Abstract
Context
Adiponectin is an abundant adipokine, which has antiinflammatory, anti-atherosclerotic and vasoprotective actions, and potential antiresorptive effects on bone metabolism. It seems to be directly involved in the improvement and control of energy homeostasis, protecting bone health and predicting osteoporotic fracture risk.
Objective
To examine the relationship between adiponectin level and bone mineral density (BMD) in post-menopausal women with metabolic syndrome (MetS) and low BMD, and to estimate the prognostic significance of adiponectin in osteoporosis.
Design
Clinical-laboratory cross-sectional study including 120 middle-aged and elder women (average 69.18±7.56 years).
Subjects and Methods
The anthropometric parameters were measured for all examinees. Lumbar spine and hip BMD, as well as body fat percentage, were measured using a Hologic DEXA scanner. In all subjects serum adiponectin concentration was measured by ELISA method.
Results
The level of adiponectin was significantly positively correlated with BMD-total, BMD of the lumbar spine and BMD of the femoral neck (r=0.618, r=0.521, r=0.567; p<0.01). Levels of adiponectin and BMD are significantly lower in post-menopausal women with MetS and osteoporosis compared to patients with osteopenia (856.87±453.43 vs. 1287.32±405.21 pg/mL, p<0.01; BMD, p<0.05), and the highest values in healthy examinees. A cut-off value of adiponectin level for osteoporosis/osteopenia was 1076.22/1392.74 pg/mL.
Conclusions
Post-menopausal women with MetS have significantly lower adiponectin level and low BMD compared to healthy examinees. Adiponectin may be an early, significant and independent predictor of developing osteoporosis in women with MetS, especially in post-menopausal period.
Keywords: adiponectin, osteoporosis, metabolic syndrome, bone mineral density
INTRODUCTION
Osteoporosis (OP) is the most frequent metabolic bone disease. It is estimated to affect approximately 70 million women aged between 50-84 years in Europe and 400 thousand women in Serbia. Osteoporosis is associated with the metabolic syndrome (MetS), which has become an epidemic of the modern world with an expected increase in fracture prevalence (1, 2). Post-menopausal status is associated with the increased risk of MetS development for 40 and 50%. However, the pathogenesis of OP and MetS is not yet completely understood (3).
Some researchers have documented that the immune-endocrine-metabolic un-regulatory mechanisms, as well as “the state of low-grade inflammation”, upregulation of receptor activators of nuclear kappa B ligand, oxidative stress, post-menopausal status, etc. are the potential pathogenetic correlation between central fat accumulation, insulin resistance, and hyperlipidemia, which precede the development of metabolic and bone disorders (4-6).
Adipocytes and osteoblasts, except for common origin from mesenchymal stem cell, have the ability to produce a hormone called adiponectin which regulates the main metabolic activites of the human body. Receptors for adiponectin are expressed in all bone cells (in both osteoblasts and osteoclasts) (7-9). In metabolically healthy individuals of normal body weight and normotensive individuals it is increased (10).
Adiponectin as a hormone has shown direct or indirect effects in bone metabolism (Fig. 1) (11), through the modulation of several signal transductions and molecules of bone remodeling: c-JunN-terminal kinases (JNK), p38 mitogen-activated protein kinases (MAPK), 5′ AMP-activated protein kinase (AMPK), Foxo-1, peroxisome proliferator-activated receptor (PPAR) (8, 9, 11-15).
Figure 1.
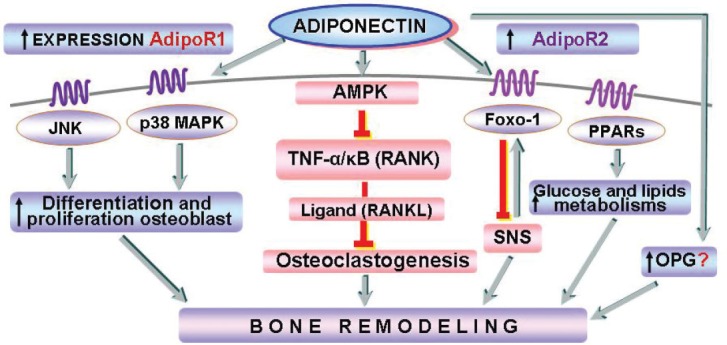
Potential role of adiponectin in bone metabolism.
Recent findings show that adiponectin directly influences osteoblast proliferation and differentiation and that it also inhibits tumor necrosis factor-alpha (TNF-α) and receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis (16, 17). On the other hand, according to some data adiponectin may have detrimental effects on bone metabolism by stimulating the RANKL pathway and inhibiting the production of the decoy receptor for RANKL, osteoprotegerin (18).
Changes in the level of adiponectin may reflect the progression of the metabolic disease. It is easily measurable, stable in circulation, and its concentrations are inversely correlated with all other circulating adipokines, lipids and hormones concentrations (8, 19). Some authors suggested that adiponectin may be a potential prognostic marker of hypertension and a novel therapeutic target for MetS (20, 21).
In recent years, beside the standard procedures in osteoporosis, diagnosis (measuring BMD), the application of FRAX algorhythm has been introduced as a procedure for estimating the patients’ 10-year risk of bone fractures (22). Recent evidence suggests different results (23-26). Higher and/or lower level of adiponectin has been associated with lower values of BMD in healthy post-menopausal women, and/or women with MetS.
Based on the conflicting results of experimental and epidemiologic studies of the role of adiponectin on the bone, the main objective of this study is to examine and determine the relationship between adiponectin level and BMD in post-menopausal women with MetS and lower BMD, as well as to estimate the prognostic significance of adiponectin as a potential biomarker of osteoporosis.
MATERIALS AND METHODS
A clinical-laboratory cross-sectional study involved 100 post-menopausal women with MetS and lower BMD (osteopenic, no=60; osteoporotic, no=40) and 20 healthy post-menopausal women without MetS and normal BMD (age range 40-78 yrs.). The Ethics Committee of “Niska Banja” Institute for Treatment and Rehabilitation approved the study. The detailed information was obtained from the patients’ written consent given prior to the investigation. The post-menopausal status was defined as the cessation of menstruation for at least 1 year. All examinees were measured for MetS components using the criteria of MetS proposed by The International Diabetes Federation (27).
The clinical examination of patients, measurements for parameters of MetS and osteodensitometry determination of BMD were performed at the Institute “Niska Banja”, and the determination of adiponectin concentration was performed at the Center for Molecular Medicine and Stem Cell Research of the Faculty of Medical Sciences, University of Kragujevac between February and December 2013.
The exclusion criteria referred to subjects with liver or renal diseases, inflammatory diseases, vascular diseases (i.e., peripheral vascular disease, cerebro-vascular disease), evident endocrine disorders, or subjects who at that moment were being on any form of drug treatment that could possibly have effects on bone metabolism like bisphosphonate, or estrogen replacement therapy, oral contraceptives, statins, aspirin, antioxidants, vitamin D or calcium supplementations.
The group with MetS was consecutively divided into two different categories based on the measured bone density, according to the World Health Organization consensus (28), and were denominated as normal BMD, osteopenia and osteoporosis. Osteodensitometric determination of BMD was assessed with a DXA (dual energy X-ray absorptiometry scan) device – Densitometer Choplogic Discovery QDR-C. BMD was measured in g/cm2, according to the standard protocol for the anterior-posterior lumbar spine L1-L4 (BMDL2-L4), mean of right and left femur neck (BMD hip), and total-body bone mineral content (BMD-total). Measurements were compared as T-scores expressed in standard deviations (SD) using the peak bone mass from the manufacturer’s reference population; Z-score indicates deviation from the normal age and sex-matched mean in SD. Osteoporosis and osteopenia were defined in accordance with the WHO (28). WHO Fracture Risk Assessment (FRAX) scores for 10-year risks of hip and major osteoporotic fracture for post-menopausal women was performed using a FRAX-algorithm, (version 3.0) from the WHO (29).
The determination of the concentration of adiponectin in the fasting serum were performed using Enzyme-linked immunosorbent assay (ELISA); a method that uses a commercial ELISA kit specific for human adiponectin (Human Adiponectin Duo Set ELISA Development kit, R & D systems, USA). The absorbance was measured using a Microplate Reader (Zenyth, Anthos, US) set at 450 nm. The measuring range of the method was 62.5 - 4000 pg/mL and intra- and inter-assay coefficients of variation were 4.5 and 5.0 % .
Statistical analyses
All the data were presented as means ± standard deviations (SD), as absolute numbers and percentages, dependent on the statistical method used. To compare the means of the two examinee groups the Student T test was applied. The comparison of the means of adiponectin level, BMD, and other parameters was achieved using a one-way and two-factor analysis of variance (ANOVA). The relationship among adiponectin levels, BMD and other parameters was determined by Pearson’s correlation coefficient (r). Univariate and multivariate logistic regressions were used to calculate Odds Ratio (OR) and 95% Confidence Intervals (CI) for the development of osteoporosis. A cut-off value of adiponectin for predicting the risk of osteoporosis and osteopenia was defined by using ROC-curve. All statistical tests were two-tailed, and the statistical significance was defined as p<0.05. The statistical analyses were performed with SPSS version 20.0 (SPSS, Chicago, IL, United States).
RESULTS
The baseline characteristics of the study population, changes and established differences of adiponectin levels and BMD in post-menopausal women with MetS (clinical group) compared with the control group of healthy examinees, are presented in Table 1.
Table 1.
Basic, clinical and biochemical characteristics in examined groups
| CLINICAL GROUP N=100 | ||||
| Mean values | Osteoporosis | Osteopenia | Control group | p values |
| Number (%) | 40 (33%) | 60 (50%) | 20 (17%) | P<0.01 |
| Age (year) | 61.44±7.25 | 56.62±6.32 | 53.18±6.02 | P>0.05 |
| BMD-total (g/cm2) | 1.012±0.102* | 1.087±0.068 | 1.209±0.115 | P=0.041 |
| BMDL2-L4 (g/cm2) | 0.803±0.121* | 0.897±0.083 | 1.112±0.128 | P=0.023 |
| BMD-hip (g/cm2) | 0.623±0.086* | 0.706±0.062 | 0.877±0.064 | P=0.037 |
| Adiponectin (pg/mL) | 856.87±453.43** | 1287.32±405.21 | 1785.76±444.02 | P=0.008 |
| Normoadiponectinemia | 8 (20%) | 21 (35%) | 14 (70%) | P<0.001 |
| Hypoadiponectinemia | 32 (80%) | 39 (65%) | 6 (30%) | P<0.001 |
The differences between clinical subgroups are significant to the 0.01 level
0.05 level.
BMD-Bone mineral density, BMDL2-L4-Lumbar spine BMD, BMD-hip-Femoral neck BMD.
In the clinical group of post-menopausal women with MetS the prevalence of osteoporosis was 40%, and the prevalence of osteopenia was 60%. We found a higher frequency of hypoadiponectinemia in the subgroup of examinees with osteoporosis compared to the subgroup of examinees with osteopenia and control group. In the subgroup with osteoporosis, 32 (80%) women had hypoadiponectinemia, and in the subgroup with osteopenia 39 (65%) had hypoadiponectinemia, and 70% of examinees in the control group had normoadiponectinemia. In this study the adiponectin levels and values of BMD were significantly lower in women with MetS and osteoporosis, while they were lower in women with MetS and osteopenia compared to the control group of healthy examinees with normal BMD (856.87±453.43 vs. 1287.32±405.21 vs. 1785.76±444.02, p<0.01 for all comparisons).
Moreover, the values of BMD-total were significantly lower (1.012±0.102 vs. 1.087±0.068 g/cm2), especially BMD of the lumbar spine (0.803±0.121 vs. 0.897±0.083 g/cm2), as well as BMD of the femoral neck (0.623±0.086 vs. 0.706±0.062 g/cm2, p<0.05 for all comparisons).
The correlation analysis between adiponectin level, BMD and 10-year fractures risk was determined using Pearson’s linear correlation, and is shown in Table 2.
Table 2.
Correlations between adiponectin level, BMD and 10-year fractures risk
| Pearson ‘s Correlation coefficient | ADIPONECTIN | BMD-total | BMD- L2-L4 | BMD-hip | Major osteoporotic | Hip fracture |
| ADIPONECTIN | - | 0.618** | 0.521** | 0.567** | - 0.320* | - 0.278* |
| BMD-total | 0.618** | - | 0.474* | 0.517* | - 0.247* | - 0.265* |
| BMDL2-L4 | 0.521** | 0.474* | - | 0.426* | - 0.221 | - 0.226 |
| BMD-hip | 0.567** | 0.517* | 0.426* | - | - 0.563** | - 0.489** |
| Major osteoporotic fracture | - 0.320* | -0.247* | - 0.221 | - 0.563** | - | - 0.789** |
| Hip fracture | - 0.278* | -0.265* | - 0.226 | - 0.489** | - 0.789** | - |
Correlation is significant to the level 0.01 (2-)
Correlation is significant to the level 0.05 (2-).
BMD-Bone mineral density, BMDL2-L4-Lumbar spine BMD, BMD-hip-Femoral neck BMD.
The adiponectin level was significantly positively correlated with BMD-total, BMDL2-L4 and BMD-hyp (r = 0.618, r = 0.521, r = 0.567 respectively; p<0.01 for all correlations). Weaker, but still significant positive correlations (p<0.05) were found between BMD-total and BMD-LS (r = 0.474), BMD-total and BMD-hip (r = 0.517). On the other hand, significant negative correlations (p<0.05) were found between adiponectin levels and 10-year risk of major osteoporotic fracture (r = - 0.320) and between adiponectin levels and 10-year risk of hip fracture (r = - 0.278).
The results of non/adjusted Odds Ratio (OR) for the development of osteoporosis (Univariate and Multivariate logistic regression analysis) are presented in Table 3.
Table 3.
Non/adjusted Odds Ratio (OR) and 95% confidence intervals (CI) of Risk factors for osteoporosis
| Uni-analysis | Multi-analysis | |||||
| Parameters | OR | 95% Cl | P | OR | 95% Cl | P |
| Age | 1.051 | 1.020 - 1.079 | 0.001 | 1.248 | 1.222 - 1.543 | < 0.01 |
| BMD-total | 0.952 | 0.910 - 1.008 | 0.012 | 0.988 | 0.969 - 1.019 | < 0.05 |
| BMDL2-L4 | 0.926 | 0.900 - 0.962 | 0.001 | 0.941 | 0.922 - 0.971 | < 0.01 |
| BMD-hip | 0.932 | 0.916- 0.962 | 0.001 | 0.948 | 0.923 - 0.975 | < 0.01 |
| Adiponectin | 0.977 | 0.960 - 0.982 | 0.000 | 0.980 | 0.960 - 0.998 | < 0.01 |
BMD-Bone mineral density, BMDL2-L4-Lumbar spine BMD, BMD-hip-Femoral neck BMD.
In predictive models of Univariate regression analyses, the non-adjusted OR showed that there was a significant risk of the development of osteoporosis with older age (5.1%, p<0.01), lower BMDL2-L4 (4.8%, p<0.05), BMD-hip (6.8%, p<0.01), lower serum adiponectin level (2.3%, p<0.001), higher BMI (4.8%, p<0.05), and higher HOMA-IR index (3.5%, p<0.05). Similarly, the multivariable adjusted analysis OR showed that a highly significant risk for osteoporosis (p<0.01 for all correlations) was connected with older age (24.8%), lower BMDL2-L4 (5.9%), BMD-hip (5.2%), higher BMI (3.4%), and lower serum adiponectin level (OR=0.98, 2%). Waist circumference and HOMA-IR index do not have the significance as predictors for osteoporosis after multivariable adjustment.
The results of testing values of adiponectin for predicting the risk of osteoporosis and osteopenia by using ROC-curve (Fig. 2) determined that the value of adiponectin ≤1392.74 pg/mL was associated with a higher risk of osteopenia whereas the value of adiponectin ≤1076.22 pg/mL was associated with a higher risk of osteoporosis. The level of adiponectin was distinguished as a stronger predictor of osteoporosis than of osteopenia.
Figure 2.
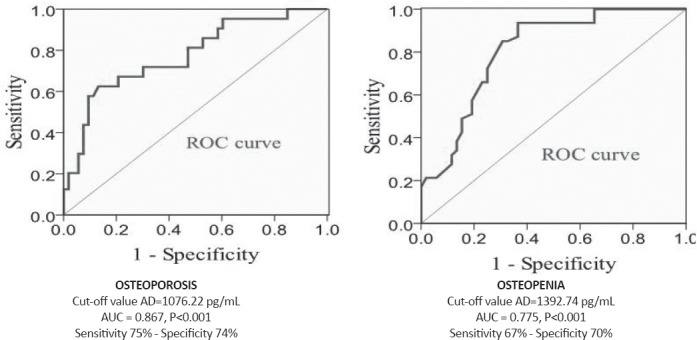
Analysis of the ROC curve for adiponectin levels for predicting the risk for development of osteoporosis (left) and osteopenia (right).
DISCUSSION
To our knowledge, a study which examines the correlation between plasma adiponectin concentration and bone mineral values in post-menopausal women with metabolic syndrome has not been conducted yet.
The results of the recent cross-sectional study, which involved a large number of middle-aged and elderly Chinese women (9930), show that the presence of MetS is accompanied by the increased risk for osteoporotic fractures (29). Our results also indicate that the MetS itself presents a risk for the occurrence of osteoporosis, opposite the traditional view that MetS has protective effects on the bone system.
Concerning the complexity of the immune/inflammatory etiopathogenesis of OS and MetS, it is important to determine circulating adipokine concentrations, namely adiponectin, as a potential biomarker for bone metabolism, which would contribute to an individually adjusted approach of estimating the risk of fractures (19). Circulating adiponectin concentration increases with age in normal-weight, middle-aged women (30). Recent data (31, 32) indicate that the level of adiponectin is significantly decreased in post-menopausal women, probably caused by the physiological deficiency of estrogens and their up-stream regulation effect on adiponectin, as well as by an increased tendency of creating the hexameric form of adiponectin. In the subgroup with osteoporosis, we have found that 80% of women had hypoadiponectinemia, and 70% of examinees in the control group had normoadiponectinemia, and that adiponectin levels and values of BMD were significantly lower in women with MetS and osteoporosis, compared to the control group of healthy examinees with normal BMD.
In post-menopausal period, low estrogen and insulin levels may cause fat storage and fat redistribution, secretion of numerous proinflammatory adipokines, when osteoprotegerin is not sufficient to stop the formation of RANK ligands and osteoclast inhibition. Additionally, the results of experimental studies show that hyperglycemia and hypoadiponectinemia influence the decreased expression of osteocalcin via PPAR receptors (33). The potential protective mechanism which would help the fulfilling of lesions is, in fact, compensatory adiponectin secretion which stimulates the binding of osteoprotegerin and RANKL.
Hypoadiponectinemia appears to be in the alarm compensatory response of insulin resistance and low BMD. Due to its insulin-sensitive and anti-inflammatory role, adiponectin stimulates the expression of osteocalcin and differentiation of osteoblasts, and it also inhibits the accelerated adipogenesis in bone marrow, known as fatty marrow.
In addition, recent observations show that bone marrow adipose tissue may have positive, protective roles, influence and adaptive functions outside the bone tissue, due to adiponectin production, as this hormone may promote human jaw bone marrow stem cell osteogenesis through the APPL1-p38 MAPK signaling pathway (11, 33, 35). One of the possible ways in which adiponectin actions can be explained is that they are stimulated by thiazolidinedione agonists of peroxisome proliferator-activated receptor γ (PPAR-γ) which is associated with the increased bone mass and reduced bone marrow adiposity number. This peptide has also been demonstrated to increase the proliferation and differentiation of osteoblasts, inhibit the activity of osteoclasts, and bone resorption (11).
Study of Kajimura et al. (35) reveals that adiponectin has the unusual ability to regulate the same function in two opposite manners depending on where it acts and that it opposes, partially, Leptin’s influence on the sympathetic nervous system. It also proposes that adiponectin regulation of bone mass occurs through a PI3-kinase-FoxO1 pathway.
Similar to our results, some authors point out that adiponectin could be an independent predictor of BMD and that it only positively correlates with biochemical bone turnover markers in post-menopausal women (23). In contrast, Iacobellis et al. (26) suggest that adiponectin levels are negatively correlated with BMD in women with MetS at the same post-menopausal stage. Richards et al. (36) also confirm the inverse correlation in a similar study and this relationship persists even after the adjustment of potential confounding factors, including BMI, serum leptin, central fat mass, and exercise. Obesity and visceral fat contribute to changes in the serum levels of leptin, adiponectin and other adipokines, the decrease of insulin sensitivity, which results in their effects on the acceleration of differentiation and proliferation of osteoclasts. On the other hand, a formed circulus viciosus is easily maintained by the “low grade” chronic inflammation as one of the most important characteristics of MetS and OS (24).
In this study we have found a significantly positive correlation between serum adiponectin and BMD and, on the other hand, a negative correlation between serum adiponectin and a 10-year risk of major osteoporotic fracture and hip fracture independent of potential confounders, such as BMD and age. Some studies suggest that there are no such correlations (37). However, the physiological significance of these results has not been explained yet.
Recent studies have mainly investigated the level of adiponectin in postmenopause, or in MetS and they show contradictory results (23-27). In contrast, this study investigated the simultaneous influence of MetS and postmenopause on the adiponectin and BMD levels. The results assessed by uni- and multivariate regression analysis indicate that adiponectin can be a predictor for low BMD, independent of age, menopausal status, fat mass and other parameters MetS.
These results show that adiponectin level was more useful than BMD for assessing the risk for osteoporosis in post-menopausal women wiht MetS after multi-variable adjustment logistic regression analysis.
The results of our study indicate that hipoadiponectinemia is an important parameter of metabolic state and decreased bone mineral density. According to the results found in numerous studies, our results also show the potential balancing role of adiponectin in bone metabolism regulation, depending on the prevalence of negative mediators in bone metabolism.
However, these conflicting results may be due to the differences between studies in the skeletal regions measured, and/or differences in the age and menopausal status of the subjects studied, insufficient hormones: insulin, leptin, adiponectin and bone markers: osteocalcin, bone alkaline phosphatase, which are related to BMD and body weight. This is presumably due to the fact that there are different influences on the BMD and level of adiponectin in healthy women, as menopause, age, duration of the post-menopausal period, and body fat percentage, as well as the lack of analysis of all bone metabolism markers and inflammation markers by different criteria for MetS and observing the different stages of disease. In the early stage of MetS, there was a real inflammatory response, the increase of proinflammatory markers, as well as the compensatory increase of adiponectin level in newly developed metabolic disorders, while in the later, stable form of MetS, the hypoadiponectinemia was caused by the chronic inflammation.
Understanding the potential antiresorptive effects of adiponectin, and pathophysiology of MetS and osteoporosis can clarify the pathophysiological correlation between adipose-vasculatory and bone axis, and introduce this hormone as a routine marker.
In conclusion, this study found a significant, positive and relatively strong correlation between adiponectin levels and BMD. Post-menopausal women with osteoporosis have significantly lower adiponectin level and decreased bone mineral density compared to women with osteopenia. Our results suggest that changes in the levels of adiponectin may be an important prognostic parameter of lower bone mineral density and osteoporotic fracture risk in women with metabolic syndrome.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int. 2017;28(5):1531–1542. doi: 10.1007/s00198-017-3909-3. [DOI] [PubMed] [Google Scholar]
- 2.Aleksandra KN, Sasa M, Suada GS. Physical activity and osteoporotic fractures in postmenopausal women with osteoporosis in Serbia. Sanamed. 2012;7(1):15–19. [Google Scholar]
- 3.Wong SK, Chin KY, Suhaimi HF, Ahmad F, Ima-Nirwana S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients. 2016;8(6) doi: 10.3390/nu8060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oros S, Ianas O, Vladoiu S, Giurcaneanu M, Ionescu L, Neacsu E, Voicu G, Stoiceanu M, Rosca R, Neamtu C, Badiu C, Dumitrache C. Does obesity protect postmenopausal women against osteoporosis? Acta Endocrinologica-Bucharest. 2012;8(1):67–76. [Google Scholar]
- 5.Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria PA, Napoli N, Pozzilli P, Manfrini S. BMI and BMD: The potential interplay between obesity and bone fragility. Int. J. Environ. Res. Public Health. 2016;13(6) doi: 10.3390/ijerph13060544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–408. doi: 10.1007/s00198-008-0712-1. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, Liu M. Adiponectin: a versatile player of innate immunity. Journal of Molecular Cell Biology. 2016;8(2):120–128. doi: 10.1093/jmcb/mjw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ZV, Scherer PE. Adiponectin, the past two decades. Journal of Molecular Cell Biology. 2016;8(2):93–100. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Koh SJ, Hyun YJ, Choi SY, Chae JS, Kim JY, Park S, Ahn CM, Jang Y, Lee JH. Influence of age and visceral fat area on plasma adiponectin concentrations in women with normal glucose tolerance. Clin Chim Acta. 2008;389(1-2):45–50. doi: 10.1016/j.cca.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhou P, Kimondo JW. Adiponectin and osteocalcin: relation to insulin sensitivity. Biochemistry and Cell Biology. 2012;90(5):613–620. doi: 10.1139/o2012-022. [DOI] [PubMed] [Google Scholar]
- 12.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, Ikeda T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 13.Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Mitsui Y, Gotoh M, Fukushima N, Shirachi I, Otabe S, Yuan X, Hashinaga T, Wada N, Mitsui A, Yoshida T, Yoshida S, Yamada K, Nagata K. Hyperadiponectinemia enhances bone formation in mice. BMC Musculoskelet Disord. 2011;12:18. doi: 10.1186/1471-2474-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YY, Cheen CY, Chen CC, Lin HJ, Mersmann HJ, Wu SC, Ding ST. The effects of Adiponectin on bone metabolism. J.Biomedical Science and Engineering. 2014;7:621–630. [Google Scholar]
- 16.Yamaguchi N, Kukita T, Li YJ, Kamio N, Fukumoto S, Nonaka K, Ninomiya Y, Hanazawae S, Yamashita Y. Adiponectin inhibits induction of TNF-α/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Letters. 2008;582(3):451–456. doi: 10.1016/j.febslet.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, Chen J. Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt. J Biol Chem. 2011;286:12542–12553. doi: 10.1074/jbc.M110.152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648–1656. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 19.Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovascular Diabetology. 2014;13:103. doi: 10.1186/1475-2840-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH, Hung YJ. Possible new therapeutic approach for obesity-related diseases: Role of adiponectin receptor agonists. Diabetes Investig. 2015;6(3):264–266. doi: 10.1111/jdi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond). 2016;40(9):1353–1359. doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanis J, McCloskey E, Johansson H, Oden A, Ström O, Borgström F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(Suppl4):S407–413. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- 23.Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–623. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Xie H, Zhao Q, Xie GQ, Wu XP, Liao EY, Luo XH. Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J Endocrinol Invest. 2010;33(10):707–711. doi: 10.1007/BF03346674. [DOI] [PubMed] [Google Scholar]
- 25.Lubkowska A, Dobek A, Mieszkowski J, Garczynski W, Chlubek D. Adiponectin as a Biomarker of Osteoporosis in Postmenopausal Women: Controversies. Disease Markers. 2014;2014:975178. doi: 10.1155/2014/975178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacobellis G, Iorio M, Napoli N, Cotesta D, Zinnamosca L, Marinelli C, Petramala L, Minisola S, D’Erasmo E, Letizia C. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J Endocrinol Invest. 2011;34(1):e12–15. doi: 10.1007/BF03346703. [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. Diabet Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. A Consensus Statement from the International Diabetes Federation. [DOI] [PubMed] [Google Scholar]
- 28.Prevention and management of osteoporosis Report of a WHO Scientific Group. Geneva, World Health Organization, 2003 (WHO Technical Report Series No. 921). [PubMed] [Google Scholar]
- 29.Qin L, Yang Z, Zhang W, Gu H, Li X, Zhu L, Lu S, Xing Y, Zhang H, Niu Y, Ning G, Su Q. Metabolic syndrome and osteoporotic fracture: a population-based study in China. BMC EndocrDisord. 2016;16(1):27. doi: 10.1186/s12902-016-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenta R, Kontogianni MD, Yiannakouris N. Association between circulating levels of adiponectin and indices of bone mass and bone metabolism in middle-aged post-menopausal women. J Endocrinol Invest. 2012;35(3):306–311. doi: 10.3275/7744. [DOI] [PubMed] [Google Scholar]
- 31.Sowers M, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X, Yosef M, Symons J. Changes in body composition in women over six years at midlife: ovarian and chronological aging. Journal of Clinical Endocrinology and Metabolism. 2007;92(3):895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurimae J, Jurimae T. Plasma adiponectin concentration in healthy pre- and post-menopausal women: relationship with body composition, bone mineral, and metabolic variables. American Journal of Physiology-Endocrinology and Metabolism. 2007;293(1):E42–E47. doi: 10.1152/ajpendo.00610.2006. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Zhu X, Wang Q, Wang L. Hyperglycemia induces endoplasmic reticulum stress-dependent CHOPexpression in osteoblasts. Exp. Ther. Med. 2013;5:1289–1292. doi: 10.3892/etm.2013.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu Y, Wu H, Lu S, Hu H, Li D, Wu Y, Tang Z. Adiponectin Promotes Human Jaw Bone Marrow Stem Cell Osteogenesis. J Dent Res. 2016;95(7):769–775. doi: 10.1177/0022034516636853. [DOI] [PubMed] [Google Scholar]
- 35.Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo XE, Mann JJ, Karsenty G. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013,4;17(6):901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 37.Ozkurt B, Ozkurt ZN, Altay M, Aktekin CN, Caglayan O, Tabak Y. The relationship between serum adiponectin level and anthropometry, bone mass, osteoporotic fracture risk in postmenopausal women. Eklem Hastalik Cerrahisi. 2009;20(2):78–84. [PubMed] [Google Scholar]


