Abstract
Context
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is a novel biomarker for cardiovascular diseases (CVD) risk estimation with high specificity for vascular inflammation. Few studies have investigated Lp-PLA2 levels in patients with metabolic syndrome (MetS) and obstructive sleep apnea syndrome (OSAS).
Objective
This study aimed to evaluate the role of Lp-PLA2 levels as a marker of vascular inflammation that contributes to cardiometabolic dysfunction in patients with MetS and OSAS.
Design
This is a prospective case-control study.
Subjects and Methods
83 men were enrolled. Following anthropometric measurements, laboratory analysis and overnight sleep study, patients were divided into three groups: MetS, OSAS with/without MetS. Serum Lp-PLA2 levels were determined by ELISA method.
Results
Serum Lp-PLA2 levels were statistically significant among the three groups and were higher in OSAS with MetS group than those without MetS. A significant positive relationship between increased Lp-PLA2 level and CRP (C-reactive protein) and apnea–hypopnea index (AHI) was found. Average oxygen saturation (AvO2) and the lowest oxygen saturation were negatively correlated with Lp-PLA2. The number of desaturation events, oxygen desaturation index, AvO2, AHI and CRP were significant predictors of Lp-PLA2.
Conclusions
Lp-PLA2 levels are associated with OSAS severity and might play an important role in predicting CVD in OSAS with/without MetS.
Keywords: lipoprotein-associated phospholipase A2, metabolic syndrome, obstructive sleep apnea, cardiovascular disease risk
INTRODUCTION
Global estimates show a substantial increase in the prevalence of metabolic syndrome (MetS) and obstructive sleep apnea syndrome (OSAS), in part due to the presence of the indisputable endemic character of obesity. According to World Health Statistics 2014, published by World Health Organization (WHO), 11% of men and 15% of women were obese. The high prevalence of overweight and obesity found in a rural adult Romanian population with no hallmark of western lifestyle (1), leads us to the growing concern that MetS is a common disease in our country and may also contribute to other disabling conditions (2). OSAS has been identified as an important public health burden (3) due to its association with cardiometabolic disease and all-cause mortality (4). OSAS is a complex disorder that is characterized by chronic intermittent hypoxia induced by respiratory events during sleep that disrupt sleep physiological architecture (5) and lead to sympathetic activation, endothelial activation and inflammation, oxidative stress, increased coagulation and metabolic dysregulation (6). Cardiovascular disease (CVD) is the leading cause of death in Europe (7) and is responsible for over 62% of all-cause mortality in Romania (8). There is growing evidence that MetS is one of the most important risk factors for CVD. OSAS has also been studied as a potential and treatable risk factor for CVD (9) considering its independent association with components of MetS, namely arterial hypertension, high fasting blood glucose levels, high triglycerides (TG) and low HDL cholesterol (HDL-C) (10, 11). Large epidemiological studies have shown the association of increased inflammatory markers with endothelial dysfunction and development of atherosclerosis in OSAS and MetS patients. Recently, lipoprotein-associated phospholipase A2 (Lp-PLA2) has emerged as a novel predictive biomarker of subclinical inflammation in cardiovascular diseases (12,13) with high specificity for vascular inflammation (14). Lp-PLA2 is a calcium-independent, 45-kDA protein, member of the phospholipases A2 superfamily of enzymes that hydrolyzes oxidized phospholipids generating proinflammatory and proatherogenic products such as lysophosphatidylcholine and non-esterified fatty acids associated with signaling pathways activation involved in alteration of the vascular wall (15). Lp-PLA2 can be found in two forms: intracellular form expressed in atherosclerotic plaque and extracellular form found in circulating plasma in association with lipoproteins (16). Over 80% of plasma Lp-PLA2 mass circulates bound to apolipoprotein B on LDL-C, while the rest is associated with very-low-density lipoprotein, HDL-C and lipoprotein A (17). Lp-PLA2 manifests dual effects: anti-inflammatory and anti-thrombotic by degrading oxidized phospholipids, including platelet-activating factor and pro-oxidative and pro-inflammatory properties, with the balance inclined towards the latter (18). Our study aims to evaluate the role of serum Lp-PLA2 levels as a marker of vascular inflammation that contribute to cardiometabolic dysfunction in patients with MetS and OSAS alone and also in individuals with coexisting syndromes. To our best knowledge, this is the first study to compare Lp-PLA2 serum levels between individuals with either MetS, OSAS or both syndromes.
MATERIALS AND METHODS
Study population
In our prospective case-control study, between January 2016 and February 2017, 102 consecutive male subjects attended our clinic because of clinical suspicion of OSAS: 83 participants and 16 healthy controls were included in the final analysis. 19 subjects met one or more of the following exclusion criteria: history of hypo- and hyperthyroidism, uncontrolled chronic medical conditions, acute or inflammatory diseases, sleep disorders other than OSAS, prior continuous positive airway pressure (CPAP) treatment, previous use of lipid-lowering therapy, sedative or hypnotic medication in the last 3 months. After anthropometric measurements, laboratory analysis and overnight sleep study, patients were divided into three groups according to MetS and OSAS criteria: MetS (group 1), OSAS without MetS (group 2) and OSAS with MetS (group 3). The control group consisted of healthy individuals with no signs of MetS or OSAS. All patients enrolled in this study gave written consent prior to participation.
Study protocol
The subjects were asked a set of detailed questions on personal habits, history of diseases and use of medication. Body mass index (BMI, kg/m2), neck (NC) and waist circumference (WC) measurements were assessed. The average of three seated blood pressure measurements was recorded. All patients underwent overnight unattended cardiorespiratory sleep study at the hospital using a portable multichannel device that recorded oral and nasal flow, chest and abdominal movements, continuous pulse oximetry, snoring and body position. The tests were validated and interpreted by a trained sleep physician according to American Academy of Sleep Medicine guidelines for diagnostic testing for adult sleep apnea (19). Venous blood samples were obtained from all patients in the morning after overnight cardiorespiratory polygraphy, after more than 12 hours of fasting. Measurements of blood glucose, total cholesterol (TC), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), triglycerides (TG), C-reactive protein (CRP) and complete blood count (CBC) analysis were performed. The monocyte count was assessed part of the routine hemogram with a reference value of 2-10%. All blood samples were determined using commercially available kits.
Lp-PLA2 measurements
Serum samples were obtained from patients using a serum separator tube and allowed to clot for two hours at room temperature before centrifugation for 20 minutes at 1000xg; they were then frozen and stored at −80° until analysis. Serum LpPLA2 level was determined spectrophotometrically by using “Cloud-Clone Corp.” Enzyme-Linked Immunosorbent Assay (ELISA) kit. LpPLA2 serum level is expressed as ng/mL. The baseline values of serum Lp-PLA2 obtained in our control group were 351.9 ± 105.3 ng/mL.
Definitions
The diagnosis of metabolic syndrome was based according to International Diabetes Federation (IDF) Guidelines (20) by following the criteria: central obesity (defined as WC ≥ 94 cm for Caucasian male) plus the presence of any two of the following: 1) TG levels ≥150 mg/dL or treatment for elevated TG; 2) low HDL-C < 40 mg/dL or specific medication; 3) High systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mm Hg or drug treatment for previously diagnosed hypertension; 4) elevated fasting plasma glucose ≥ 100 mg/dL or formerly diagnosed with type 2 diabetes. Obesity was defined (21) by using the BMI cut-off points of ≥ 30 kg/m2 established by WHO. Arterial hypertension diagnosis was assessed by using the current guidelines (22). Framingham score was calculated as risk of coronary heart disease and stroke at 10 years (23). Monocyte to high-density lipoprotein ratio (MHR) was calculated by dividing monocyte count by HDL-C level.
Statistical analysis
Statistical analysis of the data was performed using SPSS version 20.0. The normal distribution of continuous data was tested using Shapiro-Wilk test. Normally distributed data were presented as mean ± SD and non-normally distributed data were expressed as median and interquartile range. We used Student’s t test and one-way ANOVA to compare normally distributed data and Mann-Whitney U test and Kruskal-Wallis for nonparametric variables. Correlation analysis was performed using Pearson and Spearman tests. In regression models we used Lp-PLA2 levels as dependent variable to evaluate potential associations with sleep parameters and traditional risk factors for CVD. A p value of <0.05 was considered statistically significant.
RESULTS
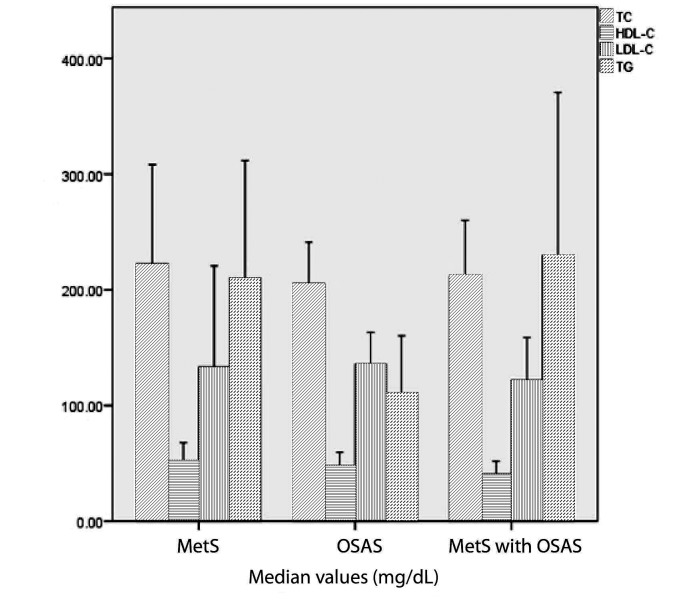
Of the total group of 83 patients, 53 (63.8%) met the criteria for MetS, of which 42 (79.2%) were diagnosed with OSAS. 13 subjects (15.7%) had normal apnea hypopnea index (AHI) <5/h, 24.1% had mild OSAS (AHI 5-14.9/h), 26.5% had moderate OSAS (AHI 15-29.9/h) and 33.7% had severe OSAS (AHI>30/h). The prevalence of severe OSAS in the study population was 34.1% and more than half of them belonged to OSAS with MetS group. The baseline demographic and clinical characteristics of study population are shown in Table 1. There were significantly higher values of BMI, NC, WC, SBP, DBP, TG, Framingham score, CRP, MHR, sleep parameters and a significantly lower HDL-C levels in all patient groups compared to their corresponding values in the control group. Although an increasing trend was observed regarding advancing age and the presence of OSAS or its association with MetS, it did not reach statistical significance. As expected, all subjects were obese with significantly higher BMI, NC and WC compared to the control group. OSAS with MetS group had higher BMI compared to OSAS without MetS (p=0.037), higher TG levels (p=0.001), increased plasma glucose level (p=0.032) and higher Framingham score (p=0.042). In regard to sleep parameters, patients with higher number of respiratory events during sleep were found in the OSAS with MetS group. There was a statistically significant difference in oxygen desaturation index (ODI) (p=0.044) and number of desaturations (p=0.044) between OSAS patients with and without MetS. When comparing the three groups, we found a statistically significant difference in NC, TG, Framingham score and all studied sleep parameters; these were significantly higher in OSAS with MetS group. Also, we found that lower HDL-C levels were associated with this group. The distribution of lipid profile among the three studied groups is shown in Figure 1. The Lp-PLA2 mass levels ranged from 187.9 to 2000 ng/mL with a median value of 728.5 ng/mL for all study participants. There were statistically significant differences in serum Lp-PLA2 levels among the three groups (Fig. 2) and also when compared to the control group. A significant difference in Lp-PLA2 levels between OSAS patients with and without MetS was also observed. In all study population, correlation analysis showed a positive association between Lp-PLA2 levels and BMI (r=0.31, p=0.046), CRP (r=0.29, p=0.047) and AHI (r=0.37, p=0.015). A negative modest association was found between Lp-PLA2 levels and average oxygen level (r=-0.33, p=0.034) and lowest oxygen saturation (r=-0.36, p=0.019). Lp-PLA2 levels had also significant positive association with TG (r=0.94, p=0.005) in MetS group and significant inverse correlation with plasma glucose (r=-0.71, p=0.004) in OSAS without MetS group. In OSAS and MetS group, Lp-PLA2 was associated with BMI (r=0.49, p=0.024), CRP (r=0.45, p=0.036) and sleep parameters: AHI (r=0.44, p=0.043), ODI (r=0.48, p=0.024) and number of desaturation events (r=0.49, p=0.022). In addition, in all study group population, we found a positive correlation between Framingham score and AHI (r=0.40, p=0.009), ODI (r=0.43, p=0.005), number of desaturation events (r=0.41, p=0.006) and an inverse association with average oxygen level (r=-0.34, p=0.026) and lowest oxygen saturation (r=-0.39, p=0.010). The correlations between serum Lp-PLA2 and other clinical variables were analyzed using a simple linear regression model (Table 2). The strongest predictors for Lp-PLA2 levels variation in MetS group were TG, ODI and number of desaturation episodes. In OSAS without MetS group, monocyte and MHR were found to be responsible for 55% and 51% of the variation, respectively. The strongest predictors of variation in Lp-PLA2 levels in OSAS with MetS group were BMI, WC, CRP, AHI, ODI, the number of desaturation events and average oxygen levels.
Figure 1.
The distribution of lipid profile among the three studied groups. Results are expressed as median and interquartile range values.
Figure 2.
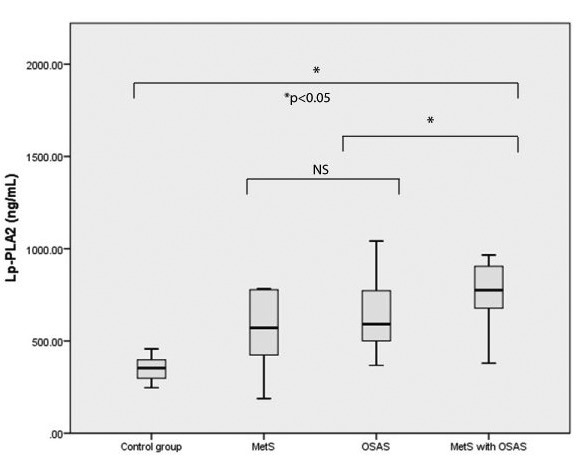
Comparison of serum Lp-PLA2 levels among the three groups. Data are expressed as median values and interquartile range (25th-75th). The median serum levels of Lp-PLA2 were significantly higher in patients with MetS and OSAS compared to MetS and OSAS alone (p<0.05). NS - not significant.
Table 1.
The baseline demographic, clinical characteristics and comparison of study population. Normally distributed data is shown as mean ± standard deviation and nonparametric values are expressed as median (quartile 1; quartile 3)
| Variables | Control group (n=16) | MetS (n=13) | OSAS (n=28) | OSAS with MetS (n=42) | p value* |
| Age, years | 50.06 ± 2.3 | 49.33 ± 2.8 | 50.92 ± 1.7 | 53.52 ± 1.0 | p=0.216 |
| BMI, kg/m2 | 20.7± 2.6 | 33.83 ± 0.9 | 33.85 ± 1.2 | 38.03 ± 1.3 | p=0.051 |
| NC, cm | 31.5 ± 1.0 | 44.0 ± 0.7 | 46.2 ± 1.4 | 46.9 ± 0.6 | p=0.027 |
| WC, cm | 80.1± 8.9 | 117.0 ± 2.7 | 116.5 ± 2.9 | 123.7 ± 2.9 | p=0.170 |
| SBP, mmHg | 108.1 ± 4.3 | 135.8 ± 4.1 | 128.0 ± 4.6 | 139.7 ± 3.5 | p=0.119 |
| DBP, mmHg | 71.9 ± 1.2 | 85.5 ± 2.7 | 78.9 ± 2.7 | 83.5 ± 2.1 | p=0.266 |
| *TC, mg/dL | 169.2 | 174.9 | 207.6 | 212.7 | p=0.990 |
| (167.5; 185.4) | (163.5; 313.2) | (169.3; 238.4) | (156.7; 252.9) | ||
| *HDL-C, mg/dL | 66.4 ± 11.4 | 52.1 ± 6.3 | 47.6 ± 3.1 | 40.6 ± 2.4 | p=0.048 |
| *LDL-C, mg/dL | 107 | 116.6 | 148.5 | 118.0 | p=0.546 |
| (86; 118.7) | (70; 209.7) | (108.2; 155.5) | (94; 154.2) | ||
| *TG, mg/dL | 88.65 | 192.6 | 98.4 | 222.2 | p=0.004 |
| (62.6; 118.1) | (137.4; 319.1) | (74.7; 127) | (125; 269.8) | ||
| *Glucose, mg/dL | 91 | 93 | 94 | 114 | p=0.110 |
| (87.5; 100.5) | (85.5; 117.2) | (91; 108.9) | (93.5; 149) | ||
| *FRS, % | 3.2 | 12.5 | 13.3 | 35.3 | p<0.001 |
| (1.8; 5.0) | (7.1; 31.6) | (10.7; 16.9) | (23.3; 52.6) | ||
| *CRP, mg/dL | 0.7 | 3.2 | 3.1 | 4.2 | p=0.454 |
| (0.42; 1.2) | (2.4; 4.1) | (1.8; 4.7) | (2.2; 5.4) | ||
| Monocyte (103 μL) | 0.42 ± 0.02 | 0.59 ± 0.05 | 0.70 ± 0.04 | 0.61 ± 0.03 | p=0.227 |
| MHR, 10-3 | 6.0 ± 1.8 | 12.0 ± 1.5 | 15.6 ± 1.6 | 16.1 ± 1.2 | p=0.299 |
| *Lp-PLA2 mass, ng/mL | 353.8 | 570.9 | 598.8 | 775.7 | p=0.046 |
| (298.0; 404.7) | (365.1; 778.7) | (473.9; 786.2) | (669.6; 904.7) | ||
| *AHI, events/h | 1.75 (1.1; 2.7) | 3.1 (2.5; 3.3) | 18.4 (12.8; 28.2) | 31.2 (10.6; 56.4) | p=0.046 |
| *ODI, events/h | 0.55 (0.2; 1.2) | 8.2 (4.7; 23.2) | 19.6 (13.1; 25.5) | 36.1 (14.1; 70.1) | p=0.025 |
| *Number of desaturations | 7.5 | 65.5 | 137.5 | 238.0 | p=0.022 |
| (4.2; 13.7) | (33; 131) | (91.7; 195.7) | (93; 82.5) | ||
| *Lowest SaO2, % | 91 (89; 92) | 83 (77.2; 89.5) | 81.5 (62.2; 85.2) | 70 (52.5; 82.5) | p=0.092 |
| *Average SaO2, % | 93 | 91.5 | 91.5 | 91 | p=0.169 |
| (94; 95) | (90.5; 94.2) | (89.7; 93.2) | (84; 92.5) |
Legend: BMI = body mass index; NC = Neck circumference; WC = Waist circumference; SBP = Systolic blood pressure; DBP = Diastolic blood pressure; TC= Total cholesterol; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; TG = Triglycerides; FRS = Framingham Risk Score; CRP = C-reactive protein; MHR = Monocyte to high-density lipoprotein ratio; Lp-PLA2 = Lipoprotein-associated phospholipase A2; AHI= apnea hypopnea index; ODI = oxygen desaturation index; SaO2 = arterial oxygen saturation. *p-value <0.05 indicates statistical significance between MetS, OSAS and OSAS with MetS patients.
Table 2.
Linear regression analysis between lipoprotein-associated phospholipase A2 and other clinical variables
| Control group | MetS | OSAS | OSAS with MetS | |||||
| Variables | r | p | r | p | r | p | r | p |
| BMI | 0.33 | 0.20 | 0.25 | 0.30 | 0.05 | 0.41 | 0.57 | 0.003 |
| WC | 0.14 | 0.58 | 0.09 | 0.86 | 0.01 | 0.63 | 0.17 | 0.06 |
| TG | 0.07 | 0.79 | 0.84 | 0.01 | 0.05 | 0.44 | 0.01 | 0.63 |
| CRP | 0.21 | 0.42 | 0.10 | 0.33 | 0.08 | 0.76 | 0.64 | 0.001 |
| Monocyte | 0.34 | 0.19 | 0.15 | 0.43 | 0.55 | 0.02 | 0.001 | 0.97 |
| MHR | 0.46 | 0.06 | 0.12 | 0.48 | 0.51 | 0.03 | 0.001 | 0.96 |
| AHI | 0.11 | 0.66 | 0.23 | 0.33 | 0.04 | 0.48 | 0.51 | 0.009 |
| ODI | 0.36 | 0.16 | -0.82 | 0.02 | 0.01 | 0.66 | 0.43 | 0.02 |
| Number of desaturation events | 0.29 | 0.26 | 0.76 | 0.03 | 0.07 | 0.79 | 0.68 | <0.001 |
| Average SaO2 | 0.07 | 0.79 | 0.05 | 0.92 | 0.18 | 0.52 | -0.59 | 0.002 |
Abbreviations: BMI = body mass index; WC = Waist circumference; TG = Triglycerides; CRP = C-reactive protein; MHR = Monocyte to high-density lipoprotein ratio; AHI= apnea hypopnea index; ODI = oxygen desaturation index; SaO2 = arterial oxygen saturation.
DISCUSSION
In the present study, serum Lp-PLA2 level was significantly elevated in subjects diagnosed with OSAS and especially in those diagnosed with MetS as well, when compared to MetS alone. Also, OSAS without MetS subjects had higher Framingham scores than MetS patients alone. The positive association found between Lp-PLA2 and BMI and WC were confirmed by other studies (24) and might be explained by the fact that macrophages are found in a high number in visceral adipose tissue and are responsible for Lp-PLA2 secretion. The high prevalence of MetS in OSAS subjects found in our study is a major cause for concern considering the burden of cardiometabolic consequences derived from both syndromes. More than half of the patients with severe OSAS belonged to OSAS with MetS group. Given the complex, bidirectional interactions and interconnected mechanisms between OSAS and MetS, as we expected, most risk factors associated with the initiation or development of CVD were found in OSAS with MetS group. The presence of both OSAS and MetS, described as syndrome Z (25) may be linked to higher risk of CVD due to cumulative and synergistic effects (26,27). Wilcox et al. suggested that if OSAS alone were linked plausibly to CVD, then OSAS therapy would be indicated for predictive reasons (25) that might lead to increased awareness of OSAS and improvement of cardiovascular risk factors in both OSAS patients and coexisting syndromes. Lp-PLA2 has emerged as an independent risk marker for cardiovascular disease and it is being used as an adjunct to clinical risk prediction (28). CRP plays an important role in atherosclerosis and numerous large-scale studies have shown that CRP can be used as an inflammatory marker and as a mediator of atherosclerosis. Our findings show strong association in MetS alone group between the two inflammatory Lp-PLA2 and CRP levels known to be associated with myocardial infarction and stroke (29). In OSAS without MetS group, monocyte and MHR accounted for more than half of the variation of serum Lp-PLA2 levels. Similarly to Lp-PLA2, MHR was identified as a novel inflammation marker used as an independent predictor of CVD severity (30, 31). MHR was also investigated in OSAS patients and found to be associated with OSAS severity and suggested to be a useful predictor for CVD in individuals with OSAS (32). In our study, although it did not meet a significant statistical value, MHR is higher in OSAS patients, especially in OSAS with MetS comparing to MetS alone. This might be explained by the fact that Lp-PLA2 is a product of monocytes and its production regulated by inflammatory mediators is exacerbated in OSAS due to hypoxic stress (33). Our study provides evidence of a strong positive correlation between serum Lp-PLA2 and studied sleep parameters such as AHI, ODI, number of desaturation events and an inverse association with a lowest oxygen saturation and average oxygen saturation. These results are in agreement with two other studies that evaluated Lp-PLA2 levels in OSAS patients that also reported a significant association between circulating Lp-PLA2 concentration and arousal index (34, 35). AHI and ODI, as well as arousal index, are good indicators for severity of respiratory events during sleep. We also found a strong association between Framingham score and studied sleep parameters. The inverse correlation between this score and average and lowest oxygen saturation and the fact that Lp-PLA2 is also negatively correlated with these variables, might suggest that OSAS severity is linked to higher risk of CVD. We observed an independent association of Lp-PLA2 with OSAS severity expressed as ODI, result that leads us to conclude that there is an independent relationship between OSAS and CVD and that one of the factors involved is hypoxia. Chronic intermittent hypoxia is the most important characteristic of OSAS pathophysiology and a distinctive feature of this condition may be the main culprit involved in endothelial dysfunction, linking OSAS to atherosclerosis (36). Our study has some limitations: the sample was small, not random and there was a lack of available data regarding cardiac assessments.
In conclusion, chronic intermittent hypoxia, a key feature of OSAS, is associated with high proinflammatory state burden that adds to global cardiometabolic risk estimated by conventional risk factors. OSAS with MetS is associated with higher number of respiratory events during sleep and higher serum Lp-PLA2 levels when compared to OSAS and MetS alone. Lp-PLA2 levels are associated with OSAS severity and might play an important role in predicting CVD in OSAS patients with or without MetS.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Mihalache L, Graur L, I, Popescu D, Boiculese L, Badiu C, Graur M. The Prevalence of the Metabolic Syndrome and Its Components in A Rural Community. Acta Endocrinologica-Bucharest. 2012;8(4):595–606. [Google Scholar]
- 2.Beyca HH, Mesci B, Caklili OT, Mutlu HH, Oguz A. Neuropathy associated with hypertriglyceridemia in patients with metabolic syndrome. Acta Endocrinologica-Bucharest. 2016;12(1):26–29. doi: 10.4183/aeb.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raşcu A, Arghir OC, Otelea M. Obstructive sleep apnea– Case report and literature review. RJLM. 2016;24(2):118–121. [Google Scholar]
- 4.Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev. 2010;14(4):239–247. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Force AO, American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. JCSM. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 6.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290(14):1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 7.European Cardiovascular Disease Statistics 2017. Available from http://www.ehnheart.org/cvd-statistics.html.
- 8.Dorobanţu MA, Darabont RO, Ghiorghe S, Babes K, Pop DA, Toma DA, Vasilescu MA, Dobreanu MI, Tăutu OA. Profile of the Romanian hypertensive patient data from SEPHAR II study. Rom J Intern Med. 2012;50(4):285–296. [PubMed] [Google Scholar]
- 9.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 10.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Gruber A, Horwood F, Sithole J, Ali NJ, Idris I. Obstructive sleep apnea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. 2006;5(1):22–29. doi: 10.1186/1475-2840-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vittos O, Toana B, Vittos A, Moldoveanu E. Lipoprotein-associated phospholipase A2 (Lp-PLA2): a review of its role and significance as a cardiovascular biomarker. Biomarkers. 2012;17(4):289–302. doi: 10.3109/1354750X.2012.664170. [DOI] [PubMed] [Google Scholar]
- 13.Moldoveanu E, Tanaseanu C, Tanaseanu S, Kosaka T, Manea G, Marta DS, Popescu LM. Plasma markers of endothelial dysfunction in type-2 diabetes patient. Eur J Intern Med. 2006;17(1):38–42. doi: 10.1016/j.ejim.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Moldoveanu E, Serban M, Marta DS, Serban I, Huica I. Lipoprotein-associated phospholipase A2 activity in patients with preserved left ventricular ejection fraction. Biomarkers. 2011;6(7):587–589. doi: 10.3109/1354750X.2011.611597. [DOI] [PubMed] [Google Scholar]
- 15.Iribarren C, Gross MD, Darbinian JA, Jacobs DR, Sidney S, Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults. Arterioscler Thromb Vasc Biol. 2005;25(1):216–221. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]
- 16.Stafforini DM, Zimmerman GA. Unraveling the PAF-AH/ Lp-PLA2 controversy. J Lipid Res. 2014;55(9):1811–1814. doi: 10.1194/jlr.E052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robins SJ, Collins D, Nelson JJ, Bloomfield HE, Asztalos BF. Cardiovascular events with increased lipoprotein-associated phospholipase A2 and low high-density lipoprotein-cholesterol. Arterioscler Thromb Vasc Biol. 2008;28(6):1172–1178. doi: 10.1161/ATVBAHA.107.160739. [DOI] [PubMed] [Google Scholar]
- 18.Serban M, Tanaseanu C, Kosaka T, Vidulescu C, Stoian I, Marta DS, Tanaseanu S, Moldoveanu E. Significance of platelet activating factor acetylhydrolase in patients with non-insulin dependent (type 2) diabetes mellitus. J Cell Mol Med. 2002;6(4):643–647. doi: 10.1111/j.1582-4934.2002.tb00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care 2017. Available from https://www.idf.org/e-library/guidelines.html. [DOI] [PubMed]
- 21.World Health Organization Obesity and overweight. Available from http://www.who.int/mediacentre/factsheets/fs311/en/.
- 22.2013 ESH/ESC Guidelines for the management of arterial hypertension. Available from https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Arterial-Hypertension-Management-of.
- 23.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care. The Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 24.Acevedo M, Varleta P, Kramer V, Valentino G, Quiroga T, Prieto C, Parada J, Adasme M, Briones L, Navarrete C. Comparison of lipoprotein-associated phospholipase A2 and high sensitive C-reactive protein as determinants of metabolic syndrome in subjects without coronary heart disease: in search of the best predictor. Int J Endocrinol. 2015;2015:934681. doi: 10.1155/2015/934681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox I, McNamara SG, Collins FL, Grunstein RR, Sullivan CE. “Syndrome Z”: the interaction of sleep apnea, vascular risk factors and heart disease. Thorax. 1998;53(suppl.3):S25–28. [PMC free article] [PubMed] [Google Scholar]
- 26.Zamarrón C, Valdés Cuadrado L, Álvarez-Sala R. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm Med. 2013;2013:521087. doi: 10.1155/2013/521087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodson BL, Wung SF, Archbold KH. Obstructive sleep apnea hypopnea syndrome and metabolic syndrome: a synergistic cardiovascular risk factor. J Am Acad Nurse Pract. 2012;24(12):695–703. doi: 10.1111/j.1745-7599.2012.00771.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefont-Rousselot D. Lp-PLA2, a biomarker of vascular inflammation and vulnerability of atherosclerosis plaques. Ann Pharm Fr. 2016;74(3):190–197. doi: 10.1016/j.pharma.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Elkind MS, Leon V, Moon YP, Paik MC, Sacco RL. High-sensitivity C-reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke. 2009;40(10):3233–3237. doi: 10.1161/STROKEAHA.109.552802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cetin MS, Cetin EH, Kalender E, Aydin S, Topaloglu S, Kisacik HL, Temizhan A. Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ. 2016;25(11):1077–1086. doi: 10.1016/j.hlc.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Kanbay M, Solak Y, Unal HU, Kurt YG, Gok M, Cetinkaya H, Karaman M, Oguz Y, Eyileten T, Vural A, Covic A. Monocyte count/ HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46(8):1619–1625. doi: 10.1007/s11255-014-0730-1. [DOI] [PubMed] [Google Scholar]
- 32.Koseoglu HI, Pazarli AC, Kanbay A, Demir O. Monocyte count/ HDL cholesterol ratio and cardiovascular disease in patients with obstructive sleep apnea syndrome: A multicenter study. Clin Appl Thromb Hemost. 2018;24(1):139–144. doi: 10.1177/1076029616677803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamaki S, Yamauchi M, Fukuoka A, Makinodan K, Koyama N, Tomoda K, Yoshikawa M, Kimura H. Production of inflammatory mediators by monocytes in patients with obstructive sleep apnea syndrome. Intern Med. 2009;48(15):1255–1262. doi: 10.2169/internalmedicine.48.2366. [DOI] [PubMed] [Google Scholar]
- 34.Bekci TT, Kayrak M, Kiyici A, Maden E, Ari H, Kaya Z, Teke T, Akilli H. The association among lipoprotein-associated phospholipase A2 levels, total antioxidant capacity and arousal in male patients with OSA. Int J Med Pharm. 2011;8(5):369–376. doi: 10.7150/ijms.8.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakka S, Siahanidou T, Voyatzis C, Pervanidou P, Kaminioti C, Lazopoulou N, Kanaka-Gantenbein C, Chrousos GP, Papassotiriou I. Elevated circulating levels of lipoprotein-associated phospholipase A2 in obese children. Clin Chem Lab Med. 2015;53(7):1119–1125. doi: 10.1515/cclm-2014-1081. [DOI] [PubMed] [Google Scholar]
- 36.Atkeson A, Jelic S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag. 2008;4(6):1327–1335. doi: 10.2147/vhrm.s4078. [DOI] [PMC free article] [PubMed] [Google Scholar]