Abstract
Context
Epidemiological data have shown that obesity increases the risk of developing colorectal cancer and also an increased body mass index (BMI) is associated with a worse prognosis. Bevacizumab based systemic therapy, an antiVEGF targeted therapy, is an important treatment option for metastatic colorectal cancer (mCRC) patients. Obesity is associated with high level of vascular endothelial growth factor (VEGF), that might provoke resistance to antiVEGF monoclonal antibody.
Objective
To evaluate the efficacy in terms of progression free survival (PFS) and overall survival (OS) of bevacizumab systemic therapy in patients with mCRC.
Design
Retrospective cohort, single center study.
Subjects and Methods
Between January 2007 and December 2012, 112 patients with mCRC, who followed bevacizumab based systemic therapy in the “Ion Chiricuta” Oncology Institute in Cluj-Napoca, were included in our analysis.
Results
Values of BMI ≥ or <27 kg/sqm was found that PFS is statistically significant superior in patients with BMI<27 kg/sqm (n=77) than in those with BMI ≥ 27 kg/sqm (n=35), 24 months versus 17.9 months (p = 0.04). Five years OS was not influenced by the BMI, 35% vs 30% (p=0.29). In patients with liver metastases with values of BMI ≥ 27 kg/sqm have PFS lower than patients with a BMI <27 kg/sqm, 17.5 months versus 24.5 months (p = 0.02). Five years OS was not influenced by the BMI, 39% (BMI <27 kg/sqm) vs. 22% (BMI ≥ 27 kg/sqm) (p = 0.09).
Conclusions
This study demonstrated the negative influence of BMI on both PFS on the entire sample of patients and in patients with liver metastases only, BMI cut-off value proved to be 27 kg/square meter and shows that the BMI may be an important prognostic factor with a high clinical relevance in patients with mCRC.
Keywords: metastatic colorectal cancer, body mass index, bevacizumab, progression free survival, overall survival
INTRODUCTION
Annually on a worldwide level, 1.35 million people receive the colorectal cancer diagnosis and about 700,000 people die because of this pathology (1). Recently, visible progress was made when it comes to analyzing the molecular and the pathogenetic mechanism of this cancer type and several mutations involved in the evolution from normal cells to adenoma and subsequently to carcinoma were discovered (2). The presence of molecular injuries, such as the RAS mutation, is being used today with the purpose of predicting the efficacy of the targeted systemic therapy. Even though recent research revealed other mutations to tumor suppressor genes involved in the pathophysiological mechanism of colorectal cancer, such as APC, p53, Smad4, the main therapeutical options are still based on monoclonal antibodies antiEGFR and antiVEGF associated to chemotherapy (3-5).
The prognostic factors of colorectal cancer are based on clinical data such as TNM staging, surgical resection margins, lymphovascular invasion, followed by molecular analysis of degree of differentiation type or microsatellites instability. From these issues the necessity of identifying new possible prognostic and predictive factors to guide the therapeutical decision in many of the clinical situations found in this pathology (6).
Obesity and overweight were the proposed factors, since it is well known that they represent an etiological factor for colorectal cancer and a real public health problem. The World Health Organization estimated that over 500 million persons over the age of 20 were obese in 2008 (7, 8).
In Romania, the epidemiological data looks as worrying as the global setting, the overweight ratio among adults being around 30-35%, while the obesity tends to affect ¼ of the entire adult population (9-12). Obesity and overweight are associated with a high risk of many cancer types: breast, colorectal, endometrial, esophageal, renal and pancreatic (13,14).
Regarding colorectal cancer, it has been established that an elevated body mass index (BMI) has a negative impact on overall survival, progression free survival and time to relapse, men being more affected than women (15).
If we refer to patients with metastatic colorectal cancer, it is well known that BMI has an impact on overall survival and progression free survival (16). Even though research has demonstrated that BMI does not influence the action of fluoropyrimidine based therapy (15), elevated levels of vascular endothelial growth factor, seen mostly in patients with excess abdominal and visceral fat (17-19), could alter the efficacy of antiVEGF (bevacizumab) monoclonal antibodies, resulting in a negative impact on progression free survival interval (17,19).
OBJECTIVE
The endpoint of the present study is to evaluate the efficacy of anti-VEGF therapies (bevacizumab) on progression free survival (PFS) and overall survival (OS) in patients with metastatic colorectal cancer and how it is influenced by BMI.
PATIENTS AND METHODS
Between January 2007 and December 2012, 112 patients with metastatic colorectal cancer, who followed bevacizumab based systemic therapy in the “Prof. Dr. Ion Chiricuta” Oncology Institute in Cluj-Napoca, were included in the study. The inclusion criteria were: age over 18 years, confirmed metastatic disease, ECOG performance status between 0-2, cardiology exam that allowed the administration of chemotherapy, laboratory tests (blood count, liver and kidney function) allowing chemotherapy, a life expectancy greater than 3 months and no prior history of other malignancies. The patients with severe cardiovascular disease, with bone metastases as only location, under anticoagulant treatment, or with chronic severe bleeding disorders were excluded.
All patients were included regardless of chemotherapy regimen followed. The dose of chemotherapy was adjusted to a maximum of 2 square meters body surface area. Bevacizumab dose was calculated based on the weight at the time of administration, the rhythm of administration being considered 5mg / kg every 14 days or 7.5 mg / kg every 21 days. Body mass index was calculated at treatment initiation. The reference values were: underweight / normal value for a BMI <25 kg / sqm and overweight / obese at values of BMI ≥25 kg/sqm.
Treatment response was assessed according to RECIST 1.1 criteria every 3 months from the beginning of treatment (20). The study was approved by the Ethics Board of the Oncology Institute “Prof. Dr. Ion Chiricuta” Cluj-Napoca.
RESULTS
Of the 112 patients included in the study, 57 were women (50.9%) and 55 were men (49.1%). Median age at diagnosis was 55.5 years (between 25 and 76 years). Primary tumors were located in the colon in 67 patients (59.82%) and in the rectum or recto-sigmoid junction in the remaining 45 patients (40.17%). At the time of diagnosis more than half of the patients (n=66, 58.92%) had locally or locoregionally advanced disease (std. III), and a total of 25 (22.3%) patients already had metastatic disease at diagnosis (std. IV) (Table 1).
Table 1.
Patients characteristics
| Sex | n | % |
| F | 57 | 50.89% |
| M | 55 | 49.11% |
| Median age | 55.5 | (25-76) |
| Histopatology | n | % |
| Colonic adenocarcinoma | 67 | 59.82% |
| Rectal adenocarcinoma | 25 | 22.32% |
| Rectosigm. adenocarcinoma | 20 | 17.86% |
| Stage | n | % |
| I | 3 | 2.68% |
| II | 18 | 16.07% |
| III | 66 | 58.93% |
| IV | 25 | 22.32% |
| Metastatic sites | n | % |
| Liver | 63 | 56.25% |
| Liver+lung | 15 | 13.39% |
| Lung | 13 | 11.61% |
| Other Sites | 21 | 18.75% |
All 112 patients had metastatic disease at the time of enrollment in the study, the main sites of metastases were: liver in 63 (56.2%) patients, lung in 13 (11.6%) patients, lung and liver in 15 (13.4%) patients. Other sites of metastases were brain, adrenal, ovarian and bones in 21 (18.75%) patients.
Regarding treatment regimens administered, all patients received bevacizumab as first-line therapy. This was associated either with capecitabine in 24 (21.42%) patients, or with an irinotecan based scheme in a total of 23 (20.53%) patients, or with an oxaliplatin based scheme in a total of 65 (58.03%) patients.
The median number of bevacizumab cycles was 27.5, ranging from a minimum of 6 to a maximum of 87 cycles. Duration of follow-up was 60 months and on completion of this period, 33% (95% CI:25-43) of patients were still alive, the median OS was 37.56 months (Fig. 1). A total of 8% (95% CI: 4-17) of the patients showed no signs of disease progression, the median PFS was 22.6 months (Fig. 2).
Figure 1.
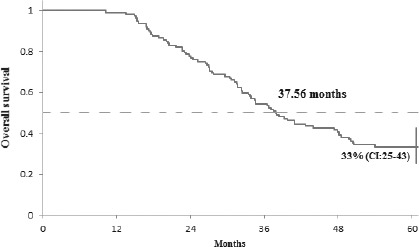
Overall survival of all registered patients. The 5-year OS was 33% (95% CI 25-43). The median OS was 37.56 months.
Figure 2.
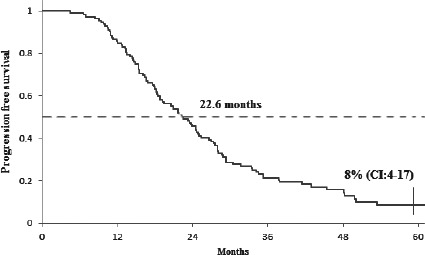
Progression free survival of all registered patiens. The 5-year PFS was 8% (95% CI 4-17). The median PFS was 22.6 months.
Regarding the body mass index value, no significant differences were found in progression free survival or overall survival between patients with BMI values over or under the threshold of 25 kg/sqm). Nevertheless, the median progression free survival was significantly higher in patients with BMI<27 kg/sqm (n=77) than in those with BMI ≥ 27 kg/sqm (n=35): 24 months versus 17.9 months (p = 0.04) (Fig. 3).
Figure 3.
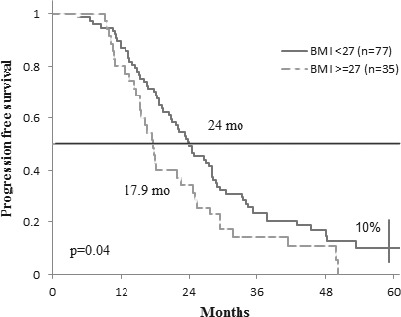
Progression free survival according to BMI.
Overall survival at 5 years was not influenced by the BMI, 35% vs 30% (p=0.29) (Fig. 4).
Figure 4.
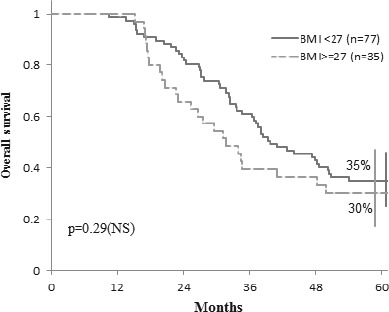
Overall survival according to BMI.
Given that more than half of the patients (56.25%) had liver as the only metastatic site, we analyzed this group of patients to see how BMI affects their evolution during treatment. We found that patients with liver metastases with values of BMI ≥ 27 kg/sqm (n=22) have a median progression free survival significantly lower than patients with a BMI <27 kg/sqm (n=41): 17.5 months versus 24.5 months (p = 0.02) (Fig. 5). The 5 years overall survival in this subset of patients was not influenced by BMI: 39% (BMI <27 kg/sqm, n=41) vs. 22% (BMI ≥ 27 kg/sqm, n=22) (p = 0.09), even though for the group with BMI <27 kg/sqm there is a trend towards an increased survival (Fig. 6).
Figure 5.
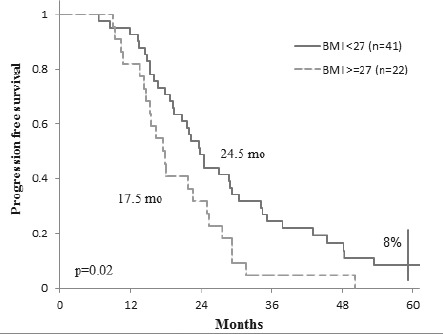
Progression free survival in patients with liver metastasis according to BMI.
Figure 6.
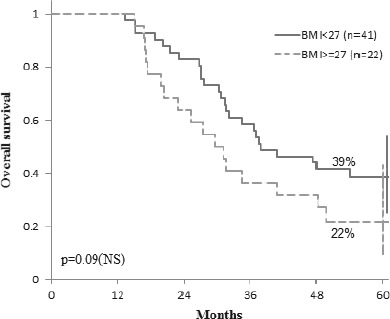
Overall survival in patients with liver metastasis according to BMI.
DISCUSSION
Phase III clinical trials demonstrated that associating bevacizumab to the classic chemotherapy protocols brought an improvement in overall survival and progression free survival (21, 22) for patients with metastatic colorectal cancer. All extensive investigations carried out to this point have not been able to establish valid predictive markers that could influence the effectiveness of targeted treatments antiVEGF. Studies on obese animals demonstrated the development of the resistance to antiVEGF treatments (23), proving that excessive visceral fat could be associated with increased levels of VEGF and subsequently with resistance to antiVEGF therapies in metastatic colorectal cancer patients (19).
Obesity is a well-known risk factor for the development of colorectal cancer (24) and is associated with a high rate of mortality through this pathology (25,26). Even though the pathophysiological mechanism is not yet fully understood, there is reason to believe that fat tissue is involved in producing adipokines and cytokines such as VEGF, with proangiogenic effect, that could disrupt the angiogenesis process (17, 27).
Most studies assessing the association between BMI and mortality from colorectal cancer were performed on localized disease at diagnosis and analyzed the influence of adjuvant treatments. Only one study investigated the way obesity (especially visceral fat) influences the outcomes of patients with metastatic colorectal cancer who received antiVEGF therapy (19). This study tried to determine how body mass index (BMI), subcutaneous fat area (SFA) and visceral fat area (VFA) influenced the outcomes of patients with mCRC treated with first line bevacizumab based systemic therapy.
One hundred and twenty patients with mCRC who received bevacizumab based treatment (n=80) or chemotherapy alone (n=40) as first line therapy were analyzed with a CT scan to measure SFA and VFA. The link between tumour response, time-to-progression (TTP) and overall survival was evaluated.
In the bevacizumab group, median follow-up was 24 months (range 3-70). BMI, SFA and VFA above the median values (BMI ≥23.6 kg/sqm, VFA≥117.58 cm2, SFA≥193.92 cm2) were associated with absence of response. TTP was shorter in patients with high BMI (9 vs. 12 months; p=0.01) or high VFA (9 vs. 14 months; p=0.0008). Overall survival was shorter in patients with high VFA (p=0.0493, median OS was not reached). In multivariate analysis VFA was independently associated with response, TTP and OS (HR=7.18, p=0.008, HR=5.79, p=0.005 and HR=2.88, p=0.027, respectively).
In the chemotherapy group, median follow-up was 30 months (range 4-84) and BMI, SFA and VFA were not associated with response, TTP and OS.
This study provides the evidence that high BMI and VFA predict poorer outcomes in patients with mCRC given bevacizumab as first line systemic therapy.
In conclusion, the present study has met its objectives and demonstrated the negative influence of BMI on both PFS in the entire group of patients and in patients with liver metastases only. BMI cut-off value proved to be 27 kg/square meters.
Our study limitations are related to the relatively small number of patients included in the analysis, and the retrospective design of the study.
In addition, the definition of obesity/overweight is quite controversial, and there are also uncertainties as to whether the BMI is a strong enough indicator for determining the obese/overweight status, as easy and noninvasive a marker as it may be (24).
Our study shows that BMI may be an important prognostic factor with a high clinical relevance in patients with metastatic colorectal cancer following systemic treatments based on antiVEGF therapies (bevacizumab), and could be a predictive marker for treatment response to guide therapeutic decision in many clinical situations.
To confirm our results, larger, prospective studies are needed, as well as perhaps a clearer definition of obesity/ overweight, and indicators other than BMI, such as visceral fat area or subcutaneous fat area based on computed tomography (24), in order to identify the source of excess VEGF and to assess to what extent it influences the response to antiVEGF treatment.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.International Agency for Research on Cancer. World Health Organization GLOBOCAN 2012: Estimated cancer incidence, mortality, and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Voutsadakis IA. Pathogenesis of colorectal carcinoma and therapeutic implications: The roles of the ubiquitin-proteasome system and Cox-2. J Cell Mol Med. 2007;11(2):252–285. doi: 10.1111/j.1582-4934.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Köhne C-H, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes H-G, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240–2247. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 6.Marzouk O, Schofield J. Review of histopathological and molecular prognostic features in colorectal cancer. Cancers. 2011;3:2767–2810. doi: 10.3390/cancers3022767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Obesity. Situation and trends. Accessed March 08, 2017 at site: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/
- 8.World Health Organization Global Status Report on noncommunicable diseases. Chapter 1. Burden: mortality, morbidity and risk factors. 2010. Accessed March 08, 2017 at site: http://www.who.int/nmh/publications/ncd_report_chapter1.pdf?ua=1.
- 9.Cinteza M, Pana B, Cochino E, Florescu M, Margulescu A, Florian A, Vinereanu D. Prevalence and control of cardiovascular risk factors in Romania cardio-zone national study. Maedica A J Clin Med. 2007;2(4):277–282. [Google Scholar]
- 10.Dorobantu M, Badila E, Ghiorghe S, Darabont RO, Olteanu M, Flondor P. Total cardiovascular risk estimation in Romania. Data from the SEPHAR study. Rom J Intern Med. 2008;46(1):29–37. [PubMed] [Google Scholar]
- 11.Roman G, Bala C, Creteanu G, Graur M, Morosanu M, Amorin P, Pircalaboiu L, Radulian G, Timar R, Achimas Cadariu A. Obesity and Health-Related lifestyle factors in the general population in Romania: A cross sectional study. Acta Endocrinologica-Bucharest. 2015;11(1):64–71. [Google Scholar]
- 12.Mihalache L, Graur L, I, Popescu D, Boiculese L, Badiu C, Graur M. The Prevalence of the Metabolic Syndrome and Its Components in A Rural Community. Acta Endocrinologica-Bucharest. 2012;8(4):595–606. [Google Scholar]
- 13.Guilbert JJ. Geneva: World Health Organization; 2002. The world health report 2002: Reducing risks, promoting healthy life. Educ Health (Abingdon) 2003; 16(2):230. [DOI] [PubMed] [Google Scholar]
- 14.World Cancer Research Fund/American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. [Google Scholar]
- 15.Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R, Goldberg RM, Degramont A, O’Connell MJ, Sargent DJ. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–1536. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renfro L, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll H-J, Douillard J-Y, Hurwitz H, Fuchs CS, Diaz-Rubio E, Porschen R, Tournigand C, Chibaudel B, Falcone A, Tebbutt NC, Punt CJ a, Hecht JR, Bokemeyer C, Van Cutsem E, Goldberg RM, Saltz LB, de Gramont A, Sargent DJ, Lenz H-J. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First-Line Clinical Trials in the ARCAD Database. J Clin Oncol. 2016;34(2):144–150. doi: 10.1200/JCO.2015.61.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46(11):1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 18.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29(11):1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 19.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil J-P, Krause D, Hillon P, Borg C, Chauffert B, Ghiringhelli F. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59(3):341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) European J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 22.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 23.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA. 2002;99(16):10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 25.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000;152(9):847–854. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 27.Oros S, Ianas O, Vladoiu S, Giurcaneanu M, Ionescu L, Neacsu E, Voicu G, Stoiceanu M, Rosca R, Neamtu C, Badiu C, Dumitrache C. Does Obesity Protect Postmenopausal Women Against Osteoporosis? Acta Endo Buc. 2012;8(1):67–76. [Google Scholar]


