Abstract
Methods
Ninety Wistar male rats were used in this study. Type 1 diabetes was induced by i.p injection of 50 mg/kg of streptozotocin in all animals. After 42 days of treatment with testosterone (2mg/kg/day) or voluntary exercise alone or in combination, the heart of the rats has been removed and MicroRNA was extracted from the heart using miRCURYTM RNA isolation kit.
Results
Our results showed that either testosterone or exercise increased miRNA-126 expression levels in the heart of diabetic rats. Treatment of diabetic rats with testosterone and exercise at the same time had a synergistic effect on miRNA-126 levels in the heart. Furthermore, in castrated diabetes group, miRNA-126 levels were significantly decreased in heart, whereas either testosterone treatment or exercise training enhanced expression of this miRNA. Also, simultaneous treatment of castrated diabetic rats with testosterone and exercise had an additive effect on miRNA-126 expression levels.
Conclusion
This study showed that testosterone and exercise promote an increase in the expression of miRNA-126 in the heart tissue and this may be related to cardiac angiogenesis. These results may indicate that testosterone and exercise can help to prevent progression of diabetic cardiomyopathy due to impaired angiogenesis in the heart.
Keywords: Diabetes, MiR-126, Heart, Testosterone, Exercise
INTRODUCTION
Diabetes mellitus (DM) is a significant healthcare concern worldwide. Diabetes-induced hyperglycemia results in macro and microvascular complications such as diabetic cardiomyopathy, nephropathy, and retinopathy (1). Diabetic heart disease is the main cause of morbidity and mortality among the people with diabetes, due to marked inhibition of angiogenesis present in coronary heart and peripheral vascular system (2).
Many investigations have demonstrated the role of microRNAs (miRNAs) in many physiological processes, as well as in the regulation of endothelial gene expression during angiogenesis (3, 4). In recent years, several studies have suggested that miR-126 participates in the angiogenesis process. MiR-126 is the most important factor for maintaining vascular integrity during ongoing angiogenesis (5, 6). On the cellular levels, knockdown of miR-126 caused defects in endothelial cell proliferation, migration, tube formation, and sprouting (7). It was shown that deletion of miR-126 in a subset of mutant mice results in vascular leakage, hemorrhaging, and embryonic lethality (8). Clinical study revealed that patients with coronary artery disease and diabetes mellitus 2 had lower circulating miR-126 levels. It was suggested that high glucose levels can lead to a decrease in the miR-126 content in the endothelial particles(9).
Testosterone is the principal male sex hormone and an anabolic steroid (10). Some studies have shown that testosterone is implicated in the regulation of angiogenesis related genes (11, 12). Testosterone deficiency is common in men with diabetes and STZ-induced diabetic male rats (13, 14). It was suggested that cardiovascular risk factors and mortality may increase in individuals with subnormal blood levels of testosterone(15).
Many studies have demonstrated the beneficial effects of exercise on cardiac performance in diabetes mellitus (16, 17). It was shown that exercise can improve blood glucose metabolism, insulin action, and cardiovascular risk factors in diabetic subjects (18). Our previous studies have shown the positive effects of voluntary exercise on cardiovascular diseases (19, 20) . In the animal model of voluntary exercise, the rats have free access to a running wheel and use the wheel according to its physiological threshold for physical activity (21).
The cardiovascular system is an important target of androgen action and exercise, but the evolved molecular mechanism of effect of testosterone and voluntary exercise in the diabetic hearts remains largely unexplored. On the other hand, it was proven that miR-126 participates in the pathogenesis of some diabetic complications (22). Therefore, the aim of this study was to investigate the effect of testosterone administration alone or in combination with exercise training on miR-126 levels in the heart of diabetic or castrated diabetic rats.
METHODS
Animals and experimental design
This research was carried out in accordance with the National Research Council’s protocol for the care and use of laboratory animals. The Ethics Committee of Tabriz University approved the experimental protocol (NO: 92198). Ninety male Wistar rats (250 - 270 g) were provided by the colony of our university and were randomly assigned to testosterone or placebo treatment and sedentary or voluntary exercise groups. Then animals were divided into nine groups (n= 10): 1- Diabetic sham castration + placebo group (Dia-S- Cas), 2-Diabetic + placebo group (Dia), 3-Diabetic + Testosterone group (Dia -T), 4-Diabetic + Exercise + placebo group (Dia-E), 5-Diabetic + Exercise + Testosterone group (Dia-T-E), 6-Diabetic + castrated + placebo group (Dia-Cas), 7- Diabetic + castrated + Testosterone group (Dia-Cas-T), 8-Diabetic + castrated + Exercise + placebo group (Dia-Cas-E) ,9-Diabetic + castrated + Testosterone+ Exercise group (Dia-Cas-T-E) .
With regard to mortality in diabetic animals, resistance to diabetes and the possibility to avoid exercising, the number of samples in each group at the start of the tests, 10 were considered at least, but the analysis was made for an average of 7 animals.
Castration and hormone replacement therapy
Sexually adult male rats were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine hydrochloride (5 mg/kg). After that, the rats subjected to orchiectomy were placed in a supine position and the testes were removed via a low-middle abdominal incision. To avoid disruption of hormonal influence, testosterone replacement began immediately after surgery (23). Testosterone propionate (UNIGEN, Life Science) dissolved in dimethyl sulfoxide (DMSO) was administered subcutaneously at a physiological dose (2 mg/kg /day) for 6 weeks (23). Rats in the Dia-S- Cas, Dia, Dia-E, Dia-Cas and Dia-Cas-E groups were injected with the same amount of DMSO vehicle.
Induction of diabetes
Type 1 diabetes was induced by a single intraperitoneal injection of streptozotocin (55 mg/kg) (Sigma, St. Louis Mo, USA) to all animals. Streptozotocin was dissolved in 10 mM sodium citrate, pH 4.5, with 0.9% NaCl. Diabetes was verified 72h later by evaluating blood glucose levels by using a glucometer (Elegance, Model: no: CT-X10 Germany). Rats with a blood glucose levels ≥ 300 mg/dL were considered to be diabetic.
Voluntary exercise
Rats in exercise groups were housed individually in cages equipped with a stainless-steel vertical running wheel (Tajhiz Gostar, Tehran) and were allowed free access to the wheel 24 h per day for 6 weeks. Rats exercised in the running wheel according to their physiological threshold for physical activity. So, voluntary exercise is considered as mild/ moderate exercise (21). Daily running distance was recorded by a permanent sensor installed on running wheel.
Total RNA extraction and real time PCR
MicroRNA was extracted from the heart tissue using the miRCURYTM RNA Isolation Kit (Exiqon, Vedbaek, Denmark) according to the manufacturer’s protocol. PCR primer sequence for miR-126 was CAUUAUUACUUUUGGUACGCG (www.mirbase.org). The procedure was performed based on spin column using a proprietary resin as a separation matrix for RNA from other cell components. RNA content and purity were measured using Nanodrop 1000 spectrophotometer (Thermo scientific, Wilmington DE 19810 USA). The expression profile of miR-126 was performed on total RNA extracts by using universal cDNA synthesis kit. Briefly, total RNA containing microRNA was polyadenylated and cDNA was synthesized using a poly(T) primer with a 30 degenerate anchor and a 50 universal tag (Exiqon, Vedbaek, Denmark). Each cDNA was used as a template for separate assay for microRNA and mRNA quantitative real-time PCR by using SYBR Green master mix (Exiqon, Vedbaek, Denmark). Real-time PCR reactions were fulfilled on a Bio-Rad iQ5 detection System (Bio-Rad, Richmond, CA, USA).The amount of PCR products were normalized with housekeeping beta-glucuronidase gene for miR-126. The 2-(ΔΔCt) method was used to determine relative quantitative levels of miR-126. The results were expressed as the fold-difference to the diabetic group. The expression profile of miR-126 was performed on total RNA extracts by using the universal complementary DNA (cDNA) synthesis kit. The amount of PCR products were normalized with housekeeping miR -1 for miR-126. The 2− (ΔΔCt) method was used to determine relative quantitative levels of miR-126. The results were expressed as the fold-difference to the relevant diabetic group.
Statistical analysis
All values were analyzed by one or two-way analysis of variance (ANOVA) and also Tukey test was used to compare quantitative data. A value less than 0.05 was considered statistically significant in all cases. Results are expressed as means ± S.E.M. Statistical analysis of data was carried out using SPSS statistical software (Version 17.0).
RESULTS
Glucose results
According to Table 1-A (analysis by two way ANOVA), testosterone or exercise significantly (p<0.001) declined blood glucose levels in Dia group. Also, combination therapy with testosterone and exercise in Dia group had a significant decreasing effect on blood glucose levels compared with Dia-T (p<0.001) and Dia-E (p<0.05) groups. Table 1-B shows that castration significantly (p<0.001) increased blood glucose levels in diabetic rats and treatment of Dia-Cas group with testosterone significantly (p<0.001) decreased blood glucose levels. Two way ANOVA showed that six weeks replacement of testosterone or exercise training significantly (p<0.001) reduced blood glucose levels in the diabetic castrated rats. Also, six weeks of simultaneous treatment of testosterone and exercise in Dia-Cas group significantly (p<0.001) diminished blood glucose levels compared to testosterone or exercise treated groups (Table 1-B). Analysis of data for sham group was not significant in comparison to diabetic group (data are not shown).
Table 1.
Effects of testosterone and exercise on blood glucose concentrations after 6 weeks in diabetic groups (A).
| A. | |||||
| Groups | |||||
| Variants | Dia | Dia-T | Dia-E | Dia-T-E | |
| After 6 weeks BG | 345±23.66 | 312±11.04*** | 313.5±8.40*** | 301±6.54***&&&@ | |
| B. | |||||
| Groups | |||||
| Variants | Dia | Dia-Cas | Dia-Cas-T | Dia-Cas-E | Dia-Cas-T-E |
| After 6 weeks BG | 345±23.66 | 353.16±22.36*** | 336.83±20.63+++ | 323.83±14.83+++ | 321.16±13.24***+++ΔΔΔ ΔΔΔ |
BG: blood glucose (mg/dL).
Effects of testosterone and exercise on body weight and blood glucose concentrations after 6 weeks in diabetic castrated groups (B). Diabetic group (Dia), Diabetic + Testosterone group (Dia -T), Diabetic + Exercise + placebo group (Dia-E), Diabetic + Exercise + Testosterone group (Dia-T-E), Diabetic + castrated + placebo group (Dia-Cas), Diabetic + castrated + Testosterone group (Dia-Cas-T), Diabetic + castrated + Exercise + placebo group (Dia-Cas-E), Diabetic + castrated + Testosterone+ Exercise group (Dia-Cas-T-E). Data are expressed as mean± SEM for 7 animals. *p<0.05 vs. the Dia group. &&& p<0.001 vs. the Dia-Tes group. @ p<0.05 vs. the Dia-Exe group. + p<0.05 vs. the Dia-Cas group. ΔΔΔ p<0.001 vs. the Dia-Cas-E group.
MiR-126 expression in the heart tissue
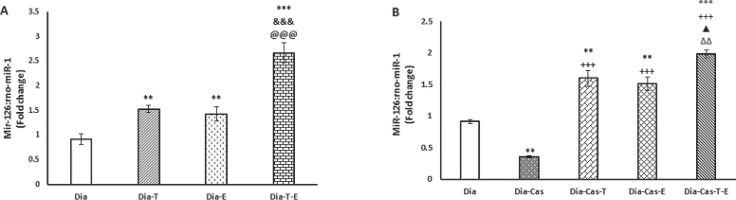
The effects of testosterone and voluntary exercise on miR-126 expression in the heart are shown in Fig. 1. According to Fig. 1-A, treatment of Dia group with testosterone and exercise significantly (P<0.01) enhanced miR-126 expression levels. Also, combination therapy with testosterone and exercise in diabetic rats showed a significant (p<0.001) increasing effect on miR-126 expression levels compared to testosterone or exercise alone treated groups. Figure 1-B shows that castration significantly (P<0.01) decreased miR-126 expression in the heart of Dia groups. Six weeks replacement of testosterone or voluntary exercise in Dia-Cas group, significantly (p<0.001) enhanced miR-126 expression in the heart compared to testosterone or exercise alone treated groups. Also, simultaneous treatment with testosterone and exercise in Dia-Cas group significantly increased miR-126 expression compared to Dia-Cas-T group (p<0.05) and Dia-Cas-E group (p<0.01). Analysis of data for sham group did not show any significant difference in comparison to diabetic group (data are not shown).
Figure 1.
Effects of testosterone and exercise on miRNA-126 expression levels in the heart after 6 weeks in diabetic groups (A). Effects of testosterone and exercise on miRNA-126 expression levels in the heart after 6 weeks in diabetic castrated groups (B). Effects of testosterone and exercise on miRNA-126 expression levels in the heart after 6 weeks in diabetic castrated groups. Diabetic group (Dia), Diabetic + Testosterone group (Dia -T), Diabetic + Exercise + placebo group (Dia-E), Diabetic + Exercise + Testosterone group (Dia-T-E), Diabetic + castrated + placebo group (Dia-Cas), Diabetic + castrated + Testosterone group (Dia-Cas-T), Diabetic + castrated + Exercise + placebo group (Dia-Cas-E) , Diabetic + castrated + Testosterone+ Exercise group (Dia-Cas-T-E). Data are expressed as mean± SEM for 7 animals. ** p<0.01, *** p<0.001 vs. the Dia group. &&& p<0.001 vs. the Dia-Tes group. @@@p<0.001 vs. the Dia-Exe group. +++ p<0.001 vs. the Dia-Cas group. ▲ p<0.05 vs. the Dia-Cas-Tes group.ΔΔ p<0.01 vs. the Dia-Cas-E group.
DISCUSSION
The present study demonstrated for the first time that testosterone and exercise increased miR-126 expression levels in the heart of diabetic rats. Our findings showed that castration impaired the expression of miR-126 in the heart of diabetic rats and testosterone-replacement therapy increased levels of miRNA. Also, we demonstrated that simultaneous treatment of diabetic and castrated diabetic rats with testosterone and exercise had enhanced effect on expression of miR-126. Our results exhibited that exercise trained groups had enhanced levels of miR-126 compared with sedentary groups. Moreover, our results displayed that the fasting blood glucose levels of diabetic and castrated diabetic rats were reduced by testosterone treatment or exercise training.
Since cardiovascular disease is a major complication of diabetes, there are several evidences to suggest that diabetes condition causes structural and functional abnormalities in the myocardium and vasculature. Although it was shown that diabetes impaired angiogenesis in the myocardial tissue of animal models of ischemia and in ischemic human hearts (24), the underlying molecular mechanisms are not being known completely.
miR-126 is one of the most abundant miRNAs in vascularized tissues that acts upon various transcripts to control angiogenesis (25). It has been shown that targeted deletion of miR-126 in mice can result in vascular leakage, hemorrhaging, and embryonic lethality (6). In addition, there are some evidences that circulating serum miR-126 levels are related to the development and severity of heart failure and circulating levels of miR-126 decrease in patients with coronary artery disease and diabetes mellitus (25). It is also suggested that high glucose levels lead to reduction in the miR-126 content of endothelial apoptotic bodies (9). MiR-126 might induce angiogenesis by repression of Spred-1 (Sprouty-related protein 1), a negative regulator of MAP kinase signaling and phosphoinositol-3 kinase regulatory subunit 2 (PI3KR2, also known as p85-A), which suppresses the activity of PIK3/Akt/eNOS pathways (6).
Testosterone regulates angiogenesis related genes and is involved in cell proliferation and angiogenesis in the myocardium (11, 12). It was proven that testosterone supplementation increased angiogenesis in the heart of castrated rats (26). Both diabetes and the metabolic syndrome are associated with a modest reduction in testosterone, in magnitude comparable to the effect of 10 years of aging (13, 27). Recent study has shown that male STZ-induced diabetic rats had lower testosterone levels compared with non-diabetic rats (28). These observations and other scientific evidence are suggesting that testosterone deficiency contributes or at least is associated with cardiovascular risk factors in diabetic subjects (28, 29). However, the molecular mechanisms of testosterone effect in cardiac angiogenesis are not yet known exactly. Our study is the first to show that testosterone therapy raised miRNA126 expression in the heart of castrated diabetic and diabetic rats. Moreover, our results displayed that testosterone therapy reduced the means of fasting blood glucose levels in the Dia and Dia-Cas groups. In agreement with our results, Rao and colleagues suggested that testosterone may improve glucose metabolism (29). Therefore, testosterone might promote cardiac angiogenesis by increasing miRNA-126 expression through glucose lowering mechanisms.
Physical activity has satisfying effect on cardiovascular function and is salutary to diabetic subjects (30). Several studies showed the impact of exercise on circulating miRNAs (31-33). It was reported that aerobic exercise training promotes an increase in the expression of miRNA-126 in the heart of rats (34). Also, it has been shown that resistance exercise enhanced circulating miRNA-126 in healthy individuals (35). Da Silva and colleagues reported that swimming exercise increased angiogenesis in the heart of rats through miRNA-126 by inhibiting negative regulators of the VEGF pathway (36). Our study revealed that voluntary exercise increased expression of miRNA-126 in the heart of Dia and Dia-Cas groups. In addition, our findings displayed that; exercise can lead to desirable changes in circulating levels of glucose in Dia and Dia-Cas groups. These data confirm previous studies that exercise decreased the means of fasting blood glucose levels (37), increased the blood flow and delivery of glucose to the skeletal muscle (38).
Furthermore, this study exhibits that testosterone treatment and exercise training have an additive effect on expression of miRNA-126 in the heart of diabetic rats. In accord with our study, it is reported that, when exercise is added to testosterone supplementation, cardiac remodelling process, as structural or biochemical changes, becomes more efficient (39). This is also in line with findings of Fry and Lohnes that physical exercise can elicit a heightening testosterone response (40).
In conclusion, this study showed that testosterone treatment and exercise training promote an increase in the expression of miRNA-126 in the heart of diabetic and castrated diabetic rats. This effect may be related to cardiac angiogenesis induced by testosterone and exercise by indirect regulation of the VEGF pathway and by direct regulation of its target genes (Spred-1 and PI3KR2), which was associated with increasing the activity of the VEGF pathway and may be through the angiogenic pathways MAPK and PI3K/Akt/eNOS. These findings suggest that miRNA-126 is a potential therapeutic target for vascular complications in diabetes.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
Support of this investigation by the Drug Applied Research Center, Tabriz University of Medical Sciences through grant, is gratefully acknowledged.
References
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in health and disease. Nature medicine. 2003;9(6):653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 3.Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascular pharmacology. 2011;55(4):79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Heusschen R, van Gink M, Griffioen AW, Thijssen VL. MicroRNAs in the tumor endothelium: novel controls on the angioregulatory switchboard. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2010;1805(1):87–96. doi: 10.1016/j.bbcan.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Developmental cell. 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Developmental cell. 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M, Vos JB, de Boer HC, Quax PH, Rabelink TJ, van Zonneveld AJ. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13(8a):1577–1585. doi: 10.1111/j.1582-4934.2008.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Solingen C, de Boer HC, Bijkerk R, Monge M, van Oeveren-Rietdijk AM, Seghers L, de Vries MR, van der Veer EP, Quax PH, Rabelink TJ, van Zonneveld AJ. MicroRNA-126 modulates endothelial SDF-1 expression and mobilization of Sca-1+/Lin− progenitor cells in ischaemia. Cardiovascular research. 2011;92(3):449–455. doi: 10.1093/cvr/cvr227. [DOI] [PubMed] [Google Scholar]
- 9.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in type 2 DiabetesNovelty and significance. Circulation research. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 10.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5(3):427–448. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Death AK, McGrath KC, Sader MA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-κB-dependent pathway. Endocrinology. 2004;145(4):1889–1897. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 12.Ng MK, Quinn CM, McCrohon JA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS, Death AK. Androgens up-regulate atherosclerosis-related genes in macrophages from males but not females: molecular insights into gender differences in atherosclerosis. J Am Coll Cardiol. 2003;42(7):1306–1313. doi: 10.1016/j.jacc.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, Zajac JD, Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, Wells CC, Garman JH, Asico L, Escano CS, Maric C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension. 2008;51(4):1218–1224. doi: 10.1161/HYPERTENSIONAHA.107.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malkin CJ, Pugh PJ, Morris PD, Kerry KE, Jones RD, Jones TH, Channer KS. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart. 2004;90(8):871–876. doi: 10.1136/hrt.2003.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodiwiss AJ, Kalk WJ, Norton GR. Habitual exercise attenuates myocardial stiffness in diabetes mellitus in rats. American Journal of Physiology-Heart and Circulatory Physiology. 1996;271(5):H2126–H33. doi: 10.1152/ajpheart.1996.271.5.H2126. [DOI] [PubMed] [Google Scholar]
- 17.Loganathan R, Bilgen M, Al-Hafez B, Zhero SV, Alenezy MD, Smirnova IV. Exercise training improves cardiac performance in diabetes: in vivo demonstration with quantitative cine-MRI analyses. Journal of Applied Physiology. 2007;102(2):665–672. doi: 10.1152/japplphysiol.00521.2006. [DOI] [PubMed] [Google Scholar]
- 18.Ljubisavijevic M. Effects of voluntary exercise on heart function in streptozotocin (STZ)–induced diabetic rat. Int J Diabetes & Metabolism. 2007;15:32–37. [Google Scholar]
- 19.Chodari L, Mohammadi M, Ghorbanzadeh V, Dariushnejad H, Mohaddes G. Testosterone and Voluntary Exercise Promote Angiogenesis in Hearts of Rats with Diabetes by Enhancing Expression of VEGF-A and SDF-1a. Canadian Journal of Diabetes. 2016;40(5):436–441. doi: 10.1016/j.jcjd.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Chodari L, Mohammadi M, Mohaddes G, Alipour MR, Ghorbanzade V, Dariushnejad H, Mohammadi S. Testosterone and Voluntary Exercise, Alone or Together Increase Cardiac Activation of AKT and ERK1/2 in Diabetic Rats. Arquivos Brasileiros de Cardiologia. 2016;107(6):532–541. doi: 10.5935/abc.20160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kizaki T, Maegawa T, Sakurai T, Ogasawara JE, Ookawara T, Oh-ishi S, Izawa T, Haga S, Ohno H. Voluntary exercise attenuates obesity-associated inflammation through ghrelin expressed in macrophages. Biochemical and biophysical research communications. 2011;413(3):454–459. doi: 10.1016/j.bbrc.2011.08.117. [DOI] [PubMed] [Google Scholar]
- 22.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circulation research. 2010;107(5):677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Fu L, Han Y, Teng Y, Sun J, Xie R, Cao J. Testosterone replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing expression of cytokines HIF-1a, SDF-1a and VEGF. European journal of pharmacology. 2012;684(1):116–124. doi: 10.1016/j.ejphar.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Abaci A, Oğuzhan A, Kahraman S, Eryol NK, Unal S, Arinç H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99(17):2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 25.Olson EN. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Science translational medicine. 2014;6(239):239ps3–ps3. doi: 10.1126/scitranslmed.3009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lissbrant IF, Lissbrant E, Persson A, Damber J-E, Bergh A. Endothelial cell proliferation in male reproductive organs of adult rat is high and regulated by testicular factors. Biology of reproduction. 2003;68(4):1107–1111. doi: 10.1095/biolreprod.102.008284. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes. Diabetes care. 2007;30(4):911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 28.Khaneshi F, Nasrolahi O, Azizi S, Nejati V. Sesame effects on testicular damage in streptozotocin-induced diabetes rats. Avicenna J Phytomed. 2013;3(4):347–355. [PMC free article] [PubMed] [Google Scholar]
- 29.Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nature Reviews Endocrinology. 2013;9(8):479–493. doi: 10.1038/nrendo.2013.122. [DOI] [PubMed] [Google Scholar]
- 30.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109(2):220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 31.Radom-Aizik S, Zaldivar F, Leu SY, Adams GR, Oliver S, Cooper DM. Effects of exercise on microRNA expression in young males peripheral blood mononuclear cells. Clinical and translational science. 2012;5(1):32–38. doi: 10.1111/j.1752-8062.2011.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makarova JA, Maltseva DV, Galatenko VV, Abbasi A, Maximenko DG, Grigoriev AI, Tonevitsky AG, Northoff H. Exercise Immunology Meets MiRNAs. Exerc Immunol Rev. 2014;20:135–164. [PubMed] [Google Scholar]
- 33.Radom-Aizik S, Zaldivar F, Jr., Oliver S, Galassetti P, Cooper DM. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J Appl Physiol. 2010;109(1):252–261. doi: 10.1152/japplphysiol.01291.2009. (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes T, Baraúna VG, Negrão CE, Phillips MI, Oliveira EM. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. American Journal of Physiology-Heart and Circulatory Physiology. 2015;309(4):H543–H52. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlemann M, Möbius-Winkler S, Fikenzer S, Adam J, Redlich M, Möhlenkamp S, Hilberg T, Schuler GC, Adams V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. European journal of preventive cardiology. 2014;21(4):484–491. doi: 10.1177/2047487312467902. [DOI] [PubMed] [Google Scholar]
- 36.Da Silva Jr ND, Fernandes T, Soci U, Monteiro A, Phillips MI, de Oliveira EM. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Medicine and science in sports and exercise. 2012;44(8):1453–1462. doi: 10.1249/MSS.0b013e31824e8a36. [DOI] [PubMed] [Google Scholar]
- 37.Oghbaei H, Asl NA, Sheikhzadeh F, Alipour MR. The Effect of Regular Moderate Exercise on miRNA-192 Expression Changes in Kidney of Streptozotocin-Induced Diabetic Male Rats. Advanced pharmaceutical bulletin. 2015;5(1):127. doi: 10.5681/apb.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren M. Physical activity: exercise prescription for the older adult with type 2 diabetes. Topics in Geriatric Rehabilitation. 2010;26(3):221–232. [Google Scholar]
- 39.Gonçalves L, de Souza RR, Maifrino LB, Caperuto ÉC, Carbone PO, Rodrigues B, Gama EF. Resistance exercise and testosterone treatment alters the proportion of numerical density of capillaries of the left ventricle of aging Wistar rats. The Aging Male. 2014;17(4):243–247. doi: 10.3109/13685538.2014.919252. [DOI] [PubMed] [Google Scholar]
- 40.Fry A, Lohnes C. Acute testosterone and cortisol responses to high power resistance exercise. Human physiology. 2010;36(4):457–461. [PubMed] [Google Scholar]