Abstract
Pollen grains and plant spores have emerged as a novel biomaterial for a broad range of applications including oral drug and vaccine delivery, catalyst support, and removal of heavy metals. However, before pollens can be used, their intrinsic biomolecules, which occupy a large part of the pollen inner cavity must be removed not only to create empty space but because they have potential to cause allergies when used in vivo. These intrinsic materials in the pollen core can be extracted through a chemical treatment to generate clean pollen shells. The commonly used method involves a series of sequential treatments with organic solvents, alkalis, and acids to remove the native pollen biomolecules. This method, though successful for treating lycopodium (Lycopodium clavatum) spores, fails for other species of pollens such as common ragweed (Ambrosia elatior) and thus prevents widespread investigation of different pollens. Herein, we report a new chemical treatment for obtaining clean pollen shells from multiple plant species. This new method involves sequential treatment with acetone, phosphoric acid, and potassium hydroxide. Scanning electron micrographs and protein quantification have shown that the new method can successfully produce clean, intact, and hollow shells from many pollen species including ragweed, sunflower, black alder, and lamb’s quarters. These results demonstrate the broad applicability of this method to clean pollens of different species, and paves the way to start investigating them for various applications.
Keywords: Pollen aperture, Pollen treatment, Pollen turgor pressure, Ragweed pollen, Sporopollenin
Introduction
Pollen grains are naturally occurring microcapsules produced by plants to transport the male gametes from the anther (male reproductive part) to stigma (female reproductive part) for fertilization.1–3 During this transportation, pollen grains protect the male gamete from environmental stresses. A typical pollen grain has a tough outer shell known as the exine and an inner shell known as the intine. The intine holds the male gamete and other biomolecules and nutrients.4–7 The intine is made of cellulose and pectin, while the exine is primarily made of a biopolymer known as sporopollenin, whose exact molecular formula remains unknown.4 Sporopollenin is known to be tough and resistant to high temperatures, various organic solvents, acids, and alkalis.4, 8–10 Because of this toughness, pollens are becoming increasingly popular for a variety of applications in oral therapeutic delivery, such as, oral vaccination,11–13 encapsulation of magnetic resonance imaging (MRI) contrast agents for oral delivery,14 encapsulation of fish oil for taste masking15, and encapsulation of other drugs for oral delivery.4, 16–17
There are several desired traits that pollens must possess before they can be used for different applications. They must be ‘intact’, i.e., uncracked or unfragmented so that their unique geometry and morphological features can be utilized to their maximum potential, such as for carrying drugs and vaccines for biomedical applications. Pollens must be ‘clean’ and devoid of biomolecules and other cellular material that naturally dwell inside them, so as to prevent allergic reactions and to produce a vacant core that can be filled with therapeutic molecules. Lastly, for processability purposes, pollens must not form clumps when dry, and must be readily dispersible in a solvent without forming aggregates. Therefore, it is important to produce pollens in an ‘unaggregated’ state.
Previous studies have largely focused on the use of spores of lycopodium (Lycopodium clavatum) for different applications.11, 16, 18 To process raw lycopodium spores, a well-established ‘conventional method’ is used. This method involves the use of organic solvents for defatting the pollens, followed by alkali treatment to remove the proteinaceous material from within pollens, and then treatment with an acid as the last step to remove residual proteins and cellulosic intine.4, 11, 19–20 This method, although successful for obtaining clean shells from lycopodium, fails for other pollen species. For example, Mundargi et al., have reported the failure of the conventional method for treating sunflower (Helianthus annuus) pollens.21 The authors have shown that sunflower pollens were damaged after potassium hydroxide (KOH) treatment, which is the second step in the conventional method. To overcome this limitation, the authors bypassed the KOH step and directly proceeded to the orthophosphoric acid treatment, which is the last step in the conventional method. However, with this approach the residual nitrogen content after treatment was still quite high (about 0.7 %), indicating lack of proper removal of proteins. Similarly, Fadiran has also previously reported failure of the conventional method in treating short ragweed (Ambrosia artemisiifolia) pollens.22 Fadiran reported pollen breakage and formation of a large amount of insoluble debris after treating ragweed pollens with KOH. To separate ragweed pollens from this debris, the author used sonication and then without washing the pollens proceeded directly to the orthophosphoric acid treatment step. Finally, to recover pollens as a product from the acid solution, a multi-step procedure was used, which comprised of: i) centrifugation in vials at very low rpm (~ 1.5 rpm) to allow pollens to float to the top of the acid solution, ii) removal of excess acid from vials by inserting long metal syringe needles into the vials through perforated caps, and iii) neutralization of the remaining acid in the vials with a base. The authors later used the treated ragweed pollens as reinforcing fillers for polymers. 23
Hence, there is a need to develop an optimized and robust treatment procedure that can produce morphologically intact, clean, and non-aggregated pollen shells from a variety of pollen species through a simple protocol that can be readily adapted for large-scale processing.
To develop such a protocol, in this study, we first investigated the cause of failure of the conventional treatment process. Common ragweed (Ambrosia elatior) pollen was used as a model pollen for this purpose. The conventional method and its variants were employed on ragweed pollens to assess their ability to produce intact pollen shells. As seen before, these methods were unsuccessful.21 Hence a new method was developed. The success of this new method in producing intact shells from ragweed was confirmed by scanning electron microscopic analysis. Elemental analysis confirmed that the resulting pollen shells had minimal residual protein content. We discovered that high turgor pressure is the mechanistic principle of the new treatment process. This new method was also found to be successful in cleaning pollens of other species.
Materials and Methods
Materials.
Raw lycopodium spores were obtained from Sigma Aldrich (MO, USA). Raw ragweed, sunflower, black alder (Alnus glutinosa), and lamb’s quarters (Chenopodium album) pollens were purchased from Pharmallerga (Lišov, Czech Republic). Acetone, KOH, orthophosphoric acid, ethanol, hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Fisher Scientific (PA, USA). Milli-Q water (Direct-Q 3 UV system, MilliporeSigma, MA, USA) with an electrical resistivity of 18.2 MΩ.cm (at 25 °C) was used in all experiments.
Pollen grain treatment.
To obtain clean-hollow ragweed pollen shells, different treatment pathways were followed as summarized in Figure 1.
Figure 1. Schematic diagram of conventional and modified conventional treatment processes.
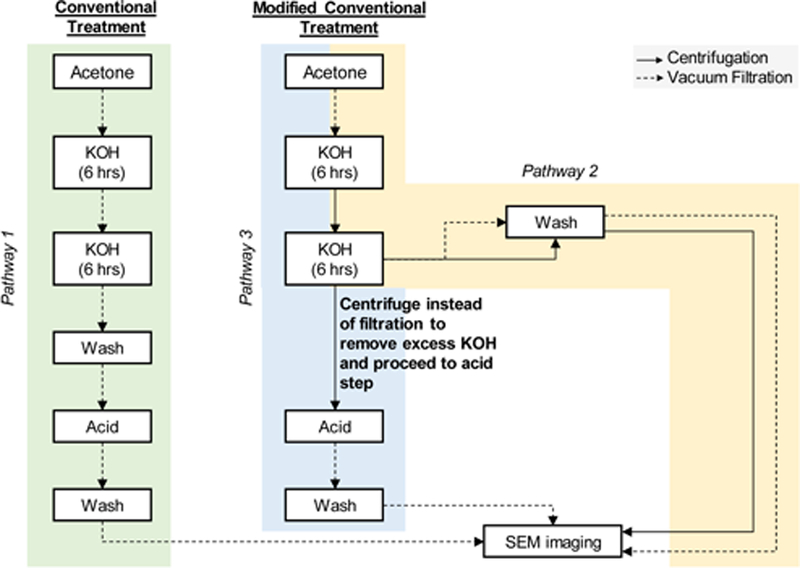
Pathway 1 shows processing steps for conventional treatment of pollen grains. Pathways 2 and 3 represent modifications to the conventional treatment and are called modified conventional treatment. Their respective steps are shown. In pathway 2 modification was made at the wash step to compare centrifugation versus vacuum filtration. Pathway 3 shows processing steps after the centrifugation process was selected based on pathway 2 as the more suitable option for washing the pollens.
Conventional treatment:
This treatment is summarized as pathway 1 in Figure 1. Lycopodium or ragweed pollens (50 g) were stirred overnight in 450 ml of acetone under reflux at 65 °C. These were then air-dried overnight, transferred to 600 ml of 6% w/v aqueous KOH, and refluxed at 120 °C for 6 h. After 6 h of reflux, the solution was cooled to room temperature, filtered, and the solids from the filter paper were added to 600 ml of 6% w/v aqueous KOH for another 6 h of reflux at 120 °C. Pollens were then filtered, washed with hot water (3 x 300 ml) and hot ethanol (3 x 300 ml), and air-dried overnight. They were next refluxed in 900 ml of orthophosphoric acid for 7 days at 160 °C. On the 8th day the solution was cooled, and pollens were filtered and washed with water (5 x 300 ml), acetone (300 ml), 2 M HCl (300 ml), 2 M NaOH (300 ml), water (5 x 300 ml), and ethanol (2 x 300 ml). Following these washings, the treated pollens were dried at 60 °C in an oven until constant weight was achieved.
Modified conventional treatment:
This treatment is summarized as pathway 2 and pathway 3 in Figure 1. Both pathways started with acetone treatment step. Next the first KOH reflux of conventional treatment was performed. After 6 h of KOH reflux, the solution was cooled to room temperature and centrifuged (unlike filtration step of conventional treatment) at 233 × g for 1 min at room temperature. The supernatant was discarded and fresh KOH solution (50 ml) was added to the tube. This mixture was transferred to a round bottom flask containing the remaining fresh KOH (550 ml) and stirred under reflux for additional 6 h. In pathway 2, filtration and centrifugation methods were compared for isolation of pollens from the KOH solution and from the wash step. In pathway 3, centrifugation method was used for isolation of pollens from the KOH solution, the supernatant was discarded, and the pollens were transferred directly to orthophosphoric acid (900ml) and refluxed at 160 °C for 7 days (same as conventional treatment). The recovered pollens were washed as mentioned above in conventional treatment using filtration method for isolation and dried at 60 °C in an oven until constant weight was achieved.
High-temperature switched treatment:
Ragweed pollens (20 g) were stirred overnight under reflux in acetone at 65 °C (as in conventional treatment). Pollens were filtered, air dried overnight, and transferred to orthophosphoric acid (400 ml) and refluxed for 7 days at 160 °C (different from conventional treatment, which has alkali treatment at this stage). On the 8th day, the pollens were separated from the acid by vacuum filtration, and washed with hot water (2 x 250 ml), acetone (250 ml), 2 M HCl (250 ml), 2 M NaOH (250 ml), water (6 x 250 ml), acetone (250 ml), and ethanol (2 x 250 ml). After overnight air drying, pollens were transferred to 6% w/v KOH solution (350 ml) and refluxed for 12 h at 120 °C with the solution renewed after 6 h. Pollens were then filtered, washed with hot water (6 x 250 ml), acetone (250 ml), and hot ethanol (2 x 250 ml) and then dried in an oven at 60 °C until constant weight was achieved.
Low-temperature switched treatment:
Acetone treatment was identical to the high-temperature switched treatment scheme. However, to study the effect of lower treatment temperatures, the orthophosphoric acid treatment was carried out at 60 °C, and the KOH treatment was carried out at 80 °C.
Scanning electron microscopy (SEM).
SEM analysis of different samples of pollens was performed using a Hitachi S-4300 E/N (FESEM) microscope (Hitachi, Japan). To image the inner cavity of pollen grains, they were frozen with dry-ice and broken with a mortar and pestle to expose the inner cavity. The samples were placed on an aluminum stub with carbon tape and coated with gold and palladium using a Technics Hummer V Sputter Coater (Anatech, CA, USA) to enable visualization. Samples were imaged at different magnifications at an accelerating voltage of 2 kV.
Elemental analysis.
Dried pollens (both raw/natural and chemically treated) were analyzed using a calibrated PerkinElmer 2400 Series II CHNS/O analyzer. All measurements were performed in triplicate with approximately 2 mg samples used for each test. Percent nitrogen values were multiplied with the Kjeldahl conversion factor of 6.25 to determine final protein percent .11
Fourier-transform infrared (FTIR) spectroscopy analysis.
FTIR spectra of natural and chemically treated ragweed pollen samples were obtained using a FTIR instrument (Spotlight 400, PerkinElmer, Inc., Waltham, MA, USA) equipped with a Universal Attenuated Total Reflectance (UATR) accessory. All FTIR spectra were collected at a spectral resolution of 4 cm-1, with 32 co-added scans over the range from 4000 to 650 cm-1. The Perkin-Elmer software was used to perform spectra normalization and baseline corrections.
Study and visualization of aperture opening phenomenon.
Natural raw pollen grains were treated (defatted) with acetone as previously described in the conventional treatment. To observe the build-up of pressure, a small sample of defatted pollen grains were placed on a glass slide to which orthophosphoric acid or water (10 μl) was added and immediately live video was captured under bright field with a Nikon Plan Fluor 40x oil lens (NA 1.3) on the Nikon DS-U2/L2 camera of the Nikon Eclipse Ti-E microscope (Nikon Instruments Inc., Melville, NY, USA). To assess the effect of solvents on pollen apertures, we visualized the pollens with SEM after exposing defatted pollen samples (5 mg) for 5 min in a microcentrifuge tube with 300 μl of water or orthophosphoric acid (85% v/v) or phosphate buffer saline (PBS) (1X and 10X) or sucrose solution (10 % w/w and 100 %w/w). The solution was then removed by centrifugation at 233 x g for 1 min, the pollens were washed with water (3 x 300μl), air-dried overnight, and SEM analysis was performed.
Statistical analysis.
All statistical analysis was performed using GraphPad Prism 7 (GraphPad software, Inc., CA, USA). Elemental analysis data was compared using a two-tailed t-test. Statistical significance was considered for p < 0.05.
Results
Conventional treatment is unable to produce clean pollen shells from ragweed pollens.
Conventional treatment involves sequential treatment of pollen grains with acetone, KOH, and orthophosphoric acid (Figure 2A). This treatment has been successfully used by us and other investigators in the past to obtain clean lycopodium spores.4, 11, 17 As expected, after conventional treatment, morphologically intact and clean spore shells were obtained from lycopodium (Figure 2B and C). However, when conventional treatment was applied to ragweed pollens, upon filtration after the first 6 h of KOH reflux they were found buried in a cake on the filter paper (Figure 2D). The cake was dense and could not be re-dissolved to perform the second KOH treatment. An inspection of the cake under SEM revealed that ragweed pollens were still present; however, they were tightly embedded in an insoluble extraneous material (Figure 2E and F).
Figure 2. Schematic diagram and images of lycopodium and ragweed pollens processed using the conventional treatment protocol.
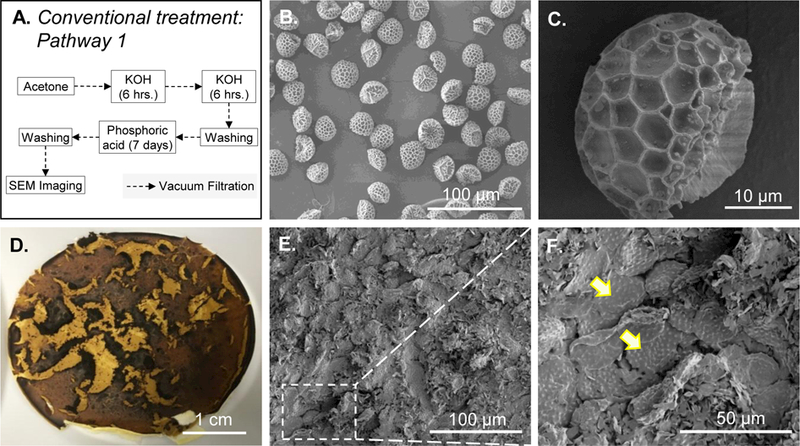
A. Schematic diagram of the processing steps of the conventional treatment protocol. B. Lycopodium after processing with conventional treatment and C. zoomed in image of a single lycopodium. Ragweed pollens after 6 hours of KOH treatment: D. photograph of the cake formed on the filter paper after vacuum filtration. E. SEM image of the cake showing pollen entrapped in extraneous matter. F. zoomed in SEM image of the cake showing more details of entrapped pollens. Some entrapped pollens are indicated by arrows.
To study the chemical nature of materials forming the insoluble cake, the solution after KOH treatment was centrifuged to separate pollens from the solution. The dark brown supernatant was carefully removed and dried at 120 ºC to evaporate the water. Dried materials were kept at 80 ºC in an oven overnight to further remove moisture and then analyzed with FTIR. The FTIR spectrum of the extract showed several distinct vibrations (1634, 1562, 1311, and 768 cm-1) as compared to the KOH spectrum (Figure 3). The absorption bands at 1634, 1562, 1311, and 768 cm-1 are due to amide I, amide II, amide III, and amide IV in proteins, respectively.24 This shows that the insoluble matter is primarily composed of proteins and peptides.
Figure 3. Fourier-transform infrared (FTIR) spectra of extracted materials from ragweed pollen after KOH treatment.
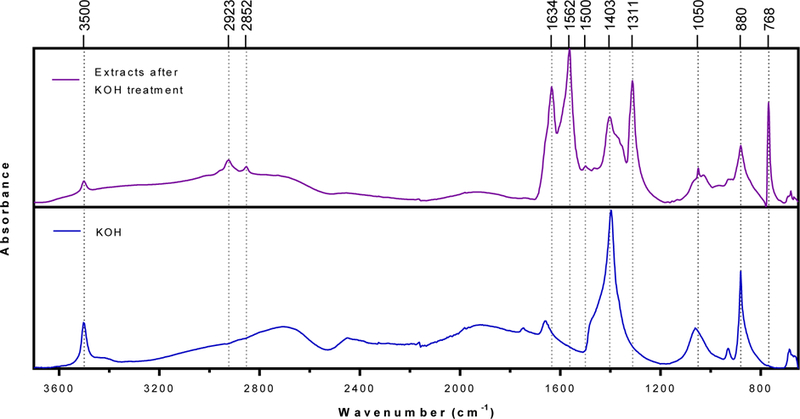
Ragweed pollens were treated with 6% w/v KOH solution for 6 h and extracts were separated from the solution via centrifugation, dried at 120 ºC, and analyzed using FTIR.
Modified schemes of conventional treatment.
To overcome the difficulty in recovering ragweed pollens after the KOH step, several modifications of the conventional treatment method were attempted. These modifications were specifically motivated to prevent the extraneous insoluble material from precipitating out. We postulated that if this material could be kept suspended in solution and not allowed to precipitate during processing, then it may be possible to complete the treatment and obtain clean ragweed shells.
i) Replacement of vacuum filtration by centrifugation.
Since the cake is a result of filtration, we hypothesized that if we could separate ragweed from the refluxed KOH solution without performing filtration, we might be able to prevent cake formation and thus enable recovery of ragweed for further processing. Therefore, after the first 6 h of KOH treatment, we separated pollens via centrifugation instead of filtration. After excess KOH supernatant was discarded, the ragweed pollen appeared to be suspended in the minimal KOH that was left behind in the tube. These ragweed were directly transferred to fresh KOH for another 6 h of treatment, following which, centrifugation was again used to wash the ragweed pollens with hot water and ethanol. After washing, to compare the effect of centrifugation and filtration, a part of ragweed pollens were separated from ethanol by vacuum filtration and the rest by centrifugation (Figure 4A). After filtration the cake was again formed (Figure 4B, C and D), while centrifugation yielded a relatively cleaner product, regardless, it still produced clumped ragweed, which could not be dissociated to obtain individual pollens (Figure 4E and F). Since we could not obtain individual ragweed, the subsequent orthophosphoric acid treatment was not performed.
Figure 4. Images of ragweed pollens processed using conventional treatment and modified conventional treatment protocols.
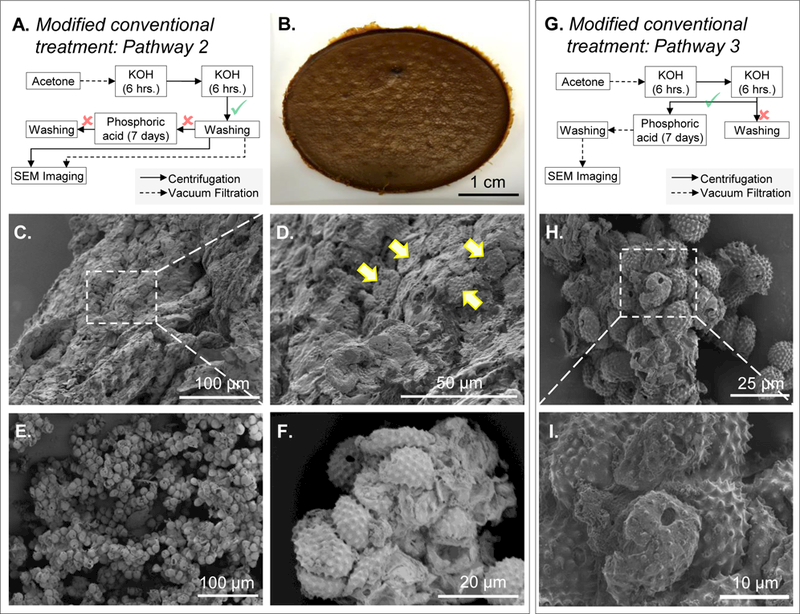
A. Schematic diagram of the processing steps for figures B to D (vacuum filtration) and E to F (centrifugation). B. Photograph of the cake formed after vacuum filtration. C. SEM image of the cake showing pollens entrapped in extraneous matter. D. Zoomed in SEM image of the cake showing entrapped pollens (indicated by arrows). E. Pollen clumps formed after centrifugation. F. Zoomed in SEM image of the clumps showing more details of clumped pollens. G. Schematic diagram of the processing steps for figures H and I. H. SEM image of pollens clumped together. I. Zoomed in SEM image of the clump showing more details of unclean pollen surfaces.
ii) Direct transfer to orthophosphoric acid without washing ragweed pollens after KOH treatment.
Next, we postulated that eliminating the wash steps after 12 h (6 h + 6 h) of KOH treatment might allow subsequent processing of ragweed pollens with orthophosphoric acid (Figure 4G). Therefore, after ragweed pollens had been centrifuged to remove excess KOH, we transferred the residual solids (with minimal KOH) directly into 85% orthophosphoric acid without washing them. During the early days (day 2 to 3) of acid reflux, we observed clumping of ragweed pollen. After 7 days of acid treatment, ragweed pollens were isolated from the acid by vacuum filtration, washed with different solvents as mentioned in the conventional treatment method section, and dried. The clumps that were formed in the early days of acid reflux remained fused. Although a better-quality product was obtained, the SEM images revealed that the pollen surfaces were still covered with extraneous material and multiple pollens were found sticking to each other. (Figure 4H and I)
Switched chemical treatment for ragweed.
Because we were unable to secure clean ragweed pollens from the extraneous matter by implementing conventional treatment protocol with modifications, we decided to refocus our processing strategy. Instead of attempting to separate ragweed from the extraneous matter, we decided to instead prevent its formation in the first place. Since we had previously seen that the material was being formed in the KOH treatment step, we decided to skip KOH treatment, and proceeded directly to treat pollens with orthophosphoric acid after acetone defatting. This rationalization led to the development of high-temperature switched treatment, a new method where the sequence of alkali and acid steps is switched (Figure 5A). We found that after orthophosphoric acid treatment ragweed pollens were intact, extraneous matter was not produced, and pollens were not clumped. We then proceeded to reflux the ragweed in KOH for 12 h with the solution renewed at 6 h. It is important to note that at each step, the pollens were separated from the solvents by vacuum filtration. Upon completion of high-temperature switched treatment protocol, ragweed pollen before (Figure 5B, C and D) and after treatment (Figure 5E, and F) were similar in morphology. Furthermore, their exterior surfaces and interior regions (Figure 5G) were clean, indicating successful removal of pollen cellular material.
Figure 5. SEM images of ragweed pollens processed using the switched treatment.
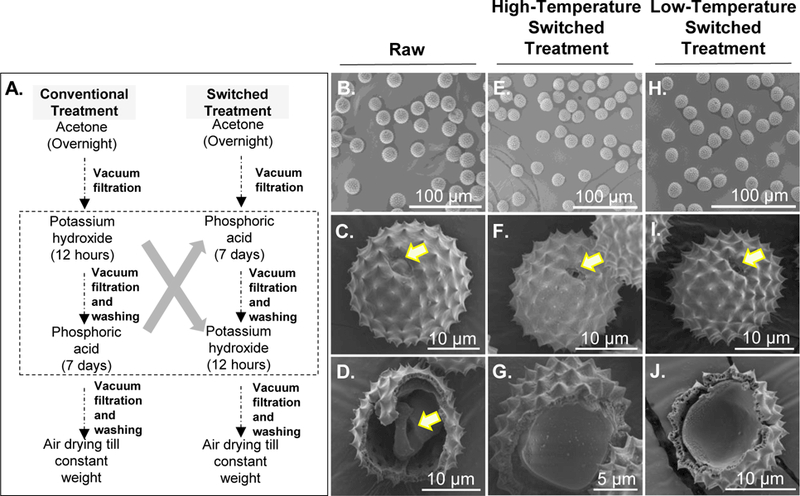
A. Comparison diagram of the conventional treatment and switched treatment steps. B. Raw ragweed pollens. C. Zoomed in image of a raw ragweed pollen. D. Interior of raw ragweed pollen showing the presence of natural biological material as indicated by the arrow. E. Ragweed pollen processed with high-temperature switched treatment. F. Zoomed in image of a ragweed pollen after high-temperature switched treatment showing an intact morphology. G. Inside of ragweed pollen after high-temperature switched treatment showing a clean interior. H. Ragweed pollen processed with low-temperature switched treatment. I. Zoomed in image of a ragweed pollen after low-temperature switched treatment showing an intact morphology. J. Inside of ragweed pollen after low-temperature switched treatment showing a clean interior. Arrow in figure C indicates a closed aperture in raw pollen, while arrows in F and I indicate open apertures after switched treatment.
To determine whether switched treatment at reduced temperatures would yield a similar product and thereby make the process simpler, we also processed ragweed pollens under low-temperature non-reflux orthophosphoric acid and KOH conditions (low-temperature switched treatment). It can be seen from Figure 5H, I and J that pollens obtained by this method also showed a clean surface and interior; and were also intact like the pollens obtained using high-temperature conditions of high-temperature switched treatment.
Comparison of effects of high- and low-temperature switched treatment protocols on ragweed pollen.
To determine any differences between ragweed pollens obtained from low- and high-temperature switched treatment protocols, we performed biochemical characterizations. Elemental analysis showed that after treatment with high- and low-tempe rature switched treatment protocols, protein content of raw ragweed (27.3 ± 0.51%) was reduced by similar amounts (p > 0.99, t-test) to 0.66 ± 0.38% and 0.66 ± 0.68%, respectively (Figure 6).
Figure 6. Protein content of ragweed shells obtained using the switched treatment protocols.
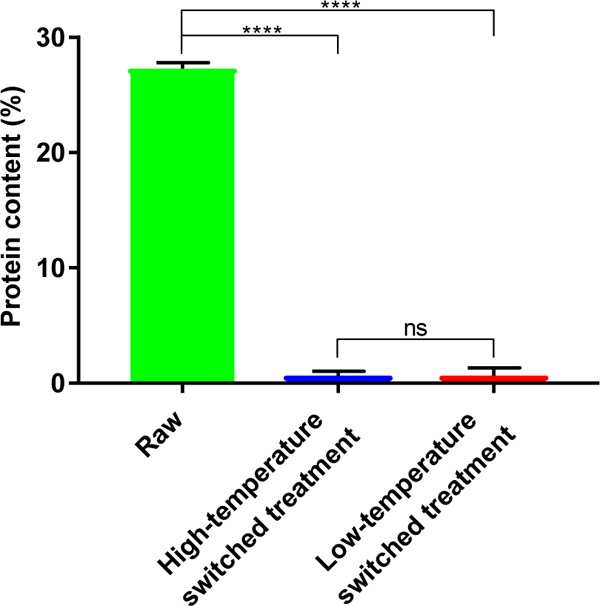
Ragweed pollens processed by high- and low-temperature switched treatment protocols showed a dramatic reduction in protein content over raw ragweed pollens, indicating success of the switched treatment process in removing native proteinaceous material. Values shown are means ± SD from three independent samples. A two-tailed t-test was used for comparison. (ns = not significant, [****] = p < 0.001)
FTIR spectra of ragweed pollens are shown in Figure 7. A decrease in intensity of the carbohydrate fingerprint region (1200–900 cm-1 due to C-O-C and C-OH stretching) was observed after high-temperature switched treatment as compared to natural ragweed pollen. However, ragweed pollen after low-temperature switched treatment showed a stronger carbohydrate vibration at 1070 cm-1. The relative abundance of cellulose (1033 cm-1)25 decreased for both high- and low-temperature switched treatments. The high-temperature switched treatment also caused less moisture to adsorb on the treated ragweed surface. This is evident from a lack of vibration at 1660 cm-1 (due to adsorbed water)26. Consistent with reduction of carbohydrate and moisture after treatment, the intensity of broad vibration at 3300 cm-1, which can be attributed to O-H stretching from adsorbed moisture or carbohydrates, also reduced.
Figure 7.
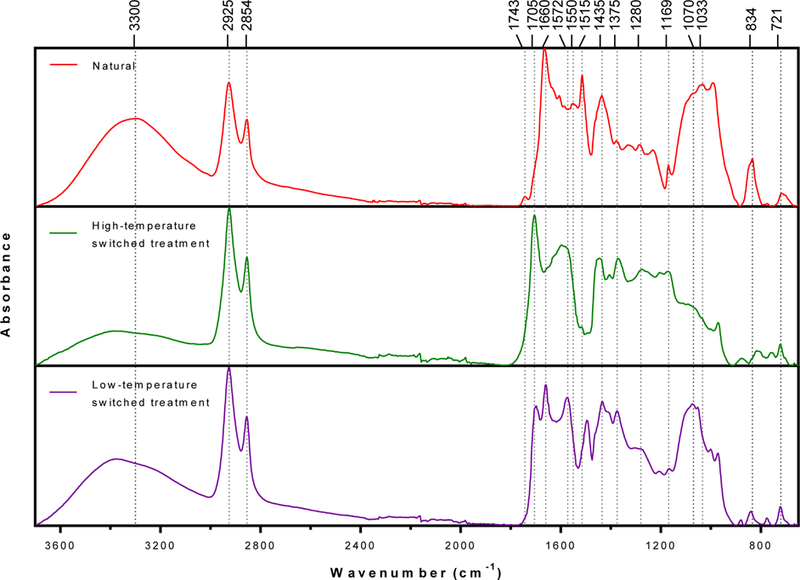
Fourier-transform infrared (FTIR) spectra of ragweed pollen treated using switched treatment protocols.
The sharp peaks at 2925 and 2854 cm-1 are associated with out-of-phase C–H stretching and in-phase C–H stretching in methylene group (CH2), respectively. The vibrations at 1375 and 721 cm-1 are due to CH3 bending and CH2 rocking, respectively. These peaks remained similar in both natural and treated ragweed pollens. Both high- and low-temperature switched treatment ragweed lacked characteristic vibrations at 1743 cm-1 (C=O stretching in lipids) and 1550 cm-1 (N–H bending and C–N stretching in amide II) indicating that treated ragweed pollens are lipid- and protein-free. Moreover, strong vibrations at 1705 cm-1 (C=O stretching in carboxylic acid) and 1572 cm-1 (asymmetric stretching of carboxylate (COO-) anions)27 appeared after chemical treatment.
The vibrations 1280 cm-1 (C–O stretching in carbohydrates or carboxylic acid) was present in natural and treated ragweed pollen spectra. Phenolic ring vibrations28 at 1515 (shifted to 1494 cm-1 in low-temperature switched treatment ragweed), 1435, and 1169 cm-1 were present in both natural and treated ragweed pollen. The intensity of the phenylpropanoid vibration at (834 cm-1)29 decreased after both treatments and shifted slightly towards the lower wavenumber after high-temperature switched treatment.
Overall, both high- and low-temperature switched treatment ragweed are lipid and protein free; elevated temperature treatment mainly affected the carbohydrates.
Ability of low-temperature switched treatment protocol to clean other pollens.
Since both low- and high-temperature switched treatments produced clean and intact pollen shells from ragweed pollen, we next evaluated, low-temperature switched treatment, the milder of the two protocols, for its ability to produce pollen shells from pollens of other species. We treated lamb’s quarters, sunflower and black alder pollens with the low-temperature switched treatment protocol. SEM analysis demonstrated that the low-temperature switched treatment protocol could produce clean and intact pollen shells from lamb’s quarters (Figure 8A, B, and C), sunflower (Figure 8D, E, and F), and black alder (Figure 8G, H and I). Furthermore, elemental analysis showed that the nitrogen content reduced from 4.40 ± 0.03 % to 0.33 ± 0.01 % for black alder pollens, from 4.56 ± 0.06 % to 0.19 ± 0.01 % for sunflower pollens, and from 3.35 ± 0.01 % to 0.22 ± 0.02 % for lamb’s quarters pollens.
Figure 8. SEM images of different species of pollens processed using the low-temperature switched treatment protocol.

A. Lamb’s quarters pollen. B. Zoomed in image of a lamb’s quarters pollen showing an intact morphology and clean exterior surface. C. Inside of lamb’s quarters pollen showing a clean interior. D. Sunflower pollen. E. Zoomed in image of a sunflower pollen showing an intact morphology and clean exterior surface. F. Inside of sunflower pollen showing a clean interior. G. Black alder pollen. H. Zoomed in image of a black alder pollen showing an intact morphology and clean exterior surface. I. Inside of black alder pollen showing a clean interior. Arrows in B, C, E, F, H, and I indicate open apertures after switched treatment.
Live imaging of pollens treated with different solvents.
We saw that apertures in the pollen shells get opened after successfully cleaning them with low-temperature switched treatment. Apertures are locations on pollen shells from which the pollen tube emerges during pollination and are as such structurally weaker than the rest of the pollen shell because they have thinner walls.30 To gain a better understanding of this phenomenon, we performed live imaging of pollens after addition of different solvents. Addition of orthophosphoric acid on lamb’s quarters pollens caused the apertures to burst open almost as soon as acid was added (Figure 9A: t = 15 s and 33 s), and the process was complete within under 120 s (Figure 9A: t = 97 s). This effect can be seen in real time in supplementary video 1. SEM image of lamb’s quarters after 5 min of acid treatment (Figure 9A) clearly shows the open aperture. Meanwhile, water, PBS (1X and 10X), and a sucrose solution (10 %w/w and 100 %w/w) did not cause this effect (Figure 9B). A similar phenomenon was observed with pollens of other species as shown in supplementary data (Figure S1 and S2).
Figure 9. Images of pollen apertures bursting open.
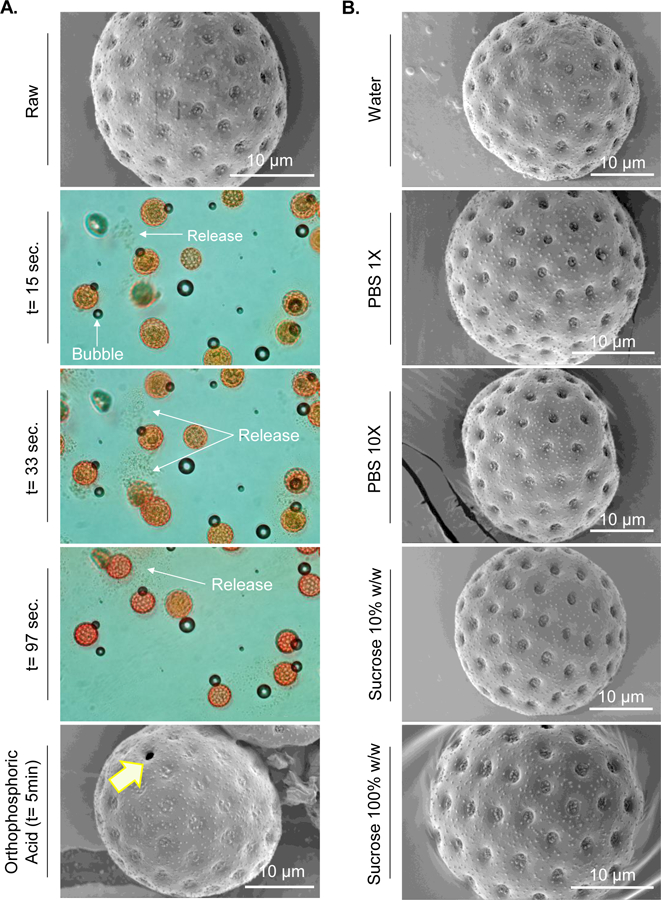
A. Lamb’s quarters pollen before and at different times after exposure to orthophosphoric acid. Arrows indicate either air bubbles, or release of matter from inside the pollen due to bursting open of apertures. Air bubbles are an artifact and are not related to bursting of apertures. B. SEM images of lamb’s quarters after exposure to other solvents that did not cause aperture to open. It should be noted that due to practical limitations of time needed to apply the cover slip and subsequent lens focusing there is a time gap of about 5 s between addition of acid to lamb’s quarters on the slide and start of video capture. The time label t = 15 s, t = 33 s, and t = 97 s represent time after video capture was started.
Discussion
In this study our objective was to develop a protocol that can be used to produce intact pollen shells of different species without clumping or breaking them, and with a low level of residual nitrogen content. Lack of such a method is currently limiting the field and preventing investigation of pollens of other species for drug delivery and other applications. The conventional treatment has previously been reported to successfully produce clean and intact spore shells from lycopodium.11 However, when we used the conventional treatment protocol on ragweed, recovery of individual ragweed pollen shells was unachievable because they were entrapped in a thick layer of extraneous matter after the KOH treatment step. When we carefully examined the extraneous matter we could see ragweed pollen in it. We therefore, postulated that KOH was not necessarily damaging the pollens, but because the extraneous matter being generated was insoluble, it was entrenching the pollens, and providing an illusion that ragweed pollens were being damaged. Indeed, when we replaced the filtration step with a centrifugation step it helped to remove some of the extraneous matter, and revealed more of the embedded ragweed pollens, which were undamaged. Mundargi et al., 21 have previously reported that sunflower pollens were getting damaged by the KOH treatment step. They based this conclusion on SEM images of the extraneous matter. However, based on our results with ragweed, we postulate that sunflower pollens were not getting damaged by KOH but were simply buried in the extraneous matter and hard to see.
We tried to extract ragweed pollens from this insoluble matter by use of different solvents such as ethanol, acetone, and water, however, our attempts were unsuccessful (data not shown). Next, instead of solubilizing the extraneous matter, we attempted to remove it by centrifugation rather than filtration. Even when majority of the extraneous matter was removed and the ragweed pollen along with a minute amount of residual KOH and matter were directly transferred in to orthophosphoric acid, clumping was still observed within 24 hours (data not shown). Despite this, we continued the acid reflux for 7 days with the expectation that prolonged acid treatment might break down the pollen clumps, however, unclumped ragweed pollens were still not obtained. Based on these findings, we hypothesized that the extraneous matter was arising due to excess native material being released from pollen grains into the surrounding solution as a result of the KOH treatment. FTIR analysis of the matter revealed that majority of this material consisted of proteins and peptides.
We then focused on a new strategy to develop clean pollens. Instead of trying to separate pollens from the extraneous matter, we attempted to prevent its formation in the first place. We had noticed that when the extraneous matter produced after KOH treatment was added to orthophosphoric acid, although it remained insoluble, additional matter was not produced. We thus hypothesized that performing the acid treatment first instead of KOH might allow us to completely eliminate the formation of the insoluble matter. Indeed, switching the orthophosphoric acid and KOH steps resulted in clean and unbroken pollens, and this gave rise to the high- and low-temperature switched treatment protocols. Elemental and FTIR analysis of the ragweed pollen from both high- and low-temperature switched treatment methods suggested that treated ragweed pollens were lipid- and protein-free. FTIR analysis further showed that after high-temperature switched treatment significant amount of carbohydrates are removed, while after low-temperature switched treatment significant amount of carbohydrate remained in the pollen wall.
To establish the validity of low-temperature switched treatment as a general chemical treatment to produce clean and intact pollens from all pollen species, we checked its ability to clean lamb’s quarters, sunflower, and black alder pollens. Lamb’s quarters, sunflower, and black alder pollens were successfully treated using the low-temperature switched treatment protocol and pollens with low nitrogen content were obtained.
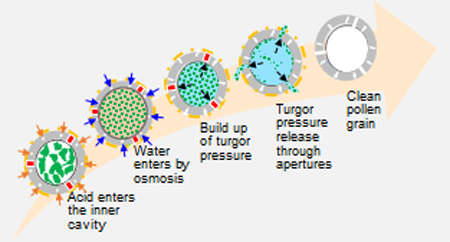
We found that pollen apertures were getting opened after switched treatment protocol. Live video imaging revealed that addition of orthophosphoric acid was causing the apertures to burst open. We propose the following hypothesis (Figure 10) to explain the mechanism. It is known that the acid treatment step is responsible for maximum removal of biological material from within pollens.20 When pollens are treated with orthophosphoric acid, it enters the pollens and causes rapid solubilization of a large amount of biological material in the pollen core. This creates a high solute (osmolyte) concentration inside the pollens relative to the outside. Consequently, due to this osmotic gradient, water from outside the pollens rapidly enters the pollen interior. This increases turgor pressure inside the pollens, and when it exceeds the rupture strength of the apertures, the apertures burst open.
Figure 10. Schematic showing mechanism of opening of aperture in pollen grains during orthophosphoric acid treatment.
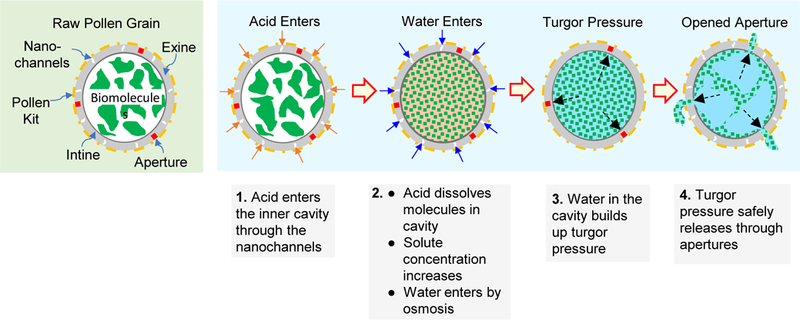
Orthophosphoric acid enters the pollen grains and dissolves the molecules contained in its interior. This causes buildup of an osmotic gradient and allows more water to enter the pollen cavity, which increases turgor pressure and bursts the pollen shell wall open at aperture sites, because they are mechanically weaker than the rest of the pollen wall.
Bursting open of pollens due to osmotic shock is a common phenomenon in nature and has been previously reported in literature.31–32 The effect of osmotic bursting on the apertures of pollens has also been reported previously which supports our hypothesis.33 At the apertures the pollen walls are naturally weak because at this location pollen tubes emerge from pollens during pollination.30 This phenomenon of emergence and growth of pollen tube is not fully understood but it is also thought to be aided by turgor pressure and other mechanisms.5, 34 We were able to observe this bursting of apertures in real time under bright field microscopy (supplementary videos). The aperture bursting occurred within tens of seconds after addition of orthophosphoric acid.
Lamb’s quarters pollens have many apertures. It was observed that only a few of these were getting opened after low-temperature switched treatment. This observation is consistent with our postulate that turgor pressure is the cause of aperture-opening and not chemical dissolution because once the turgor pressure reaches a threshold, few apertures burst open to release the turgor pressure. If chemical dissolution was the alternate mechanism, a larger number of apertures would be expected to get opened since the chemical dissolution process would more uniformly affect the apertures, especially considering that the acidolysis step is performed for 7 days.
To eliminate the case that simple water absorption by the pollens was not responsible for turgor pressure build up, we exposed raw lamb’s quarters pollens to different solutions including pure water, PBS (1X and 10X), and sucrose (100 % w/w and 10 % w/w in water). The lamb’s quarters pollen grains remained intact in all these solutions, however, apertures were seen bursting open in orthophosphoric acid even at room temperature, within few seconds. A similar phenomenon was also observed with ragweed, sunflower, and black alder pollens.
An important consequence of low-temperature switched treatment (and high-temperature switched treatment) protocol is that apertures measuring a couple of micrometers in dimensions get opened in pollens. These openings can provide entryways to fill the clean core of the pollen shells with different molecules for oral delivery, or with matrix material to achieve controlled release of these molecules.
Conclusion
Obtaining clean and intact pollen shells of species other than lycopodium has always been a challenge. Different methods have been attempted, but there is need for a robust and optimized process that can be used with a variety of pollen species to consistently produce clean and intact pollen shells with a low level of residual protein content. In this study, we first investigated the cause of failure of the conventional treatment method, which is although successful with lycopodium, but fails for ragweed and other pollens. Based on the results of this study, it was revealed that the alkali hydrolysis step results in entrapment of pollens in extraneous matter from which they are irrecoverable by solvent washing with ethanol, acetone, and water. Even several modifications to the conventional treatment method were unable to solve this problem. Guided by our observations, we developed a new method where the sequence of alkali and acid treatment steps was switched. This method was successful in producing clean and intact pollen shells of not only ragweed but several other species of pollens. This new process was able to produce clean and intact pollens even at lower processing temperatures. Based on these results we conclude that the switched protocol can be applied to pollen species that have apertures on their surface, which can facilitate the release of the turgor pressure generated in the orthophosphoric acid treatment step. This new pollen treatment method is an important finding since it provides a robust and well-optimized protocol for processing aperture-containing pollen species to obtain clean, intact, and protein-free shells for numerous applications.
Supporting Information
Figure S1. Images of apertures of different pollen grains bursting due to turgor pressure buildup after exposure to orthophosphoric acid. Figure S2. SEM images of pollen grains apertures after exposure to a variety of solvents. Videos showing bursting of apertures of different pollen grains (lamb’s quarter, black alder, sunflower and, ragweed) after exposure to orthophosphoric acid, and water as a control.
Supplementary Material
Acknowledgements
We thank Erandi Rajakaruna in the Department of Plant and Soil Science at Texas Tech University for her help with the FTIR. This research was supported by the National Institutes of Health (NIH) [grant number DP2HD075691] and the Defense Advanced Research Projects Agency (DARPA) [grant number N66001-12-1-4251]. HSG is a co-inventor on a patent related to the development of pollen grains for oral vaccines.
Footnotes
This potential conflict of interest has been disclosed and is managed by Texas Tech University.
References
- 1.Bedinger P, The remarkable biology of pollen. Plant Cell 1992, 4 (8), 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg M; Brownfield L; Twell D, Male gametophyte development: a molecular perspective. J Exp Bot 2009, 60 (5), 1465–1478. 10.1093/jxb/ern355. [DOI] [PubMed] [Google Scholar]
- 3.Mascarenhas JP, The Male Gametophyte of Flowering Plants. Plant Cell 1989, 1 (7), 657–664. 10.2307/3868955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diego-Taboada A; Beckett ST; Atkin SL; Mackenzie G, Hollow Pollen Shells to Enhance Drug Delivery. Pharmaceutics 2014, 6 (1), 80–96. 10.3390/pharmaceutics6010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlund AF; Swanson R; Preuss D, Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 2004, 16 (Suppl), S84–S97. 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roulston TH; Cane JH, Pollen nutritional content and digestibility for animals. Pl. Syst. Evol 2000, 222 (1), 187–209. 10.1007/BF00984102. [DOI] [Google Scholar]
- 7.Shivanna KR, Pollen biology and biotechnology Science Publishers, Inc.: 2003. [Google Scholar]
- 8.Ariizumi T; Toriyama K, Genetic Regulation of Sporopollenin Synthesis and Pollen Exine Development. Annu. Rev. Plant Biol 2011, 62 (1), 437–460. 10.1146/annurev-arplant-042809-112312. [DOI] [PubMed] [Google Scholar]
- 9.Bohne G; Richter E; Woehlecke H; Ehwald R, Diffusion Barriers of Tripartite Sporopollenin Microcapsules Prepared from Pine Pollen. Ann. Bot 2003, 92 (2), 289–297. 10.1093/aob/mcg136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH; Seo J; Jackman JA; Cho N-J, Inflated Sporopollenin Exine Capsules Obtained from Thin-Walled Pollen. Sci. Rep 2016, 6, 28017 10.1038/srep28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atwe SU; Ma Y; Gill HS, Pollen grains for oral vaccination. J. Control. Release 2014, 194, 45–52. 10.1016/j.jconrel.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uddin MJ; Gill HS, Ragweed pollen as an oral vaccine delivery system: Mechanistic insights. J. Control. Release 2017, 268, 416–426. 10.1016/j.jconrel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin MJ; Gill HS, From allergen to oral vaccine carrier: A new face of ragweed pollen. Int. J. Pharm 2018, 545 (1), 286–294. 10.1016/j.ijpharm.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorch M; Thomasson MJ; Diego-Taboada A; Barrier S; Atkin SL; Mackenzie G; Archibald SJ, MRI contrast agent delivery using spore capsules: controlled release in blood plasma. Chem. Commun 2009, (42), 6442–6444. 10.1039/B909551A. [DOI] [PubMed] [Google Scholar]
- 15.Barrier S; Rigby AS; Diego-Taboada A; Thomasson MJ; Mackenzie G; Atkin SL, Sporopollenin exines: A novel natural taste masking material. LWT - Food Science and Technology 2010, 43 (1), 73–76. 10.1016/j.lwt.2009.07.001. [DOI] [Google Scholar]
- 16.Mundargi RC; Tan E-L; Seo J; Cho N-J, Encapsulation and controlled release formulations of 5-fluorouracil from natural Lycopodium clavatum spores. J Ind Eng Chem 2016, 36, 102–108. 10.1016/j.jiec.2016.01.022. [DOI] [Google Scholar]
- 17.Diego-Taboada A; Maillet L; Banoub JH; Lorch M; Rigby AS; Boa AN; Atkin SL; Mackenzie G, Protein free microcapsules obtained from plant spores as a model for drug delivery: ibuprofen encapsulation, release and taste masking. J. Mater. Chem B 2013, 1 (5), 707–713. 10.1039/C2TB00228K. [DOI] [PubMed] [Google Scholar]
- 18.Barrier S; Rigby AS; Diego-Taboada A; Thomasson MJ; Mackenzie G; Atkin SL, Sporopollenin exines: A novel natural taste masking material. Food sci technol 2010, 43 (1), 73–76. 10.1016/j.lwt.2009.07.001. [DOI] [Google Scholar]
- 19.Mackenzie G, et al. , Chapter 24 - Pollen and Spore Shells—Nature’s Microcapsules, in Microencapsulation in the Food Industry Academic Press: San Diego: 2014; p 283–297. [Google Scholar]
- 20.Mundargi RC; Potroz MG; Park JH; Seo J; Tan E-L; Lee JH; Cho N-J, Eco-friendly streamlined process for sporopollenin exine capsule extraction. Sci. Rep 2016, 6, 19960 DOI: 10.1038/srep1996010.1038/srep19960https://www.nature.com/articles/srep19960#supplementary-informationhttps://www.nature.com/articles/srep19960#supplementary-information . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundargi RC; Potroz MG; Park JH; Seo J; Lee JH; Cho N-J, Extraction of sporopollenin exine capsules from sunflower pollen grains. RSC Adv 2016, 6 (20), 16533–16539. 10.1039/C5RA27207F. [DOI] [Google Scholar]
- 22.Fadiran O Characterization And Use Of Pollen As A Biorenewable Filler For Polymer Composites. Dissertation, Georgia Institute of Technology, Atlanta, GA, 2015. [Google Scholar]
- 23.Fadiran OO; Meredith JC, Surface treated pollen performance as a renewable reinforcing filler for poly(vinyl acetate). Journal of Materials Chemistry A 2014, 2 (40), 17031–17040. 10.1039/C4TA03219E. [DOI] [Google Scholar]
- 24.Kong J; Yu S, Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin 2007, 39 (8), 549–559. [DOI] [PubMed] [Google Scholar]
- 25.Stuart BH, Infrared spectroscopy: fundamentals and applications. Ando DJ, Ed. John Wiley and Sons: New York, 2005; pp 137–165. [Google Scholar]
- 26.Dreischmeier K; Budke C; Wiehemeier L; Kottke T; Koop T, Boreal pollen contain ice-nucleating as well as ice-binding ‘antifreeze’ polysaccharides. Sci. Rep 2017, 7, 41890 DOI: 10.1038/srep4189010.1038/srep41890https://www.nature.com/articles/srep41890#supplementary-informationhttps://www.nature.com/articles/srep41890#supplementary-information . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Max J-J; Chapados C, Infrared spectroscopy of aqueous carboxylic acids: comparison between different acids and their salts. J. Phys. Chem. A 2004, 108 (16), 3324–3337. [Google Scholar]
- 28.Jardine PE; Abernethy FAJ; Lomax BH; Gosling WD; Fraser WT, Shedding light on sporopollenin chemistry, with reference to UV reconstructions. Rev. Palaeobot. Palynol 2017, 238, 1–6. [Google Scholar]
- 29.Zimmermann B; Bağcıoğlu M; Sandt C; Kohler A, Vibrational microspectroscopy enables chemical characterization of single pollen grains as well as comparative analysis of plant species based on pollen ultrastructure. Planta 2015, 242 (5), 1237–1250. [DOI] [PubMed] [Google Scholar]
- 30.Walker JW, Aperture evolution in the pollen of primitive angiosperms. American Journal of Botany 1974, 61 (10), 1112–1137. 10.2307/2441929. [DOI] [Google Scholar]
- 31.Human H; Nicolson SW, Digestion of maize and sunflower pollen by the spotted maize beetle Astylus atromaculatus (Melyridae): is there a role for osmotic shock? J. Insect Physiol 2003, 49 (7), 633–643. 10.1016/S0022-1910(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 32.Bohne G; Woehlecke H; Ehwald R, Water Relations of the Pine Exine. Ann. Bot 2005, 96 (2), 201–208. 10.1093/aob/mci169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matamoro-Vidal A; Raquin C; Brisset F; Colas H; Izac B; Albert B; Gouyon P-H, Links between morphology and function of the pollen wall: an experimental approach. Bot. J. Linn. Soc 2016, 180 (4), 478–490. 10.1111/boj.12378. [DOI] [Google Scholar]
- 34.Hill AE; Shachar-Hill B; Skepper JN; Powell J; Shachar-Hill Y, An Osmotic Model of the Growing Pollen Tube. PLOS ONE 2012, 7 (5), e36585 10.1371/journal.pone.0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


