Abstract
Context
Despite that the Triglycerides/High Density Lipoprotein Cholesterol (TG/HDL-C) ratio has been associated with insulin resistance and cardiovascular disease, some outcomes differ between populations.
Objective
The objective of this study was to evaluate the association between TG/HDL-C ratio and cardio-metabolic risk factors in both obese and normal weight women.
Design
Cross sectional, from January to December of 2015.
Subjects and Methods
Two hundred and fifty three women aged 40 to 60 years. Anthropometric and laboratory measurements were performed. Insulin resistance was measured by the homeostasis model assessment for insulin resistance (HOMA-IR). All participants underwent a Doppler ultrasound to measure intima-media thickness of carotid artery (cIMT).
Results
TG/HDL-C ratio correlated with body mass index (r=0.194, p=0.01), and visceral adipose tissue (r=0.193, p=0.002). Additionally, TG/HDL-C correlated with glucose (r=0.367, p=0.001), insulin (r=0.354, p=0.001) and HOMA-IR (r=0.396 p=0.001). TG/HDL-C was associated with prediabetes, Odds Ratio (OR) was 1.83 (95%CI 1.07-3.13) and insulin resistance 3.27 (95%CI 1.78-6.01), and this risk remains in normal weight women 4.7 (95%CI 1.2-17.81) for prediabetes and 4.38 (95%CI 1.42-13.84) for insulin resistance. No significant risk for cIMT.
Conclusion
A TG/HDL-C ratio ≥ 3.0 is a potential risk factor for prediabetes and insulin resistance in women 40-60 years, even in normal weight women.
Keywords: TG/HDL-C, Insulin Resistance, Carotid Intima-Media Thickness, Prediabetes
INTRODUCTION
It is well known that obesity correlates with the risk of developing diabetes or cardiovascular disease (1). The prevalence of overweight and obesity in Mexican women is 73% (2) and is estimated that every 15 minutes a woman dies secondary to a cardiovascular disease in the country (3). Despite this association, there are obese patients without metabolic abnormalities who are described as “metabolically healthy obese patients” (4). Also, there are normal weight subjects with metabolic abnormalities, “metabolically unhealthy normal weight” (5). Therefore, finding risk factors to target in order to prevent or reduce the risk of mortality has become necessary even in normal weight women. However, there are no universal criteria for distinguishing metabolically healthy from metabolically unhealthy (6).
Some identified factors associated with cardio-metabolic risk are hyperinsulinemia, insulin resistance (IR)(7) and carotid intima-media thickness (cIMT)(8). However, the accurate measurement of insulin or IR parameters is complicated in the clinical practice. Recently it has been observed that the Triglycerides/High Density Lipoprotein Cholesterol (TG/HDL-C) ratio can identify IR, cardio-metabolic risk and cardiovascular disease (9, 10). Nevertheless, there are discrepancies in the discriminatory power and the cutoff points among populations (11-13). As such, it is expected that a commonly available and standardized measurement of TG/HDL-C ratio could help clinicians to identify patients who are not only IR but also display the characteristic of dyslipidemia (14). In some studies, TG/HDL-C ratio has demonstrated a similar or better sensibility than the metabolic syndrome criteria(13, 15), but with variations in the cutoff points within the population. Interestingly, some prospective studies have been developed in order to identify if TG/HDL-C ratio could predict a major cardiovascular outcome, or is just a useful parameter in hypertensive subjects (10). To our knowledge, only a few studies have compared the TG/HDL-C ratio with IR, or independent markers of cardiovascular disease such as carotid intima-media thickness (cIMT) and prediabetes, even in normal weight women.
The present study was performed to address this issue by evaluating the efficacy of TG/HDL-C ratio as a simple way to identify cardiometabolic risks in both obese and normal weight women.
SUBJECTS AND METHODS
A cross-sectional comparative study was performed in 253 women aged 40 to 60 years who consecutively attended the Endocrine Medical Research Unit, of the National Medical Center, Mexican Social Security Institute (Unidad de Investigación Médica en Enfermedades Endocrinas, Centro Medico Nacional IMSS). The exclusion criteria included women with an established diagnosis of diabetes mellitus, renal or liver failure, chronic infections, endocrine or blood disorders, history of cardiovascular disease or thrombosis. Participants who were found to be under hormone treatment were also excluded. The protocol was approved by the Scientific Committee of the Instituto Mexicano del Seguro Social. Participants were informed about the study and provided their written informed consent.
Clinical assessment
The medical history of the participants was taken and their anthropometric parameters were also recorded. Heights and weights were measured without shoes and with light clothing using a Bame weight and height scale. With the participant standing, the waist circumference was measured at the midpoint of the distance between the iliac crest and the inferior border of the last rib. This measurement was performed at the end of expiration. Systolic and diastolic blood pressures were recorded using an aneroid sphygmomanometer. Body Mass Index (BMI) in kg/m2 was calculated. For analysis, subjects were separated into two categories by BMI: BMI < 25 for normal weight women and ≥25 for overweight and/or obese.
We defined prediabetes as Impaired Fasting Glucose (IFG) 100-125mg/dL and/or HbA1c (5.7-6.4%) in accordance with ADA criteria(16).
Body composition analysis
Body composition was assessed with a 353 ioiJAWON Body Composition analyzer. Bioelectrical impedance was analyzed in the morning after a 12-hour fast and adequate hydration. Bioelectrical impedance was measured with the patient upright, wearing light clothing, and without shoes. The analyzer measured weight within an accuracy of 0.1kg, as well as body impedance (in ohms), with calculation of the Visceral Adipose Tissue (VAT) and the percentage of Total Body Fat.
Carotid intima-media thickness assessment
The cIMT was measured with the patient in supine position with neck slightly in extension, both common carotid arteries were assessed using a high resolution B-Mode ultrasonography device (Aloka-α7 lineal multifrequency transductor). The average IMT of the posterior wall of the right and left common carotid arteries and the presence of atheromatous plaque were evaluated according to the Mannheim Consensus (17). All measurements were taken by the same operator. At-risk group was defined for those women with cIMT >0.70 mm and/or the presence of atheromatous plaque (18).
Laboratory procedures
Venous blood samples were collected after an overnight fast of 10 hours between 7.30 am and 8 am. After centrifuging at 3500 rpm for 15 minutes to separate the serum, 500 μL-aliquots were prepared and stored at −70°C until assayed. Serum glucose, cholesterol, triglycerides and HDL-C were determined using enzymatic methods in the semiautomatic chemical Ekem Kontrol Lab Analyzer.
Insulin was measured by a solid-phase radioimmunoassay (Millipore, Billerica; Mississippi, USA); the sensitivity of this assay was 2 μU/ mL and the intra- and inter-assay coefficient of variation was 4.0% and 8.6%, respectively. IR was evaluated through homeostasis model assessment (HOMA) according to the method of Matthews et al. (19): HOMA-IR = insulin (mU/L) X fasting glucose (mmol/L) / 22.5. Using a cutoff of 3.0, the subjects were classified as IR if HOMA-IR ≥3.0 and non-IR if <3.0(20).
To identify the optimal cut-off for TG/HDL-C ratio, Receiving Operating Characteristics Curve (ROC) was performed to examine the discriminatory power of TG/HDL-C ratio for IR with an Area Under the Curve (AUC) of 0.716 (95%IC 0.649-0.783), using a 3.0 cut-point equal with the one used in a previous Mexican study (20). The sensitivity and specificity were 66 and 69% respectively for predicting IR. In this way we made two groups: low risk group <3.0 and high risk group ≥3.0.
Statistical analysis
Prediabetes, IR, and cIMT were considered as nominal variables. All the other variables were considered as quantitative.
In order to identify the distribution of the variables, skewness, kurtosis and Kolmogorov-Smirnov were conducted. These parametric variables are represented as mean and standard deviation (M+SD), while non-parametric variables are shown as median and interquartile ranges 25-75.
To determine differences between groups according to TG/HDL-C ratio, t-Student test for independent variables and Mann-Whitney U test were performed for parametric or non-parametric variables respectively. For nominal variables, Chi-Square test was performed. Therefore, to define correlations, we used the Spearman correlation coefficient and for associations, Odds Ratio (OR) and confidence interval (95%CI). A p value < 0.05 was considered statistically significant. Analysis was performed using the software program SPSS 21.
RESULTS
A total of 253 women were evaluated with a mean age of 52.3 ± 5.85 years. 76.3% were overweight or obese (mean BMI 28.84 Kg/m2 ± 5.08). The median of IR was 3.57 assessed by HOMA, 62.7% had IR with a cut-off of 3.0 and 54.3% had high risk of cardiovascular disease assessed by cIMT. The median of TG/HDL-C ratio was 2.70 with an interquartile range (1.82 - 4.13). The other characteristics of the participants are as shown in Table 1.
Table 1.
Characteristics of study participants
| Variable | n | |
| Age (years) | 253 | 52.3 ± 5.8 |
| Weight (Kg) | 253 | 68.8 ± 12.5 |
| Body Mass Index (Kg/m2) | 253 | 28.8 ± 5 |
| Normal | 60 | 23.7 % |
| Overweight | 99 | 39.1 % |
| Obese | 94 | 37.2 % |
| Waist (cm) | 253 | 105.5 ± 10.4 |
| Visceral Adipose Tissue (cm2) | 253 | 138.4 ± 57.8 |
| Total Fat (%) | 253 | 37.7 ± 4.9 |
| Systolic Blood Pressure (mmHg) | 253 | 111 ± 14 |
| Diastolic Blood Pressure (mmHg) | 253 | 74 ± 9 |
| Glucose (mg/dL) | 253 | 86.7 ± 19.6 |
| Normal | 221 | 87.4% |
| Impaired Fasting Glucose | 32 | 12.6% |
| HbA1C (%) | 247 | 5.54 ± 0.5 |
| Normal | 158 | 64% |
| Prediabetes | 89 | 36% |
| Insulin (mU/L)* | 253 | 17.0 (13.0 - 24.8) |
| HOMA-IR * | 253 | 3.57 (2.60 - 5.32) |
| Normal | 94 | 37.2% |
| Insulin Resistance | 159 | 62.8% |
| Triglycerides (mg/dL)* | 253 | 140.0 (110.0 - 193.0) |
| Total-Cholesterol (mg/dL) | 253 | 233.70 ± 47.9 |
| HDL-C (mg/dL) | 253 | 55.0 ± 15.0 |
| LDL-C (mg/dL) | 253 | 145.6 ± 42.9 |
| cIMT (mm) | 188 | 0.69 ± 0 .11 |
| Normal Risk | 86 | 45.7 % |
| High Risk | 102 | 54.3 % |
| TG/HDL-C ratio* | 253 | 2.7 (1.8 - 4.1) |
| Low Risk | 159 | 62.8% |
| High Risk | 94 | 37.2% |
Parametric variables are represented as mean + standard deviation, HOMA-IR, homeostasis model assessment of insulin resistance, TG/HDL-C ratio Low Risk: <3.0, High Risk: >3.0
Non parametric variables are represented as median and interquartile ranges.
There was no significant difference between groups by age, systolic and diastolic blood pressure, HDL-C, or LDL-C. In comparison to women with TG/HDL-C ratio <3.0, the group with TG/HDL-C ratio >3.0 had an increased BMI, IR, VAT (Table 2).
Table 2.
Characteristics of participants according to TG/HDL-C ratio > 3.0 cut-off point
| TG/HDL-C ratio | p-Value | |||
| <3.0 (62.8%) | ≥3.0 (37.2%) | |||
| Age (years) | 52.4 ± 5.9 | 52.2 ± 5.6 | NS | |
| Weight (kg) | 67.6 ± 12 | 70.9 ± 13 | 0.045 | |
| Body Mass Index (Kg/m2) | 28.2 ± 4.9 | 29.9 ± 5.1 | ||
| Normal | 26.4% | 19.1% | 0.009 | |
| Overweight | 43.4% | 32.0% | 0.012 | |
| Obesity | 30.2% | 48.9% | ||
| Waist (cm) | 90.4 ± 11.5 | 95 ± 12.1 | 0.003 | |
| Visceral Adipose Tissue (cm2) | 132.7 ± 59.2 | 148.1 ± 54.2 | 0.041 | |
| Total Fat (%) | 37.5 ± 4.8 | 38 ± 5.1 | NS | |
| Systolic Blood Pressure (mmHg) | 111.1 ± 13.4 | 112.8 ± 15.1 | NS | |
| Diastolic Blood Pressure (mmHg) | 74.8 ± 9.2 | 75.2 ± 9.3 | NS | |
| Glucose (mg/dL) | 83.3 ± 18.6 | 92.6 ± 20 | ||
| Normal Glucose | 93.7% | 76.6% | <0.001 | |
| Impaired Fasting Glucose | 6.3% | 23.4.% | <0.001 | |
| HbA1c (%) | 5.4 ± 0.4 | 5.6 ± 0.3 | ||
| <5.7 | 67.1% | 58.7% | 0.044 | |
| (5.7-6.4) | 32.9% | 41.3% | 0.184 | |
| Insulin (mU/L)* | 15.9 (12.1 - 22.5) | 19.9 (15.4 - 29.6) | <0.001 | |
| HOMA-IR* | 3.2 (2.3 - 4.7) | 4.4 (3.1 - 6.8) | ||
| Normal | 46.5% | 20.9% | <0.001 | |
| Insulin Resistance | 53.5% | 79.1% | <0.001 | |
| Triglycerides (mg/dL)* | 118 (94 - 142) | 209 (177 - 257.5) | <0.001 | |
| Total Cholesterol (mg/dL) | 233.3 ± 47.2 | 234.3 ± 49.1 | NS | |
| HDL–C (mg/dL) | 61.3 ± 13.2 | 44.4 ± 11.5 | <0.001 | |
| LDL–C (mg/dL) | 147 ± 43.6 | 143.3 ± 41.6 | NS | |
| cIMT (mm) | 0.69 ± 0.10 | 0.7 ± 0.11 | ||
| Normal Risk | 51.9% | 36.8% | NS | |
| High Risk | 48.1% | 63.2% | 0.81 | |
HOMA-IR, homeostasis model assessment of insulin resistance; cIMT, Carotid intima-media thickness. Parametric variables are represented as mean and standard deviation.
Non-parametric variables are represented as median and interquartile range.
A positive correlation was found of TG/HDL-C ratio with BMI (r= 0.194, p= 0.01), waist circumference (r=0.203, p=0.001), VAT (r= 0.193, p=0.002), Total Fat (r= 0.183, p=0.004), also with glucose, HbA1c, insulin and HOMA-IR, but not with cIMT (Table 3).
Table 3.
Correlations between TG/HDL-C ratio and metabolic variables
| Variable | r | p-Value |
| Glucose (mg/dL) | 0.367 | <0.001 |
| HbA1c (%) | 0.167 | 0.009 |
| Insulin (mU/dL) | 0.354 | <0.001 |
| HOMA-IR | 0.396 | <0.001 |
| Total Fat (%) | 0.183 | 0.004 |
| Visceral Adipose Tissue (cm2) | 0.193 | 0.002 |
| Total Cholesterol (mg/dL) | 0.38 | NS |
| LDL-C (mg/dL) | -0.010 | NS |
| cIMT (mm) | 0.002 | NS |
HOMA-IR: Homeostasis model assessment of insulin resistance, cIMT: Carotid intima-media thickness.
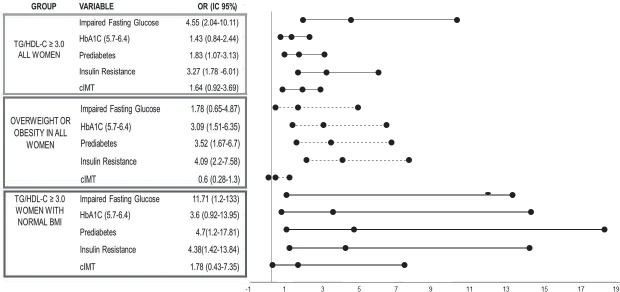
Women with a TG/HDL-C ratio of ≥3.0 had a statistically significantly increased risk of prediabetes (OR 1.83, 95%CI 1.07-3.13) and IR (OR 3.27, 95%CI 1.78-6.01). However, no statistical significance was found for cIMT. Meanwhile, overweight and/or obese women had a higher OR of prediabetes 3.52 (95%CI 1-67-6.7) and IR 4.09 (95%CI 2.27-7.58), however, cIMT remained with no statistical significance. Moreover, a subgroup of women with normal weight and TG/HDL-C ratio ≥3.0 has 4.7 (95%CI 1.2-17.81) risk of prediabetes and 4.38 (95%CI 1.48-13.84) risk of IR. Nonetheless, there was no significance with cIMT (Fig. 1).
Figure 1.
Forest Plot using association by Odds Ratio (OR) of cardiometabolic risk factors among three groups 1) All women with TG/HDL ratio ≥ 3.0, 2) Women with overweight and obesity and 3) Women with normal BMI and TG/HDL-C ratio ≥ 3.0.
cIMT: Carotid intima-media thickness.
DISCUSSION
This study demonstrated that TG/HDL-C ratio is a predictor of insulin resistance and prediabetes in women aged 40-60 years, even in normal weight women. Previous studies have shown that TG/HDL-C ratio is able to identify IR and increased cardiometabolic risk, including the development of type 2 diabetes mellitus in populations of different ages (21, 22). However, studies in women aged 40 to 60 years are limited.
In a study conducted by Salazar et al., which included both women and men aged 17 to 80 years, TG/HDL-C ratio had the ability to identify IR and cardiometabolic risk comparable with that achieved using the harmonized version of the metabolic syndrome(15),(23). Another study by González-Chavez et al. conducted in a 177 apparently healthy Mexican subjects, confirmed that TG/HDL-C ratio >3.0 was associated with presence of IR (OR of 2.64, 95%CI 1.12-6.29)(20). In a younger Mexican population it was found that TG/HDL-C ratio identified a greater number of IR subjects than the metabolic syndrome criteria, suggesting that TG/HDL-C ratio >2.5 for women is clinically useful in detecting IR in apparently healthy young individuals (24). In the present study, the cut-off point was identified by ROC curve. This cut-off was similar to that reported by Gonzalez-Chavez et al. (20), in the same way as the BMI. By contrast, in the study by Murguía et al. (24), the population was younger and had a lower BMI.
Different from other studies that include men and women (10, 15, 20, 22, 24), the present study focused on women, not only because there are important differences in the distribution of risk factors between the genders, but also because women at this age increases their cardiometabolic risk.
TG/HDL-C ratio has been proposed as atherogenic marker (25, 26). This marker correlates with LDL phenotype and small HDL particles (27, 28). TG/HDL-C ratio strongly predicts risk of myocardial infarction (26, 29). Moreover, among women with suspected ischemia, the TG/HDL-C ratio is a powerful independent predictor of all cause of mortality and cardiovascular events (26). Recently, in other large population survey, with participants of 20 to 90 years old, TG/HLD-C ratio was associated with cardiovascular disease and all cause of mortality, included coronary heart disease (30).
Carotid IMT is considered a surrogate marker for atherosclerotic cardiovascular disease (31). In the present study carotid IMT was measured for identifying women at future risk for cardiovascular disease, nonetheless, TG/HDL-C ratio was not associated with IMT. By contrast, in a clinical trial conducted by Maki et al. (32), TG/HDL-C ratio was predictor of IMT progression, however, this study enrolled patients at moderate cardiovascular risk, higher carotid IMT and did not report gender-specific risk ratios. Further studies are required to clarify the relationships between cIMT and TG/HDL ratio.
One limitation of this study is the lack of glucose tolerance curve to identify prediabetes. Nonetheless, in the present study prediabetes criteria were defined using but the impaired fasting glucose and HbA1c levels (16), which may have a close correlation to identify prediabetes (33). The main strength of this study is that it was conducted in women among 40 to 60 years old. Study of women is important because during this stage, some risk factors, such as insulin resistance and abdominal fat increase, which, together with other changes, such as those occurring in lipid profile, increase cardio-vascular risk (34). Although in this study the participants were not grouped as premenopausal and postmenopausal women. In a previous study it was found that TG/HDL-C ratio was different between premenopausal and postmenopausal women (35). The authors suggested that the difference could be attributed to age and abdominal visceral fat modification (35). These variables were analyzed in the present study. However, it is necessary to design studies to evaluate whether the menopausal state affects the efficacy of TG/HDL-C ratio to identify cardio-metabolic risk.
In conclusion, TG/HDL-C ratio was associated with cardio-metabolic risk factors, even in normal weight women who are insulin resistant and who go on to develop diabetes. This is important, since this marker has the ability to identify equally lean and obese women before development of manifest disease.
Conflict of interest
Authors declare no conflict of interest.
Funding
This work was supported in part by a scientific grant from Consejo Nacional de Ciencia y Tecnología (2013-01-201874).
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Rojas-Martínez R1, Aguilar-Salinas CA, Jiménez-Corona A, Gómez-Pérez FJ, Barquera S, Lazcano-Ponce E. Prevalence of obesity and metabolic syndrome components in Mexican adults without type 2 diabetes or hypertension. Salud Publica Mex. 2012;54(1):7–12. [PubMed] [Google Scholar]
- 3.INEGI Causas de Mortalidad, Defunciones generales de mujeres por principales causas de mortalidad, 2013 2015 [cited 2015 10/September/2015]. Available from: http://www3.inegi.org.mx/sistemas/sisept/Default.aspx?t=mdemo125&s=est&c=23589. [Google Scholar]
- 4.Sims EA. Are there persons who are obese but metabolically healthy? 2001;50(12):1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 5.Shaharyar S, Roberson LL, Jamal O, Younus A, Blaha MJ, Ali SS, Zide K, Agatston AA, Blumenthal RS, Conceição RD, Santos RD, Nasir K. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy Brazilian population. J Obes. 2015;2015:178526. doi: 10.1155/2015/178526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bala C, Craciun AE, Hancu N. Updating the Concept of Metabolically Healthy Obesity. Acta Endocrinologica-Bucharest. 2016;12(2):197–205. doi: 10.4183/aeb.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zavaroni I, Bonini L, Gasparini P, Barilli AL, Zuccarelli A, Dall’Aglio E, Delsignore R, Reaven GM. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: the Barilla factory revisited. Metabolism. 1999;48(8):989–994. doi: 10.1016/s0026-0495(99)90195-6. [DOI] [PubMed] [Google Scholar]
- 8.Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid artery intima-media thickness: the carotid atherosclerosis progression study. Stroke. 2004;35(9):2150–2154. doi: 10.1161/01.STR.0000136720.21095.f3. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin T, Reaven G, Abbasi F, Lamendola C, Saad M, Waters D, Simon J, Krauss RM. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? The American J Cardiol. 2005;96(3):399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 10.Salazar MR, Carbajal HA, Espeche WG, Aizpurua M, Maciel PM, Reaven GM. Identification of cardiometabolic risk: visceral adiposity index versus triglyceride/HDL cholesterol ratio. Am J Med. 2014;127(2):152–157. doi: 10.1016/j.amjmed.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Arthur FK, Adu-Frimpong M, Osei-Yeboah J, Mensah FO, Owusu L. Prediction of metabolic syndrome among postmenopausal Ghanaian women using obesity and atherogenic markers. Lipids Health Dis. 2012;11:101. doi: 10.1186/1476-511X-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arthur FK, Adu-Frimpong M, Osei-Yeboah J, Mensah FO, Owusu L. The prevalence of metabolic syndrome and its predominant components among pre-and postmenopausal Ghanaian women. BMC Res Notes. 2013;6:446. doi: 10.1186/1756-0500-6-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soska V, Jarkovsky J, Ravcukova B, Tichy L, Fajkusova L, Freiberger T. The logarithm of the triglyceride/HDL-cholesterol ratio is related to the history of cardiovascular disease in patients with familial hypercholesterolemia. Clin Biochem. 2012;45(1-2):96–100. doi: 10.1016/j.clinbiochem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol. 2008;7:4. doi: 10.1186/1475-2840-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar MR, Carbajal HA, Espeche WG, Leiva Sisnieguez CE, March CE, Balbín E, Dulbecco CA, Aizpurúa M, Marillet AG, Reaven GM. Comparison of the abilities of the plasma triglyceride/high-density lipoprotein cholesterol ratio and the metabolic syndrome to identify insulin resistance. Diabetes Vascular Dis Res. 2013;10(4):346–352. doi: 10.1177/1479164113479809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The American Diabetes Association Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33(2):97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. doi: 10.1159/000343145. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escobedo J, Schargrodsky H, Champagne B, Silva H, Boissonnet CP, Vinueza R, Torres M, Hernandez R, Wilson E. Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: the CARMELA cross sectional study. Cardiovasc Diabetol. 2009;8:52. doi: 10.1186/1475-2840-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Chavez A, Simental-Mendia LE, Elizondo-Argueta S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cir Cir. 2011;79(2):126–131. [PubMed] [Google Scholar]
- 21.Armato J, Reaven G, Ruby R. Triglyceride/High-Density Lipoprotein Cholesterol Concentration Ratio Identifies Accentuated Cardio-Metabolic Risk. Endocrine Practice. 2015:1–18. doi: 10.4158/EP14479.OR. [DOI] [PubMed] [Google Scholar]
- 22.Salazar MR, Carbajal HA, Espeche WG, Dulbecco CA, Aizpurúa M, Marillet AG, Echeverría RF, Reaven GM. Relationships among insulin resistance obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diabetes Vasc Dis Res. 2011;8(2):109–116. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 23.Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Leiva Sisnieguez CE, Leiva Sisnieguez BC, March CE, Stavile RN, Balbín E, Reaven GM. Use of the plasma triglyceride/high-density lipoprotein cholesterol ratio to identify cardiovascular disease in hypertensive subjects. J Am Soc Hypertens. 2014;8(10):724–731. doi: 10.1016/j.jash.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC, Espinoza-Camacho MA, Jiménez-Morales M, Piña E, Méndez-Cruz AR, Villalobos-Molina R, Reaven GM. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res. 2013;54(10):2795–2799. doi: 10.1194/jlr.M040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34(7):583–588. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 26.Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, Bairey-Merz CN, Sopko G. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2009;157(3):548–555. doi: 10.1016/j.ahj.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanak V, Munoz J, Teague J, Stanley A, Jr., Bittner V. Accuracy of the triglyceride to high-density lipoprotein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. The Am J Cardiol. 2004;94(2):219–222. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 28.Jia L, Long S, Fu M, Yan B, Tian Y, Xu Y, Gou L. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolism. 2006;55(9):1141–1148. doi: 10.1016/j.metabol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96(8):2520–2525. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 30.Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. Journal Investig Med. 2014;62(2):345–349. doi: 10.2310/JIM.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 31.Negi SI, Nambi V. The role of carotid intimal thickness and plaque imaging in risk stratification for coronary heart disease. Curr Atheroscler Rep. 2012;14(2):115–123. doi: 10.1007/s11883-012-0225-4. [DOI] [PubMed] [Google Scholar]
- 32.Maki KC, Davidson MH, Dicklin MR, Bell M, Witchger M, Feinstein SB. Predictors of anterior and posterior wall carotid intima media thickness progression in men and women at moderate risk of coronary heart disease. J Clin Lipidol. 2011;5(3):141–151. doi: 10.1016/j.jacl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, Bainbridge KE, Fradkin JE. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernández Muñoz MJ, Basurto Acevedo L, Córdova Pérez N, Vázquez Martínez AL, Tepach Gutiérrez N, Vega García S, Rocha Cruz A, Díaz Martínez A, Saucedo García R, Zárate Treviño A, González Escudero EA, Degollado Córdova JA. Epicardial adipose tissue is associated with visceral fat, metabolic syndrome, and insulin resistance in menopausal women. Rev Esp Cardiol. 2014;67(6):436–441. doi: 10.1016/j.rec.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Arthur FKN, Adu-Frimpong M, Osei-Yeboah J, Mensah FO, Owusu L. Prediction of metaboic syndrome among postmenopausal Ghanaian women using obesity and atherogenic markers, women. Lipids in Health and Dis. 2012:101–114. doi: 10.1186/1476-511X-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]