Abstract
Introduction
Vitamin D (VD) levels were correlated with different health conditions, including reproductive disorders in males. Vitamin D action is mediated through vitamin D receptor (VDR), which acts as a transcription factor. VDR gene promoter is embedded in a GC-rich island. The VDR gene has been shown to have several polymorphisms that affect the receptor function.
Aim
To examine the relationship between Cdx-2 polymorphism (rs17883968), the methylation status of VDR’s promoter and serum levels of 25-hydroxyvitamin D in male infertility.
Patients and Methods
A total of 69 infertile men and 37 age-matched controls were enrolled in this study. Vitamin D level assessments were detected using the electrochemiluminescent method. Cdx-2 VDR polymorphism identification was performed by PCR on DNA samples from blood, followed by restriction. Methylation of VDR gene promoter was assessed by qMS-PCR using bisulfite-treated DNA from fresh sperm.
Results
Vitamin D levels was found to be significantly decreased in infertile groups compared the controls (p=0.0279). The GG genotype was found in a higher percentage in controls and the AA genotype was higher in infertile group (p=0.0056). Infertile homozygote (GG) and heterozygote (GA) individuals had significantly higher vitamin D levels than AA homozygote. Methylation is higher in individuals with lower vitamin D levels and AA genotype is characterized by higher methylation values.
Conclusion
The results provide new insights of Cdx-2 polymorphism is involved in vitamin D deficiency, highlighting the important role of epigenetic modification of vitamin D receptor and male infertility along with the genetic context.
Keywords: epigenetics, male infertility, vitamin D, VDR Cdx-2 polymorphism
INTRODUCTION
The role of vitamin D as an endocrine effector is supported by its expression in each cell type (1-3). Most types of cells are able to produce the active hormone in an autocrine manner by local expression and activity of CYP27B1. Vitamin D action is mediated through vitamin D receptor (VDR), which acts as a transcription factor. VDRs belong to the steroid receptor gene family and are intracellular polypeptides (50–60 kDa). VDR specifically bind 1,25-dihydroxycholecalciferol and exhibit zinc-finger containing DNA binding domain that facilitates the interaction with vitamin D response elements in the target genes (4). VDR recruits large and diverse coregulatory machines, which contain chromatin remodeling complexes. Three regulatory complexes were found to interact with the VDR: SWI/SNF an ATPase-containing, chromatin remodeling complex, a histone acetylation complex containing histone acetyltransferases (HAT) and a mediator complex that facilitates the activation of RNA pol II through its C-terminal domain (CTD) (5). Therefore, a large number of genes is regulated by 1,25 (OH)2D (1).
The human VDR gene and its promoter were characterized in 1997 by Miyamoto et al. (8). Located in 12q12-q14 region in humans, VDR gene contains 11 exons and spans approximately 75 kb (10, 11). In males, VDR and VD metabolizing enzymes are expressed in Sertoli cells, germ cells, Leydig cells, mature spermatozoa and in the epithelial cells lining the male reproductive tract (2, 3, 6, 7).
Vitamin D signaling pathway alterations, especially those involving VDR may provide new insights about its abnormal uptake, metabolism, and the role in disease development. The VDR gene has been shown to have several polymorphisms. VDR-FokI (rs2228570; C>T), VDR-BsmI (rs1544410; A>G), VDR-TaqI (rs731236; C>T) and VDR-ApaI (rs7975232; A>C) (10) being the most studied. Arai et al. (12) have described a Cdx2 polymorphism in a promoter region of VDR gene. The (rs17883968) polymorphism G→A exists at a binding site for an intestinal-specific transcription factor called CDX2 (caudal type homeobox 2). The Cdx2 polymorphism in the promoter region of VDR gene modulates its activity, and the allele G of Cdx2 (the significant allele) exhibits 30% less transcriptional activity compared to allele A (13).
Taking into account that the promoter of the VDR gene is embedded in a GC-rich island (14) with five binding motifs for the transcription factor SP1 (lying between nucleotide (nt) −72 and −34 relative to the transcription start site) (15), its transcriptional activity could be affected also by epigenetic mechanisms such as promoter hypermethylation. SP1 is known to interact with both cellular and viral promoters (8).
Studies regarding the role of hypermethylation of the VDR promoter on gene silencing are contradictory. While one study demonstrates the epigenetic silencing of VDR in breast cancer (16), another one does not confirm an association between VDR promoter hypermethylation and reduced gene expression in parathyroid adenomas (14).
The aim of this study was to examine the relationship between Cdx-2 polymorphism (rs17883968), the methylation status of VDR’s promoter region and serum levels of 25-hydroxyvitamin D (25(OH)D) in infertile men.
SUBJECTS AND METHODS
A total of 69 infertile men (aged between 23 and 45 years) and 37 age-matched controls (22-46 years) were enrolled in this study at the andrology outpatient in the National Institute of Endocrinology, Bucharest, Romania. Subject evaluations included andrology examination, semen analysis, sperm culture, hormonal profile (FSH, LH, testosterone, prolactin and estradiol), testicular and prostate ultrasonography, vitamin D level and VDR Cdx-2 polymorphism assessment. The study was performed in the Research Laboratory at “C.I. Parhon” National Institute of Endocrinology, Bucharest, approved by the Ethical Committee and subjects were recruited in accordance to Helsinki Declaration, after signing the informed consent.
Exclusion criteria: all enrolled subjects were free of pelvic radiotherapy and/or chemotherapy (for the last 6 months), of known genetic aberrations, urogenital infections, bilateral orchiectomy, vasectomy, and occupational exposure to noxious hydrocarbons organophosphates, ionizing radiation, heavy metals. Infertile men were selected based on strict inclusion criteria: aged over 18 years with infertility status, with ART (assisted reproductive technology) and reproductive failure over 1 year. The inclusion criteria for the control group were: normal semen parameters (according to the WHO standards of 2010 (Word Health Organization, 2010) (17), matched for age. All patients were of Caucasian origin and came from different Romanian regions.
Semen analysis and vitamin D level assessment
Semen analysis was performed on fresh samples (harvested after 3-5 days of abstinence) using a Leica microscope and a Mackler chamber.
Vitamin D level assessments were performed on fasting blood collected in tubes with separating gel for hormonal analyses. 25(OH)D was measured using the electrochemiluminescence system Roche Cobas E601 with complete reagent kit (Roche Diagnostics, Basel, Switzerland). The intra- and inter-batch variation coefficients were below 5%.
DNA isolation
DNA for genomic investigation (Cdx-2 polymorphism) was purified from whole blood using a Promega Wizard® Genomic DNA Purification Kit (Promega Corporation, Madison, USA), according to manufacturer instructions. The blood samples were harvested on EDTA anticoagulant (Ethylenediaminetetraacetic acid).
High Pure PCR Template Preparation Kit (Roche Molecular Biochemicals, Mannheim, Germany) was used for DNA isolation from the sperm after seminal fluid samples were centrifuged. DNA’s concentration and purity were evaluated with Infinite 200 PRO NanoQuant Plate spectrophotometer (Tecan Group Ltd, Mannedorf, Switzerland); the samples were stored at -20°.
PCR-RFLP for VDR Cdx-2 amplicons
PCR mixture consists of 50ng/μL of DNA, 200 mM of dNTP, 10 μM of each primer (forward and reverse), and 1.25 U of Taq polymerase in a total volume of 20 μL per tube, with 10X PCR buffer and 2.5 mM magnesium chloride. PCR was performed using 5’-CAGCATGCCTGTCCTCAGC-3’ as the forward primer and 5’- CCAGTACTGCCAGCTCCCA-3’ as the reverse primer (18), to produce a 135 bp amplicon. PCR conditions: 5 min. of denaturation at 94°C, followed by 35 cycles that include denaturation (94°C for 40 s), annealing (61°C for40 s), and polymerization (72°C for 30 s), and one final cycle of extension at 72°C for 7 min.
The PCR products were digested 3 hours with restriction endonuclease Bpu10I that cuts the 135 bp amplicon into two fragments of 72 bp and 63 bp VDR genotypes of each subject were identified based on the digestion pattern and the alleles, according to the absence (‘‘A’’) or presence (‘‘G’’) of the Bpu10I site. The digested products were analyzed by 2% agarose gel electrophoresis and staining with ethidium bromide.
Vitamin D receptor methylation assay
Unmethylated C residues conversion was performed using bisulfite treatment (EpiTect kit, Qiagen, Valencia, California, USA). 700ng/µL of each isolated DNAs (from semen) were bisulfite treated along with positive (CpGenome Universal Methylated DNA) and negative (CpGenome Universal Unmethylated DNA) controls (Millipore, Billerica, MA, USA).
MS-PCR (Methylation Specific PCR) was performed to identify methylated and unmethylated sequences. Primers were designed using Primer3 Blast for gene expression and Methprimer (19) to distinguish between methylated and unmethylated DNA. The primers were synthesized by Invitrogen Corporation (Carlsbad, California, USA).
Primers for methylated sequence:
forward 5’-AAGTTAAGATGGTTGTAGCG TTAAC-3’ reverse 5’-CGACGAATAAACAAACTAT TCCG-3’; (product size: 196, Tm: 58°C), Primers for unmethylated sequence: forward 5’- TTAAGATGGTTGTAGTGTTAATGG-3’reverse 5’ - CCAACAAATAAACAAACTATTCCAC-3’; (product size: 194, Tm: 58°C).
Direct Q-MSP of Genomic DNA (a quantitative method) was used to evaluate the methylation degree of the VDR gene promoter in each sample. Standard curves were designed using positive controls (fully methylated DNA) and negative controls (fully unmethylated DNA). Positive and negative controls were diluted in order to obtain a serial dilution of 10 pg, 100 pg, 1 ng and 10 ng, according to Absolute Quantitation Using Standard Curve Getting Started Guide (Applied Biosystems 7300/7500/7500 Fast Real-Time PCR System, Foster City, CA). All DNA samples were diluted to a final concentration of 10 ng/μL. 25 μL FastStart Universal SYBR Green Master (ROX) (Roche Molecular Biochemicals, Mannheim, Germany) 0.3 μM primers and 5 μL DNA were used. Direct Q-MSP was performed in 50 μL final volume and the parameters used were: 95°C-10 min (1 cycle), and 40 cycles at 95°C for 15 sec and 60 sec at the specific annealing temperature (58°C).
qPCR experiments were performed in duplicate (ΔCt≈1.2 between replicates) and mean values were used for calculations. In order to establish the quality of DNA samples we used ACTB gene as reference (20). All negative samples for methylation presented a Ct for ACTB below 35, excluding false negative results. ACTB methylation independent primers:
forward5’-TGGTGATGGAGGAGGTTT AGTAAGT -3’, reverse 5’-ACCAATAAAACCTACT CCTCCCTTAA-3’.
Methylation percentage calculation method was done according to Fackler et al. (20). The concentration of unmethylated and methylated VDR’s promoter for each patient sample was extrapolated using the standard curves. The percentage of methylation was calculated according to the formula: % M = 100 X [ng methylated gene A/ (ng methylated gene A+ unmethylated gene A)], where total target gene DNA was taken as the sum of U +M.
Statistical data analysis
Polymorphisms are expressed as percentages to show their distribution. The genotype and allele frequency distributions were compared by χ2 test and computed Hardy-Weinberg equilibrium to the expected genotype distribution and risk alleles. For odd ratio calculation Fisher’s exact test was used. Differences between groups were analysed using ANOVA. The variance between two groups was analysed using t-test. p-value <0.05 was considered statistically significant, as posthoc test in order to decide which p-values are small enough to be considered, while controlling the False Discovery Rate (FDR) at 5%. Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli test was used. All analyses were performed with GraphPad Prism 6.0.
RESULTS
A total of 69 infertile subjects and 37 control individuals were included in the study. Phenotype features suggesting androgen deficiency (such as cryptorchidism, poor virilization, hypospadias, gynecomastia, enunchoid proportions or testicular hypotrophy) were not present at our infertile subjects.
The differences between the control group and infertile group are presented in Table 1.
Table 1.
Sperm parameters in infertile group vs. control
| Control group N=37 Median, range | Infertile group N=69 Median, range | P value | |
| Sperm concentration | 3.8 x107 | 2.0x106 | 0.0122 |
| (spermatozoa no. /mL) | 1.5x107-1.03 x108 | 1.2x105-8.0x106 | |
| 50% | 14.7% | ||
| Motility | 44-52% | 0-31% | 0.0008 |
| 60% | 2% | ||
| Morphology | 32-90% | 0-4% | 0.0014 |
According to semen parameters and considering WHO guidelines (normal sperm concentration 1,5x107) (17) the infertile patients were divided into two groups: (I) 42 patients with a sperm concentration under 5x106/mL, motility under 25% and without normal morphology; (II) 27 patients with sperm concentration greater than 5x106/mL, motility 25-31% and morphology between 1-4%.
Vitamin D levels
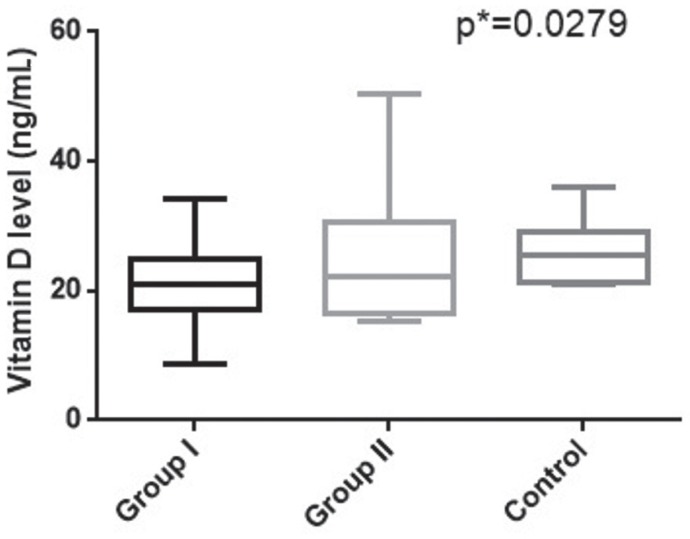
Vitamin D levels were found to be significantly decreased in the infertile patients groups versus controls (p=0.0279) (Fig. 1). Vitamin D levels were found to be lower in group I of infertile patients (median=20.86 ng/mL, range=8.79-34.16 ng/mL), whereas controls presented higher levels (median=25.45 ng/mL, range=20.9-36.10 ng/mL). Group II of infertile patients presented intermediary vitamin D values (median=22.30 ng/mL, range=15.26-50.29 ng/mL).
Figure 1.
Vitamin D levels in studied groups.
PCR-RFLP detection of the Cdx-2 polymorphism
Distribution of Cdx-2 VDR genotypes in studied groups is shown in Table 2. The analyses for Cdx-2 VDR polymorphism in infertile patients showed that 6 cases were homozygote (GG), 28 heterozygote (GA) and 35 homozygote (AA). The frequency of G allele was 0.29 and for A allele 0.71, χ2=0.014. The same investigation in control group revealed that 10 cases were homozygote for G allele (GG), 19 were heterozygote (GA) and 8 were homozygote (AA). The frequency of G allele in population was 0.53 and 0.47 for A allele, χ2=0.033. The controls and infertile groups were compared using χ2 test and the difference was significant (p=0.0229). In infertile patients vs. control group we found lower percentage of GG genotype (8.7% vs. 27.03%) and higher percentage of AA genotypes (50.72% vs. 21.62%). The statistical significance increases (p=0.0056) when comparing the infertile group with controls. Association test for the presence of A allele in the infertile group showed an OR=2.730 (p=0.0006) (Table 2).
Table 2.
Cdx-2 VDR genotypes of studied groups
| Cdx-2 VDR genotypes | Group I | Group II | Control | χ2 | |||
| No. | % | No. | % | No. | % | p-value | |
| GG | 3 | 9.68 | 3 | 7.89 | 10 | 27.03 | |
| GA | 16 | 51.61 | 12 | 31.58 | 19 | 51.35 | |
| AA | 12 | 38.71 | 23 | 60.53 | 8 | 21.62 | 0.0056 |
Despite numerous publications, the influence of Vitamin D on reproductive health remains ambiguous; however, our study showed that infertile men present low levels of Vitamin D. According to our results, the A allele may represent a potential risk for infertility.
VDR promoter methylation levels
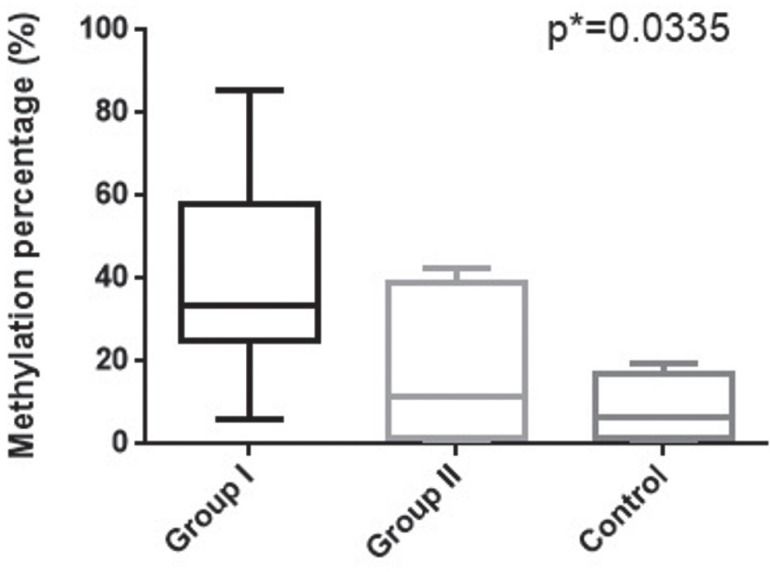
VDR gene promoter presents three CpG islands (Island 1- 192 bp, Island 2- 187 bp, Island 3- 160 bp) that are subjected to DNA methylation. To evaluate the link between VDR1 gene promoter methylation and the infertile status we quantified the methylation levels by qMS-PCR in the investigated groups. As shown in Figure 2, VDR methylation percentage increases with the severity of the diagnosis. The differences in the methylation percentage between the infertile men and control groups are significant (p=0.0335), the control group presenting lower VDR methylation (median= 6.41%, range: 1.12-8.32%) than infertile groups (Group I median=33.70%, range: 5.85-85.46%, Group II median=11.27%, range: 2.36-42.67%) (Fig. 2).
Figure 2.
Methylation percentage of VDR gene promotor in studied groups.
Further we compared the VDR promoter methylation percentage with sperm parameters and we found significant differences related to sperm count, motility and morphology (p=0.0026 for sperm count; p=0.0396 for motility and p=0.0264 for morphology), (Table 3).
Table 3.
VDR gene promoter methylation percentage correlated with sperm count, motility and morphology
| Sperm concentration | p-value | ||||
| Infertile 1.2x105-8.0x106 | Control 1.5x107-1.03 x108 | ||||
| Methylation percentage (%) | 33.27% | 7.53% | 0.0026 | ||
| Median (Range) | 10.47-75.20% | 1.12-19.32% | |||
| Motility | |||||
| 0-10% | 20-30% | >30% | |||
| Methylation percentage (%) | 35.33% | 19.41% | 10.24% | 0.0396 | |
| Median (Range) | 5.85-75.20% | 1.12-42.67% | 2.36-19.32% | ||
| Morphology | |||||
| 0-1% | 2-5% | 6-40% | >40% | ||
| Methylation percentage (%) | 40.04% | 25.36% | 19.32% | 9.22% | 0.0264 |
| Median (Range) | 23.41-75.20% | 10.47-34.9% | 1.12-42.67% | 2.36-11.27 | |
Correlation of Association between VDR promoter methylation and vitamin D levels
Despite the large number of studies published on vitamin D, there is still discussion on the threshold of desired 25-hydroxyvitamin D [25(OH)D] concentration advising minimal levels 50 nmol/L (20 ng/mL) (21). Vitamin D deficiency is defined by most experts as a serum 25(OH)D level of less than 20 ng/mL (50 nmol/L). Vitamin D insufficiency is defined as 21-29 ng/mL (52-72 nmol/L) and vitamin D sufficiency as 30 ng/mL (75 nmol/L). Vitamin D toxicity is observed when serum 25(OH)D levels are higher than 150 ng/mL (374 nmol/L) (22).
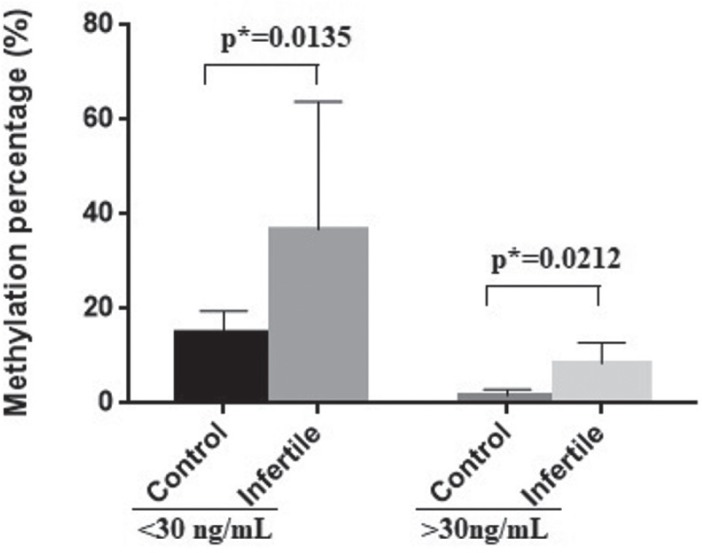
Therefore, in our study we establish as cut-off a value of 30 ng/mL and we evaluate the correlation between VDR gene promoter methylation and vitamin D levels.
Vitamin D levels are significantly influenced by the VDR gene promoter methylation. As we showed in figure 3 the methylation percentage is higher in individuals with lower vitamin D levels (p=0.0135). The VDR gene promoter methylation is also higher in infertile subjects (<30ng/mL - control-median: 14.90%, range: 10.47- 19.32% vs. infertile range: median: 34.90%, 10.47- 85.46%). Individuals with higher vitamin D levels presented lower methylation percentage in both groups (>30ng/mL-control median: 1.62%, range: 0.12- 3.12% vs. infertile median: 9.22%, range: 2.36- 13.46%) (Fig. 3).
Figure 3.
VDR gene promoter methylation percentage correlated with vitamin D level. The plot was represented as mean with SD.
Association between VDR promoter methylation and Cdx-2 VDR genotypes
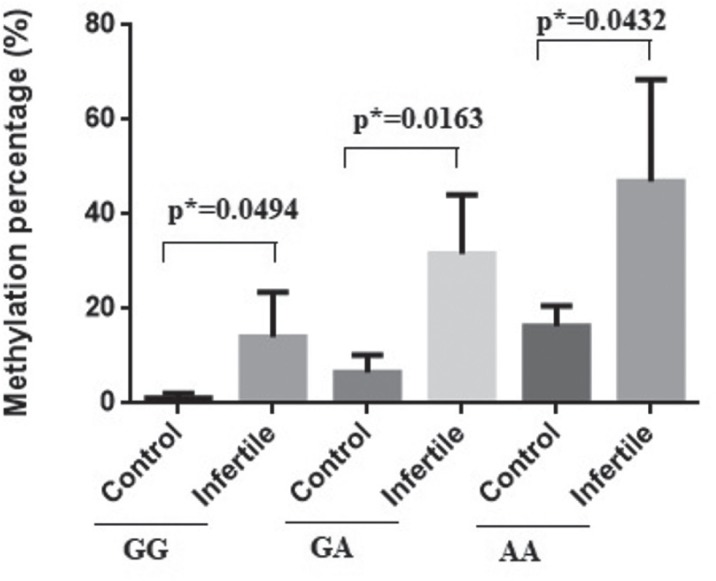
Further we evaluated in infertile patients and controls a possible connection between the methylation levels of VDR promoter and other investigated parameters. In Figure 4 we can observe that AA genotype is characterized by higher methylation percentage values, infertile individuals presenting higher values (p=0.0432) (control-median: 18.32%, range: 11.47-19.32%, vs. infertile-median: 39.21%, range: 25.36-85.46%). The lowest methylation percentages were observed in GG homozygote infertile individuals control-median: 1.12%, range: 0.12-2.12%, vs. infertile-median: 10.87%, range: 2.36-30.55%).
Figure 4.
VDR gene promotor methylation according to Cdx-2 genotypes.The plot was represented as mean with SD.
Association between Cdx-2 VDR genotypes and Vitamin D levels
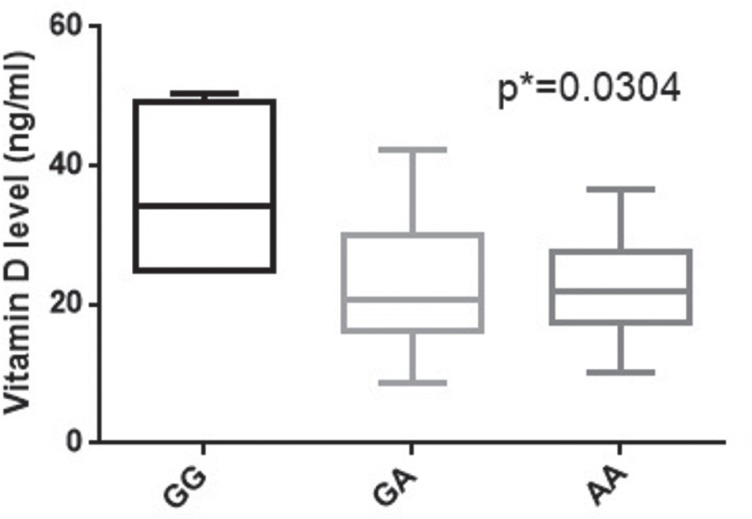
Interestingly, infertile homozygote (GG) and heterozygote (GA) individuals presented significantly higher vitamin D levels than AA homozygote (p=0.0304). The GG patients exhibit the highest vitamin D levels (median: 34.25 ng/mL, range: 24.96-50.29 ng/mL), comparing with GA individuals (median: 20.66 ng/mL, range: 8.79-42.23 ng/mL) and AA individuals (median: 22 ng/mL, range: 10.23-36.71 ng/mL) (Fig. 5).
Figure 5.
Vitamin D levels correlated with Cdx-2 VDR genotypes in infertile patients.
DISCUSSION
Vitamin D deficiency and insufficiency have been correlated with various human disorders including bone strength, cardiovascular, diabetes, infectious, oncologic, musculoskeletal, neuropsychologic and reproductive disorders (23-30).
Animal studies showed that vitamin D deficiency in male rats may affect spermatogenesis (32). Therefore, sperm count, motility, histological structure of testis and spermatogenesis are more dependent on normal serum calcium and phosphorus levels (31). Bloomberg et al. (2011, 2014) in a study performed on 300 men suggested that 1,25(OH)D3 increased intracellular calcium concentration and sperm motility and induced the acrosome reaction in mature spermatozoa (3, 33).
Our study is in consensus with previously presented data of the influence of vitamin D levels on sperm parameters (3, 33). We showed that infertile patients presented the lowest levels of vitamin D. One of the most interesting targets in the research of the role of vitamin D and infertility is the vitamin D receptor. The vitamin D receptor is present in male reproductive system and has been found in human sperm using immunohistochemistry, especially in the spermatozoa head (nucleus) and mid piece (34, 35). It was shown that Vitamin D receptor null mice presented gonadal insufficiency with hypergonadotropic hypogonadism (36).
Vitamin D receptor polymorphism was associated with vitamin D deficiency and several human disorders. Five well-known SNPs of human VDR, FokI (C/T), BsmI (A/G), ApaI (A/C), TaqI (T/C), and Cdx-2 (A/G), were extensively studied for their association with cancer risk (37, 38).
Cdx-2 polymorphism in the VDR gene (a single base change G to A, located upstream in the 5’ UTR of the gene) that significantly alters the transcriptional activity of the VDR promoter region may affect the serum 25(OH)D concentrations and the risk of osteoporosis and fracture in middle-aged and elderly Chinese women (39).
Torkko reported that the Cdx2 polymorphism significantly increased the prostate cancer risk among Hispanic populations carrying the SRD5A2 V89L VV genotype (40). However, a study conducted by Rowland found no relationship between prostate cancer and ApaI and Cdx-2 SNPs (41).
Previously results showed that VDR Cdx-2 polymorphism is significantly associated with risk of breast cancer in Africans, but there was not a significant association in Caucasians (42).
Currently there are no known studies to associate Cdx-2 polymorphism with male infertility. By our knowledge, this is the first study that shows an association between the male infertility, vitamin D deficiency and Cdx-2 polymorphism. Vitamin D levels were found to be significantly decreased in infertile groups compared the controls. The GG genotype was found in a higher percentage in controls and the percentage of AA genotype was higher in infertile group. According with our results it seems that A allele may represent a potential risk for infertility. In addition, we have found that the infertile homozygote (GG) and heterozygote (GA) individuals had significantly higher vitamin D levels than AA homozygote. On the other hand, VDR gene promoter methylation level was associated with lower sperm motility, lower sperm concentration, and with poor sperm morphology as well. Methylation percentage is higher in individuals with lower vitamin D levels, especially in infertile group. AA genotype is characterized by higher methylation percentage values.
Importantly, this study is the first to report the methylation status of vitamin D receptor gene promoter and the correlation with male infertility.
The promoter of VDR gene presents a GC-rich island that contains many regulatory elements important in transcription (8). Disruption of promoter activity by DNA methylation is an epigenetic mechanism frequently observed in cancer by inactivating tumor-suppressor genes (43); by contrast hypomethylation was associated with oncogenes activation.
Therefore, our study showed that methylation of vitamin D receptor gene promoter has an important effect not only on the sperm count, but also it was associated with poor sperm morphology and motility. Higher methylation percentage was also related to vitamin D deficiency. Infertile individuals presenting AA genotype (Cdx-2 polymorphism) are characterized by higher methylation percentage values.
In summary, this study provides the evidence that A allele of the Cdx-2 polymorphism is significantly associated with infertility and higher methylation percentage of VDR gene promoter in individuals presenting AA genotype. These results suggest that epigenetic mechanisms may underlie the role that vitamin D plays in male infertility. Further functional and genetic studies will help to better understand the biological significance and importance of the vitamin D in infertility.
In conclusion, the results provide new insights regarding the involvement of Cdx-2 polymorphism in impact of vitamin D deficiency to male infertility, bringing new genetic evidences of the role of vitamin D receptor in the pathogenesis of male infertility. Considering all the results obtained in this study, we have to highlight the important role of epigenetic changes of vitamin D receptor upon the vitamin D deficiency and male infertility along with the genetic context.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
Acknowledgement
The study was partially performed on infrastructure developed through Project 433/ID 929/SMIS code 14049, SOP-IEC, Operation 2.2.1, POSCCE 2.2.1, ID 918, SMIS 14045
This work received financial support through the project entitled “CERO – Career profile: Romanian Researcher”, grant number POSDRU/159/1.5/S/135760, cofinanced by the European Social Fund for Sectoral Operational Programme Human Resources Development 2007-2013.
References
- 1.Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab. 2015;29(4):621–631. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Blomberg JM, Nielsen JE, Jorgensen A, Rajpert-De ME, Kristensen DM, Jorgensen N, Skakkebaek NE, Juul A, Leffers H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25(5):1303–1311. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 3.Blomberg JM. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10(3):175–186. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- 4.Wan LY, Zhang YQ, Chen MD, Liu CB, Wu JF. Relationship of structure and function of DNA-binding domain in vitamin D receptor. Molecules. 2015;20(7):12389–12399. doi: 10.3390/molecules200712389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3) Endocrinol Metab Clin North Am. 2010;39(2):255–269. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nangia AK, Hill O, Waterman MD, Schwender CE, Memoli V. Testicular maturation arrest to testis cancer: spectrum of expression of the vitamin D receptor and vitamin D treatment in vitro. J Urol. 2007;178(3 Pt 1):1092–1096. doi: 10.1016/j.juro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Alzoubi A, Mandi H, Al Bashir S, Halalsheh O, Al Ebbini M, Alzarir M, Al-Ahmar K, Alfaqih M, Al-Hadidi AH. Normalization of Serum Vitamin D Improves Semen Motility Parameters in Patients with Idiopathic Male Infertility. Acta Endocrinologica-Bucharest. 2017;13(2):180–187. doi: 10.4183/aeb.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, Inoue Y, Morita K, Takeda E, Pike JW. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol. 1997;11(8):1165–1179. doi: 10.1210/mend.11.8.9951. [DOI] [PubMed] [Google Scholar]
- 9.Szpirer J, Szpirer C, Riviere M, Levan G, Marynen P, Cassiman JJ, Wiese R, DeLuca HF. The Sp1 transcription factor gene (SP1) and the 1,25-dihydroxyvitamin D3 receptor gene (VDR) are colocalized on human chromosome arm 12q and rat chromosome 7. Genomics. 1991;11(1):168–173. doi: 10.1016/0888-7543(91)90114-t. [DOI] [PubMed] [Google Scholar]
- 10.Labuda M, Fujiwara TM, Ross MV, Morgan K, Garcia-Heras J, Ledbetter DH, Hughes MR, Glorieux FH. Two hereditary defects related to vitamin D metabolism map to the same region of human chromosome 12q13-14. J Bone Miner Res. 1992;7(12):1447–14453. doi: 10.1002/jbmr.5650071212. [DOI] [PubMed] [Google Scholar]
- 11.Sarkissyan M, Wu Y, Chen Z, Mishra DK, Sarkissyan S, Giannikopoulos I, Vadgama JV. Vitamin D receptor FokI gene polymorphisms may be associated with colorectal cancer among African American and Hispanic participants. Cancer. 2014;120(9):1387–1393. doi: 10.1002/cncr.28565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai H, Miyamoto K-I, Yoshida M, Yamamoto H, Taketani Y, Morita K, Kubota M, Yoshida S, Ikeda M, Watabe Fumiko, Kanemasa Yasuhiro, Takeda Eiji. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res. 2001;16(17):1256–1264. doi: 10.1359/jbmr.2001.16.7.1256. [DOI] [PubMed] [Google Scholar]
- 13.Ntais C, Polycarpou A, Ioannidis JP. Vitamin D receptor gene polymorphisms and risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1395–1402. [PubMed] [Google Scholar]
- 14.Varshney S, Bhadada SK, Sachdeva N, Arya AK, Saikia UN, Behera A, Rao SD. Methylation status of the CpG islands in vitamin D and calcium-sensing receptor gene promoters does not explain the reduced gene expressions in parathyroid adenomas. J Clin Endocrinol Metab. 2013;98(10):E1631–E1635. doi: 10.1210/jc.2013-1699. [DOI] [PubMed] [Google Scholar]
- 15.Dynan WS, Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- 16.Marik R, Fackler M, Gabrielson E, Zeiger MA, Sukumar S, Stearns V, Umbricht CB. DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer Biol Ther. 2010;10(1):44–53. doi: 10.4161/cbt.10.1.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper TG, Noonan E, von ES, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 18.Flugge J, Krusekopf S, Goldammer M, Osswald E, Terhalle W, Malzahn U, Roots I. Vitamin D receptor haplotypes protect against development of colorectal cancer. Eur J Clin Pharmacol. 2007;63(11):997–1005. doi: 10.1007/s00228-007-0367-4. [DOI] [PubMed] [Google Scholar]
- 19.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18(11):1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 20.Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, Lapides A, Garrett E, Argani P, Sukumar S. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64(13):4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- 21.Sohl E, de Jongh RT, Heymans MW, van Schoor NM, Lips P.J. Thresholds for serum 25(OH)D concentrations with respect to different outcomes. Clin Endocrinol Metab. 2015;100(6):2480–2488. doi: 10.1210/jc.2015-1353. [DOI] [PubMed] [Google Scholar]
- 22.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010;95(6):2630–2633. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osei K. 25-OH vitamin D: is it the universal panacea for metabolic syndrome and type 2 diabetes? J Clin Endocrinol Metab. 2010;95(9):4220–4222. doi: 10.1210/jc.2010-1550. [DOI] [PubMed] [Google Scholar]
- 25.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward KA, Das G, Roberts SA, Berry JL, Adams JE, Rawer R, Mughal MZ. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010;95(10):4643–4651. doi: 10.1210/jc.2009-2725. [DOI] [PubMed] [Google Scholar]
- 27.Ramlau-Hansen CH, Hakonsen LB, Christensen M, Bonde JP, Olsen J, Thulstrup AM. Maternal shift work during pregnancy and biomarkers of reproductive function among the male offspring-a pilot follow-up study. Scand J Work Environ Health. 2011;37(6):533–538. doi: 10.5271/sjweh.3164. [DOI] [PubMed] [Google Scholar]
- 28.Faserl K, Golderer G, Kremser L, Lindner H, Sarg B, Wildt L, Seeber B. Polymorphism in vitamin D-binding protein as a genetic risk factor in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2011;96(1):E233–E241. doi: 10.1210/jc.2010-1532. [DOI] [PubMed] [Google Scholar]
- 29.Cigerli O, Parildar H, Unal AD, Tarcin O, Kut A, Eroglu H, Guvener N. Vitamin Deficiency and Insulin Resistance in Nondiabetic Obese Patients. Acta Endocrinologica-Bucharest. 2016;12(3):319–327. doi: 10.4183/aeb.2016.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ristic S, Kocic SS, Milovanovic DR, Mihajlovic G, Mihailovic N, Lucic AT, Zivanovic S. Vitamin D Status in Patients with Mental Disorders: A Cross-Sectional Analysis of Single Cohort from Routine Practice. Acta Endocrinologica-Bucharest. 2017;13(1):40–46. doi: 10.4183/aeb.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sood S, Reghunandanan R, Reghunandanan V, Marya RK, Singh PI. Effect of vitamin D repletion on testicular function in vitamin D-deficient rats. Ann Nutr Metab. 1995;39(2):95–98. doi: 10.1159/000177848. [DOI] [PubMed] [Google Scholar]
- 32.Sun W, Chen L, Zhang W, Wang R, Goltzman D, Miao D. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015;308(1):E51–E62. doi: 10.1152/ajpendo.00076.2014. [DOI] [PubMed] [Google Scholar]
- 33.Blomberg JM, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, Petersen JH, Juul A, Dissing S, Jorgensen N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26(6):1307–1317. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 34.Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006;68(6):1345–1349. doi: 10.1016/j.urology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, Ando S. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009;7:140. doi: 10.1186/1477-7827-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141(4):1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- 37.Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29(9):3511–3536. [PubMed] [Google Scholar]
- 38.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30(7):1170–1180. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 39.Ling Y, Lin H, Aleteng Q, Ma H, Pan B, Gao J, Gao X. Cdx-2 polymorphism in Vitamin D Receptor gene was associated with serum 25-hydroxyvitamin D levels, bone mineral density and fracture in middle-aged and elderly Chinese women. Mol Cell Endocrinol. 2016;427:155–161. doi: 10.1016/j.mce.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Torkko KC, van BA, Mai P, Beuten J, Balic I, Byers TE, Hokanson JE, Norris JM, Baron AE, Lucia MS, Thompson IM, Leach RJ. VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res. 2008;14(10):3223–3229. doi: 10.1158/1078-0432.CCR-07-4894. [DOI] [PubMed] [Google Scholar]
- 41.Rowland GW, Schwartz GG, John EM, Ingles SA. Calcium intake and prostate cancer among African Americans: effect modification by vitamin D receptor calcium absorption genotype. J Bone Miner Res. 2012;27(1):187–194. doi: 10.1002/jbmr.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou ZC, Wang J, Cai ZH, Zhang QH, Cai ZX, Wu JH. Association between vitamin D receptor gene Cdx2 polymorphism and breast cancer susceptibility. Tumour Biol. 2013;34(6):3437–3441. doi: 10.1007/s13277-013-0919-4. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17(3):330–339. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]