The main treatment for valvular disease consists in surgical replacement with mechanical or biological artificial devices; these are excellent mid-term replacements which greatly improve the patient’s quality of life. However, long-term use of these devices (beyond 10-15 years) is associated with bleeding risks in the case of mechanical valves and degeneration, for biological valves (1). Heart valve tissue engineering, the science of combining scaffolds and cells has the potential to revolutionize the field of valve surgery by providing functional, durable, and viable valves. To date, several approaches have been tested using synthetic and natural scaffolds and a variety of cells and bioreactors, but overall most approaches have not been successful on the long term when tested in animals or human patients as pulmonary or aortic implants (1–4). We hypothesized that one potential approach would be based on well characterized xenogeneic acellular aortic valve root scaffolds seeded with autologous adipose-tissue derived stem cells (ASCs). To test this hypothesis, we prepared porcine acellular aortic roots, injected them with autologous ASCs and implanted them in the right ventricular outflow tract (RVOT) of juvenile sheep.
Porcine valve roots were decellularized with detergents and enzymes using a pressurized perfusion system and then stabilized with polyphenols as described before (5). Male juvenile sheep weighing about 35 kg were used for this study. After acclimation, we collected inter-scapular adipose tissue from each animal, isolated ASCs using a published collagenase-based procedure (6) and propagated them in culture for 3-4 passages. We then mounted each acellular aortic root within a decellularized bovine pericardial tube and seeded each cusp with 4 million autologous ASCs in 200 ul PBS by injection into the base of each cusp. ASC-seeded valved conduits were then implanted under general anesthesia in the right ventricle outflow tract (RVOT) with full ligation of the native pulmonary artery. Six animals (n=6) underwent implantation with autologous ASC-seeded valves and one with a non-cell seeded valve as control. Postoperatively antalgics, antibiotics, and diuretics were administered for 5 days. Anticoagulant (Fraxiparine 2 × 0.3 ml) therapy was maintained for the first 30 days and anti-aggregant treatment (Aspenter 75 mg/day orally) for the entire length of the study with a target follow-up of 4 months. Monthly transthoracic echography was performed under mild sedation, monitoring the valve hemodynamics, leaflet mobility and thickness and evidence for right ventricular remodeling. All animal procedures were performed in accordance to the “Guide for the care and use of laboratory animals”, published by the NIH (NIH Publication No. 85-23, revised 1996) under an animal use protocol approved by the University of Medicine and Pharmacy Targu Mures Ethical Committee (AUP #8/04.02.2012).
Clinical follow up and analysis of explained valves
The early post-operatory evolution of all animals was favorable, with rapid recovery and without immediate complications or early deaths (7/7 survival). The valve which has been prepared without ASC injection before implantation performed very well hemodynamically and exhibited minimal echographic changes on follow-up. The explanted valve did not reveal any macroscopic changes, with the leaflets intact and mobile (Figure 1A). Histological analysis of the cusps showed complete absence of infiltrated cells, inflammation, calcification or thrombi and lack of fibrous tissue overgrowth (Figure 1A).
Figure 1.
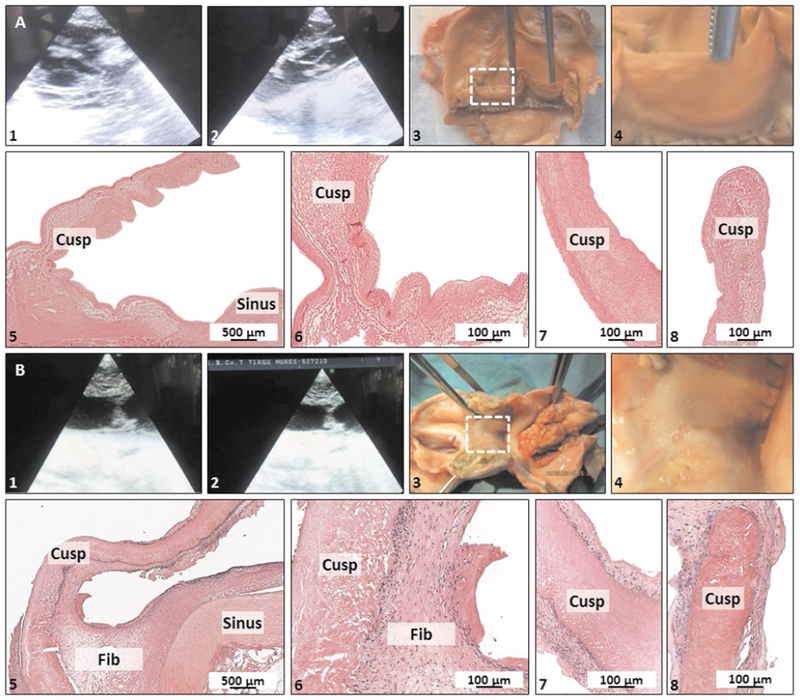
Clinical follow up, macroscopy and histology of explanted valves. Panel A, non-stem cell seeded control valve, Panel B, ASC-seeded valve. (1, 2) Echography, (3) Macroscopic overview of whole conduit and (4) a zoomed-in macroscopic detail of a single cusp (dashed white box in 3). (5-8) histology with H&E staining at low magnification (5), portion of the sinus and the cusp base (6), middle portion (7) and tip of cusp (8). Fib, fibrous tissue overgrowth.
The six ASC-seeded valves had a different evolution. Progressively, at about 1 month post-implantation, animals started to present symptoms of right ventricular failure. These included dyspnea, tachypnea and ascites, which were correlated with echographic aspects of cusp thickening and reduced mobility, resulting in valvular insufficiency and right ventricular dilatation (Figure 1B). The pathology progressed slowly with time towards the same end-point (right ventricular failure), but not at the same rate for all animals. Considering the time-line of this pathology and due to humane considerations, we euthanized animals at 1, 3 and 4 months followup (n=2 per time point). Upon macroscopic evaluation, fibrous tissue overgrowth was observed in all valves, covering both distal and proximal anastomoses, the pericardial segments and also slowly enclosing the cusps. In advanced stages, the fibrous overgrowth completely covered all three cusps, essentially creating a non-valved conduit for the right outflow tract (Figure 1B). Histology confirmed these results, showing the presence of a 100-300 um thick, well developed fibrous tissue rich in fibroblasts and connective tissue (Figure 1B) covering intact cusp tissues. No cells of any kind (seeded or infiltrated) could be detected in any of the cusps, including lack of any infiltrated inflammatory cells or fibroblasts and no thrombosis occurred at any cusp surface. Calcihcation was absent from all cusp tissues analyzed and only 2 explanted aortic wall samples exhibited small nodules typical of calcification (Alizarin Red stain, data not shown).
Discussions, Conclusions and Limitations
In order to generate living tissue engineered valves we chose to harness the outstanding hemodynamics and 3D structures of the porcine aortic root and the regenerative potential of autologous adult stem cells. By combining the two components and subjecting them to mechanical stimuli typical of the valve milieu, we expected that ASCs would be able to differentiate into valve cells and maintain valvular matrix homeostasis. To our knowledge this is among the first attempts to revitalize the cusp interstitium with autologous ASCs. We then mounted the acellular aortic roots within pericardial conduits and used them for extra-anatomic implantation of valves in the RVOT. Overall, acellular valve roots proved to be hemodynamically adequate scaffolds for valve tissue engineering in the sheep model and were not affected by immune rejection, calcification or thrombosis in this animal model.
Implantation of ASC-seeded valves however, was associated with progressive and persistent chronic covering of valve tissues with host fibrous tissue overgrowth, which eventually lead to right ventricular insufficiency. Although not initially developed for tissue engineering, sheep implantation as recommended by the FDA, continues to be the animal model of choice for testing of acellular valve scaffolds, despite the fact that the sheep is known for its propensity to develop variable degrees of hbrotic responses to implants (7–9). It is not clear at this point what initiated the hbrotic reaction in our study and whether that was associated directly with the seeded ASCs. The absence of any seeded ASCs in the interstitium of the valves could possibly be due to presumptive vulnerability of ASCs to mechanical forces, but this has to be tested more extensively.
The major limitation of this study is the small number of animals tested. Although we started with n=6 for the ASC-seeded valves, their progressive covering with fibrous tissue, development of right ventricular insufficiency, and humane considerations forced us to euthanize several animals before the targeted time point. Similar results have been obtained recently by our group using ASC-seeded acellular pulmonary valves (10), pointing to a causal relationship between stem cell seeding and tissue overgrowth in the sheep. Clearly, additional studies with larger sample sizes and more detailed tracking of the fate of ASCs after implantation are needed to answer some of the above-mentioned questions.
Acknowledgments
This project was funded in part by NIGMS of the National Institutes of Health under award number 5P20GM103444-07, by the Harriet and Jerry Dempsey Associate Professorship Award and by a grant from the Romanian National Authority for Scientific Research, CNCS-UEFISCDI, project number PNII-ID-PCCE-2011-2-0036.
References
- 1.Simionescu DT, Chen J, Jaeggli M, Wang B, Liao J. Form Follows Function: Advances in Trilayered Structure Replication for Aortic Heart Valve Tissue Engineering. J Healthc Eng. 2012. June;3(2):179–202. DOI: 10.1260/2040-2295.3.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sohier J, Carubelli I, Sarathchandra P, Latif N, Chester AH, Yacoub MH. The potential of anisotropic matrices as substrate for heart valve engineering. Biomaterials. 2014. February;35(6):1833–44. DOI: 10.1016/j.biomaterials.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 3. Weber B, Dijkman PE, Scherman J, Sanders B, Emmert MY, Grunenfelder J, et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013. October;34(30):7269–80. DOI: 10.1016/j.biomaterials.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 4.Tudorache I, Calistru A, Baraki H, Meyer T, Hoffler K, Sarikouch S, et al. Orthotopic replacement of aortic heart valves with tissue-engineered grafts. Tissue Eng Part A. 2013. August;19(15-16):1686–94. DOI: 10.1089/ten.tea.2012.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sierad LN, Shaw EL, Bina A, Brazile B, Rierson N, Patnaik SS, et al. Functional Heart Valve Scaffolds Obtained by Complete Decellularization of Porcine Aortic Roots in a Novel Differential Pressure Gradient Perfusion System. Tissue Eng Part C Methods. 2015. December;21(12):1284–96. DOI: 10.1089/ten.tec.2015.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–9. DOI: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 7.Schoen FJ. Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol. 2011. October;22(5):698–705. DOI: 10.1016/j.copbio.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Vesely I Heart valve tissue engineering. Circ Res. 2005. October 14;97(8):743–55. DOI: 10.1161/01.RES.0000185326.04010.9f. [DOI] [PubMed] [Google Scholar]
- 9. Schoen FJ. Pathologic findings in explanted clinical bioprosthetic valves fabricated from photooxidized bovine pericardium. J Heart Valve Dis. 1998. March;7(2):174–9. [PubMed] [Google Scholar]
- 10.Harpa MM, Movileanu I, Sierad LN, Cotoi OS, Suciu H, Sircuta C, et al. Pulmonary heart valve replacement using stabilized acellular xenogeneic scaffolds; effects of seeding with autologous stem cells. Rev Romana Med Lab. 2015;23(4):415–29. DOI: 10.1515/rrlm-2015-0046. [DOI] [Google Scholar]


