Abstract
Purpose
Minimally invasive follicular thyroid carcinomas (MIFCs) are uncommon; literature offers limited guidance on their natural history and management. Starting January 2015 we measured circulating tumor cells (CTCs) in patients with MIFC (n=22) or benign thyroid tumors with follicular features (n=4).
Methods
In a retrospective analysis, we assessed detectability of and serial changes in CTC, compared demographic/clinical differences between CTC-positive versus CTC-negative subgroups using Student’s t-test, and examined correlations between CTC status and serum thyroglobulin using Spearman’s test. CTCs were quantitated via immunomagnetic separation/microscopic inspection.
Results
Thirteen patients (50%: 12/22 MIFC, 1/4 benign tumor) were initially CTC-positive; 3 remained CTC-positive in ≥1 subsequent measurement. CTC-positive patients had larger tumors and more frequent multifocality and vascular invasion versus CTC-negative patients (n=13). However, no tested variable differed significantly between the subgroups. After 17.2±10.5 months, neither subgroup showed evidence of disease. Significant correlation was absent (p ≥ 0.263) between CTC and Tg negativity (r = 0.243; n=13 evaluable) or initial CTC positivity and Tg positivity (r = -0.418; n=9 evaluable).
Conclusions
In the studied settings, CTC measurement is feasible, has unclear clinical/outcome implications, but may provide different information versus thyroglobulin testing. Lengthier assessment is warranted in larger series.
Keywords: circulating tumor cells (CTCs), differentiated thyroid carcinoma (DTC), minimally invasive follicular thyroid carcinoma (MIFC), risk stratification
INTRODUCTION
Historically, minimally invasive follicular thyroid carcinoma (MIFC) generally has been understood to refer to grossly encapsulated follicular thyroid tumors lacking nuclear features of papillary thyroid carcinoma (PTC), and exhibiting microscopic invasion of the thyroid capsule, of a limited number of blood vessels, or of both (1, 2). MIFCs are relatively uncommon; based on published series, they constitute only some 3%–13% of differentiated thyroid carcinomas (DTCs)(1). European cancer registry data suggest an annual incidence for MIFC of one case per one million inhabitants (1).
The heterogeneity, relatively small population, and disparate reported outcomes of patients diagnosed with the condition mean that literature offers limited guidance regarding the natural history or optimal management of MIFC. To our knowledge, only five published series (3-7) have contained >100 MIFC patients (1). The largest of these series (6) was a population-level analysis of 1200 American patients, of whom 73.2% underwent total thyroidectomy and 53.3% received post-surgical radioiodine therapy (RAIT). In this sample, with patients followed for 4–13 years, just 2 individuals died of MIFC, and overall survival did not differ from that of the US general population. In contrast, the third largest MIFC series, comprising 292 Japanese patients (4), had a 29% recurrence rate (20/70 cases) after 5–10 years’ follow-up among M0 patients not given post-surgical radioiodine therapy (RAIT).
As embodied by the American series (6), in Western countries, aggressive treatment traditionally has been common in patients with MIFC. Such individuals often have undergone total thyroidectomy, RAIT, and suppressive thyroid hormone therapy (7). However, based on predominantly favorable outcomes of these patients in some series (6, 7), consensus has been building that less radical treatment, e.g., lobectomy, withholding of RAIT, and replacement thyroid hormone therapy, often may be appropriate (1, 2, 4, 6). This approach increases the importance of dynamic assessment of individuals’ recurrence and mortality risks (2). Age ≥45 years at the time of surgery, primary tumor size ≥40 mm, vascular invasion, and distant metastasis have been shown to predict worse prognosis in patients with MIFC (1, 4, 7). Nonetheless, additional risk markers could be helpful in determining individuals’ treatment and follow-up intensity.
Because they presumably are involved in cancer persistence and metastasis, circulating tumor cells (CTCs) are emerging as a potential prognostic biomarker in a variety of epithelial neoplasia (8-11), including DTC (12-20). CTC measurement is conceptually attractive in DTC for at least three reasons. First, unlike serum thyroglobulin (Tg), currently the most frequently-used blood biomarker in managing this neoplasm, CTCs, when measured accurately, reflect only malignant thyroid cells. Second, unlike Tg determination or scintigraphy, CTC counts do not depend on TSH levels, and unlike the former modality, they are unaffected by anti-Tg antibodies (TgAb). Third, CTC counts might provide actionable information more rapidly than do serial Tg determinations, since increases in Tg concentrations, but presumably not CTC counts, largely depend on macroscopic tumor growth (21). With these rationales, between January 1st 2015 and 31 October 2016, we initiated routine serial CTC measurement in our patients with MIFC. We also ordered serial CTC determinations in selected patients with benign thyroid tumors with follicular features, a possible MIFC precursor (1).
We now report a retrospective analysis of our pilot experience with such testing. The analysis sought to answer three questions: 1) are CTCs detectable and do their counts change over time in patients with MIFC or benign tumors with follicular features; 2) do such patients with CTC positivity differ demographically or clinically from such patients who are CTC-negative, and if so, how; and 3) does CTC positivity correlate with Tg positivity, i.e., are CTC potentially offering different information than is Tg?
METHODS
Patients and ethics
This analysis involved 26 patients with a histological diagnosis of MIFC (n=22) or of benign thyroid tumors with follicular features (n=4) who underwent at least one CTC determination at the Prof. Dr. Ion Chiricuţă Institute of Oncology, Cluj-Napoca, Romania (IOCN) starting January 1st 2015. The 22 MIFC patients comprised the entire cohort with this histotype that was seen at our institution in 2015-2016. The latest follow-up in the study sample was 31 October 2016. IOCN is Romania’s second largest DTC tertiary referral center, with a database including over 7500 thyroid cancer patients as of the end of 2016 (22, 23).
The study sample encompassed four subgroups, patients with: A) MIFC with ≤4 sites of vascular invasion noted in their histopathology reports (“definite MIFC subgroup,” n=14); B) benign thyroid tumors with follicular features (“benign subgroup”, n=4); C) a histological classification of MIFC but >4 or a “large” number of sites of vascular invasion noted in their histopathology reports (“borderline MIFC/widely invasive follicular thyroid carcinoma (WIFC) subgroup”; n=5) and 4) a thyroid tumor with a histological diagnosis of MIFC plus at least one other thyroid tumor type in the contralateral lobe (“MIFC+ subgroup”, n=3).
Table 1 summarizes patient characteristics by subgroup. On average, age, follicular tumor size, and prevalence and extent of follicular tumor invasiveness increased along the subgroup continuum “ benign ” < “ MIFC ” < “ MIFC + ” < “ borderline MIFC/ WIFC”. This observation suggests that these classifications reflected an advancing natural history of the follicular thyroid tumor. Of interest, this hierarchy was not reflected by mean stimulated Tg values, which were higher in the “benign” subgroup than in the MIFC subgroup, and were markedly the highest in the “MIFC+” subgroup. Also of interest, no patient was TgAb-positive at any time during the study period.
Table 1.
Patient characteristics
| Variable | Patient subgroup | |||
| Definite MIFC (n = 14) | Benign (n = 4) | Borderline MIFC/WIFC (n = 5 ) | MIFC+ (n = 3) | |
| Histology | MIFC with ≤4 vessels invaded | 3 FTUMP, 1 Hürthle cell adenoma | Classified as MIFC, but >4 (n = 2), 5 (n = 1), 7 (n = 1) or a “large” number of vessels invaded | MIFC, but 1 each with PTC, PDTC, or MTC in contralateral lobe; patient with MIFC + PDTC had 6 vessels invaded |
| Age, years, mean ± SD | 47 ± 15 | 35 ± 10 | 57 ± 19 | 52 ± 15 |
| Females, % (n) | 79% (11) | 75% (3) | 100% (5) | 100% (3) |
| Follicular tumor characteristics | ||||
| Size, mean ± SD, mm <10.0 mm, % (n) Multifocality, % (n) |
36 ± 20 14% (2) 7% (1) |
27 ± 15 0 0 |
41 ± 33 0 0 |
30 ± 22a 0 0b |
| Tumor invasion, % (n) | ||||
| None Capsular invasion only Vascular invasion only Vascular invasion + capsular invasion Vessels invaded, mean ± SD |
0 57% (8) 0 43% (6) 1.8 ± 1.4 (n = 6) |
100% (4) 0 0 0 0 |
0 0 0 100% (5) 6.0 ± 1.4 (n = 2)c |
0 67% (2) 0 33% (1) 6 ( n = 1) |
| Lymph node involvement, % (n) | ||||
| N0 N1a N1b Nxd |
36% (5) 7% (1) 0 57% (8) |
50% (2) 0 0 50% (2) |
20% (1) 0 0 80% (4) |
0 0 33% (1)e 67% (2) |
| Distant metastasis, n (%) | ||||
| Any Lung Bone Other |
0 0 0 0 |
0 0 0 0 |
20% (1) f 20% (1) 0 0 |
0 0 0 0 |
| Stimulatedh serum Tg, μg/L, at baseline, mean | ||||
| ± SD ≥1.0 μg/L, % (n) |
0.5 ± 0.7 (n = 10) 30% (3) (n = 10) |
23 ± 11 100% (4) |
36 ± 69 100% (5) |
294 ± 509 33% (1) |
| RAIT | ||||
| None, % (n) Any, % (n) Cumulative activity, GBq, mean ± SD |
36% (5) 64% (9) 1.3 ± 1.1 |
75% (3) 1% (3) 1.1 |
60% (3) 40% (2) 1.6 ± 2.7 |
33% (1) 67% (2) 4.2 ± 5.3 |
| Follow-up, months, mean ± SD | 18 ± 13 | 19 ± 1 | 13 ± 10 | 18 ± 1 |
Due to rounding, percentages may not add to 100%.
FTC, follicular thyroid carcinoma; FTUMP, follicular tumor of uncertain malignant potential; MIFC, minimally invasive follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; SD, standard deviation; Tg, thyroglobulin; TgAb, anti-thyroglobulin antibodies.
Primary tumor sizes for the other thyroid cancers were PTC, 3 mm, MTC, 7 mm, PDTC, data not available.
All 3 patients had a single follicular tumor, but had another thyroid cancer in the contralateral lobe.
Average excludes patients with “>4” vessels invaded (n = 2) or a “large” number of vessels invaded (n = 1)
No Nx patient was found to have lymph node metastasis on the post-RAIT scan.
This patient’s lymph node metastases were from the co-existing PDTC.
This patient’s distant metastases were diffuse lung metastases of FTC.
The subgroups respectively include 1 patient each with a history of colon or breast cancer.
All Tg testing was performed following ≥2 weeks of thyroid hormone withdrawal, with serum thyrotropin >40 mIU/L.
TgAB titers ≥115 IU/mL were considered to be TgAb-positive.
The CTC measurement protocol was approved by the IOCN Ethics Committee. All patients provided written informed consent for all CTC determinations and for participation in anonymized analyses such as this one. Patients were informed about all CTC results, and those results factored into joint patient-physician decision-making regarding treatment radicality and follow-up intensity.
MIFC management
Until 2011, our institution managed MIFC aggressively, with total thyroidectomy, post-surgical RAIT, generally with 1.85–3.70 Gbq (50–100 mCi) activities, and long-term thyroid hormone suppressive therapy (target TSH <0.1 mIU/L). We also recommended twice-yearly follow-up visits, including serum Tg and TgAb testing, neck ultrasonography, and physical exam, during the first 5 years post-primary treatment.
Since 2011, we have attempted to more selectively apply radical treatment. In particular, in patients with no evidence of persistent disease post-surgery, we usually administer lower activities for post-surgical RAIT or even omit RAIT; additionally, we now typically use thyroid hormone therapy regimens tailored more closely to the patient’s risk factors, sometimes giving suppressive therapy for only a limited time. Further, we recommend twice-yearly comprehensive follow-up exams only in higher-risk patients.
CTC isolation/quantification
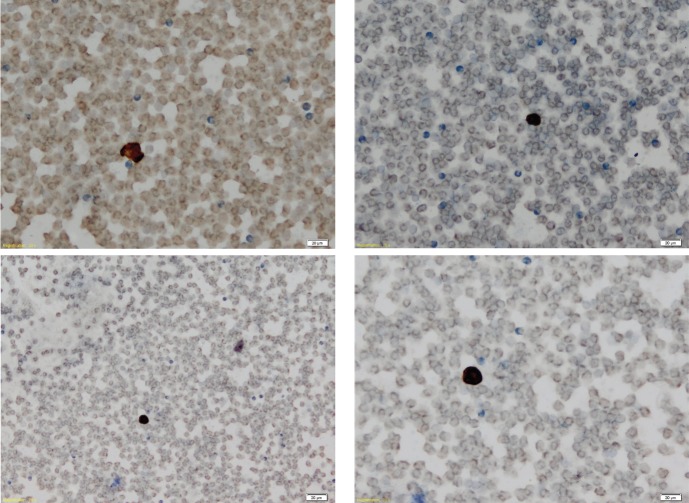
We tested a locally-developed, manual method of counting CTCs that were isolated through immunomagnetic separation via a gentle hemolysis using a buffered hypotonic solution. Our method is based on visual detection of immunostaining for both cytokeratin 8 expression and cytokeratin 19 expression on the CTC surface, complemented by visual identification, on microscopy, of malignant morphology based on pre-defined criteria (Fig. 1). These criteria are useful in allowing an experienced cytologist to identify CTCs.
Figure 1.
Representative images of microscope slides showing CTC negativity (A and B) and CTC positivity (C and D), as represented by presence or absence of immunostaining for cytokeratins 8 and 19. Magnification x 20, calibration bar 20 μm. (A and B) Negative findings (CTC count = 0/7.5 mL) from a female patient, age 36 years, with vascular and capsular invasion. (C and D) Positive slides (CTC count = 13/7.5 mL) from a male patient, age 85 years, with capsular invasion only.
Briefly, 7 mL venous blood samples were collected in ethylene diamine triacetic acid (EDTA) tubes, with the first mL of each sample discarded to avoid contamination with epidermal cells. Blood samples were preserved on ice/refrigerated at 4°C for ≤4 hours. Samples were divided in two equal portions (3 mL). Each portion was placed into a 50 mL lidded centrifuge tube, and 40 mL of red blood cell (RBC) working solution (RBC stock solution [ammonium chloride, 1.55 M; ammonium bicarbonate, 0.1 M; 2 mL EDTA, 0.5M] diluted 1:10 with distilled water) was added.
The samples were incubated for 10 minutes on ice, followed by isolation of nucleated cells via centrifugation at 2000 rotations per minute (RPM) for 5 minutes. The resulting pellets each were combined with 3 mL of cold Dulbecco’s phosphate-buffered saline (PBS). A second isolation step was performed: centrifugation for 10 min at 1300 RPM, followed by re-suspension of pellets in cold PBS. The pellets then were cytocentrifuged on histologic slides for 5 minutes at 600 RPM, followed by fixation in methanol at 4°C for 15 minutes.
Then, a standard immunocytochemical staining method was applied, consisting in the following steps: washing with tris base solution (TBS); blocking of any nonspecific reactions with Protein Block (Novolink Polymer Detection System, Newcastle, UK); incubation with primary antibodies to cytokeratins 8 and 19, which are highly expressed on thyroid carcinoma cells (anti-cytokeratin 8.18 5D3 clone, Thermo Scientific, Waltham, MA, USA; anti-cytokeratin 19, Leica Biosystems, Inc., Buffalo Grove, IL, USA); washing twice more with TBS; incubation with 100μl Novolink Polymer; washing twice further with TBS; incubation with 3′3-diaminobenzidine hydrochloride; and washing with distilled water. The slides were counterstained with hematoxylin and rinsed once with fresh water, twice with 96% alcohol solution, and once with pure alcohol, after which Xylene (Chemical Laboratory, Iasi, Romania) was applied.
CTC counting was performed simultaneously by two pathologists (among them, NIB and RB) viewing slides under 10x, 20x, and 40x magnifications using a BX43 upright light microscope (Olympus Life Science Solutions, Shinjuku, Tokyo, Japan), at an accredited laboratory, Santomar Oncodiagnostic, Cluj-Napoca, Romania. The criteria for classifying a cell as malignant were: enlarged nuclei, increased nucleus-cytoplasm ratio, granular appearance of chromatin with prominent nucleoli. Each pathologist photographed all cells that he/she believed to show such morphology; final classification of cells as CTCs was made by consensus by the two pathologists.
Tg and TgAb testing
Serum Tg and serum TgAb measurements were carried out using immunochemical methods with electrochemoiluminescence detection, Roche kits, and Cobas instruments (both from Roche Diagnostics, Basel, Switzerland), in the same ISO 15189-certified accredited laboratory. Samples for Tg quantitation were taken after thyroid hormone withdrawal of ≥2 weeks with a TSH rise to 40 mIU/L. The Tg assay had a lower limit of detection of <0.04 μg/L, an intra-run coefficient of variation (CV) of 1.8%, and an inter-run CV of 3.0%. The TgAb assay had an intra-run CV of 5.6% and an inter-run CV of 8.7%. TgAb determinations <115 IU/mL were considered to be negative.
Statistics
Data are presented as means ± standard deviations (SDs), proportions, or both. Subgroup comparisons regarding characteristics of patients who were CTC-negative at all determinations versus patients who were CTC-positive at the first measurement were made using Student’s t-test. Respective correlations between CTC negativity or CTC positivity at the first determination or CTC positivity at the first and second determinations and stimulated Tg negativity or stimulated Tg positivity (threshold 0.04 μg/L) were assessed using Spearman’s test. P≤0.05 was deemed to be significant. Statistical analyses were performed using SPSS version 19 or Statistica version 10 for Windows (SPSS Institute, Cary, NC, USA and StatSoft, Tulsa, OK, USA, respectively).
RESULTS
CTC were detectable at the initial (1-month) determination in half the patients (13/26), including 12/22 with MIFC (55%) and 1/4 (25%) with benign thyroid tumors with follicular features. For the CTC-positive patients, values in the first CTC determination ranged from 1–13/7.5 mL of blood (mean ± SD 5.1±3.3/7.5 mL) (Table 2). CTC remained detectable in 2 patients in the 6-month, 12-month, and 18-month measurements, and in 1 additional patient in the 6-month determination, but not subsequently. In all 3 individuals with multiple positive CTC determinations, values generally declined over time. Of the 13 patients positive for CTC in at least one measurement, none had evidence of disease post-primary treatment, after a mean follow-up of 20.2±10.4 months.
Table 2.
Serial CTC counts and outcomes by patient
| Pat. # | Sex, age at time of first CTC measurement, yr | MIFC primary tumor size, mm | MIFC Invasion | N and M status; other disease |
CTC count, cells/7.5 mL blood Stimulateda
serum Tg, μg/L Time post-last surgery |
Treatment and outcome at latest follow-up | |||
| 1 mo | 6 mos | 12 mos | 18 mos | ||||||
| 1 | F, 51 | 20 | C+V+ | NxM0 | 9 0.9 |
0 UD |
ND UD |
0 ND |
TT; 1 RAIT x 1.85 GBq; NED at 23 months |
| 2 | F, 29 | 25 | C+ | N0M0 | 3 1.9 |
0 1.4 |
0 0.4 |
0 ND |
TT; NED at 20 months |
| 3 | F, 77 | 6 | C+V+ | N0M0; colon cancer | 0 818 |
0 5.7 |
0 UD |
0 ND |
TT; 1 RAIT x 1.85 GBq; NED at 20 months |
| 4 | F, 46 | 50 | C+V+ | N0M0 | 3 2.3 |
0 2.1 |
0 2.0 |
0 ND |
TT; 1 RAIT x 1.11 GBq; NED at 17 months |
| 5 | M, 28 | 50 | C+V+ | N0M0 | 4 10 |
0 UD |
0 UD |
0 ND |
TT; 1 RAIT x 2.55 GBq; NED at 19 months |
| 6 | F, 37 | 12 | C+ | N0M0 | 0 15 |
0 0.3 |
0 1.3 |
0 ND |
Lob; NED at 39 months |
| 7 | F, 66 | 12 | C+ | NxM0 | 4 1.3 |
0 1 |
0 1 |
0 ND |
TT; NED at 18 months |
| 8 | F, 61 | 25 | C+ | NxM0 | 5 4.6 |
ND 0.6 |
ND 0.4 |
ND ND |
TT; NED at 50 months |
| 9 | F, 36 | 55 | C+V+ | NxM0 | 0 10 |
0 11 |
0 0.3 |
0 ND |
TT; 1 RAIT x 1.85 GBq; NED at 17 months |
| 10 | F, 47 | 60 | C+ | NxM0 | 0 0.4 |
0 UD |
0 UD |
0 ND |
TT; 1 RAIT x 1.85 GBq; NED at 14 months |
| 11 | M, 42 | 55 | C+ | N1aM0 | 0 4.7 |
0 ND |
0 ND |
0 ND |
TT; 1 RAIT x 3.3 GBq; NED at 4 months |
| 12 | F, 29 | 20 | C+ | NxM0 | 0 1.1 |
0 ND |
0 ND |
0 ND |
TT; 1 RAIT x 1.85 GBq; NED at 5 months |
| 13 | M, 61 | 25 | C+ | NxM0 | 0 1.7 |
0 ND |
0 ND |
0 ND |
TT; 1 RAIT x 1.85 GBq; NED at 6 months |
| 14 | F, 52 | 55 | C+V+ | NxM0 | 0 11.3 |
0 ND |
0 ND |
0 ND |
TT; NED at 3 months |
| 15 | F, 36 | 33 | None | N0M0; FTUMP | 1 35 |
0 20 |
0 0.2 |
0 ND |
CT2; 1 RAIT x 1.11 GBq; NED at 20 months |
| 16 | M, 37 | 13 | None | NxM0; FTUMP | 0 12 |
0 13 |
0 13 |
0 ND |
TT; NED at 18 months |
| 17 | F, 22 | 17 | None | NxM0; FTUMP | 2 30 |
0 1.1 |
0 0.7 |
0 ND |
CT2; NED at 17 months |
| 18 | F, 45 | 45 | None | N0M0; Hürthle cell adenoma with follicular pattern | 13 14.7 |
2 2.3 |
0 1.1 |
ND ND |
TT; NED at 19 months |
| 19 | F, 75 | 55 | C+EV+ | N0M1 (diffuse interstitial lung) | 5 158 |
2 101 |
1 2.5 |
1 ND |
TT, L; 1 RAIT x 6.3 GBq; PerD at 21 months |
| 20 | F, 34 | 14 | C+EV+ | NxM0; history of breast cancer | 0 16.6 |
0 13.6 |
0 0.4 |
0 ND |
TT; NED at 20 months |
| 21 | F, 39 | 10 | C+EV+ | NxM0 | 3 0.6 |
0 UD |
0 UD |
0 ND |
TT; 1 RAIT x 1.85 GBq; at 18 months |
| 22 | F, 67 | 38 | C+EV+ | NxM0 | 0 1.6 |
0 ND |
0 ND |
0 ND |
TT; NED at 3 months |
| 23 | F, 69 | 90 | C+EV+ | NxM0 | 7 1.5 |
ND ND |
ND ND |
ND ND |
TT; NED at 1 month |
| 24 | F, 35 | 54 | C+EV+ (right lobe) | N1bM0; PDTC (left lobe) | 7 882 |
5 145 |
2 51 |
2 ND |
TT, L; 3 RAITs totaling 10.2 GBq; PerD at 19 months |
| 25 | F, 60 | 24 | C+ (right lobe) | NxM0; PTC (left lobe) | 0 0.2 |
0 UD |
0 UD |
0 ND |
TT; 1 RAIT x 2.38 GBq; NED at 18 months |
| 26 | F, 61 | 3 | C+ (right lobe) | NxM0; MTC (left lobe) | 0 0.5 |
0 0.3 |
0 UD |
0 ND |
TT; NED at 18 months |
The 6-month, 12-month, and 18-month CTC and Tg tests were performed within 1 month before or after the putative testing time. To convert GBq into mCi, divide by 0.037. Due to rounding, percentages may not add to 100%.
C+, capsular invasion; CT2, completion thyroidectomy, total of 2 surgeries; EV+, extensive vascular invasion (>4 or “large” number of vessels invaded); FTUMP, follicular tumor of unknown malignant potential; L, lymphadenectomy; Lob, lobectomy; MIFC, minimally invasive thyroid carcinoma; MTC, medullary thyroid carcinoma; ND, not done; NED, no evidence of disease according to physical examination, serum Tg and TgAb, ultrasonography, and whole-body scintigraphy; PDTC, poorly differentiated thyroid carcinomal; PerD, persistent disease; PTC, papillary thyroid carcinoma; RAIT, radioiodine therapy; Tg, thyroglobulin; TT, total thyroidectomy; UD, undetectable (<0.04 μg/L); V+, vascular invasion; WIFC, widely invasive follicular thyroid carcinoma.
All Tg testing was performed following ≥2 weeks of thyroid hormone withdrawal, with serum thyrotropin >40 mIU/L.
The 13 remaining patients were consistently CTC-negative throughout their mean 14.2±10.2 months of follow-up. This subgroup also had no evidence of disease post-primary treatment.
Compared to their CTC-negative counterparts, patients with positive initial CTC determinations had larger mean tumor size and greater prevalences of primary MIFC tumors or benign tumors >10 mm in diameter, of multifocality, and of vascular invasion (Table 3). However, neither these nor any other tested differences between the subgroups attained statistical significance.
Table 3.
Patient characteristics by baseline CTC status
| Variable | CTC-negative (n = 13) | CTC-positive (n = 13) | P |
| Histology, % (n)[% of histological subgroup] | |||
| Definite MIFC Benign Borderline MIFC/WIFC MIFC+ |
62% (8) [57%] 8% (1) [25%] 15% (2) [40%] 15% (2) [67%] |
46% (6) [43%] 23% (3) [75%] 23% (3) [60%] 8% (1) [33%] |
0.61 0.43 0.69 0.65 |
| Age, years, mean ± SD | 49 ± 15 | 46 ± 17 | 0.36 |
| Females, % (n) | 77% (10) | 92% (12) | 0.33 |
| Follicular tumor characteristics | |||
| Size, mean ± SD, mm <10.0 mm, % (n) Multifocality, % (n) |
29 ± 21 14% (2) 0 |
40 ± 22 0 8% (1) |
0.20 NA NA |
| Tumor invasion, % (n) | |||
| None Capsular invasion only Vascular invasion only Vascular invasion + capsular invasion Vessels invaded, mean ± SD |
8% (1) 54% (7) 0 38% (5) 0.9 ± 1.7 (n = 12)a |
23% (3) 23% (3) 0 54% (7) 1.6 ± 2.6 (n = 11)b |
0.30 0.111 NA 0.422 0.44 |
| Lymph node involvement, % (n) | |||
| N0 N1a N1b Nx |
15% (2) 8% (1) 0 77% (10) |
46% (6) 0 8% (1) 46% (6) |
0.46 NA NA 0.22 |
| Distant metastasis, n (%) | |||
| Any Lung Bone Other |
0 0 0 0 |
8% (1) 8% (1) 0 0 |
NA NA NA NA |
| RAIT | |||
| None, % (n) 1, % (n) 3, % (n) Cumulative activity, GBq, mean ± SD |
46% (6) 54% (7) 0 1.1 ± 1.2 |
46% (6) 46% (6) 8% (1) 1.9 ± 3.0 |
1.00 0.77 NA 0.38 |
| Stimulated serum Tg, μg/L, at baseline, mean | |||
| ± SD ≥1.0 μg/L, % (n) |
69 ± 225 77% (10) |
23 ± 11 100% (4) |
0.46 0.31 |
| Duration of follow-up, months, mean ± SD | 14 ± 10 | 19 ± 1 | 0.08 |
To convert GBq into mCi, divide by 0.037. Due to rounding, percentages may not add to 100%.
FTUMP, follicular tumor of uncertain malignant potential; MIFC, minimally invasive follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; NA, not [statistically] assessable; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; RAIT, radioiodine therapy; SD, standard deviation; Tg, thyroglobulin; TgAb, anti-thyroglobulin antibodies.
Due to rounding, percentages may not add to 100%
Excludes 1 patient with “>4” vessels invaded.
Excludes 1 patient with “>4” and 1 patient with a “large” number of vessels invaded
cIncludes 1 patient each with a history of colon cancer or breast cancer
Two patients were excluded from the analyses of correlation between CTC and Tg statuses, due to their having ambiguous stimulated Tg values (0.604 and 0.918 μg/L absent post-operative RAIT. Hence 24 patients were evaluable for these analyses.
There was no statistically significant correlation between CTC negativity, defined as a CTC count of 0/7.5 mL of blood, and stimulated Tg negativity, defined as Tg <0.04 μg/L in patients receiving post-operative RAIT, and <1.0 μg/L in patients not receiving post-operative RAIT (r = 0.243, p = 0.423, n=13). A statistically significant correlation also was absent between CTC positivity and stimulated Tg positivity at the first CTC determination (r = -0.418, p = 0.263, n=9). However, in the very limited number of evaluable patients (n=2) with CTC positivity in their first two measurements, there was a strong and statistically significant positive correlation between CTC positivity and stimulated Tg positivity (r = 1.00, p = 0.001).
DISCUSSION
To our knowledge, this is the first report focusing on CTC testing in patients with MIFC and benign thyroid tumors with a follicular pattern, although the modality has been investigated for at least a decade in DTC (12-20). Our analysis had three main findings. First, notwithstanding the mostly relatively limited tumor burden in our sample, CTC were detectable in at least 1 determination in substantial proportions of patients with MIFC (54%, 12 of 22 individuals) and even benign thyroid tumors with a follicular pattern (25%, 1 of 4 individuals). Additionally, CTC measurements varied relatively widely (from 1–13 CTCs/7.5 mL of blood) and in some patients, were present in up to 4 measurements extending over 18 months. These observations suggest feasibility of CTC testing in the studied settings.
Second, there were no statistically significant differences in any tested characteristic between the subgroups of CTC-negative versus CTC-positive patients (n = 13 each). However, tumor size and prevalence of multifocality and vascular invasion seemed to be greater in CTC-positive patients. Hence it may be that CTC positivity reflects more advanced disease, greater tumor cell access to the circulation, or both factors. However, our analysis, with a relatively small sample, lacked statistical power to verify these relationships.
Arguing against a relationship between CTC positivity and tumor burden, though, was the lack of a clearcut pattern of CTC positivity in our small sample of patients (n=5) with metastatic disease, co-existence of other types of thyroid tumors, or both characteristics.
Nor did CTC status show any relationship with clinical outcome in our analysis: none of our patients had evidence of disease at the latest visit. However, detection of such a relationship may have been obscured by short follow-up (on average, <18 months) and generally intense treatment (e.g., total thyroidectomy in 25/26 patients, RAIT in 14/26).
Third, in most of our patients (22/26, 85%), there appeared to be no significant correlation between CTC status and stimulated Tg status. This finding suggests that CTC determination may be providing different information than is Tg testing, although as alluded to above, the nature of that information remains unclear.
Our analysis focused on different thyroid tumors and used different CTC measurement methods than did earlier studies of CTC in DTC or benign thyroid tumors. We used immunomagnetic separation and manual visual inspection of cytokeratin 8 and 19 staining and of cell morphology, while other investigators quantitated circulating mRNA of the thyrotropin receptor with/without mRNA of other thyroid-specific proteins (12-15,18,19), or quantitated circulating cells that were positive for epithelial cell adhesion molecule (16,17,20). Therefore, our results are not directly comparable with previously published observations. Nonetheless, earlier studies (12-20), many also preliminary, tended to suggest absent or unclear relationships of CTC status with patient/disease characteristics or clinical outcome in DTC, as does our study.
This analysis had a number of limitations. First, reflecting the rarity of these neoplasia, the study sample was small. Second, our analysis was single-center, decreasing generalizability. However, these limitations may have been somewhat mitigated by our MIFC patients comprising a consecutive, unselected cohort.
A third limitation of our study was that CTC positivity was seen in 10/13 patients only in the initial determination, made 1 month after total thyroidectomy or completion total thyroidectomy. It therefore cannot be excluded that in our cohort, CTC positivity was strongly influenced by surgical handling. This hypothesis is supported by the significant correlation noted in our analysis between at least two consecutive positive CTC determinations and Tg positivity, while correlation with Tg positivity was absent when only the 1-month post-surgery CTC test was positive. However, the significant correlation was seen only in 2 evaluable patients, so its interpretation requires extreme caution. Nonetheless, it would be of interest to investigate using as the baseline CTC value a determination made later after surgery, e.g., at 6 months.
A fourth limitation of our study is that we did not evaluate possible statistical relationships between CTC status and demographic and clinical risk factors characterized elsewhere (1, 4, 7). Also, we lacked CTC values for healthy subjects or for patients with other forms of DTC or other thyroid disorders; such comparators would have provided useful context. Moreover, we analyzed CTC data categorically, not continuously, and did not assess CTC results as part of a multi-variable model, which may be the optimal way to deploy these data in everyday practice. Lastly, the CTC testing methodology that we used, entailing multi-step cell preparation and isolation, could lead to loss of or damage to tumor cells, reducing accuracy. More importantly, the methodology’s time-consuming, labor-intensive nature may render such a protocol unsuitable for widespread application; likely, this modality would need to be reserved for ambiguous or challenging cases. Conversely, the combination of biochemical testing (for surface cytokeratins) and microscopic inspection characterizing our technique could increase reliability compared to fully-automated CTC quantitation techniques.
In conclusion, this preliminary, hypothesis-generating work suggests that CTC testing is feasible in, and may provide otherwise uncaptured prognostic or other clinical information regarding patients with MIFC or benign thyroid tumors with a follicular pattern. Further study, preferably prospective and multi-center, in larger samples with longer follow-up is warranted.
Conflict of interest
No author has any potential conflict of interest associated with this research.
References
- 1.Dionigi G, Kraimps JL, Schmid KW, Hermann M, Sheu-Grabellus SY, De Wailly P, Beaulieu A, Tanda ML, Sessa F. Minimally invasive follicular thyroid cancer (MIFTC)-a consensus report of the European Society of Endocrine Surgeons (ESES) Langenbeck Arch Surg. 2014;399(2):165–184. doi: 10.1007/s00423-013-1140-z. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asari R, Koperek O, Scheuba C, Riss P, Kaserer K, Hoffmann M, Niederle B. Follicular thyroid carcinoma in an iodine-replete endemic goiter region: a prospectively collected, retrospectively analyzed clinical trial. Ann Surg. 2009;249(6):1023–1031. doi: 10.1097/SLA.0b013e3181a77b7b. [DOI] [PubMed] [Google Scholar]
- 4.Sugino K, Kameyama K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Yano Y, Uruno T, Akaishi J, Suzuki A, Masaki C, Ito K. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid. 2012;22(8):798–804. doi: 10.1089/thy.2012.0051. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Hirokawa M, Masuoka H, Yabuta T, Kihara M, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Prognostic factors of minimally invasive follicular thyroid carcinoma: extensive vascular invasion significantly affects patient prognosis. Endocrine J. 2013;60(5):637–642. doi: 10.1507/endocrj.ej12-0419. [DOI] [PubMed] [Google Scholar]
- 6.Goffredo P, Cheung K, Roman SA, Sosa JA. Can minimally invasive follicular thyroid cancer be approached as a benign lesion?: a population-level analysis of survival among 1,200 patients. Ann Surg Oncol. 2013;20(3):767–772. doi: 10.1245/s10434-012-2697-4. [DOI] [PubMed] [Google Scholar]
- 7.Goffredo P, Jillard C, Thomas S, Scheri RP, Sosa JA, Roman S. Minimally invasive follicular carcinoma: predictors of vascular invasion and impact on patterns of care. Endocrine. 2016;51(1):123–130. doi: 10.1007/s12020-015-0649-z. [DOI] [PubMed] [Google Scholar]
- 8.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Canc Research. 2007;13(3):920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 9.Hong B, Zu Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics. 2013;3(6):377–394. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 11.Freidin MB, Tay A, Freydina DV, Chudasama D, Nicholson AG, Rice A, Anikin V, Lim E. An assessment of diagnostic performance of a filter-based antibody-independent peripheral blood circulating tumour cell capture paired with cytomorphologic criteria for the diagnosis of cancer. Lung Cancer. 2014;85(2):182–185. doi: 10.1016/j.lungcan.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Milas M, Mazzaglia P, Chia SY, Skugor M, Berber E, Reddy S, Gupta M, Siperstein A. The utility of peripheral thyrotropin mRNA in the diagnosis of follicular neoplasms and surveillance of thyroid cancers. Surgery. 2007;141(2):137–146. doi: 10.1016/j.surg.2006.12.002. discussion 146. [DOI] [PubMed] [Google Scholar]
- 13.Milas M, Barbosa GF, Mitchell J, Berber E, Siperstein A, Gupta M. Effectiveness of peripheral thyrotropin receptor mRNA in follow-up of differentiated thyroid cancer. Ann Surg Oncol. 2009;16(2):473–480. doi: 10.1245/s10434-008-0211-9. [DOI] [PubMed] [Google Scholar]
- 14.Novosel T, Ritter HE, Gupta M, Harvey A, Mitchell J, Berber E, Siperstein A, Milas M. Detection of circulating thyroid cancer cells in patients with thyroid microcarcinomas. Surgery. 2009;146(6):1081–1089. doi: 10.1016/j.surg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Milas M, Shin J, Gupta M, Novosel T, Nasr C, Brainard J, Mitchell J, Berber E, Siperstein A. Circulating thyrotropin receptor mRNA as a novel marker of thyroid cancer: clinical applications learned from 1758 samples. Ann Surg. 2010;252(4):643–651. doi: 10.1097/SLA.0b013e3181f5ba51. [DOI] [PubMed] [Google Scholar]
- 16.Winkens T, Pachmann K, Freesmeyer M. Circulating epithelial cells in patients with thyroid carcinoma. Nuklearmedizin. 2013;52(1):7–13. doi: 10.3413/Nukmed-0524-12-08. Can they be identified in the blood? [DOI] [PubMed] [Google Scholar]
- 17.Winkens T, Pachmann K, Freesmeyer M. The influence of radioiodine therapy on the number of circulating epithelial cells (CEC) in patients with differentiated thyroid carcinoma - a pilot study. Exper Clin Endocrinol Diabetes. 2014;122(4):246–253. doi: 10.1055/s-0034-1370921. [DOI] [PubMed] [Google Scholar]
- 18.Aliyev A, Gupta M, Nasr C, Hatipoglu B, Milas M, Siperstein A, Berber E. Circulating thyroid-stimulating hormone receptor messenger RNA as a marker of tumor aggressiveness in patients with papillary thyroid microcarcinoma. Endocr Prac. 2015;21(7):777–781. doi: 10.4158/EP14425.OR. [DOI] [PubMed] [Google Scholar]
- 19.Sorg S, Pachmann K, Brede-Hekimian K, Freesmeyer M, Winkens T. Determining tissue origin of circulating epithelial cells (CEC) in patients with differentiated thyroid cancer by real-time PCR using thyroid mRNA probes. Canc Letters. 2015;356(2 Pt B):491–495. doi: 10.1016/j.canlet.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Xu JY, Handy B, Michaelis CL, Waguespack SG, Hu MI, Busaidy N, Jimenez C, Cabanillas ME, Fritsche HA, Cote GJ, Sherman SI. Detection and prognostic significance of circulating tumor cells in patients with metastatic thyroid cancer. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2016-2567. jc20162567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachelot A, Cailleux AF, Klain M, Baudin E, Ricard M, Bellon N, Caillou B, Travagli JP, Schlumberger M. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12(8):707–711. doi: 10.1089/105072502760258686. [DOI] [PubMed] [Google Scholar]
- 22.Piciu D, Piciu A, Irimie A. Thyroid cancer in children: a 20-year study at a Romanian oncology institute. Endocrine J. 2012;59(6):489–496. doi: 10.1507/endocrj.ej11-0397. [DOI] [PubMed] [Google Scholar]
- 23.Piciu D, Irimie A, Piciu A. Investigation of thyroid carcinoma over 40 years, using the database of the Ion Chiricuta Institute of Oncology Cluj-Napoca. J BUON. 2014;19(2):524–529. [PubMed] [Google Scholar]