Abstract
Background
Neuroendocrine neoplasms (NENs) can arise in most of the epithelial organs of the body and are not a rare condition in the gastrointestinal tract (GIT). The presence of NENs in GIT associated with other secondary primary malignancies (SPM) has been considered an exotic event. This study aims to describe the case reports of NENs accompanied by synchronous primary tumors.
Methods and findings
We performed a systematic literature search of the databases Scopus, PubMed, Scielo and LILACS to identify case reports that described the presence of NENs in GIT with SPM. 78 case reports were included. The mean of age of the cases was 60.2 years. 60% were male. 95.4% were NENs G1. 17 cases of NENs had metastasis. 80% of SPM were recognized in the GIT (36% in stomach, 27% in large intestine, 11.2% in small intestine, and 5.6% in esophagus). The most common type of SPM was adenocarcinoma (49.4%), followed by GIST (13.5%), other NENs in different GIT segment (7.9%), lymphoma (6.8%), and squamous cell carcinoma (4.5%). The most common tumor in GIT was adenocarcinoma (97.6%) and the presence of adenocarcinoma in the same segment of GIT was found in 68.4% of the cases. Association between adenocarcinomas and NENs in GIT (p:<0.0001) and adenocarcinoma and tumor in the same segment of GIT location were observed (p<0.001).
Conclusion
These results demonstrate that NENs with SPM are not a rare condition. Several theories have been proposed to explain this association; one of these is the ability of NENs to generate synchronous tumors by autocrine and paracrine effect. We observed an association between adenocarcinomas and NENs in the same segment of GIT.
Introduction
Neuroendocrine neoplasms (NENs) can arise in most of the epithelial organs of the body. NENs are a diverse group of tumors and have been found predominantly in the lung, tubular gastrointestinal tract, and pancreas. The terminology for NENs has been problematic for various reasons [1–3]. The current classification (2018) aims to stratify NENs by their prognosis. This classification adapted by World Health Organization (WHO) is divided according to the anatomic site (tubular gastrointestinal tract (GIT), pancreas, uterus, and lung); family (Neuroendocrine tumors (NETs) or Neuroendocrine carcinoma (NEC)); and grade. In GIT the current terminology of NETs are grade 1 (G1), grade 2 (G2), and grade 3 (G3), while the NECs are classified as small cell neuroendocrine carcinoma and large cell neuroendocrine carcinoma [3].
NENs account for about of 0.5% of newly diagnosed neoplasms [4]. Their incidence has increased, possibly due to improved diagnostic techniques [5]. Their prevalence in the United States is estimated at 103.312 cases, which is twice the prevalence of pancreatic and gastric cancers combined [5,6]. These tumors have a female preponderance of around 2.5:1, and the most frequent primary sites are the gastrointestinal tract (62%-67%) and the lung (22%-27%). 12% to 22% of patients are metastatic at presentation [4]. The most commonly documented NENs locations in GIT are colon and rectum (69%), followed by small intestine (36%), stomach (10%), appendix (5%) and esophagus (0.4–2%) [7,8]
The majority of NENs arise sporadically, but an association with Multiple Endocrine Neoplasia Syndrome type 1 and familial clustering is recognized [4]. NENs can be found synchronous (occurring at the same time) with other secondary primary malignancies (SPM) or metachronous (occurring at different times) [9–12], even without genetic predisposition syndrome [12]. The case reports of these occurrences have increased over time and have been considered as unusual. Therefore, the aim of this systematic review is to describe the cases of NENs accompanied with synchronous tumors reported in the literature.
Methods
Search strategy
We carried out a systematic review of case reports published in Scopus, PubMed, Scielo and LILACS, which includes BIREME, and many other LA sources. No limits regarding language or publication period were placed on the search. The search was done using the following terms: synchronous AND (neuroendocrine tumors OR carcinoid tumors OR small cell carcinoma OR large cell carcinoma). The PRISMA guidelines were followed during data extraction, analysis, and reporting [13]. The search was done during June 2018. We considered papers available in the following languages: English and Spanish.
Study selection, data extraction and quality assessment
The inclusion criteria for the systematic review were the following: case reports with the presence of NENs confirmed by histopathology in the GIT with the presence of synchronous malignant tumors in any location. SPM were defined as any primary malignant tumor which occurred at the same time with the other tumor. The exclusion criteria were the presence of mixed adenoneuroendocrine carcinomas (MANECs) or neuroendocrine carcinoma of unknown primary site.
Two reviewers screened all the titles and abstracts from the publications performed an eligibility assessment. Retrieved articles were rejected if the eligibility criteria were not met. A third reviewer was consulted when eligibility criteria were unclear. Hand-searches were performed from the articles that seemed to be relevant.
The extracted data from each article were: author name, year of publication, age, gender, localization of NENs (esophagus, stomach, small intestine (duodenum, jejunum and ileum) and colorectum (appendix, colon and rectum), family of NENs, grade of NENs and localization and type of SPM, and presence of metastasis of NENs and SPM.
To assess the article quality we used a modified tool version that has been used in previous studies [14,15]. Three researchers independently evaluated the assessments of all case reports. We used three items: (1) Patients were described adequately (clinical history, laboratory and radiological findings); (2) accurate diagnoses were provided (Diagnosis of NENs and SPM were confirmed by pathology); (3) convincing evidence in support of the diagnosis was presented (NENs and SPM were confirmed using immunohistochemistry or the pathological images showed in the article, the images were interpreted by a pathologist (RPM)). Data extracted by the abstract were not included in the quality assessment.
Data synthesis and analysis
Univariate analysis was applied to determine distribution of clinical and pathological findings. Type and location of SPM were analyzed as a different case report in patients with more than one SPM. Patients with multiple NENs tumors in the same localization were analyzed as one only case report; this was done in order to avoid overestimation. Chi-square test was employed to determine statistically significant differences between localization of NENs and tumor subtype. A p-value <0.05 was considered statistically significant. Statistical analysis was performed with the STATA 13.
Results
Systematic review literature
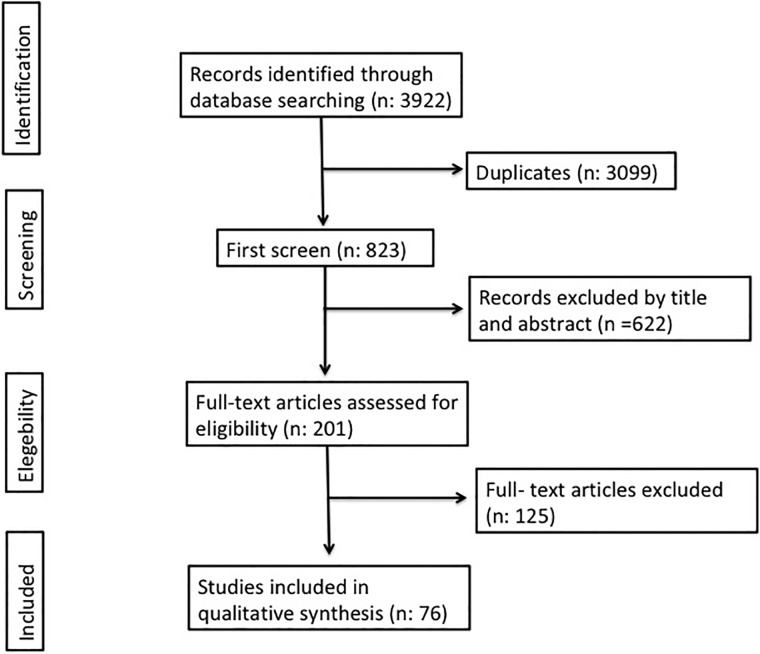
We identified 3922 articles in the databases. Of these, 3099 were identified as duplicates. A total of 201 full text articles were assessed for eligibility. Finally, 76 articles that contained interpretable data and fulfilled the eligibility criteria were included (Fig 1). [16–90] In 16 articles the data extraction was made from its abstract [21,22,24,33–35,37,45,64,70,71,73,74,80]. The flowchart for systematic literature review and articles included in the analysis is shown in Fig 1. Detailed information is shown in the S1 Table.
Fig 1. Flow chart of the systemic literature review.
Quality appraisal
The overall quality of the cases was good. Most cases reported an adequate description of patient past medical history, laboratory and radiology findings (98%). Accurate diagnosis was provided in 95% and convincing evidence of diagnosis was provided in 70% (S2 Table).
Patient characteristics
The mean of age of the cases was 60.2 years old (standard deviation 14 years). 60% were male. 77 cases reports had NENs of the GIT (S1 Table). Three in the esophagus, 16 in the stomach, 36 in the small intestine, 22 in the large intestine (16 in the colon and rectum and 6 in the appendix). 56 cases were NET G1, two cases were NET G2, 3 cases were NET G3, and in one case the grade of NET was not available. 15 cases were NEC (10 small cell neuroendocrine carcinoma and 5 large cell neuroendocrine carcinoma). 17 cases of NENs had metastasis (11 cases of NETs and 6 NEC) (Table 1). And six cases had metastasis of the SPM (4 adenocarcinomas, 1 glioblastoma and 1 NET G1). Eight cases had more than one SPM [18,32,34,35,53,57,68,70] (these cases were added to the statistical analysis as a single case report) (S3 Table). And six cases had more than one NENs in the same GIT (these cases were analysis as one only case report) [26,37,41,49,51,61]. In total 89 case reports were included in the statistical analysis.
Table 1. Characteristic of patients with NENs.
| NET G1 | NET G2 | NET G3 | NET grade non available | NEC (small cell) | NEC (large cell) | Total | |
|---|---|---|---|---|---|---|---|
| Total | 56 | 2 | 3 | 1 | 10 | 5 | 77 |
| Age (mean) | 60.2 years | 62.5 years | 74 years | 52 years | 48.6 years | 62.4 years | 60.2 years |
| Gender | |||||||
| Male | 31 | 1 | 2 | 1 | 6 | 5 | 46 |
| Female | 21 | 1 | 1 | 0 | 4 | 0 | 27 |
| Non available | 4 | 0 | 0 | 0 | 0 | 0 | 4 |
| Location | |||||||
| Esophagus | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| Stomach | 13 | 0 | 0 | 0 | 0 | 3 | 16 |
| Small intestine | 30 | 2 | 2 | 0 | 2 | 2 | 36 |
| Appendix | 6 | 0 | 0 | 0 | 0 | 0 | 6 |
| Large intestine | 7 | 0 | 1 | 1 | 5 | 0 | 16 |
| Metastasis | |||||||
| Yes | 8 | 1 | 1 | 1 | 3 | 3 | 17 |
| No | 43 | 1 | 2 | 0 | 6 | 1 | 53 |
| Non available | 5 | 0 | 0 | 0 | 1 | 1 | 7 |
From the rare cases with atypical location and patients with more than one SPM (S3 Table), we also found cases with malignant tumors and benign tumors [59,86]. Furthermore, we also found cases with other conditions such as microsatellite instability [50,63], intestinal inflammatory disease [42,50,80] and neurofibromatosis [53,60].
Location and type of tumors in the SPM
We found that 80% of SPM were recognized in the GIT (36% in the stomach, 27% in the large intestine, 11.2% in the small intestine, and 5.6% in the esophagus). The other SPM were present in different sites (4.5% in the lung, 3.4% in the kidney, 2.2% in brain and pancreas, and 1.1% case in ovary, lymph node, cervix, bone marrow, ureter, liver, and prostate) (Table 2 and S1 Table).
Table 2. Location and type of tumor of SPM.
| Location | Adenocarcinoma | GIST | NENs | SCC | Lymphoma | Unclassifiable Carcinoma | RCC | Glioblastoma | Non small cell lung cancer | Rhabdomyosarcoma | Steroid cell tumor | Angiosarcoma | HCC | Heary cell leukemia | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esophagous | 1 | 3 | 1 | 5 | |||||||||||
| Stomach | 17 | 10 | 2 | 1 | 2 | 32 | |||||||||
| Small intestine | 3 | 2 | 2 | 3 | 10 | ||||||||||
| Large intestine | 21 | 1 | 2 | 24 | |||||||||||
| Bone marrow | 0 | 1 | 1 | ||||||||||||
| Brain | 0 | 2 | 2 | ||||||||||||
| Cervix | 0 | 1 | 1 | ||||||||||||
| Lung | 1 | 1 | 1 | 1 | 4 | ||||||||||
| Lymph node | 0 | 1 | 1 | ||||||||||||
| Ovary | 0 | 1 | 1 | ||||||||||||
| Pancreas | 0 | 1 | 1 | 2 | |||||||||||
| Liver | 0 | 1 | 1 | ||||||||||||
| Kidney | 0 | 3 | 3 | ||||||||||||
| Prostate | 1 | 1 | |||||||||||||
| Ureter | 1 | 1 | |||||||||||||
| Total | 44 | 12 | 7 | 4 | 6 | 5 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 89 |
Abbreviations: HCC: Hepatocellular carcinoma; GIST: Gastrointestinal Stromal Tumor; RCC: Renal cell carcinoma; SCC: Squamous cell carcinoma.
The most common type of SPM were adenocarcinomas (49.4%), followed by GIST (13.5%), other NENs in different GIT segments (7.9%), lymphomas (6.8%), squamous cell carcinoma (4.5%), unclassifiable carcinomas (5.6%), renal cell carcinomas (3.4%), glioblastomas (2.2%), angiosarcoma (1.1%), non-small cell lung (1.1%), rhabdomyosarcoma (1.1%), ovarian steroid cell tumor (1.1%) and heary cell leukemia (1.1%), and hepatocellular carcinoma (1.1%) (Table 2 and S1 Table).
The adenocarcinomas were detected in the 95.4% of tumors of the GIT (the colon (47.7%), the stomach (38.6%), the small intestine (6.8%), the esophagus (2.3%)) and some cases in the lung (n: 2.3%), and the prostate (2.3% (Table 3)). While, the GISTs were present in the stomach (83%) and the small intestine (17%), other NENs were observed in the stomach (28%), the small intestine (28%), the colon (14%), the lung (14%) and the pancreas (14%) (Table 4). Lymphomas were present in the small intestine (50%) (MALT (n:1), Follicular lymphoma (n:1) and T-cell lymphoma (n:1)), stomach (33%) (MALT (n:2), and lymph node (17%) (Lymphoplasmacytic lymphoma). Squamous cell carcinomas were present in the esophagus (75%) and the stomach (25%) (Table 2 and S1 Table). On the other hand, the presence of NENs and SPM in the same GIT segment was observed in 43.2% of the cases. 68.4% of the adenocarcinomas were present in the same GIT segment.
Table 3. Characteristics of the adenocarcinomas cases.
| NENs location | Author | NENs grade | Location of the adenocarcinomas cases | ||||
|---|---|---|---|---|---|---|---|
| Esophagus | Stomach | Small intestine | Large intestine | Lung | |||
| Esophagus | Saw E. | NEC (Small cell NE carcinoma) | 1 | ||||
| Stomach | Rassidakis GZ. | NETs G1 | |||||
| Cunha P. | NETs G1 | 1 | |||||
| Kim EY. | NETs G1 | 1 | |||||
| Olinici CD. | NETs G1 | 1 | |||||
| Yang L. | NETs G1 | 1 | |||||
| Yasuda K. | NETs G1 | 1 | |||||
| Sawalakhe NR. | NETs G1 | 1 | |||||
| Moya Valverde E. | NETs G1 | 1 | |||||
| Nakayama Y. | NETs G1 | 1 | |||||
| Herreros-Villanueva M. | NEC (Large cell NE carcinoma) | 1 | |||||
| Muto M | NEC (Large cell NE carcinoma) | 1 | |||||
| Small intestine | Ott RA. | NETs G1 | 1 | ||||
| Rivadeneira D. | NETs G1 | 1 | |||||
| Tse V. | NETs G1 | 1 | |||||
| McCabe H. | NETs G1 | 1 | |||||
| Cioffi U. | NETs G1 | 1 | |||||
| Srilatha PS. | NETs G1 | 1 | |||||
| Reim D. | NETs G1 | 1 | |||||
| Aslam M. | NETs G1 | 1 | |||||
| McHugh S. | NETs G1 | 1 | |||||
| Boltin D. | NETs G1 | 1 | |||||
| Wohadlo Ł. | NETs G1 | 1 | |||||
| Martínez MM. | NETs G1 | 1 | |||||
| Cokmert S. | NETs G3 | 1 | |||||
| Fukaya M | NEC (Small cell NE carcinoma) | 1 | |||||
| Waldon K | NETs G1 | 1 | |||||
| Almajano EA. | NETs G1 | ||||||
| Kim SH. | NETs G1 | 1 | 2 | ||||
| Large intestine | Park JS. | NEC (Large cell NE carcinoma) | 1 | ||||
| Gemeinhardt M. | NEC (Small cell NE carcinoma) | 1 | |||||
| Nakayama Y. | Non available | 1 | |||||
| Lipka S. | NEC (Small cell NE carcinoma) | 1 | |||||
| Zhu JG. | NETs G1 | 1 | |||||
| Meeks MW. | NETs G1 | 1 | |||||
| Mohapatra M. | NETs G1 | 1 | |||||
| Vootla V. | NETs G1 | 1 | |||||
| Zukanović G. | NEC (Small cell NE carcinoma) | 1 | |||||
| Winn JN. | NETs G1 | 1 | |||||
| Winn JN. | NETs G1 | 1 | |||||
| Total | 1 | 17 | 3 | 21 | 1 | ||
Table 4. Characteristics of the GISTs cases.
| NENs location | Author | NENs grade | Location of the GISTs cases | |||
|---|---|---|---|---|---|---|
| Esophagus | Stomach | Small intestine | Large intestine | |||
| Stomach | Ott Ra | NETs G1 | 1 | |||
| Cirillo F. | NETs G1 | 1 | ||||
| Samaras VD. | NETs G1 | 1 | ||||
| Duman DG. | NETs G1 | 1 | ||||
| Ding J. | NEC (Large cell NE carcinoma) | 1 | ||||
| Small intestine | Karatzas G. | NETs G1 | 1 | |||
| Buragas M. | NETs G1 | 1 | ||||
| Koçer NE. | NETs G1 | 1 * | ||||
| Pusiol T. | NETs G1 | 1 | ||||
| Kaur R. | NETs G1 | 1 | ||||
| Total | 0 | 8 | 2 | 0 | ||
* GIST rich in osteoclast-like giant cells.
The univariate analysis confirmed association between adenocarcinomas and NENs in the GIT (p:<0.0001), and adenocarcinoma and tumor in the same segment of GIT location (p:0.001).
Discussion
The association of NENs with synchronic tumors is not a rare condition. In 1994 Pearson and Fitzgerald reported, for the first time, a high incidence of carcinoid tumors with SPM in an autopsy series [91]. Large series of cases have documented the presence of NETs G1 (carcinoids) in GIT with synchronous SPM in a range between 62–88% and metachronous tumor between 12–38% [11]. Godwin [92], observed 62% with ≥ 1 synchronic tumors in 2471 patients, while Saha et al [93] found in 67% the secondary malignancy in 112 patients with gastrointestinal NETs G1 (carcinoids). Of such lesions, 53% were adenocarcinoma of the GIT (39% in colon and rectum; 7% in small bowel and 7% in stomach); the remainder occurred in the lung (7%), prostate (7%), cervix (7%), and other diverse sites. We found that NETs G1 associated with SPM in GIT were present in 54% (S1 Table). 80% of SPM were recognized in the GIT (45% in stomach, 34% in large intestine, 14% in small intestine and 7% in esophagus). The remainder occurred in other sites (lung (4.5%), kidney (3.4%) brain (2.2%), pancreas (2.2%), ovary (1.1%), lymph node (1.1%), cervix (1.1%), bone marrow (1.1%), ureter (1.1%), liver (1.1%), and prostate (1.1%)). In addition, the most frequent SPM was adenocarcinoma (colon (48%), stomach (38%), small intestine (7%), esophagus (2.2%), lung (2.2%), and prostate (2.2%)).
The most common NENs in the GIT are located in the colon and rectum (69%), followed by small intestine (36%), stomach (10%), appendix (5%) and, less frequently, in the esophagus (0.4–2%) [7,8] We found most cases in the small intestine (46%), followed by the colon and rectum (21.8%), the stomach (20.5%), appendix (7.7%), and the esophagus (3.8%) (Table 1). All of NECs of esophagus were small cell neuroendocrine carcinoma and co-existence with adenocarcinoma or SCC in the esophagus [8,94] Huang et al. reported that over 80% of NEC of the esophagus has synchronous SCC including tumor in situ [94]. We found two cases of small cell neuroendocrine carcinoma with SCC synchronic in esophagus: one case with adenocarcinoma [16] and the other case GIST and adenocarcinoma in stomach [18].
Very rare cases of SPM were observed in the present study. For example the presence of poorly differentiated SCC in stomach [83] (the annual incidence rate is 0.04–0.07% [95]); We observed the following: a case with angiosarcoma of the lung, GIST in the stomach, adenocarcinoma in the prostate and rectum, and NET G1 in the small intestine [35]; a case of a 59-year-old woman with NET G2 of the terminal ileum with metastasis to a mesenteric lymph node and a steroid cell tumor of the left ovary [65]; a case of 43-year-old woman with embryonal rhabdomyosarcoma of the cervix and incidental NET G1 in the appendix [78]. Also, we found a rare histological subtype in one case of a GIST with osteoclast-like giant cells and NET G1 in Ampulla of Vater [43].
Six cases of GIT lymphoma were observed (four MALT, one Follicular lymphoma and one T-cell lymphoma). And in three cases the lymphomas were present in the same intestinal segment where NEN was. We found a case with Follicular lymphoma and NET G1 in the Ampulla of Vater [52]. Two cases had MALT, one case in small intestine with NET G1 [58], and other case in stomach with NEC (Large cell NE carcinoma) and adenocarcinoma [32]. The presence of synchronic adenocarcinoma and lymphoma in the GIT is a rare condition [96,97].
The pathogenesis of NENs associated with SPM remains unclear. Diverse theories have been developed like the existence of a common carcinogenic effect that stimulates the growth of NETS and secondary primary tumor [11] or a common stem cell which may undergo similar genetic mutations (e.g. c-kit or p53) and give rise to different types of gastrointestinal malignancies [8,98,99]. Curiously, positivity of NENs markers has been observed in other carcinomas [100–102].
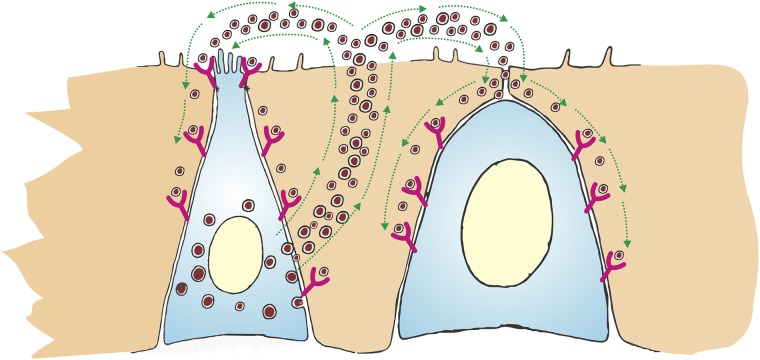
Other theories propose that the paracrine or autocrine growth loop effect by secretory peptides by neuroendocrine cell tumors (bombesin, glucagon, somatostatin, cholecystokinin and gastrin) or growth factors (platelet-derived growth factor, epidermal growth factor, transforming growth factor, insulin-like growth factor and fibroblast growth factor) produced by neuroendocrine cell tumors. These secretory peptides and growth factors are able to influence tissue growth with subsequent transformation into neoplastic cells [46,103,104] (Fig 2).
Fig 2. Schematic of paracrine and autocrine model.
The secretory peptides (bombesin, glucagon, somatostatin, cholecystokinin and gastrin) or growth factors (platelet-derived growth factor, epidermal growth factor, transforming growth factor, insulin-like growth factor and fibroblast growth factor) produced by neuroendocrine cell tumors (Left cell) influence tissue growth with subsequent transformation into neoplastic cells (Right cell).
Several neuroendocrine cell secretory peptides have been described over the years [105]. The expression of these secretory peptides receptors has been reported in normal tissue and in diverse tumors as the SPM found in our study, such as GIT adenocarcinomas (esophagus, gastric, small intestine, colorectal and pancreatic), GIST, hepatocellular carcinoma, non-small cell lung cancer, thyroid cancer, and NENs [106–116]. Our findings may support the hypothesis of paracrine effect of secretory peptides by neuroendocrine cell tumors with the SPM. We found association between adenocarcinomas and NENs in the GIT (p:<0.0001) and adenocarcinomas and NENs in the same GIT segment (p<0.001).
We would like to acknowledge the limitations of our study. First, in some articles the final diagnosis of SPM was not made with immunohistochemistry. Second, in some articles patients’ follow up was not enough to determine the progression of disease. Finally, systematic reviews of case reports delineate a number of challenges to research on rare and heterogeneous conditions. However we consider them as important tools for initial data sources of such conditions and move from the anecdote to the evidence [117].
Conclusion
Our systematic review provides a comprehensive review of SPM associated with NENs. With our data we cannot conclude the actual prevalence of NENs with SPM synchronic due to type of study, but we can conclude that the presence of SPM synchronic with NENs is not a rare condition. Large numbers of cases have documented the presence of NETs G1 (carcinoids) in GIT with synchronous SPM in a range between 62–88%. Prospective studies are necessary to determinate the relationship between paracrine effect by NENs and develop of SPM.
Supporting information
(XLSX)
(PDF)
(PDF)
(DOC)
Abbreviations
- GIT
Gastrointestinal tract
- NEC
Neuroendocrine carcinoma
- NEN
Neuroendocrine neoplasms
- NETs
Neuroendocrine tumors
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analysis
- SPM
Secondary primary malignancies
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Klimstra DS. Pathologic Classification of Neuroendocrine Neoplasms. Hematol Oncol Clin North Am. Elsevier Inc; 2016;30: 1–19. 10.1016/j.hoc.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 2.Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gönen M, et al. Pathology Reporting of Neuroendocrine Tumors: Application of the Delphic Consensus Process to the Development of a Minimum Pathology Data Set. Am J Surg Pathol. 2010;34: 300–313. 10.1097/PAS.0b013e3181ce1447 [DOI] [PubMed] [Google Scholar]
- 3.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. Springer US; 2018; 10.1038/s41379-018-0110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80 Suppl 1: 3–7. 10.1159/000080731 [DOI] [PubMed] [Google Scholar]
- 5.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26: 3063–72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Chaya C. Clinical Presentation and Diagnosis of Neuroendocrine Tumors. Hematol Oncol Clin North Am. 2016;30: 21–48. 10.1016/j.hoc.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Kilickap S, Hayran KM. Epidemiology of Neuroendocrine Tumors Neuroendocrine Tumours. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. pp. 23–33. 10.1007/978-3-662-45215-8_3 [DOI] [Google Scholar]
- 8.Egashira A, Morita M, Kumagai R, Taguchi K-I, Ueda M, Yamaguchi S, et al. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017;12: e0173501 10.1371/journal.pone.0173501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97: 934–59. 10.1002/cncr.11105 [DOI] [PubMed] [Google Scholar]
- 10.Prommegger R, Ensinger C, Steiner P, Sauper T, Profanter C, Margreiter R. Neuroendocrine tumors and second primary malignancy—a relationship with clinical impact? Anticancer Res. 2004;24: 1049–51. [PubMed] [Google Scholar]
- 11.Habal N, Sims C, Bilchik AJ. Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol. 2000;75: 310–6. [DOI] [PubMed] [Google Scholar]
- 12.Clift AK, Drymousis P, Al-Nahhas A, Wasan H, Martin J, Holm S, et al. Incidence of Second Primary Malignancies in Patients with Neuroendocrine Tumours. Neuroendocrinology. 2015;102: 26–32. 10.1159/000381716 [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62: e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 14.Haneline M. Evidence-based chiropractic practice. Sudbury, MA: Jones and Bartlett Publishers; 2006. [Google Scholar]
- 15.Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus. 2016;25: 1520–1531. 10.1177/0961203316640912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saw EC, Yu GS, Wagner G, Heng Y. Synchronous primary neuroendocrine carcinoma and adenocarcinoma in Barrett’s esophagus. J Clin Gastroenterol. 1997;24: 116–9. [DOI] [PubMed] [Google Scholar]
- 17.Deepak P, Devi R, Pillai H. Esophageal small cell carcinoma with synchronous renal cell carcinoma: a case report with review of the literature. Case Rep Gastroenterol. 2011;5: 196–200. 10.1159/000326958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan H, Lu P, Xu L, Qin Y, Li J. Synchronous occurrence of hereditary gastric adenocarcinoma, gastrointestinal stromal tumor, and esophageal small cell and squamous carcinoma in situ: an extremely rare case report. BMC Cancer. 2017;17: 720 10.1186/s12885-017-3736-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rassidakis GZ, Delladetsima JK, Letsos SP, Polyzos A, Yannopoulos A. Hepatoid adenocarcinoma of the stomach with extensive neuroendocrine differentiation and a coexisting carcinoid tumour. Histopathology. 1998;33: 186–8. [DOI] [PubMed] [Google Scholar]
- 20.Cunha P, Cunha JF, Burnay MO, Galhordas A, Fernandes R, Calinas F. [Gastric adenocarcinoma and carcinoid]. Acta Med Port. 1998;11: 577–80. [PubMed] [Google Scholar]
- 21.Kim EY, Park KC, Kwon JG. [A case of double primary cancer: early gastric adenocarcinoma associated with adenocarcinoma and carcinoid]. Korean J Gastroenterol. 2003;42: 533–8. [PubMed] [Google Scholar]
- 22.Olinici CD, Crişan D, Racu I. Synchronous gastric adenocarcinoma and carcinoid. Rom J Gastroenterol. 2004;13: 135–7. [PubMed] [Google Scholar]
- 23.Yang L. Synchronous occurrence of carcinoid, signet-ring cell carcinoma and heterotopic pancreatic tissue in stomach: A case report and literature review. World J Gastroenterol. 2006;12: 7216 10.3748/wjg.v12.i44.7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasuda K, Fujiwara H, Nomura H, Sogo Y, Sakai K, Kazuhiro T. A Case of Neuroendocrine Cell Carcinoma and Poorly Differentiated Adenocarcinoma of the Stomach in Synchronous Multiple Cancer. Japanese J Gastroenterol Surg. 2006;4: 446–451. [Google Scholar]
- 25.Sawalakhe NR, Nistala SU, Sasidharan M, Narendran RT, Amrapurkar AD, Joshi RM, et al. Synchronous gastric carcinoid and gastric adenocarcinoma with Plummer-Vinson syndrome: A case report and literature review. Ann Gastroenterol. 2009;22: 52–55. [Google Scholar]
- 26.Moya Valverde E, Moral Cebrián I, Costero Pastor B, Poves Martínez E. [Synchronous multiple gastric carcinoid tumors and gastric adenocarcinoma]. Rev Esp Enferm Dig. 2009;101: 147–8. [DOI] [PubMed] [Google Scholar]
- 27.Cirillo F. Neuroendocrine tumors and their association with rare tumors: observation of 4 cases. Eur Rev Med Pharmacol Sci. 2010;14: 577–88. [PubMed] [Google Scholar]
- 28.Samaras VD, Foukas PG, Triantafyllou K, Leontara V, Tsapralis D, Tsompanidi EM, et al. Synchronous well differentiated neuroendocrine tumour and gastrointestinal stromal tumour of the stomach: a case report. BMC Gastroenterol. 2011;11: 27 10.1186/1471-230X-11-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duman DG, Eren F, Yeğin EG, İkinci A, Yeğen C. Synchronous appearance of gastrointestinal stromal tumor and neuroendocrine tumor in stomach: review of the literature and management strategies. Turk J Gastroenterol. 2012;23: 258–61. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama Y, Higure A, Shibao K, Sato N, Matayoshi N, Yamaguchi K. Synchronous occurrence of early neuroendocrine carcinoma and tubular adenocarcinoma in the stomach. Clin J Gastroenterol. 2012;5: 307–11. 10.1007/s12328-012-0320-7 [DOI] [PubMed] [Google Scholar]
- 31.Ding J, Sun P, Cai X-Y, Fei S-H, Wu J, Qi Y-K, et al. Synchronous poorly-differentiated neuroendocrine carcinoma and gastrointestinal stromal tumor of the stomach: a case report with immunohistochemical and molecular genetic analyses of KIT and PDGFRA. Int J Clin Exp Pathol. 2014;7: 9076–80. [PMC free article] [PubMed] [Google Scholar]
- 32.Herreros-Villanueva M, Bujanda L, Gil I, Caballero MC, Cosme A. Triple synchronous gastric tumors: A rare combination diffuse adenocarcinoma, B-cell MALT lymphoma and large cell neuroendocrine carcinoma. Gastroenterol Hepatol. 2017;40: 675–677. 10.1016/j.gastrohep.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 33.Muto M, Muto M, Ichiki K, Ishikawa C, Hosoki T, Inoue M, et al. A case of synchronous quintuple gastric cancer with large-cell endocrine carcinoma. Nihon Shokakibyo Gakkai Zasshi. 2017;114: 1845–1852. 10.11405/nisshoshi.114.1845 [DOI] [PubMed] [Google Scholar]
- 34.Welter HF, Duswald KH, Welter G, Ruëff FL. [Malignant triple tumor of the gastrointestinal tract. Synchronous occurrence of a small intestinal carcinoid, a colonic and a pancreatic carcinoma]. MMW Munch Med Wochenschr. 1980;122: 131–2. [PubMed] [Google Scholar]
- 35.Ott RA, Eugene J, Kollin J, Kanas RJ, Conston DE, Chi JC. Primary pulmonary angiosarcoma associated with multiple synchronous neoplasms. J Surg Oncol. 1987;35: 269–76. [DOI] [PubMed] [Google Scholar]
- 36.Rivadeneira DE, Tuckson WB, Naab T. Increased incidence of second primary malignancy in patients with carcinoid tumors: case report and literature review. J Natl Med Assoc. 1996;88: 310–2. [PMC free article] [PubMed] [Google Scholar]
- 37.Tse V, Lochhead A, Adams W, Tindal D. Concurrent colonic adenocarcinoma and two ileal carcinoids in a 72-year-old male. Aust N Z J Surg. 1997;67: 739–41. [DOI] [PubMed] [Google Scholar]
- 38.Karatzas G, Kouraklis G, Karayiannakis A, Patapis P, Givalos N, Kaperonis E. Ampullary carcinoid and jejunal stromal tumour associated with von Recklinghausen’s disease presenting as gastrointestinal bleeding and jaundice. Eur J Surg Oncol. 2000;26: 428–9. 10.1053/ejso.1999.0911 [DOI] [PubMed] [Google Scholar]
- 39.McCabe HL. Adenocarcinoma of the gastro-oesophageal junction with a synchronous carcinoid of the duodenum. Postgrad Med J. 2001;77: 255–256. 10.1136/pmj.77.906.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tessier DJ, Harris E, Johnson DJ. Synchronous carcinoid tumor of the small bowel. J Am Coll Surg. 2002;195: 890–1. [DOI] [PubMed] [Google Scholar]
- 41.Buragas M, Kidd M, Modlin IM, Cha C. Multiple gastrointestinal stromal tumors and synchronous ileal carcinoids. Nat Clin Pract Oncol. 2005;2: 166–70; quiz 1 p following 170. 10.1038/ncponc0108 [DOI] [PubMed] [Google Scholar]
- 42.Cioffi U, De Simone M, Ferrero S, Ciulla MM, Lemos A, Avesani EC. Synchronous adenocarcinoma and carcinoid tumor of the terminal ileum in a Crohn’s disease patient. BMC Cancer. 2005;5: 157 10.1186/1471-2407-5-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koçer NE, Kayaselçuk F, Calişkan K, Ulusan S. Synchronous GIST with osteoclast-like giant cells and a well-differentiated neuroendocrine tumor in Ampula Vateri: coexistence of two extremely rare entities. Pathol Res Pract. 2007;203: 667–70. 10.1016/j.prp.2007.04.012 [DOI] [PubMed] [Google Scholar]
- 44.Srilatha PS. Concurrence of duodenal carcinoid and diffuse gastric adenocarcinoma: a rare phenomenon. Saudi J Gastroenterol. 2007;13: 197–9. 10.4103/1319-3767.36754 [DOI] [PubMed] [Google Scholar]
- 45.Chemli S, Dhouib RS, Mrad K, Ben Mansour Z, Cheour H, Ben Romdhane K, et al. [Synchronous association of ileal carcinoid and colorectal carcinoma. A case report]. Tunis Med. 2007;85: 607–9. [PubMed] [Google Scholar]
- 46.Reim D, Weirich G, Neu B, Bajbouj M, Brücher BLDM. Synchronous adenocarcinoma of the lung and neuroendocrine carcinoma of the ileum. Int J Colorectal Dis. 2008;23: 325–7. 10.1007/s00384-007-0412-x [DOI] [PubMed] [Google Scholar]
- 47.Sen N, Calli Demirkan N, Aksoy Altinboğa A, Bolat H, Erdem E. Synchronous endocrine tumors of small intestine: report of a case. Turk J Gastroenterol. 2008;19: 193–6. [PubMed] [Google Scholar]
- 48.Aslam MI, Ben Salha I, Muller S, Jameson JS. Synchronous ileal carcinoid and primary colonic neoplasms: a case report. Cases J. 2009;2: 8317 10.4076/1757-1626-2-8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McHugh SM, O’Donnell J, Gillen P. Synchronous association of rectal adenocarcinoma and three ileal carcinoids: a case report. World J Surg Oncol. 2009;7: 21 10.1186/1477-7819-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boltin D, Levi Z, Halpern M, Fraser GM. Concurrent small bowel adenocarcinoma and carcinoid tumor in Crohn’s disease—case report and literature review. J Crohns Colitis. 2011;5: 461–4. 10.1016/j.crohns.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 51.Wohadlo Ł, Darasz Z, Wysocki W. Multifocal colorectal adenocarcinoma with a synchronous multifocal carcinoid of the small intestine—case report and literature review. Pol Przegl Chir. 2011;83: 562–7. 10.2478/v10035-011-0089-2 [DOI] [PubMed] [Google Scholar]
- 52.Athanasopoulos PG, Arkadopoulos N, Stafyla V, Tympa A, Kairi E, Ryzman-Louloudis C, et al. A rare combination of an endocrine tumour of the common bile duct and a follicular lymphoma of the ampulla of Vater: a case report and review of the literature. World J Surg Oncol. 2011;9: 4 10.1186/1477-7819-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martínez M M, Norero M E, Lezcano G FG, González B S, Jarufe C N. [Combined periampullary adenocarcinoma and neuroendocrine tumor in type 1 neurofibromatosis: report of one case]. Rev Med Chil. 2011;139: 84–8 [PubMed] [Google Scholar]
- 54.Pusiol T, Zorzi MG, Morichetti G, Piscioli I, Scialpi M. Synchronous nonfunctional duodenal carcinoid and high risk gastrointestinal stromal tumour (GIST) of the stomach. Eur Rev Med Pharmacol Sci. 2011;15: 583–5. [PubMed] [Google Scholar]
- 55.Cokmert S, Demir L, Akder Sari A, Kucukzeybek Y, Can A, Akyol M, et al. Synchronous appearance of a high-grade neuroendocrine carcinoma of the ampulla vater and sigmoid colon adenocarcinoma. Case Rep Oncol Med. 2013;2013: 930359 10.1155/2013/930359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur R, Bhalla S, Nundy S, Jain S. Synchronous gastric gastrointestinal stromal tumor (GIST) and other primary neoplasms of gastrointestinal tract: report of two cases. Ann Gastroenterol. 2013;26: 356–359. [PMC free article] [PubMed] [Google Scholar]
- 57.Fukaya M, Abe T, Yokoyama Y, Itatsu K, Nagino M. Two-stage operation for synchronous triple primary cancer of the esophagus, stomach, and ampulla of Vater: report of a case. Surg Today. 2014;44: 967–71. 10.1007/s00595-013-0549-x [DOI] [PubMed] [Google Scholar]
- 58.Berroa de la Rosa E, Casadiego Matarranz L, Aparicio Duque R, Peñarrubia Ponce MJ, Ortiz de Solórzano Aurusa J, Fernández Salazar L. [Synchronic MALT lymphoma and carcinoid tumor of the ileum]. Gastroenterol Hepatol. 2014;37: 95–96. 10.1016/j.gastrohep.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 59.Grace S, Muzaffar R, Veerapong J, Alkaade S, Poddar N, Phillips N, et al. Synchronous quadruple primary neoplasms: glioblastoma, neuroendocrine tumor, schwannoma and sessile serrated adenoma in a patient with history of prostate cancer. Anticancer Res. 2015;35: 2121–7. [PubMed] [Google Scholar]
- 60.Hsu A, Han S. Synchronous neuroendocrine tumor and non-small-cell lung cancer in neurofibromatosis type 1. Clin case reports. 2015;3: 990–6. 10.1002/ccr3.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng XF, Liu QX, Hou B, Min JX, Dai JG. Esophageal Cancer with a Synchronous Multiple Carcinoid of the Duodenal Bulb. Indian J Surg. 2015;77: 543–544. 10.1007/s12262-015-1338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waldon K, Abbas JR, Shakir S, Afify S. Four tumours including neuroendocrine tumour of the ileum. BMJ Case Rep. 2015;2015 10.1136/bcr-2014-207135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alfaro Almajano E, Bengochea Martínez L, Mateos Barrionuevo FJ, Almajano Martinez C. [Synchronous well differentiated ileal neuroendocrine tumour and colonic adenocarcinoma. A case report and a review of the literature]. Rev Española Patol. 2016; 10.1016/j.patol.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 64.Buffone A, Cavallaro D, Lo Bianco S, Puzzo L, Caglià P, Cannizzaro MA. Synchronous ileal neuroendocrine tumor: diagnosis and treatment. A case report and review of the literature. Ann Ital Chir. 2016;87: 92–6. [PubMed] [Google Scholar]
- 65.Gray S, Chen Y, Litton T, Jallad B, Poddar N, Hoff JT, et al. Synchronous Ileal Neuroendocrine Tumor and Ovarian Steroid Cell Tumor Present in a Female With Hyperandrogenism. Int J Gynecol Pathol. 2016;35: 554–560. 10.1097/PGP.0000000000000285 [DOI] [PubMed] [Google Scholar]
- 66.Tsunenari T, Aosasa S, Ogata S, Hoshikawa M, Nishikawa M, Noro T, et al. Synchronous neuroendocrine tumors in both the pancreas and ileum: A case report. Int J Surg Case Rep. 2016;22: 47–50. 10.1016/j.ijscr.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dafashy TJ, Ghaffary CK, Keyes KT, Sonstein J. Synchronous Renal Cell Carcinoma and Gastrointestinal Malignancies. Case Rep Urol. 2016;2016: 7329463 10.1155/2016/7329463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim S-H, Park B-S, Kim HS, Kim JH. Synchronous quintuple primary gastrointestinal tract malignancies: Case report. World J Gastroenterol. 2017;23: 173–177. 10.3748/wjg.v23.i1.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shan B, Zhang Q, Li Y, Han F. Synchronous multiple carcinoma with small intestine and pulmonary neuroendocrine involvement: A case report. Medicine (Baltimore). 2017;96: e8623 10.1097/MD.0000000000008623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takasima S, Misumi T, Yoshizawa J, Morita M, Uyama T, Moriwaki S. [Case of rectal carcinoid associated with carcinomas of the esophagus and ureter]. Gan No Rinsho. 1983;29: 931–5. [PubMed] [Google Scholar]
- 71.Ohmori T, Okada K, Arita N, Tabei R. Multiple ileal carcinoids and appendiceal endocrine carcinoma in association with Meckel’s diverticulum. A histochemical and immunohistochemical study. Arch Pathol Lab Med. 1994;118: 283–8. [PubMed] [Google Scholar]
- 72.Zirkin H, Levy J, Katchko L. Small cell undifferentiated carcinoma of the colon associated with hepatocellular carcinoma in an immunodeficient patient. Hum Pathol. 1996;27: 992–996. [DOI] [PubMed] [Google Scholar]
- 73.Lindboe CF, Holm SE, Lie AK. Synchronous occurrence of carcinoid tumour of the appendix and T-cell lymphoma of the ileum. A case report with review of the literature. APMIS. 1999;107: 523–8. [DOI] [PubMed] [Google Scholar]
- 74.McGregor DH, Cherian R, Weston AP, Lawson L, McAnaw MP. Adenocarcinoid of ileum and appendix, incidentally discovered during exploratory laparotomy for gastric MALT lymphoma, with subsequent diffuse prostatic metastases: report of a case with light, immunohistochemical, and electron microscopic studies. Dig Dis Sci. 1999;44: 87–95. [DOI] [PubMed] [Google Scholar]
- 75.Ganguly S, Cunningham MT. A case of synchronous lymphoplasmacytic lymphoma and rectal carcinoid. Am J Clin Oncol. 2006;29: 104–5. 10.1097/01.coc.0000162442.54674.46 [DOI] [PubMed] [Google Scholar]
- 76.Park J-S, Kim L, Kim CH, Bang BW, Lee DH, Jeong S, et al. Synchronous large-cell neuroendocrine carcinoma and adenocarcinoma of the colon. Gut Liver. 2010;4: 122–5. 10.5009/gnl.2010.4.1.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salemis NS, Pinialidis D, Tsiambas E, Gakis C, Nakos G, Sambaziotis D, et al. Synchronous occurrence of neuroendocrine colon carcinoma and hairy cell leukemia. J Gastrointest Cancer. 2011;42: 131–6. 10.1007/s12029-010-9167-7 [DOI] [PubMed] [Google Scholar]
- 78.Adams BN, Brandt JS, Loukeris K, Holcomb K. Embryonal rhabdomyosarcoma of the cervix and appendiceal carcinoid tumor. Obstet Gynecol. 2011;117: 482–4 [DOI] [PubMed] [Google Scholar]
- 79.Lendzion R, Phan-Thien K-C. Synchronous small intestinal and appendiceal neuroendocrine tumours: a rare case. ANZ J Surg. 2017;87:E-159–E-160. 10.1111/ans.13053 [DOI] [PubMed] [Google Scholar]
- 80.Gemeinhardt M, Türck J, Piper B, Helmberger T, Nerlich A, Schepp W. [Adenocarcinoma of the stomach and neuroendocrine carcinoma of the colon in a 45-year-old male patient suffering from common variable immunodeficiency (CVID) and ulcerative colitis]. Z Gastroenterol. 2012;50: 1292–5. 10.1055/s-0032-1313181 [DOI] [PubMed] [Google Scholar]
- 81.Nakayama Y, Torigoe T, Minagawa N, Uehara T, Yamaguchi K. Synchronous Occurrence of Advanced Neuroendocrine Carcinoma and Tubular Adenocarcinoma of the Rectum. Case Rep Gastroenterol. 2013;7: 117–121. 10.1159/000350252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lipka S, Hurtado-Cordovi J, Avezbakiyev B, Freedman L, Clark T, Rizvon K, et al. Synchronous Small Cell Neuroendocrine Carcinoma and Adenocarcinoma of the Colon: A Link for Common Stem Cell Origin? ACG case reports J. 2014;1: 96–9. 10.14309/crj.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu F, Feng G-S, Wang Z-J, Zhang K-N. Synchronous double cancers of colonic large cell neuroendocrine carcinoma and gastric squamous-cell carcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2014;7: 5177–80. [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu J, Zhang Z, Wu G, Han W, Wang K. Synchronous Collision Neuroendocrine Tumor and Rectal Adenocarcinoma: a Case Report. Indian J Surg. 2015;77: 185–187. 10.1007/s12262-015-1256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Athiyappan K, Ramachandran R, Rajendiran S, Thangam V. Incidental Detection of Neuroendocrine Carcinoma of Rectum During Staging Workup of Renal Cell Carcinoma. World J Oncol. 2015;6: 491–494. 10.14740/wjon949w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meeks MW, Grace S, Chen Y, Petterchak J, Bolesta E, Zhou Y, et al. Synchronous Quadruple Primary Neoplasms: Colon Adenocarcinoma, Collision Tumor of Neuroendocrine Tumor and Schwann Cell Hamartoma and Sessile Serrated Adenoma of the Appendix. Anticancer Res. 2016;36: 4307–11. [PubMed] [Google Scholar]
- 87.Mohapatra S, Ibrarullah M, Mohapatra A, Baisakh MR. Synchronous adenocarcinoma and neuroendocrine carcinoma of the colon: a case report. J Surg Case Reports. 2016;2016 10.1093/jscr/rjw042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vootla V, Ahmed R, Niazi M, Balar B, Nayudu S. Synchronous Adenocarcinoma of the Colon and Rectal Carcinoid. Case Rep Gastroenterol. 2016;10: 600–604. 10.1159/000450677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zukanović G, Glavić Ž, Šantak G, Crnković T, Frančina M, Zukanović M. Synchronous caecal small-cell neuroendocrine carcinoma and adenocarcinoma of the rectum. Med Jad. 2016;46: 109–114. [Google Scholar]
- 90.Winn JN, Sathyamurthy A, Kneib JL, Ibdah JA, Tahan V. Synchronous Gastrointestinal Carcinoid Tumor and Colon Adenocarcinoma: Case Reports and Literature Review. Am J Case Rep. 2017;18: 626–630. [DOI] [PubMed] [Google Scholar]
- 91.Pearson C, Fitzegerald P. Carcinoid tumors; a re-emphasis of their malignant nature; review of 140 cases. Cancer. 1949;2: 1005–26, illust. [DOI] [PubMed] [Google Scholar]
- 92.Godwin JD. Carcinoid tumors. An analysis of 2,837 cases. Cancer. 1975;36: 560–9. [DOI] [PubMed] [Google Scholar]
- 93.Saha S, Hoda S, Godfrey R, Sutherland C, Raybon K. Carcinoid tumors of the gastrointestinal tract: a 44-year experience. South Med J. 1989;82: 1501–5. [DOI] [PubMed] [Google Scholar]
- 94.Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013;37: 467–83 [DOI] [PubMed] [Google Scholar]
- 95.Boswell JT, Helwig EB. Squamous Cell Carcinoma And Adenoacanthoma Of The Stomach. A Clinicopathologic Study. Cancer. 1965;18: 181–92. [DOI] [PubMed] [Google Scholar]
- 96.Parra-Medina R, López Correa P, Castro Quiroga P, Castro Arbelaez A, Yaspe Costa E. Synchronous Adenocarcinoma and Lymphoma of the Gallbladder: A Case Report. J Gastrointest Cancer. 2016;47: 474–477. 10.1007/s12029-015-9775-3 [DOI] [PubMed] [Google Scholar]
- 97.Nakamura S, Aoyagi K, Iwanaga S, Yao T, Tsuneyoshi M, Fujishima M. Synchronous and metachronous primary gastric lymphoma and adenocarcinoma: a clinicopathological study of 12 patients. Cancer. 1997;79: 1077–85. [PubMed] [Google Scholar]
- 98.Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, et al. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89: 1448–53. [DOI] [PubMed] [Google Scholar]
- 99.Kato T, Terashima T, Tomida S, Yamaguchi T, Kawamura H, Kimura N, et al. Cytokeratin 20-positive large cell neuroendocrine carcinoma of the colon. Pathol Int. 2005;55: 524–9. 10.1111/j.1440-1827.2005.01864.x [DOI] [PubMed] [Google Scholar]
- 100.Yao G-Y, Zhou J-L, Lai M-D, Chen X-Q, Chen P-H. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol. 2003;9: 858–61. 10.3748/wjg.v9.i4.858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romeo R, Pellitteri R, Mazzone V, Marcello MF. Chromogranin A expression in human colonic adenocarcinoma. Ital J Anat Embryol. 2002;107: 177–83. [PubMed] [Google Scholar]
- 102.Helman LJ, Gazdar AF, Park JG, Cohen PS, Cotelingam JD, Israel MA. Chromogranin A expression in normal and malignant human tissues. J Clin Invest. 1988;82: 686–90. 10.1172/JCI113648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zucker KA, Longo WE, Modlin IM, Bilchik AJ, Adrian TE. Malignant diathesis from jejunal-ileal carcinoids. Am J Gastroenterol. 1989;84: 182–6. [PubMed] [Google Scholar]
- 104.Thomas RP, Hellmich MR, Townsend CM, Evers BM. Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues. Endocr Rev. 2003;24: 571–99. 10.1210/er.2002-0028 [DOI] [PubMed] [Google Scholar]
- 105.Sundler F, Bouttcher G, Ekblad E, Haukanson R. The Neuroendocrine System of the Gut. Acta Oncol (Madr). 1989;28: 303–314. 10.3109/02841868909111198 [DOI] [PubMed] [Google Scholar]
- 106.Cassano G, Resta N, Gasparre G, Lippe C, Guanti G. The proliferative response of HT-29 human colon adenocarcinoma cells to bombesin-like peptides. Cancer Lett. 2001;172: 151–7. [DOI] [PubMed] [Google Scholar]
- 107.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer J-C, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14). Clin Cancer Res. 2002;8: 1139–46. [PubMed] [Google Scholar]
- 108.Roy J, Putt KS, Coppola D, Leon ME, Khalil FK, Centeno BA, et al. Assessment of cholecystokinin 2 receptor (CCK2R) in neoplastic tissue. Oncotarget. 2016;7: 14605–15. 10.18632/oncotarget.7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hur K, Kwak MK, Lee H-J, Park DJ, Lee HK, Lee HS, et al. Expression of gastrin and its receptor in human gastric cancer tissues. J Cancer Res Clin Oncol. 2006;132: 85–91. 10.1007/s00432-005-0043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savage K, Waller HA, Stubbs M, Khan K, Watson SA, Clarke PA, et al. Targeting of cholecystokinin B/gastrin receptor in colonic, pancreatic and hepatocellular carcinoma cell lines. Int J Oncol. 2006;29: 1429–35. [PubMed] [Google Scholar]
- 111.Chave HS, Gough AC, Palmer K, Preston SR, Primrose JN. Bombesin family receptor and ligand gene expression in human colorectal cancer and normal mucosa. Br J Cancer. 2000;82: 124–30. 10.1054/bjoc.1998.0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bold RJ, Lowry PS, Ishizuka J, Battey JF, Townsend CM, Thompson JC. Bombesin stimulates the in vitro growth of a human gastric cancer cell line. J Cell Physiol. 1994;161: 519–25. 10.1002/jcp.1041610315 [DOI] [PubMed] [Google Scholar]
- 113.Lee Y, Urbanska AM, Hayakawa Y, Wang H, Au AS, Luna AM, et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett’s-like esophagus. Oncotarget. 2017;8: 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith JP, Nadella S, Osborne N. Gastrin and Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2017;4: 75–83. 10.1016/j.jcmgh.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuiper P, Verspaget HW, Biemond I, de Jonge-Muller ES, van Eeden S, van Velthuysen M-LF, et al. Expression and ligand binding of bombesin receptors in pulmonary and intestinal carcinoids. J Endocrinol Invest. 2011;34: 665–70 [DOI] [PubMed] [Google Scholar]
- 116.Seretis E, Gavrill A, Agnantis N, Golematis V, Voloudakis-Baltatzis IE. Comparative study of serotonin and bombesin in adenocarcinomas and neuroendocrine tumors of the colon. Ultrastruct Pathol. 2001;25: 445–54. [DOI] [PubMed] [Google Scholar]
- 117.Naik HB, Abuabara K. Systematic reviews of case reports and case series: from anecdote to evidence. Br J Dermatol. 2018;178: 317–318. 10.1111/bjd.16073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(PDF)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.