | 0.046 | * | 0.3 | 0.857 | |
| Canopy | 7.1 | 0.006 | ** | 18.9 | < 0.001 | *** | 17.8 | <0.001 | *** |
| Tree age × Canopy | 1.5 | 0.260 | 3.4 | 0.0501 | * | 3.5 | 0.053 | . | |
| Site × Canopy | 3.5 | 0.004 | ** | 2 | 0.0501 | * | 0.9 | 0.516 | |
| Tree age × Site × Canopy | 2.1 | 0.057 | . | 1.4 | 0.180 | 1.3 | 0.287 | ||
| Year × Canopy | 2.8 | 0.086 | . | 1.5 | 0.248 | 2.6 | 0.100 | ||
| Tree age × Year × Canopy | 2.8 | 0.086 | . | 2.0 | 0.153 | 0.2 | 0.785 | ||
| Site × Year × Canopy | 0.4 | 0.923 | 0.7 | 0.736 | 0.2 | 0.992 | |||
| Tree age × Site × Year × Canopy | 0.3 | 0.944 | 1.5 | 0.159 | 0.8 | 0.636 | |||
The tree age x canopy and site x canopy interaction terms were not statistically significant (p-value = 0.19 and 0.50, respectively) when the foliage retention data for Soapgrass Mountain and Toad Creek were excluded from the MANOVA, indicating that canopy differences at the five lower elevation sites were similar but different than at the higher elevation sites.
Including sites Cascade Head, Woods Creek, and Klickitat Mountain, Moose Mountain, and Falls Creek. Soapgrass Mountain and Toad Creek were excluded because almost all SNC values from those sites were zeros.
Including sites Cascade Head, Woods Creek, and Klickitat Mountain, Moose Mountain, Falls Creek, Soapgrass Mountain, and Toad Creek.
Table 4.
Results of MANOVA by individual sites with N. gaeumannii. Because site is a crucial factor involved in most interactions, the MANOVA was re-run by site to clarify the effects from other factors. Tree age was another key factor in SNC severity index analysis and involved in many interactions. Canopy position contributed to foliage retention when considering the site effect.
| SNC Severity Index | Foliage retention | ||||||
|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | ||||
| CH1 | Tree age | 26.0 | 0.007 | ** | 0.8 | 0.413 | |
| Year | 6.0 | 0.070 | . | 0.4 | 0.567 | ||
| Tree age × Year | 0.8 | 0.413 | 2.0 | 0.229 | |||
| Canopy | 20.9 | 0.017 | * | 4.1 | 0.137 | ||
| Tree age × Canopy | 5.3 | 0.105 | 2.2 | 0.256 | |||
| Year × Canopy | 5.5 | 0.100 | . | 6.3 | 0.084 | . | |
| Tree age × Year × Canopy | 4.9 | 0.115 | 0.8 | 0.519 | |||
| KT1 | Tree age | 35.6 | 0.009 | ** | 0.4 | 0.596 | |
| Year | 45.9 | 0.007 | ** | 0.0 | 0.938 | ||
| Tree age × Year | 44.1 | 0.007 | ** | 2.0 | 0.252 | ||
| Canopy | 0.0 | 0.988 | 338.3 | 0.003 | ** | ||
| Tree age × Canopy | 0.1 | 0.948 | 46.4 | 0.021 | * | ||
| Year × Canopy | 0.2 | 0.864 | 1.8 | 0.361 | |||
| Tree age × Year × Canopy | 0.3 | 0.783 | 7.4 | 0.120 | |||
| WC1 | Tree age | 136.7 | < 0.001 | *** | 23.9 | 0.008 | ** |
| Year | 17.5 | 0.014 | * | 0.4 | 0.549 | ||
| Tree age × Year | 17.3 | 0.014 | * | 0.4 | 0.566 | ||
| Canopy | 14.6 | 0.028 | * | 9.3 | 0.052 | .* | |
| Tree age × Canopy | 15.0 | 0.027 | * | 0.9 | 0.498 | ||
| Year × Canopy | 3.7 | 0.154 | 0.0 | 0.996 | |||
| Tree age × Year × Canopy | 3.8 | 0.151 | 0.7 | 0.577 | |||
| MM1 | Tree age | 17.12 | 0.014 | ** | 1.8 | 0.251 | |
| Year | 284.6 | < 0.001 | *** | 0.9 | 0.392 | ||
| Tree age × Year | 258.5 | < 0.001 | *** | 12.9 | 0.023 | * | |
| Canopy | 4.8 | 0.117 | 257.3 | < 0.001 | *** | ||
| Tree age × Canopy | 3.9 | 0.148 | 5.3 | 0.105 | |||
| Year × Canopy | 1.4 | 0.379 | 1.5 | 0.349 | |||
| Tree age × Year × Canopy | 0.9 | 0.490 | 1.3 | 0.396 | |||
| FC1 | Tree age | 98.0 | 0.001 | *** | 0.0 | 0.942 | |
| Year | 0.4 | 0.565 | 4.4 | 0.105 | |||
| Tree age × Year | 0.4 | 0.585 | 2.0 | 0.227 | |||
| Canopy | 4.2 | 0.134 | 4.6 | 0.122 | |||
| Tree age × Canopy | 4.5 | 0.127 | 4.9 | 0.114 | |||
| Year × Canopy | 42.8 | 0.006 | ** | 0.3 | 0.766 | ||
| Tree age × Year × Canopy | 34.4 | 0.009 | ** | 0.8 | 0.528 | ||
| SG1 | Tree age | - | - | 0.2 | 0.700 | ||
| Year | - | - | 1.4 | 0.310 | |||
| Tree age × Year | - | - | 0.2 | 0.651 | |||
| Canopy | - | - | 1.5 | 0.358 | |||
| Tree age × Canopy | - | - | 0.0 | 0.980 | |||
| Year × Canopy | - | - | 0.2 | 0.869 | |||
| Tree age × Year × Canopy | - | - | 1.1 | 0.443 | |||
| TC1 | Tree age | - | - | 8.3 | 0.045 | * | |
| Year | - | - | 0.0 | 0.968 | |||
| Tree age × Year | - | - | 1.1 | 0.361 | |||
| Canopy | - | - | 14.6 | 0.029 | * | ||
| Tree age × Canopy | - | - | 13.4 | 0.032 | * | ||
| Year × Canopy | - | - | 0.0 | 0.972 | |||
| Tree age × Year × Canopy | - | - | 0.4 | 0.687 | |||
The abbreviation of study sites is Cascade Head (CH), Woods Creek (WC), and Klickitat Mountain (KT), Moose Mountain (MM), Falls Creek (FC), Soapgrass Mountain (SG), and Toad Creek (TC).
3. Results
3.1. SNC incidence patterns
Graphical exploration showed that the percentage of needles with pseudothecia (i.e., SNC incidence) varied by site, tree age, and canopy position (Fig. 2). SNC incidence was least in mature trees at high-elevation sites in the Cascades (Soapgrass Mountain and Toad Creek), and greatest in young and mature trees at Cascade Head in the Coast Range. At all study sites SNC incidence was greater in young stands than in the adjacent old forest stands. At all sites excluding Soapgrass Mountain and Toad Creek, nearly 100% of the two-year-old and older needles in young trees had N. gaeumannii present, whereas the peak incidence in mature trees was observed in 3–5-year-old needles except for Cascade Head, followed by a decline with increasing needle age. Also, there was more variation in SNC incidence among old trees than in the adjacent young trees, which implies the young trees were more evenly infected by N. gaeumannii than were old trees.
Graphical exploration also showed that in young trees, N. gaeumannii was present on almost all needles older than two years in all three canopy positions. In mature trees, the percentage of needles with N. gaeumannii present was greater in the middle and lower canopy than in the upper canopy, At Soapgrass Mountain and Toad Creek, most needles except for lower and mid-canopy needles in young trees at Soapgrass did not have any pseudothecia present (Fig. 2). SNC incidence pattern was unique for mature trees at most sites: 3–5-year-old needles had the greatest SNC incidence (Fig. 2). These were the needle cohorts that emerged in 2011–2014.
3.2. SNC severity index
The SNC severity index on two-year-old needles varied by site, tree age, canopy position, and year (Fig. 3). The SNC severity index was nearly 0% for young trees at Soapgrass Mountain and Toad Creek and for old trees at all sites except Cascade Head and Klickitat Mountain. The MANOVA excluded the data for Soapgrass Mountain and Toad Creek due to lack of variation. Differences in SNC severity between sites, tree ages, canopy positions, and years were all significant (p<0.05, Table 3). SNC severity was greater in young trees than in mature trees (p<0.001). Several interactions involving tree age were noted but were of minor importance because their F-values were an order of magnitude less than that for tree age (Snedecor and Cochran, 1967). Several interactions involving site, year, and/or canopy position were also statistically significant (p<0.05, Table 3). Based on the mean separation test on site with Bonferroni adjustment, the SNC severity index was different in Cascade Head than in the other four sites (Fig. 4). Consequently, MANOVA was performed on the SNC severity index by site to test for the main effects of tree age, canopy, and year, and their interactions (Table 4).
Figure 4.
Least square means of SNC severity index by site. Only five sites were present in MANOVA and mean comparison. Soapgrass Mountain and Toad Creek, the most continental sites, were excluded from the analysis because most all values were zeros. The error bar represents mean ± 1standard error. Letters represent groups. The least square means of SNC severity index between any two groups is statistically different if the letters are different
Differences in SNC severity index between canopy positions were statistically significant at Cascade Head (p=0.017) and Woods Creek (p=0.029) (Table 4). In young and old trees at Cascade Head, the SNC severity index was significantly greater in the upper canopy than in the lower and middle canopies in 2016 and 2017 (Fig. 3). At all sites excluding Cascade Head, SNC severity index values for young trees were greater than for old trees. For young trees at Woods Creek, the SNC severity index was significantly lower in the upper canopy in 2016 than in the lower and middle canopies but was uniformly low in all three canopy layers in 2017. For young trees at Woods Creek, Klickitat Mountain, and Moose Mountain, the mean SNC severity index was greater in 2016 than in 2017 (Fig. 3).
3.3. Foliage retention
Mean foliage retention for the first four years of age classes differed among sites (p<0.001) and canopy positions (p<0.001), and included several two- and three-factor interactions (Fig. 5, Table 3). When foliage retention data for Soapgrass Mountain and Toad Creek were excluded from the MANOVA, the tree age x canopy and site x canopy interactions were not statistically significant (p=0.19 and 0.50, respectively), indicating that foliage retention at the high elevation sites differed from the other areas examined (Table 3). Because the F-values for the interaction terms with canopy were about an order of magnitude less than those for the constituent main effects and not statistically significant when data for the two high-elevation sites were excluded, the interactions were considered as either not important or important only for Soapgrass Mountain and Toad Creek.
Figure 5.
Foliage retention (in year) for mature and young trees at three canopy positions across seven sites in western Oregon in 2016–2017. Foliage retention was determined by evaluating 1–4 year-old foliage on 4 year-old, or older lateral twigs. The whiskers represented the range of mean values.
Post-MANOVA, we proceeded to examine the differences in foliage retention between the main effects for site and canopy based on the Bonferroni mean separation test. We also considered the importance of the significant interactions and tested for the effects of age, canopy, and year and their interactions on foliage retention based on MANOVA for individual sites focusing on the two high-elevation sites. Based on the mean separation test on site with Bonferroni adjustment, the seven sites were partitioned into two distinct groups (Fig. 6). Foliage retention was significantly less in young and mature trees at Cascade Head and Klickitat Mountain than at the five inland sites (Fig. 5 & 6). Furthermore, the mean foliage retention in the upper canopy was significantly less than that in the lower and middle canopies (Fig. 6). However, in the analysis of individual sites, canopy and age differences in foliage retention were not statistically significant at Soapgrass Mountain (Table 4). In contrast, there was evidence that mean foliage retention differed in young and old trees at Woods Creek and Toad Creek as well as a significant main effect for canopy and an age x canopy interaction at Toad Creek (Table 4). At Toad Creek, foliar retention was least in the upper canopy of young Douglas-fir whereas no canopy differences were evident in mature trees (Fig. 5).
Figure 6.
Least square means of foliage retention in western Oregon A) by site and B) by canopy position. All seven sites were present in MANOVA and mean comparison. The error bar represents mean ± 1standard error. Letters represents groups. The least square means of foliage retention between any two groups is statically different if the letters are different.
3.4. Foliage total nitrogen
Mean total nitrogen concentration differed among years, canopy positions, and sites, but was not different between older and younger trees (Fig. 7 & 8, Table 3). In addition, there was a significant site × year interaction that we did not consider important because its F-value was an order of magnitude less than the F-value for year (Snedecor and Cochran, 1967). Mean total nitrogen concentration was significantly greater in 2016 than in 2017 and was greatest in the upper canopy and least in the lower canopy (Fig. 8). Based on the mean separation test on site with Bonferroni adjustment, mean foliage total nitrogen concentration was different at Cascade Head and Klickitat Mountain than at most other sites, and was greater in the upper canopy than in the middle and lower canopy (Fig. 8).
Figure 7.
Total foliage nitrogen concentration for mature and young trees at three canopy positions across seven sites in western Oregon in 2016–2017. Only one-year-old needles were used for foliage nitrogen measurement. The whiskers represented the range of mean values.
Figure 8.
Least square means of foliage total nitrogen concentration for seven study sites in western Oregon A) by site, B) by canopy considering site and year effects, and C) by year considering canopy and site effects. All seven sites were included in MANOVA and mean comparisons. The error bar represents mean ± 1standard error. Letters represents groups. The least square means of foliage nitrogen concentration between any two groups is statically different if the letters are different.
3.5. Leaf wetness data
Leaf surfaces were often wet in all canopy positions in all sites during May and June and mostly dry in July and August at Falls Creek, Moose Mountain, and Soapgrass Mountain (Fig. 9). There were no obvious patterns in leaf wetness among canopy positions. July 2017 was drier than 2016, but May, June, and August did not show strong differences in leaf wetness between years. Leaf wetness did not differ between sites or tree age classes during May-August except at Cascade Head in July and August. However, young trees at Cascade Head had higher leaf wetness than most other sites in nearly all months. Unfortunately, we did not have leaf wetness data from the other two coastal sites so the sample was insufficient for comparisons between coastal and inland sites.
Figure 9.
Wet hour ratio per month for May, June, July and August for one mature and one young tree at each site at Cascade Head (CH), Falls Creek (FC), Moose Mountain (MM), Soapgrass Mountain (SG), and Toad Creek (TC). We set up the wet/dry threshold as 280 mV based on the manual description as well as sensor performance on site and counted it as a wet hour if the raw number >280mV. The leaf wetness duration was presented by counting the ratio of total wet hours per month / total hours per month. Due to technical issues of sensors, May 2016 and June 2016 in Toad Creek young plot, and August 2016 in Soapgrass Mountain young plot were missed in the figure and marked as “NA”.
4. Discussion
Swiss needle cast disease severity (incidence of needles with pseudothecia × percentage of stomates occluded by pseudothecia, for two-year-old needles) was less in old trees than in young trees except for the two high-elevation Cascade Range sites where evidence of N. gaeumannii infection was negligible in both old and young trees. Our measurements of leaf nitrogen and leaf wetness were not different between young and older trees except at Cascade Head, and therefore did not explain the differences in disease severity between mature and young trees. However, we found that incidence of N. gaeumannii, which is the percentage of infected needles with pseudothecia present, peaked in second year foliage for young trees and 3–5 year foliage in older trees, except at Cascade Head. At Cascade Head, although disease severity was different for mature trees and young trees, incidence of N. gaeumannii both peaked in second year foliage for mature and young trees.
Young trees had more stomates occluded by pseudothecia on two-year-old needles than older trees. This may help explain why disease is more severe in young trees. In addition, it appears that foliage in young stands is more fully colonized than older stands because pseudothecia density is correlated with the biomass of the fungus in the needle (Manter et al. 2003), and pseudothecia density was always greater in young trees. This is significant because if a tree has over 3.2 years of foliage retention on average, tree growth will be normal even if the fungus is present within needles (Maguire et al. 2011). If needle chlorosis and casting occur sooner in young trees, due to earlier peak infection incidence and severity, then the chance for foliage retention to drop below three years is much greater in young than older trees.
We hypothesized that foliar nitrogen was positively associated with SNC disease severity. Our hypothesis was not supported by our data, which indicated no difference in foliar nitrogen in the samples of young and old trees at individual sites (Fig. 7). El-Hajj et al. (2004) noted that N. gaeumannii might acquire nitrogen and carbon from apoplastic spaces within Douglas-fir needles and disease severity could be influenced by fertilization. However, Mulvey et al. (2013) did not demonstrate any change in disease severity after fertilization with nitrogen. Perakis et al. (2006) showed a correlation between increased soil nitrogen and disease occurrence at the landscape scale, following a pattern of increasing disease east to west in the Oregon Coast Range. Perakis et al. (2006) suggested that greater nitrogen could be associated with increased foliage disease. It may be that nitrogen is important in the epidemic that is occurring along the coast, but that it does not differ enough between young and mature trees to influence differences in disease expression at the tree scale.
Leaf wetness did not explain differences in disease severity between young and mature Douglas-fir trees in this study because young and old stands were consistently wet most of the time and did not differ (Fig. 9). However, vertical and within crown canopy complexity may influence thermal properties of trees and stands, which could influence leaf temperature and fungal growth rates. Hansen et al. (2000) noted that in forest plantations disease severity was greatest in the upper crown, and on southern aspects, which are both more likely to have warmer temperatures in winter, a key period of fungal development (Manter et al. 2005). The thermal dynamics of young canopies and older canopies of Douglas-fir is not well documented, and we can only speculate if differences could account for differences in disease.
Incidence and severity of N. gaeumannii were different between adjacent mature and young trees, here we speculate the factors we did not measure which could explain this. One of possibility could be pseudothecia develop sooner on young trees and need more time to mature on old trees. There are only few studies that address differences between needles of old and young trees. In conifers, older trees have needles that are morphologically different from needles of young trees (Apple et al. 2002; Day et al. 2001; England and Attiwill 2006). These differences may contribute to old Douglas-fir trees being more resistant or tolerant to N. gaeumannii infection or development within the leaf. In addition, there are also age-related differences in defensive chemicals (Erwin et al. 2001). Secondary chemical compounds are important in plant defense against pathogens (Espinosa-Garcia and Langenheim 1991; Cook and Hain 1986) and could play a role in the observed differences in disease severity. Further, old trees that have been exposed to N. gaeumannii for much longer than young trees may have lived so long due to their tolerance to the fungus.
Hansen et al. (2000) found that SNC disease severity was greatest in the upper canopy of young trees in plantations. We found similar trends at Cascade Head, Klickitat Mountain, and Falls Creek, but disease severity was so low at other sites that we could show no patterns relative to disease severity at different canopy positions of young trees. In older trees we found some evidence that SNC severity was most pronounced in the middle and upper crown layers, but the disease severity was so low in old trees in most areas that we were unable to detect consistent patterns. In our study, N. gaeumannii was also rare at two high elevation sites. This is consistent with Manter et al. (2005) who found that warm winter temperatures were positively associated with SNC, and Ritóková et al. (2016) who showed that SNC severity decreased with increasing elevation in the western Cascades.
Our study is the first to directly compare mature and young trees for N. gaeumannii caused disease severity. Mildrexler et al. (2019) compared private lands versus public lands in the Oregon Coast Range using aerial detection data, and found that private lands with young forests tended to have more visible disease symptoms than public lands which have more older stands. It appears that mature and older trees are not as likely to express disease impacts to N. gaeumannii as young plantation trees in western Oregon. Although N. gaeumannii is still present in older tree crowns, disease severity was always lower than young trees. The conclusions of our study should be confirmed by a much larger sample size from throughout the epidemic area because of the implications for landscape management.
The current SNC epidemic area is the region where foliage retention averages less than 3 years and visible symptoms of disease such as needle chlorosis, sparse crowns, and reduced growth occur (Ritóková et al. 2016). The weather conditions within the epidemic area of SNC near the coast are distinct from the Cascade Mountains sites, and this influences the geographical distribution of the epidemic (Rosso and Hansen 2003, Shaw et al. 2011, Ritóková, et al. 2016). The three Oregon Coast Range sites used in our study are within the epidemic area, while the four Cascade Mountain sites are outside the epidemic area, and the trees were generally healthy, yet at two lower elevations sites the disease was abundant in the young stands. If we only focused on stands in the epidemic area of the Oregon Coast Range, we may have seen different patterns. For example, on the Oregon coast, Tillamook area is considered as one of the regions in which forests are most severely infected with SNC historically (Hansen et al. 2000), and in this region even mature Douglas-fir forests (~80-year-old) are often infected (Black et al. 2010).
The epidemiology of N. gaeumannii has focused on winter temperature and leaf wetness during spore dispersal (May to August) (Rosso and Hansen 2003, Manter et al. 2005). Variability in climate will influence infection success, and short-term climate trends are associated with disease intensification (Mildrexler et al. 2019). Land surface temperature trended cooler, and water balance increased from 2003–2012 during June and July. Consequently, Swiss needle cast aerial survey area went from a low of 71,465 ha in 2004 to 210,184 ha in 2012 (Ritóková et al. 2016). Lee et al. (2013, 2016, 2017) have noted that the exact climate factors associated with impacts by N. gaeumannii likely vary with geographic location. Given that our seven stands had distinct climate conditions, it is significant that patterns of infection between mature and young trees remained consistent.
In conclusion, we found that disease severity is higher in young plantation trees than in mature forest trees and that this may be a result of emergence of pseudothecia in stomates of younger needles in young trees. Although we do not know why disease severity and timing of stomatal occlusion would be different, our data suggest that leaf nitrogen and leaf wetness differences are not the reason. Therefore, future research to understand epidemiology of N. gaeumannii would investigate why there is higher pseudothecia density in two-year-old needles of younger stands, and to determine the drivers of leaf colonization by the fungus. Possibly including dynamics inside the needle, needle temperature during winter, and other factors that potentially influence pseudothecia development.
5. Acknowledgements
The authors thank Swiss Needle Cast Cooperative for providing student assistant funding and technical support during the research. Thanks to the EPA long term ecological monitoring network team for weather data and for providing support for tree climbing, and sampling. Field sites were located on the Siuslaw and Willamette National Forests, US Forest Service. We really appreciate the tree climbing and ground support of Mike Bollman, Steve Cline, Gabi Ritóková, Rong Fang, Rebecca Hsu, Brian Chiu, Eric Forsman, Dave Woodruff, Jimmy Swingle, Chia-Yun Hsu, Mark Ko, Hans Song. Thanks to Alexis Danley, Shannon Burton, and Lori Lewis for lab work, Jeff Hatten and Yvan Alleau for the chemical analysis, and Ariel Muldoon for statistical consulting. Thanks to Eric Forsman for editorial advice.
7. Appendices
Appendix A.
Summary of SNC incidence (in %) of needles with pseudothecia for all needle age classes in 2016, across seven study sites in western Oregon for mature and young trees in the upper, middle and lower crowns. The number in front of ± is average of three trees and the number followed is standard error.
| 2016 | yr1 | yr2 | yr3 | yr4 | yr5 | yr6 | yr7 | yr8 | yr9 | yr10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH1 | Mature | Upper | 10.0 ± 4.2 | 88.7 ± 5.8 | 67.2 ± 27.0 | 80.0 ± 2.0 | 63.7 ± 30.3 | 86.0 ± NA | - | - | - | - |
| Middle | 4.7 ± 4.7 | 98.0 ± 0.0 | 96.0 ± 3.1 | 85.2 ± 7.8 | 96.1 ± 1.9 | - | - | - | - | - | ||
| Lower | 1.3 ± 1.3 | 88.7 ± 1.3 | 96.0 ± 4.0 | 69.3 ± 2.7 | 90.2 ± 3.1 | 81.8 ± NA | - | - | - | - | ||
| Young | Upper | 95.3 ± 2.4 | 99.3 ± 0.7 | - | - | - | - | - | - | - | - | |
| Middle | 68.7 ± 13.8 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± NA | - | - | - | - | - | ||
| Lower | 8.0 ± 5.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± NA | - | - | - | - | ||
| KT1 | Mature | Upper | 2.7 ± 1.8 | 34.7 ± 21.7 | 60.7 ± 21.5 | 65.8 ± 13.7 | 12.0 ± NA | - | - | - | - | - |
| Middle | 0.0 ± 0.0 | 24.7 ± 8.7 | 74.7 ± 7.7 | 27.6 ± 10.1 | 17.6 ± 10.0 | 34.1 ± 17.9 | 10.9 ± 0.9 | 7.7 ± NA | - | - | ||
| Lower | 0.7 ± 0.7 | 20.0 ± 7.2 | 74.0 ± 3.1 | 29.4 ± 6.9 | 17.0 ± 7.0 | 12.5 ± 6.5 | 0.0 ± NA | - | - | - | ||
| Young | Upper | 14.0 ± 7.2 | 96.7 ± 2.4 | - | - | - | - | - | - | - | - | |
| Middle | 15.3 ± 4.8 | 96.0 ± 4.0 | 100.0 ± NA | 100.0 ± NA | - | - | - | - | - | - | ||
| Lower | 2.0 ± 2.0 | 96.0 ± 3.1 | 92.7 ± 5.5 | 89.3 ± 9.7 | 99.0 ± 1.0 | - | - | - | - | - | ||
| WC1 | Mature | Upper | 0.0 ± 0.0 | 0.7 ± 0.7 | 6.0 ± 5.0 | 22.0 ± 10.6 | 5.3 ± 2.9 | - | - | - | - | - |
| Middle | 0.0 ± 0.0 | 1.3 ± 1.3 | 29.3 ± 5.8 | 26.7 ± 6.4 | 7.0 ± 1.5 | 6.0 ± 2.0 | 4.3 ± NA | 0.0 ± NA | 4.0 ± NA | 0.0 ± NA | ||
| Lower | 0.0 ± 0.0 | 4.7 ± 1.8 | 55.3 ± 6.4 | 52.7 ± 1.3 | 12.0 ± 3.1 | 14.7 ± 4.4 | 12.0 ± 6.1 | 2.0 ± 2.0 | 0.0 ± NA | - | ||
| Young | Upper | 0.7 ± 0.7 | 85.3 ± 7.7 | - | - | - | - | - | - | - | - | |
| Middle | 0.0 ± 0.0 | 99.3 ± 0.7 | 99.3 ± 0.7 | 61.0 ± 19.0 | - | - | - | - | - | - | ||
| Lower | 0.7 ± 0.7 | 100.0 ± 0.0 | 100.0 ± 0.0 | 96.0 ± 4.0 | - | - | - | - | - | - | ||
| MM1 | Mature | Upper | 0.7 ± 0.7 | 17.3 ± 12.5 | 27.3 ± 17.1 | 28.0 ± 26.0 | 63.2 ± NA | - | - | - | - | - |
| Middle | 5.3 ± 3.5 | 25.3 ± 19.5 | 66.7 ± 30.4 | 63.3 ± 23.7 | 55.0 ± 30.7 | 50.0 ± 42.0 | 20.0 ± NA | 10.0 ± NA | 38.5 ± NA | - | ||
| Lower | 8.7 ± 4.8 | 24.7 ± 5.5 | 70.7 ± 23.4 | 58.0 ± 19.4 | 44.0 ± 16.0 | 29.0 ± 15.0 | 12.0 ± NA | 20.0 ± NA | - | |||
| Young | Upper | 20.0 ± 2.0 | 92.7 ± 3.7 | 100.0 ± 0.0 | 91.0 ± 9.0 | - | - | - | - | - | - | |
| Middle | 17.3 ± 1.8 | 98.7 ± 1.3 | 100.0 ± 0.0 | 97.3 ± 2.7 | 95.3 ± 4.7 | - | - | - | - | - | ||
| Lower | 24.0 ± 4.0 | 99.3 ± 0.7 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 86.4 ± 13.6 | 100.0 ± NA | - | - | - | ||
| FC1 | Mature | Upper | 4.7 ± 1.8 | 4.0 ± 1.2 | 40.0 ± 26.4 | 42.5 ± 12.8 | 27.0 ± 5.0 | 18.4 ± NA | - | - | - | - |
| Middle | 2.0 ± 1.2 | 6.7 ± 3.7 | 78.7 ± 12.7 | 48.0 ± 12.5 | 59.5 ± 4.6 | 40.0 ± 4.0 | - | - | - | - | ||
| Lower | 8.7 ± 5.9 | 12.7 ± 5.3 | 69.3 ± 5.5 | 64.7 ± 12.0 | 73.3 ± 12.8 | 51.5 ± 18.6 | 40.2 ± 4.5 | 48.9 ± 23.9 | - | - | ||
| Young | Upper | 12.0 ± 7.2 | 98.0 ± 1.2 | 97.4 ± 2.6 | - | - | - | - | - | - | - | |
| Middle | 19.3 ± 3.3 | 100.0 ± 0.0 | 98.7 ± 1.3 | 100.0 ± 0.0 | 99.3 ± 0.7 | 97.8 ± NA | - | - | - | - | ||
| Lower | 38.0 ± 7.6 | 98.7 ± 1.3 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± NA | - | - | - | - | ||
| SG1 | Mature | Upper | 0.0 ± 0.0 | 2.0 ± 1.2 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.00 | 0.0 ± NA | 0.0 ± NA | - | |
| Middle | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.00 | 0.0 ± 0.0 | 2.9 ± 2.9 | 0.0 ± NA | - | ||
| Lower | 1.3 ± 1.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Young | Upper | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.7 ± 2.7 | 2.7 ± 0.7 | 2.0 ± 2.0 | 0.0 ± NA | - | - | - | - | |
| Middle | 0.0 ± 0.0 | 0.7 ± 0.7 | 8.7 ± 1.3 | 8.7 ± 1.8 | 12.7 ± 10.7 | 4.7 ± 4.7 | 4.0 ± 4.0 | 0.0 ± NA | - | - | ||
| Lower | 0.0 ± 0.0 | 4.0 ± 2.3 | 46.7 ± 23.3 | 39.3 ± 15.7 | 34.7 ± 9.3 | 34.0 ± 30.0 | 28.7 ± 24.8 | 13.8 ± 10.3 | 90.0 ± NA | - | ||
| TC1 | Mature | Upper | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± NA | 0.0 ± NA |
| Middle | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Lower | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Young | Upper | 0.7 ± 0.7 | 2.7 ± 1.8 | 2.0 ± 2.0 | 2.0 ± 2.0 | - | - | - | - | - | - | |
| Middle | 3.3 ± 2.4 | 3.3 ± 2.4 | 2.0 ± 1.2 | 2.7 ± 1.8 | 1.0 ± 1.0 | 6.0 ± NA | 2.0 ± NA | 2.9 ± NA | - | - | ||
| Lower | 8.0 ± 2.0 | 1.3 ± 0.7 | 2.0 ± 1.2 | 2.0 ± 1.2 | 1.3 ± 1.3 | 0.7 ± 0.7 | 0.0 ± 0.0 | - | - | - | ||
The abbreviation of study sites is Cascade Head (CH), Woods Creek (WC), and Klickitat Mountain (KT), Moose Mountain (MM), Falls Creek (FC), Soapgrass Mountain (SG), and Toad Creek (TC).
NA means there was only one sample so it lacked of standard error.
“-” means sample wasn’t present.
Appendix B.
Summary of SNC incidence (in %) of needles with pseudothecia for all needle age classes in 2017, across seven study sites in western Oregon for mature and young trees in upper, middle and lower crowns. The number in front of ± is average of three trees and the number followed is standard error.
| 2017 | yr1 | yr2 | yr3 | yr4 | yr5 | yr6 | yr7 | yr8 | yr9 | yr10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH1 | Mature | Upper | 40.7 ± 15.3 | 96.7 ± 3.3 | 80.7 ± 15.5 | 100.0 ± NA | 100.0 ± NA | 100.0 ± NA | - | - | - | - |
| Middle | 35.3 ± 10.1 | 99.3 ± 0.7 | 98.7 ± 0.7 | 98.7 ± 1.3 | 100.0 ± NA | - | - | - | - | - | ||
| Lower | 12.7 ± 3.5 | 97.3 ± 2.7 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± NA | - | - | - | - | - | ||
| Young | Upper | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | - | - | - | - | - | - | - | |
| Middle | 80.7 ± 11.1 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 000 | 100.0 ± NA | - | - | - | - | ||
| Lower | 45.3 ± 14.3 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± NA | - | - | - | - | ||
| KT1 | Mature | Upper | 0.0 ± 0.0 | 12.0 ±0.0 | 28.9 ± 8.9 | 41.7 ± 41.7 | - | - | - | - | - | - |
| Middle | 2.0 ± 2.0 | 20.0 ± 20.0 | 44.0 ± 16.0 | 44.0 ± 12.0 | 27.0 ± 17.0 | 0.0 ± NA | 7.1 ± NA | 6.8 ± NA | - | - | ||
| Lower | 3.0 ± 1.0 | 3.0 ± 1.0 | 28.0 ± 12.0 | 57.0 ± 1.0 | 28.7 ± 4.7 | 29.0 ± 21.0 | 10.0 ± NA | 6.3 ± NA | - | - | ||
| Young | Upper | 8.7 ± 1.3 | 91.3 ± 4.8 | 99.3 ± 0.7 | 100.0 ± NA | - | - | - | - | - | - | |
| Middle | 6.7 ± 3.3 | 85.3 ± 3.5 | 98.0 ± 1.2 | 97.4 ± 2.6 | 97.2 ± 2.8 | 100.0 ± NA | - | - | - | - | ||
| Lower | 13.3 ± 3.7 | 80.7 ± 11.6 | 95.3 ± 4.7 | 100.0 ± 0.0 | 95.9 ± 1.4 | - | - | - | - | - | ||
| WC1 | Mature | Upper | 0.0 ± 0.0 | 0.7 ± 0.7 | 1.3 ± 0.7 | 4.7 ± 2.7 | 20.0 ± 8.1 | 0.0 ± 0.0 | - | - | - | - |
| Middle | 0.0 ± 0.0 | 1.3 ± 1.3 | 6.7 ± 3.7 | 32.0 ± 0.0 | 23.3 ± 4.7 | 6.8 ± 0.8 | 7.4 ± 4.6 | 10.0 ± NA | - | - | ||
| Lower | 1.3 ± 0.7 | 2.0 ± 1.2 | 17.3 ± 4.1 | 30.7 ± 4.7 | 40.0 ± 3.1 | 10.0 ± 5.3 | 15.3 ± 4.4 | 11.5 ± 3.5 | 1.6 ± 1.6 | - | ||
| Young | Upper | 0.7 ± 0.7 | 30.0 ± 16.0 | 80.0 ± 13.6 | - | - | - | - | - | - | - | |
| Middle | 2.0 ± 1.2 | 50.7 ± 10.9 | 94.0 ± 5.0 | 94.0 ± 6.0 | - | - | - | - | - | - | ||
| Lower | 2.0 ± 2.0 | 52.7 ± 15.8 | 99.3 ± 0.7 | 98.7 ± 0.7 | 57.9 ± NA | - | - | - | - | - | ||
| MM1 | Mature | Upper | 0.0 ± 0.0 | 3.3 ± 2.4 | 16.0 ± 11.4 | 31.4 ± 13.7 | 29.4 ± NA | - | - | - | - | - |
| Middle | 0.7 ± 0.7 | 0.7 ± 0.7 | 34.7 ± 17.5 | 51.9 ± 23.2 | 26.8 ± 4.8 | 18.0 ± NA | 2.0 ± NA | 11.8 ± NA | 6.5 ± NA | - | ||
| Lower | 0.7 ± 0.7 | 1.3 ± 1.3 | 52.7 ± 25.8 | 56.0 ± 28.7 | 53.3 ± 27.5 | 33.0 ± 27.0 | 25.0 ± 23.0 | 0.0 ± NA | 6.8 ± NA | - | ||
| Young | Upper | 0.0 ± 0.0 | 60.0 ± 23.2 | 89.9 ± 8.1 | 83.5 ± 8.5 | - | - | - | - | - | - | |
| Middle | 1.3 ± 1.3 | 67.3 ± 18.0 | 99.3 ± 0.7 | 96.0 ± 4.0 | 100.0 ± NA | - | - | - | - | - | ||
| Lower | 3.3 ± 0.7 | 78.7 ± 14.9 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | - | - | - | - | ||
| FC1 | Mature | Upper | 0.0 ± 0.0 | 2.0 ± 1.2 | 33.3 ± 11.8 | 40.0 ± 15.6 | 60.0 ± NA | 38.0 ± NA | 40.4 ± NA | - | - | - |
| Middle | 0.0 ± 0.0 | 3.3 ± 2.4 | 34.7 ± 18.0 | 68.7 ± 22.5 | 55.3 ± 4.4 | 57.7 ± 14.3 | 50.2 ± 11.8 | - | - | - | ||
| Lower | 0.0 ± 0.0 | 2.0 ± 1.2 | 52.7 ± 10.0 | 88.7 ± 5.7 | 73.0 ± 10.6 | 74.0 ± 12.2 | 39.9 ± 11.9 | - | - | - | ||
| Young | Upper | 36.0 ± 30.1 | 97.3 ± 1.8 | 98.7 ± 1.3 | 96.0 ± NA | 100.0 ± NA | - | - | - | - | - | |
| Middle | 22.0 ± 17.1 | 99.3 ± 0.7 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | - | - | - | - | ||
| Lower | 32.0 ± 25.1 | 95.3 ± 2.9 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± NA | - | - | - | - | ||
| SG1 | Mature | Upper | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.2 ± 3.0 | 2.4 ± 0.4 | 0.0 ± NA | - | - | - |
| Middle | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA | ||
| Lower | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Young | Upper | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 1.3 | 2.7 ± 1.8 | 7.0 ± 3.0 | 4.0 ± NA | 3.3 ± NA | - | - | - | |
| Middle | 0.7 ± 0.7 | 0.0 ± 0.0 | 19.3 ± 10.4 | 18.7 ± 11.8 | 13.3 ± 4.7 | 10.0 ± 3.1 | 4.0 ± 4.0 | 4.0 ± 4.0 | - | - | ||
| Lower | 1.3 ± 1.3 | 4.0 ± 3.1 | 31.3 ± 14.3 | 43.3 ± 25.4 | 40.7 ± 21.9 | 44.4 ± 21.6 | 58.0 ± 42.0 | 75.0 ± NA | 19.2 ± NA | - | ||
| TC1 | Mature | Upper | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± NA | - |
| Middle | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Lower | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | ||
| Young | Upper | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | - | - | - | - | - | - | |
| Middle | 0.0 ± 0.0 | 0.7 ± 0.7 | 0.7 ± 0.7 | 0.7 ± 0.7 | 2.0 ± 1.2 | 0.0 ± 0.0 | - | - | - | - | ||
| Lower | 0.0 ± 0.0 | 0.7 ± 0.7 | 1.3 ± 1.3 | 0.7 ± 0.7 | 2.7 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± NA | - | - | ||
The abbreviation of study sites is Cascade Head (CH), Woods Creek (WC), and Klickitat Mountain (KT), Moose Mountain (MM), Falls Creek (FC), Soapgrass Mountain (SG), and Toad Creek (TC).
NA means there was only one sample so it lacked of standard error.
“-” means sample wasn’t present.
Appendix C.
Results of preliminary MANOV test for the main effects tree age, site, year and canopy position alone and their interaction on SNC incidence in study areas in western Oregon. Only one- and two-year-old needles were used for statistical analysis because three- and older needles had limited data for testing covariance.
| F-value | p-value | |||
|---|---|---|---|---|
| Needle age =1 | (Intercept) | 191.6 | < 0.001 | *** |
| Tree age | 81.4 | < 0.001 | *** | |
| Site | 55.3 | < 0.001 | *** | |
| Tree age × Site | 18.8 | < 0.001 | *** | |
| Year | 1.1 | 0.307 | ||
| Tree age × Year | 0.2 | 0.654 | ||
| Site × Year | 6.7 | 0.002 | ** | |
| Tree age × Site × Year | 1.1 | 0.375 | ||
| Canopy | 8.3 | 0.003 | ** | |
| Tree age × Canopy | 3.6 | 0.048 | * | |
| Site × Canopy | 4.3 | 0.001 | *** | |
| Tree age × Site × Canopy | 3.1 | 0.008 | ** | |
| Year × Canopy | 0.0 | 0.977 | ||
| Tree age × Year × Canopy | 2.4 | 0.120 | ||
| Site × Year × Canopy | 0.7 | 0.722 | ||
| Tree age × Site × Year × Canopy | 1.4 | 0.220 | ||
| NeedleAge =2 | (Intercept) | 1320.0 | < 0.001 | *** |
| Tree age | 403.5 | < 0.001 | *** | |
| Site | 47.6 | < 0.001 | *** | |
| Tree age × Site | 24.1 | < 0.001 | *** | |
| Year | 23.7 | < 0.001 | *** | |
| Tree age × Year | 7.9 | 0.011 | * | |
| Site × Year | 6.3 | 0.002 | ** | |
| Tree age × Site × Year | 4.1 | 0.015 | * | |
| Canopy | 5.2 | 0.017 | * | |
| Tree age × Canopy | 0.7 | 0.494 | ||
| Site × Canopy | 1.4 | 0.234 | ||
| Tree age × Site × Canopy | 1.6 | 0.161 | ||
| Year × Canopy | 0.8 | 0.460 | ||
| Tree age × Year × Canopy | 1.8 | 0.193 | ||
| Site × Year × Canopy | 0.7 | 0.666 | ||
| Tree age × Site × Year × Canopy | 0.7 | 0.678 |
Appendix D.
Results of MANOVA by individual sites testing significance of SNC incidence. Because eligible needle age classes were different in each site, the MANOVA was re-run by site and by needle age to clarify the effects from other factors. The MANOVA results for SNC incidence varied by needle age class and site. Tree age was a crucial factor in SNC incidence analysis across most of study sites. SNC incidence for one- and two-year-old needles differed by canopy position at Cascade Head, and possibly two-year-old needles at Woods Creek but not the other sites.
| Needle age = 1yr | Needle age = 2yr | Needle age = 3yr | Needle age = 4yr | Needle age = 5yr | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |||||||
| CH1 | (Intercept) | 105.6 | 0.001 | *** | 7432.2 | < 0.001 | *** | 286.8 | 0.003 | ** | - | - | - | - | ||
| Tree age | 36.0 | 0.004 | ** | 5.1 | 0.086 | . | 0.8 | 0.457 | - | - | - | - | ||||
| Year | 16.9 | 0.015 | * | 60.3 | 0.001 | ** | 0.1 | 0.826 | - | - | - | - | ||||
| Tree age × Year | 0.4 | 0.577 | 52.0 | 0.002 | ** | 0.1 | 0.826 | - | - | - | - | |||||
| Canopy | 42.4 | 0.006 | ** | 18.0 | 0.021 | * | 1.6 | 0.491 | - | - | - | - | ||||
| Tree age × Canopy | 14.3 | 0.029 | * | 20.7 | 0.018 | * | 1.6 | 0.491 | - | - | - | - | ||||
| Year × Canopy | 0.6 | 0.608 | 132.6 | 0.001 | ** | 0.1 | 0.896 | - | - | - | - | |||||
| Tree age × Year × Canopy | 5.2 | 0.106 | 126.4 | 0.001 | ** | 0.1 | 0.896 | - | - | - | - | |||||
| KT | (Intercept) | 10.6 | 0.047 | * | 234.8 | 0.001 | *** | - | - | - | - | - | - | |||
| Tree age | 6.2 | 0.089 | . | 130.1 | 0.001 | ** | - | - | - | - | - | - | ||||
| Year | 0.0 | 0.976 | 30.3 | 0.012 | * | - | - | - | - | - | - | |||||
| Tree age × Year | 0.1 | 0.831 | 8.1 | 0.065 | . | - | - | - | - | - | - | |||||
| Canopy | 0.3 | 0.750 | 0.7 | 0.585 | - | - | - | - | - | - | ||||||
| Tree age × Canopy | 0.5 | 0.653 | 0.3 | 0.773 | - | - | - | - | - | - | ||||||
| Year × Canopy | 7.7 | 0.116 | 2.5 | 0.282 | - | - | - | - | - | - | ||||||
| Tree age × Year × Canopy | 5.9 | 0.146 | 0.1 | 0.887 | - | - | - | - | - | - | ||||||
| WC | (Intercept) | 5.5 | 0.079 | . | 75.2 | 0.001 | *** | - | - | - | - | - | - | |||
| Tree age | 2.2 | 0.210 | 67.9 | 0.001 | ** | - | - | - | - | - | - | |||||
| Year | 2.5 | 0.193 | 20.2 | 0.011 | * | - | - | - | - | - | - | |||||
| Tree age × Year | 0.5 | 0.539 | 18.8 | 0.012 | * | - | - | - | - | - | - | |||||
| Canopy | 0.6 | 0.624 | 9.2 | 0.053 | . | - | - | - | - | - | - | |||||
| Tree age × Canopy | 0.4 | 0.713 | 4.8 | 0.116 | - | - | - | - | - | - | ||||||
| Year × Canopy | 1.2 | 0.414 | 2.5 | 0.234 | - | - | - | - | - | - | ||||||
| Tree age × Year × Canopy | 7.2 | 0.072 | . | 2.3 | 0.244 | - | - | - | - | - | - | |||||
| MM | (Intercept) | 86.9 | 0.001 | *** | 76.5 | 0.001 | *** | 51.0 | 0.002 | ** | - | - | - | - | ||
| Tree age | 32.3 | 0.005 | ** | 42.4 | 0.003 | ** | 7.2 | 0.055 | . | - | - | - | - | |||
| Year | 64.9 | 0.001 | ** | 6.7 | 0.061 | . | 12.9 | 0.023 | * | - | - | - | - | |||
| Tree age × Year | 24.9 | 0.008 | ** | 0.2 | 0.710 | 6.4 | 0.065 | . | - | - | - | - | ||||
| Canopy | 3.9 | 0.148 | 1.6 | 0.333 | 5.1 | 0.108 | - | - | - | - | ||||||
| Tree age × Canopy | 0.8 | 0.541 | 0.4 | 0.677 | 4.7 | 0.119 | - | - | - | - | ||||||
| Year × Canopy | 0.9 | 0.496 | 0.1 | 0.884 | 0.3 | 0.735 | - | - | - | - | ||||||
| Tree age × Year × Canopy | 3.4 | 0.170 | 1.0 | 0.469 | 1.5 | 0.346 | - | - | - | - | ||||||
| FC | (Intercept) | 55.7 | 0.002 | ** | 3566.3 | < 0.001 | *** | 163.8 | < 0.001 | *** | - | - | - | - | ||
| Tree age | 37.9 | 0.004 | ** | 2894.9 | < 0.001 | *** | 16.4 | 0.015 | * | - | - | - | - | |||
| Year | 0.0 | 0.917 | 4.6 | 0.098 | . | 16.0 | 0.016 | * | - | - | - | - | ||||
| Tree age × Year | 0.6 | 0.495 | 1.4 | 0.304 | 18.6 | 0.013 | * | - | - | - | - | |||||
| Canopy | 11.9 | 0.037 | * | 4.1 | 0.139 | 2.4 | 0.242 | - | - | - | - | |||||
| Tree age × Canopy | 4.6 | 0.123 | 2.7 | 0.216 | 1.7 | 0.319 | - | - | - | - | ||||||
| Year × Canopy | 0.3 | 0.751 | 2.0 | 0.279 | 14.1 | 0.030 | * | - | - | - | - | |||||
| Tree age × Year × Canopy | 0.1 | 0.917 | 0.6 | 0.608 | 17.0 | 0.023 | * | - | - | - | - | |||||
| SG | (Intercept) | 3.6 | 0.132 | 36.6 | 0.004 | ** | 6.1 | 0.069 | . | 8.7 | 0.042 | * | 12.6 | 0.038 | * | |
| Tree age | 0.1 | 0.725 | 14.3 | 0.019 | * | 5.8 | 0.073 | . | 8.3 | 0.045 | * | 9.3 | 0.055 | . | ||
| Year | 0.1 | 0.725 | 0.2 | 0.651 | 0.2 | 0.713 | 0.8 | 0.409 | 1.1 | 0.373 | ||||||
| Tree age × Year | 3.6 | 0.132 | 0.1 | 0.819 | 0.2 | 0.713 | 0.8 | 0.409 | 0.3 | 0.629 | ||||||
| Canopy | 1.7 | 0.319 | 2.9 | 0.199 | 3.5 | 0.166 | 2.5 | 0.227 | 6.2 | 0.138 | ||||||
| Tree age × Canopy | 0.4 | 0.686 | 8.5 | 0.058 | . | 2.8 | 0.204 | 2.6 | 0.223 | 3.5 | 0.221 | |||||
| Year × Canopy | 0.4 | 0.686 | 0.4 | 0.716 | 1.5 | 0.347 | 0.2 | 0.798 | 10.1 | 0.090 | . | |||||
| Tree age × Year × Canopy | 1.7 | 0.319 | 1.6 | 0.342 | 1.5 | 0.347 | 0.1 | 0.874 | 22.3 | 0.043 | * | |||||
| TC | (Intercept) | 15.4 | 0.017 | * | 13.0 | 0.023 | * | - | - | - | - | - | - | |||
| Tree age | 15.4 | 0.017 | * | 13.0 | 0.023 | * | - | - | - | - | - | - | ||||
| Year | 15.4 | 0.017 | * | 3.0 | 0.158 | - | - | - | - | - | - | |||||
| Tree age × Year | 15.4 | 0.017 | * | 3.0 | 0.158 | - | - | - | - | - | - | |||||
| Canopy | 2.9 | 0.199 | 0.2 | 0.835 | - | - | - | - | - | - | ||||||
| Tree age × Canopy | 2.9 | 0.199 | 0.2 | 0.835 | - | - | - | - | - | - | ||||||
| Year × Canopy | 2.9 | 0.199 | 1.5 | 0.354 | - | - | - | - | - | - | ||||||
| Tree age × Year × Canopy | 2.9 | 0.199 | 1.5 | 0.354 | - | - | - | - | - | - | ||||||
The abbreviation of study sites is Cascade Head (CH), Woods Creek (WC), and Klickitat Mountain (KT), Moose Mountain (MM), Falls Creek (FC), Soapgrass Mountain (SG), and Toad Creek (TC).
“-” means result wasn’t present because of the limited data points.
6. Literature Cited
- Apple M, Tiekotter K, Snow M, Young J, Soeldner A, Phillips D, Tingey D, Bond BJ, 2002. Needle anatomy changes with increasing tree age in Douglas-fir. Tree Physiology 22, 129–136. [DOI] [PubMed] [Google Scholar]
- Beedlow PA, Lee EH, Tingey DT, Waschmann RS, Burdick CA, 2013. The importance of seasonal temperature and moisture patterns on growth of Douglas-fir in western Oregon, USA. Agriculture and Forest Meteorology 169, 174–185. [Google Scholar]
- Black BA, Shaw DC, Stone JK, 2010. Impacts of Swiss needle cast on overstory Douglas-fir forests of the western Oregon Coast Range. Forest Ecology and Management 259, 1673–1680. [Google Scholar]
- Capitano B, 1999. The infection and colonization of Douglas-fir by Phaeocryptopus gaeumannii M.Sc. thesis Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon. [Google Scholar]
- Cook SP, Hain FP, 1986. Defensive mechanisms of loblolly and shortleaf pine against attack by southern pine beetle, Dendroctonus frontalis Zimmermann, and its fungal associate, Ceratocystis minor (Hedgecock) Hunt. Journal of Chemical Ecology 12(6), 1397–1406. [DOI] [PubMed] [Google Scholar]
- Day ME, Greenwood MS, White AS, 2001. Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiology 21(16), 1195–1204. [DOI] [PubMed] [Google Scholar]
- El-Hajj Z, Kavanagh K, Rose C, Kanaan-Atallah Z, 2004. Nitrogen and carbon dynamics of a foliar biotrophic fungal parasite in fertilized Douglas-fir. New Phytologist 163(1), 139–147. [DOI] [PubMed] [Google Scholar]
- England JR, Attiwill PM, 2006. Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees 20, 79–90. [DOI] [PubMed] [Google Scholar]
- Erwin EA, Turner MG, Lindroth RL, Romme WH, 2001. Secondary Plant Compounds in Seedling and Mature Aspen (Populus tremuloides) in Yellowstone National Park, Wyoming. The American Midland Naturalist 145(2), 299–308. [Google Scholar]
- Espinosa-Garcia FJ, Langenheim JH, 1991. Effect of some leaf essential oil phenotypes in coastal redwood on the growth of several fungi with endophytic stages. Biochemical Systematics and Ecology 19(8), 629–642. [Google Scholar]
- Ewers FW, and Schmid R. 1981. Longevity of needle fascicles of Pinus longaeva (Bristlecone pine) and other North American pines. Oecologia 51:107–115. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S, 2011. An {R} Companion to Applied Regression, Second Edition Thousand Oaks CA: Sage; http://socserv.socsci.mcmaster.ca/jfox/Books/Companion (accessed 19 July 2018). [Google Scholar]
- Hansen EM, Stone JK, Capitano BR, Rosso P, Sutton W, Kanaskie A, McWilliams MG, 2000. Incidence and impact of Swiss needle cast in forest plantations of Douglas-fir in coastal Oregon. Plant Disease 84, 773–779. [DOI] [PubMed] [Google Scholar]
- Lee EH, Beedlow PA, Waschmann RS, Burdick CA Shaw DC, 2013. Tree-ring analysis of the fungal disease Swiss needle cast in western Oregon coastal forests. Canadian Journal of Forest Research 43, 677–690. [Google Scholar]
- Lee EH, Beedlow PA, Waschmann RS, Tingey DT, Wickham C, Cline S, Bollman M, Carlile C, 2016. Douglas-fir displays a range of growth responses to temperature, water, and Swiss needle cast in western Oregon, USA. Agricultural and Forest Meteorology 221(1), 176–188. [Google Scholar]
- Lee EH, Beedlow PA, Waschmann RS, Tingey DT, Wickham C, Cline S, Bollman M, Carlile C, 2017. Regional patterns of increasing Swiss needle cast impacts on Douglas-fir growth with warming temperatures. Ecology and Evolution 7(24), 11176–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R, 2018. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.1.3. https://CRAN.R-project.org/package=emmeans (accessed 19 July 2018).
- Maguire DA, Kanaskie A, Voelker W, Johnson R, Johnson G, 2002. Growth of young Douglas-fir plantations across a gradient in Swiss Needle Cast severity. Western Journal of Applied Forestry 17(2), 86–95. [Google Scholar]
- Maguire DA, Mainwaring DB, Kanaskie A, 2011. Ten-year growth and mortality in young Douglas-fir stands experiencing a range in Swiss needle cast severity. Canadian Journal of Forest Research 41, 2064–2076. [Google Scholar]
- Manter DK, Bond BJ, Kavanagh KL, Rosso PH, Filip GM, 2000. Pseudothecia of Swiss needle cast fungus, Phaeocryptopus gaeumannii, physically block stomata of Douglas-fir, reducing CO2. New Phytologist 3, 481–491. [DOI] [PubMed] [Google Scholar]
- Manter DK, Winton LM, Filip GM, Stone JK, 2003. Assessment of Swiss needle cast disease: temporal and spatial investigations of fungal colonization and symptom severity. Journal of Phytopathology 151, 344–351. [Google Scholar]
- Manter DK, Reeser PW, Stone JK, 2005. A climate-based model for predicting geographic variation in Swiss needle cast severity in the Oregon Coast Range. Phytopathology 95, 1256–1265. [DOI] [PubMed] [Google Scholar]
- Mildrexler DJ, Shaw DC, Cohen WB, 2019. Short-term climate trends and the Swiss needle cast epidemic in Oregon’s public and private coastal forestlands. Forest Ecology and Management 432, 501–513. [Google Scholar]
- Michaels E, Chastagner GA, 1984. Seasonal availability of Phaeocryptopus gaeumannii ascospores and conditions that influence their release. Plant Disease 68(11), 942–944. [Google Scholar]
- Mulvey RL, Shaw DC, Maguire DA, 2013. Fertilization impacts on Swiss needle cast disease severity in western Oregon. Forest Ecology and Management; 287, 147–158. [Google Scholar]
- Perakis SS, Maguire DA, Bullen TD, Cromack K, Waring RH, Boyle JR, 2006. Coupled nitrogen and calcium cycles in forests of the Oregon coast range. Ecosystems 9(1), 63–74. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team, 2017. nlme: Linear and Nonlinear Mixed Effects Models. R package version 31–131. https://cran.r-project.org/web/packages/nlme/index.html (accessed 19 July 2018). [Google Scholar]
- R Core Team, 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/ (accessed 19 July 2018). [Google Scholar]
- Reich PB, Koike T, Gower ST, and Schoettle AW. 1995. Causes and consequences of variation in conifer leaf life-span Pages 225–254, in Smith WK and Hinckley TM (editors), Ecophysiology of Coniferous Forests, Academic Press, New York, USA. [Google Scholar]
- Ritóková G, Shaw DC, Filip G, Kanaskie A, Browning J, Norlander D, 2016. Swiss Needle Cast in western Oregon Douglas-Fir plantations: 20-year monitoring results. Forests 7, 155. [Google Scholar]
- Rosso PH, and Hansen EM. 2003. Predicting Swiss needle cast disease distribution and severity in young Douglas-Fir plantations in coastal Oregon. Phytopathology 93:790–798. [DOI] [PubMed] [Google Scholar]
- Shaw DC, Filip GM, Kanaskie A, Maguire DA, Littke WA, 2011. Managing an epidemic of Swiss Needle Cast in the Douglas-Fir region of Oregon: The role of the Swiss Needle Cast Cooperative. Journal of Forestry 109(2), 109–119. [Google Scholar]
- Shaw DC, Woolley T, Kanaskie A, 2014. Vertical foliage retention in Douglas-fir across environmental gradients of the western Oregon coast range influenced by Swiss needle cast. Northwest Science 88, 23–32. [Google Scholar]
- Snedecor GW, Cochran WG, 1967. Statistical Methods, sixth ed The Iowa State University Press, IA, USA. [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, Braun U, Barreto RW, de Wit PJGM, Crous PW, 2017. Mycosphaerellaceae – Chaos or clarity? Studies in Mycology 87, 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2009. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York. [Google Scholar]
- Wickham H, Francois R, Henry L, Müller K, 2017. dplyr: A Grammar of Data Manipulation. R package version 0.7.4 https://CRAN.R-project.org/package=dplyr (accessed 19 July 2018).
- Zhao J, Maguire DA, Mainwaring DB, Kanaskie A, 2012. Climatic influences on needle cohort survival mediated by Swiss needle cast in coastal Douglas-fir. Tree 26, 1361–1371. [Google Scholar]