Abstract
Objective
Gestational diabetes mellitus (GDM) is a common endocrine complication in pregnancy. There are few risk factors that clearly correlate with GDM. Fibroblast growth factor 21 (FGF21) is a metabolic hormone that can regulate glucose metabolism. It has been recognized that serum levels of FGF21 are significantly increased in diabetes and insulin resistance states. The objective of this study was to determine the serum FGF21 levels in women with GDM compared with non-GDM women and its correlation with insulin resistance.
Methods
Thirty GDM patients and 60 healthy pregnant controls that matched for maternal and gestational age were selected. Women with previous history of GDM, hypertension, polycystic ovary syndrome, renal or liver failure and drug consumption with effects on glucose or insulin levels were excluded. FGF21 was determined and correlated with biochemical parameters of glucose metabolism and insulin resistance.
Results
FGF21 concentration was significantly higher in GDM (264.5±196.2 ng/L) as compared with control groups (59.1±36.5ng/L). Correlation of FGF21 with insulin resistance was not significant. A cut-off 82.07 ng/L of FGF21 had sensitivity of 100% and specificity of 85% for prediction of GDM.
Conclusion
FGF21 is increased in GDM and it is independent of insulin resistance. We suggest that FGF21 resistance could be directly involved in pathophysiology of GDM.
Keywords: GDM, FGF21, pregnancy, insulin resistance
BACKGROUND
Gestational diabetes mellitus (GDM) is one of the most common endocrine complications during pregnancy, resulting in perinatal and neonatal problems (1). GDM is a known predisposing factor for future type 2 diabetes and obesity for both mother and child (2). According to a meta- analysis in 2015, the prevalence of GDM in IRAN was 3.41% (lowest and highest rates were 1.3, 18.6% respectively) (3) and it has markedly been increasing in recent years (4). Insulin resistance and reduction in the pancreatic beta cell reserve for insulin secretion contribute to the development of GDM (3, 5). Fibroblast growth factor 21 (FGF21) is a metabolic hormone produced by the liver, adipose tissue, skeletal muscle, and pancreas that is related to glucose metabolism and insulin resistance (6-7). It has been recently reported that serum level of FGF21 is higher in insulin resistance status and obesity (FGF21 resistance) (8-9). Several studies reported that FGF21 is a strong independent predictor for type 2 diabetes (10-11). Association of FGF21with GDM has not been evaluated so far. As we reviewed the literature, there was no similar study in Iran and this study is performed the first time. In this study, we aimed to evaluate whether maternal FGF21 is altered in GDM compared to non-GDM women.
METHODS AND MATERIALS
Thirty women with GDM diagnosis (18 of GDM patients controlled with diet and 12 of them underwent insulin therapy) and 60 normoglycemic (control) women matched for gestational age were recruited for this study. Fasting blood glucose (FBG) had been obtained after 8 hours of fasting, for all participants at the first prenatal visit in the first trimester to exclude undiagnosed preexisting diabetes. Women with previous history of GDM, polycystic ovary syndrome, hypertension, preeclampsia, renal or liver disease and who were taking drugs with effects on glucose metabolism were excluded from the study. After a complete explanation of the project, demographic characteristics were recorded in first prenatal visit, including age, height, weight, blood pressure and body mass index (BMI). BMI was calculated according to the formula: Weight (kg)/height (m2). Blood pressure of the right arm was taken in a sitting position after resting for ten minutes. OGTT was done at 24-28 weeks of gestation.
GDM was defined as at least one glucose level greater than following thresholds during 75 gram, 2 hour oral glucose tolerance test (OGTT): fasting≥ 92 mg/dL (5.1 mmol/L), 1 h≥ 180 mg /dL (1 0. 0 mmol/L), 2 h≥ 153 mg /dL (8.5 mmol/L) (on a recommendation of the international Association of the Diabetes and Pregnancy Study Groups (IADPSG). Serum FGF21 levels were measured in fasting samples at the time of OGTT by FGF-21 Human ELISA Kit (ab125966). Insulin level was assessed by immunoradiometric assay and Insulin resistance was estimated with the homeostasis model assessment-insulin resistance (HOMA-IR) index (fasting blood sugar (mmol/lit) * insulin (mIU/L)/22.5). Clinical and laboratory variables of patients were classified into two categories according to results of OGTT and then these groups were compared together.
Statistical analysis
Statistical analysis was done by using SPSS version 11.5. Data was expressed as mean ± SD. Student t. test or ANOVA was used for variables with normal distribution and quantitative variables with non-normal distribution were compared with Mann-Withney. Qualitative variables between the groups were analyzed by chi-square. Based on quartiles of FGF21 concentration participants were divided into 4 groups and GDM prevalence compared between groups. Receiver operating characteristic (ROC) curves were used to investigate FGF21 cut-off points to identify GDM. Sensitivity, specificity, positive predictive value and negative predictive value of FGF21 were calculated for estimation of GDM development.
Ethical statement
The study protocol was approved by the local Ethics Committee (The research ethics committee of Mashhad University of Medical Sciences), and was conducted according to the principles of Helsinki Declaration. Written informed consent was obtained from all patients.
RESULTS
Baseline clinical and paraclinical characteristics of 90 patients are shown in Table 1.
Table 1.
Baseline characteristics of patients (n= 90)
| Characteristics | Mean± SD |
| Age (years) | 25.4±5.1 |
| Body mass index ( kg/m2) | 24.6± 4.9 |
| Systolic blood pressure( mm Hg) | 110.2 ± 14.5 |
| Diastolic blood pressure(mm Hg) | 67.2± 8.3 |
| Fgf21(ng/L) | 130.8±153.9 |
Data are expressed as means ± SD.
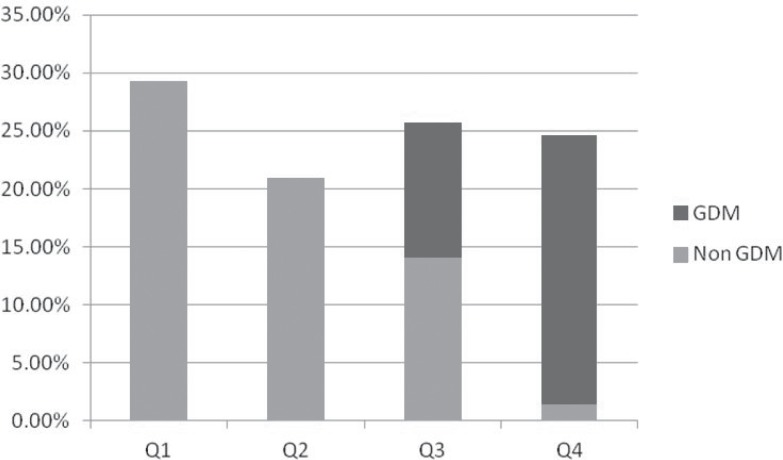
Comparison of laboratory and clinical parameters between GDM and non- GDM is shown in Table 2. Mean FGF21 level was significantly higher in patients with GDM and subjects without GDM. Correlation between FGF21 level with blood pressure and BMI was not significant. We divided FGF21 level into quartiles: 29.1% of all subjects had FGF21 level ≤42.69 (quartile 1), 20.9% of patients had FGF21 level between 42.7-83.44 ng/L (quartile 2), in 25.6 % of patients FGF21 level was between 83.45-154.1 ng/L (quartile 3) and 24.4 % of patients were in quartile 4 with FGF21 more than 154.1 ng/L. Figure 1 shows the distribution of GDM in FGF21 quartile.
Figure 1.
Prevalence of GDM by FGF21 quartile.
Table 2.
Comparison of clinical and paraclinical characteristics between GDM and non-GDM
| Variable | GDM | Non-GDM | P |
| Age (year) | 26.8±6.3 | 24.8±4.3 | 0.08 |
| Body mass index(kg/m2) | 26.0 ±5.9 | 23.9±4.3 | 0.07 |
| Systolic blood pressure (mm Hg) | 120.9±11.4 | 104.4±12.6 | <0.001 |
| Diastolic blood pressure (mm Hg) | 72.9±6.8 | 64.1±7.4 | <0.001 |
| FBG (mg/dL) | 90.0±13.3 | 72.1±6.8 | <0.001 |
| 1 hour after 75 g glucose (mg/dL) | 158.6±33.7 | 115.4±20.0 | <0.001 |
| 2 hours after 75 g glucose (mg/dL) | 142.9±37.3 | 95.5±14.8 | <0.001 |
| Insulin (m IU/L) | 11.8±5.8 | 10.7±4.2 | 0.27 |
| HOMA-IR | 2.6±1.3 | 2.3±1.2 | 0.32 |
| FGF21(ng/L) | 189.2(110.5-1087.7) | 54.9(3.12-166.0) | <0.001 |
FBS, fasting blood glucose;HOMA-IR, homeostasis model assessment-insulin resistance FGF21, Fibroblast growth factor 21:
- Data are expressed as means ± SD and median;
- P value <0.05 is considered significant;
- T. test was used for analysis of normal distribution variable and Kruskal Wallis for non normal distribution variable.
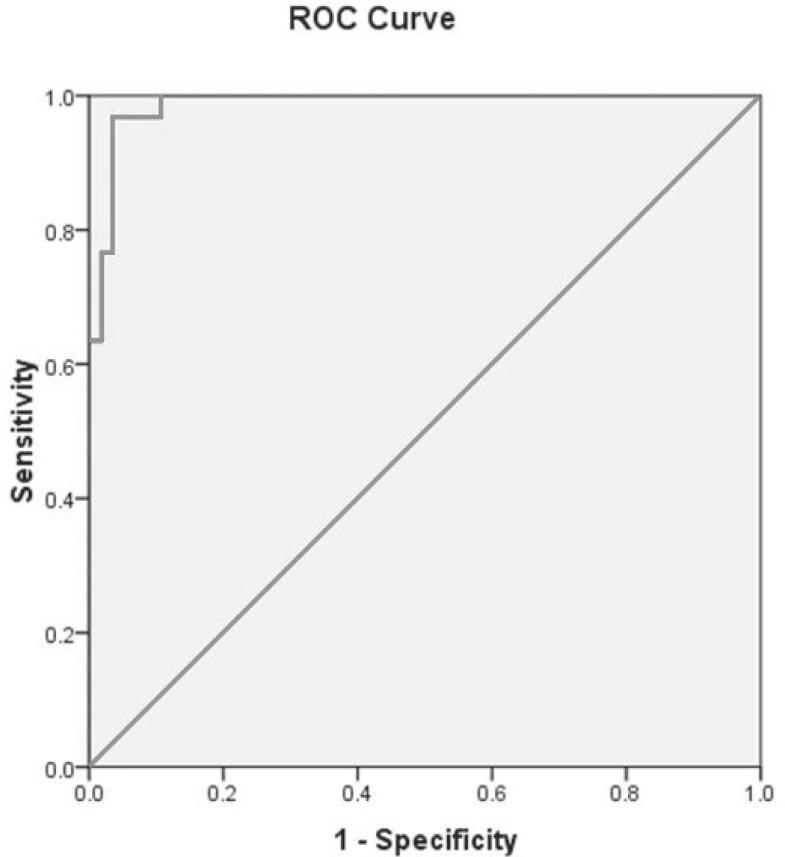
According to this figure, patients with higher level of FGF21 had a significant higher rate of GDM. A ROC curve for the prediction of GDM is based on the FGF21 (Fig. 2). Cut-off point of 82.07 had C statics of 0.95 (95% CI: 0.96-1.005, P<0.001). Sensitivity of this parameter was 100% and specificity was 75%. Positive predictive value of this test and negative predictive value were 68.2% and 100% respectively.
Figure 2.
ROC curves for the prediction of GDM based on the FGF21 concentration.
DISCUSSION
In this study, serum level of FGF21 is determined for the first time in pregnant patients in Iran, to our knowledge. Our results showed that serum FGF21 level is significantly higher in GDM patients as compared with healthy pregnant controls. There was not any significant difference in maternal and gestational ages between two groups and we excluded pregnant women with risk factors for GDM. Our results are consistent with some previous studies. Wang et al. in 30 patients with GDM and 60 healthy control groups found that FGF21 levels were significantly higher in GDM patients (12). Dekker Nitert et al. showed that FGF21 is expressed in placenta and is increased in GDM compared with healthy pregnant subjects (13). There are several studies that showed association between FGF21 and diabetes. In a large Chinese prospective by Chen et al., FGF21 levels increased progressively with dysglycemia and this author concluded that FGF21 predicted the development of diabetes (14).FGF21 improves the function of pancreatic beta cell and induces glucose uptake in fat cells (15). It is suggested that FGF21 is a mediator for metabolic function of peroxisome proliferator –activated receptor α agonists (6, 16). It seems that in metabolic imbalance states, FGF21 resistance and compensatory up-regulation of this growth factor has occurred. Inflammation, metabolic dysregulation and hyperglycemia may cause defective FGF21 signaling. Glucotoxicity impairs FGF21 actions in pancreatic islet cells (17). High blood pressure is a component of metabolic syndrome. In our study, blood pressure was significantly higher in GDM patients compared with non-GDM women. This difference may be related to changes in FGF21 level in two groups. Semba et al. showed that serum FGF21 levels are independently associated with hypertension in community-dwelling adults (18).
In the current study, insulin resistance is not significantly different between GDM subjects with control groups and we failed to find a significant relation between FGFG21 and insulin resistance. This finding is in contrast to several previous studies indicating that FGF21 correlated positively with insulin resistance (19). On the other hand this finding is in accordance with a previous study by Chen et al. that found FGF21 is an independent predictor for type 2 diabetes after adjustment for insulin levels. It seems that FGF21 is an independent causal factor for GDM.
There are several limitations in our study. Cross sectional design of this study, low sample size and not considering other known biomarkers for GDM are limitations of this study.
In conclusion, we demonstrated that serum levels of FGF21 were significantly increased in GDM compared with healthy pregnant women and FGF21 is not correlated with insulin resistance. Clearly, prospective large studies are needed to better elucidate this association and pathophysiological significance of FGF21 in GDM.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank the Vice Chancellor of Research (MUMS) for financial support of this study. We are also grateful to all co-workers who helped us in the procedure of this study which was a dissertation of an MD degree in medicine.
References
- 1.Larijani B. A review on the prevalence of gestational diabetes mellitus (GDM) in different regions of Iran. J Diabetes Metab Disord. 2009;8:7. [Google Scholar]
- 2.Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. Am J Obstet Gynecol. 2012;207(4):322. doi: 10.1016/j.ajog.2012.06.049. e1-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jafari-Shobeiri M, Ghojazadeh M, Azami-Aghdash S, Naghavi-Behzad M, Piri R, Pourali-Akbar Y, Nasrolah-Zadeh R, Bayat-KHajeh P, Mohammadi M. Prevalence and Risk Factors of Gestational Diabetes in Iran: A Systematic Review and Meta-Analysis. Iran J Public Health. 2015;44(8):1036–1044. [PMC free article] [PubMed] [Google Scholar]
- 4.Manafi M, Khadem-Ansari M. Gestational diabetes mellitus in Iranian women: a rising rate. Acta Endocrinologica (Buch) 2013;9(1):71–78. [Google Scholar]
- 5.Khosrowbeygi A, Shiamizadeh N, Taghizadeh N. Maternal circulating levels of some metabolic syndrome biomarkers in gestational diabetes mellitus. Endocrine. 2015:1–11. doi: 10.1007/s12020-015-0697-4. [DOI] [PubMed] [Google Scholar]
- 6.Woo Y, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clinical endocrinology. 2013;78(4):489–496. doi: 10.1111/cen.12095. [DOI] [PubMed] [Google Scholar]
- 7.Panahi Y, Bonakdaran S, Yaghoubi MA, Keramati MR, Haratian M, Sahebkar A. Serum Levels of Fibroblast Growth Factor 21 in Type 2 Diabetic Patients. Acta Endocrinologica (Buch) 2016;12(3):257–261. doi: 10.4183/aeb.2016.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou Z-G, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 9.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, DeFronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng X, Zhu B, Jiang F, Fan H. Serum FGF-21 levels in type 2 diabetic patients. Endocrine research. 2011;36(4):142–148. doi: 10.3109/07435800.2011.558550. [DOI] [PubMed] [Google Scholar]
- 11.Su SL, Kuo CL, Huang CS, Liu CS. Fibroblast growth factor 21 predicts type 2 diabetes in Taiwanese. Changhua J Med. 2013;11:42–47. [Google Scholar]
- 12.Wang D, Zhu W, Li J, An C, Wang Z. Serum concentrations of fibroblast growth factors 19 and 21 in women with gestational diabetes mellitus: association with insulin resistance, adiponectin, and polycystic ovary syndrome history. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0081190. e81190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker Nitert M, Barrett HL, Kubala MH, Scholz Romero K, Denny KJ, Woodruff TM, McIntyre HD, Callaway LK. Increased placental expression of fibroblast growth factor 21 in gestational diabetes mellitus. J Clin Endocrinol Metab. 2014;99(4):E591–E8. doi: 10.1210/jc.2013-2581. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Cheung BM, Tso AW, Wang Y, Law LS, Ong KL, Wat NM, Xu A, Lam KS. High plasma level of fibroblast growth factor 21 is an independent predictor of type 2 diabetes a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care. 2011;34(9):2113–2115. doi: 10.2337/dc11-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J-J, Foo JP, Liu S, Lim SC. The role of fibroblast growth factor 21 in diabetes and its complications: a review from clinical perspective. Diabetes Res Clin Pract. 2015;108(3):382–389. doi: 10.1016/j.diabres.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Ishida N. Role of PPAR in the Control of Torpor through FGF21-NPY Pathway: From Circadian Clock to Seasonal Change in Mammals. PPAR Res. 2009 doi: 10.1155/2009/412949. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol. 2009;71(3):369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Crasto C, Strait J, Sun K, Schaumberg DA, Ferrucci L. Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J Hum Hypertens. 2013;27(6):397–399. doi: 10.1038/jhh.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein S, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, Lössner U, Blüher M, Stumvoll M, Fasshauer M. Serum fibroblast growth factor 21 levels in gestational diabetes mellitus in relation to insulin resistance and dyslipidemia. Metabolism. 2010;59(1):33–37. doi: 10.1016/j.metabol.2009.07.003. [DOI] [PubMed] [Google Scholar]