Abstract
Objective
To investigate the relationship between cerebrospinal fluid (CSF) β-amyloid peptide (Aβ42) and CSF Tau in a large population of patients referred to memory clinics for investigation of cognitive dysfunction.
Methods
We analyzed Alzheimer’s disease (AD) biomarkers in CSF taken from 3565 patients referred to 18 French memory clinics. Patients were classified into four profiles according to levels of CSF biomarkers (A: amyloidosis, N: neurodegeneration). The association between CSF Tau and CSF Aβ42 were analyzed using general linear regression models, in the overall population and stratified by biomarkers profiles. We compared linear and quadratic models using Akaike information criterion. We also assessed change in biomarker profiles in a subset of patients who had 2 assessments of biomarkers.
Results
CSF Tau was negatively associated with CSF Aβ42 in the overall population, following a non-linear quadratic model. However, the nature of this association was different in the 4 profiles: positive association in A-N- profile, negative association in A-N+ and A+N+ profiles, lack of association in A+N- patients. When considering patients with longitudinal data on profiles, 36% of those initially classified as A-N+ evolved to an A+N+ profile.
Conclusions
The nature of the association between CSF Aβ42 and CFS Tau depends on the A/N profiles of patients. These results suggest an increase in CSF Aβ42 early in the disease before its decline while tau pathology progresses, this pattern is particularly observed in non-APOE4 subjects. This phenomenon may explain why some patients with neurodegeneration only markers convert to an AD profile (A+N+) over time.
Introduction
Alzheimer’s disease (AD), the most common cause of dementia, is neuropathologically characterized by extracellular β-amyloid peptide (Aβ42) deposits, neurofibrillary tangles composed of intraneuronal abnormally phosphorylated Tau, and neuronal and synaptic loss [1]. According to the “amyloid cascade” hypothesis [2], extracellular accumulation of Aβ is the first step, successively followed by deposits of neurofibrillary tangles, neuronal death and finally the clinical onset of symptoms [3]. A competing hypothesis is that neurofibrillary tangles appear first, particularly in sporadic AD [4, 5].
The development of AD biomarkers during the past decade allows new opportunities for studying the in-vivo kinetics of amyloid and tau lesions [6, 7]. Cerebrospinal fluid (CSF) measurement of Aβ42 has been shown to be inversely correlated with total brain Aβ load [8], while CSF tau levels positively correlated with the presence of neocortical neurofibrillary tangles [9]. Based on the amyloid hypothesis, a temporal chronology of AD biomarkers has been proposed [10], and in 2011 the National Institute on Aging-Alzheimer’s Association (NIA-AA) proposed biomarker based recommendations for the staging of preclinical AD [11]. Using evidence of amyloidosis (A) and neurodegeneration (N), Stage 1 corresponds to presence of Aβ deposits and the absence of neurodegeneration (A+N-), while stage 2/3 corresponds presence of both markers (A+N+), without or with subtle cognitive decline. One year after the publication of the NIA-AA recommendations, Jack et al. reported an additional category of subjects, characterized by positivity for only neurodegeneration markers (A-N+) and proposed the designation of SNAP, for Suspected Non-Alzheimer’s disease Pathophysiology for these subjects [12].
The concept of SNAP (A-N+) remains the subject of debate [13]. Several studies have shown accelerated cognitive decline in these persons [14–17], with poorer clinical prognosis than A+N- subjects [18]. However, other studies found no differences between SNAP and A-N- subjects in cognitive decline, while the A+N- and A+N+ groups declined faster [19–21]. It is worth noting that the precise biomarkers used to define SNAP remains heterogeneous across studies, some use CSF Tau or phosphorylated Tau, or T1-weighted structural MRI, and/or FDG PET imaging data.
CSF allows assessment of both Aβ42 and Tau, the hallmarks of AD, but their relationship remains poorly understood. A recent study based on data on 766 subjects from 3 independent cohorts found a non-linear quadratic association between CSF Tau and Aβ42 [22]. In the present study, we aim to extend these analyses using data on 3,565 patients in order to cover a wide spectrum of AD pathology. We test the hypothesis that the nature of the association between these biomarkers depends on the AD “profile” of patients. In further analysis on patients who had 2 lumbar punctures we analyzed the longitudinal change in biomarkers profiles to test the hypothesis that persons initially classified as SNAP may evolve to an AD profile.
Methods
Subjects
Following national recommendations, CSF analyses are routinely undertaken in patients with cognitive signs referred to hospital-based memory centers in France [23, 24]. For the present study, CSF biomarkers from 18 hospitals in and around Paris, France, were aggregated in one biochemistry department. All patients seen between January 1st 2008 and January 1st 2018 for whom AD biomarkers from CSF were available were included in the analysis; methods and characteristics of the population have been previously reported elsewhere [25, 26]. The study was approved by the Ethical Committee of Paris University Hospitals. Patients under legal protection were excluded. Written consent was signed in priority by the patients, and in case of inability to sign, by their caregivers. The consent procedure was approved by the local ethical committee.
CSF analysis
Lumbar puncture was performed on patients in a fasting state, typically between 9 and 12 am. All centers used the same model of 10 mL polypropylene tube to collect CSF (January 2008 to November 2012: CML model TC10PCS; December 2012 to January 2018: Sarstedt catalog no. 62.610.201). CSF samples were centrifuged at 1,000 g for 10 minutes at 4°C within 4 hours of collection, and then aliquoted in 0.5 mL polypropylene tubes and stored at -80°C for further analysis. CSF levels of Aβ42, total Tau, and pTau-181 were measured with the commercially available sandwich ELISA INNOTEST, using the manufacturer’s procedures (Fujirebio Europe NV, formely Innogenetics NV). The Alzheimer’s Association quality control program for CSF biomarkers validated the quality of CSF evaluations of the hospital biochemistry department [27].
Profiles based on CSF biomarkers
The CSF biomarker profiles were used to determine the “profile” of every subject using the NIA-AA staging framework [11]. The amyloidosis profile (A+N-) was characterized by a low level of CSF Aβ42 and normal levels of CSF Tau and CSF p-Tau 181. The amyloidosis combined with neurodegeneration profile (A+N+) was defined by a low level of CSF Aβ42 and increased level of CSF Tau and/or CSF p-Tau 181. Subjects with high levels of CSF Tau and/or CSF p-Tau 181 but normal Aβ42 were classified as SNAP (A-N+). Those with normal levels of all three biomarkers were classified as “normal” (A-N-).
In this study we chose not to use the recently proposed A/T/N classification[18, 28] as we have previously showed that some profiles (A-T+N-, A+T+N-) were quite rare, representing less than 1% of the sample [25]. Furthermore, in our data CSF Tau and CSF p-Tau 181 were highly correlated (Spearman coefficient correlation = 0.84, P<0.001).
Abnormal values of CSF biomarkers were defined using thresholds used routinely in clinical practice based on higher Youden index in order to discriminate AD patients from non-AD patients (i.e. best compromise for sensitivity and specificity). The type of the polypropylene tube changed in November 2012, and the ELISA INNOTEST sandwich changed on May 1st 2016, requiring adjustment of thresholds at these 2 dates. The cut-offs for CSF Aβ42, Tau and p-Tau 181 moved respectively from 500/300/65 pg/mL [29] to 730/300/58 pg/mL [25] after the change of tube.
Covariates
These included age, sex, and performance on the 30-item Mini Mental State Examination (MMSE) for an assessment of general cognitive status. APOEe4 status was known for a sub-sample of 1034 individuals.
Statistical analysis
Participant characteristics were presented overall and by profiles defined using NIA-AA CSF biomarkers (A-N-, A+N-, A+N+, A-N+). Proportions were calculated for categorical variables, while means and standard deviations were computed for continuous variables. Differences as a function of profiles were assessed using a χ2 test or Student t-test as appropriate.
The association between CSF Tau and Aβ42 was examined using general linear regression models with CSF Aβ42 as the dependent variable (PROC GLM of SAS). The analysis was initially adjusted only for the type of collection tube (unadjusted model), and then further adjusted for age, sex, and APOEe4 status. Analysis were first undertaken in the total sample and then stratified by the 4 profiles (A-N-, A+N-, A+N+, A-N+). The difference in the association between CSF Tau and Aβ42 in the 4 profiles was tested formally using interaction terms in the linear regression models.
We compared the linear model (Aβ42 = α + β xTau) to the quadratic model (Aβ42 = α + β xTau + γ x Tau2) following the Akaike information criterion (AIC), with lower AIC values designating better model fit [30]. Linear and quadratic models were compared in the overall population and then separately in each profile (A-N-, A+N-, A+N+, A-N+).
In the sub-sample with APOEe4 data (N = 1034) we examined the association between CSF Aβ42 and CSF Tau by first plotting the mean values of CSF Aβ42 as a function of deciles of CSF Tau in those with an APOEe4 allele versus none. These associations were also examined in linear regression analysis, adjusted for collection tube, age and sex, in two groups based on median CSF Tau.
Finally, in persons with 2 lumbar punctures with CSF AD biomarkers, we examined the evolution of their biomarkers profiles to assess conversion in stage of AD pathology.
The CSF biomarkers were not normally distributed and we followed the approach used by other teams by log-transforming CSF Tau and using CSF Aβ42 without any transformation in the statistical models [22]. CSF Tau and CSF phosphorylated Tau were highly correlated in our sample leading us to use CSF Tau in the main analyses; use of CSF p-Tau 181 in place of CSF Tau led to similar findings and conclusions.
All p-values were two-tailed and p≤0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
CSF biomarker data were available on 3565 persons, assessed between January 1st, 2008 and January 1st, 2018 in one of the 18 memory clinics included in the study. Their characteristics are summarized in Table 1. The mean (SD) age was 69.8 (10.3) years, 50% were women, and the mean (SD) MMSE at the time of the lumbar puncture was 21.7 (6.0). Of the 3565 persons in the sample, 27% had a normal biomarker profile (A-N-), 36% had an AD profile (A+N+), 22% were positive only for amyloid (A+N-), and 15% had the SNAP profile—positive only for neurodegeneration (A-N+). Compared to the normal profile, patients with an AD profile were older, more often women, had a lower MMSE scores and were more likely to carry at least one APOE ε4 allele.
Table 1. Characteristics of the study population, overall and as a function of profiles defined by CSF biomarkers.
Profiles are based on CSF Aβ42, CSF Tau, and CSF p-Tau (NIA-AA classification): A-N-: normal levels of all three biomarkers; A+N-: low level of CSF Aβ42 and normal levels of CSF Tau and CSF p-Tau 181; A+N+: lowe level of CSF Aβ42 and increased level of CSF Tau and/or CSF p-Tau 181; A-N+: high CSF Tau and/or CSF p-Tau 181 but normal Aβ42.
| Profiles defined by CSF biomarkers | ||||||
|---|---|---|---|---|---|---|
| Overall | A-N- | A+N- | A+N+ | A-N+ | ||
| Characteristics | (N = 3565) | (N = 947) | (N = 789) | (N = 1299) | (N = 527) | P value |
| Age, years, mean (SD) | 69.8 (10.3) | 66.0 (11.1) | 69.9 (10.5) | 72.2 (9.1) | 70.7 (9.7) | <0.001 |
| Women, n (%) | 1770 (49.7) | 437 (46.2) | 348 (44.1) | 739 (56.9) | 246 (46.7) | <0.001 |
| MMSE, mean (SD) | 21.7 (6.0) | 23.7 (5.1) | 21.6 (6.2) | 20.0 (6.2) | 22.5 (5.1) | <0.001 |
| Education levels, years, mean (SD) | 10.1 (4.5) | 10.1 (4.6) | 10.2 (4.6) | 10.0 (4.4) | 9.9 (4.3) | 0.78 |
| APOEε4 carriers, n (%)a | 430 (41.6) | 61 (19.2) | 57 (50.4) | 267 (60.5) | 45 (27.8) | <0.001 |
| CSF biomarkers (pg/ml), Mean (SD) | ||||||
| Aβ42 | 682.1 (291.4) | 942.1 (198.8) | 517.7 (161.4) | 479.6 (138.1) | 961.3 (275.2) | <0.001 |
| Tau | 404.9 (326.3) | 199.2 (56.6) | 179.7 (67.8) | 645.3 (344.6) | 519.1 (327.2) | <0.001 |
| p-Tau 181 | 60.4 (36.0) | 38.8 (10.6) | 33.0 (12.1) | 88.5 (38.4) | 70.9 (26.6) | <0.001 |
aAPOE genotype determined in a sub sample of 1034 patients.
There was an inverse association between CSF Tau and CSF Aβ42 when the study population was considered together (β[SE] = -82.1[6.7], p<0.001, Table 2). In analysis stratified by profiles, CSF Tau was positively associated with CSF Aβ42 in A-N- subjects (β[SE] = 153.6[17.7]), and negatively associated with CSF Aβ42 among A+N+ (β[SE] = -19.7[7.2]) and A-N+ subjects (-125.2[22.9]), while no significant association was observed in A+N- patients. These associations were robust to adjustment for age, sex, and APOE4 status. The test for interaction was highly significant (<0.001) suggesting that the association between CSF Aβ42 and CSF Tau differed in the four profile groups.
Table 2. Association between CSF Tau (log transformed) and CSF Aβ42, linear regression analysis.
Profiles are based on CSF Aβ42, CSF Tau, and CSF p-Tau (NIA-AA classification).
| Unadjusteda | Adjustedb | ||||
|---|---|---|---|---|---|
| Population | N | β(SE) | P | β(SE) | P |
| Overall | 3565 | -82.1 (6.7) | <0.001 | -65.1 (6.7) | <0.001 |
| Profiles | |||||
| A-N- | 947 | 153.6 (17.7) | <0.001 | 178.5 (17.6) | <0.001 |
| A+N- | 789 | 18.7 (11.9) | 0.12 | 22.9 (12.1) | 0.06 |
| A+N+ | 1299 | -19.7 (7.2) | 0.006 | -20.0 (7.1) | 0.005 |
| A-N+ | 527 | -125.2 (22.9) | <0.001 | -116.5 (22.5) | <0.001 |
aAdjusted for nature of the tube.
bAdjusted for the tube, age, gender, APOE4 status.
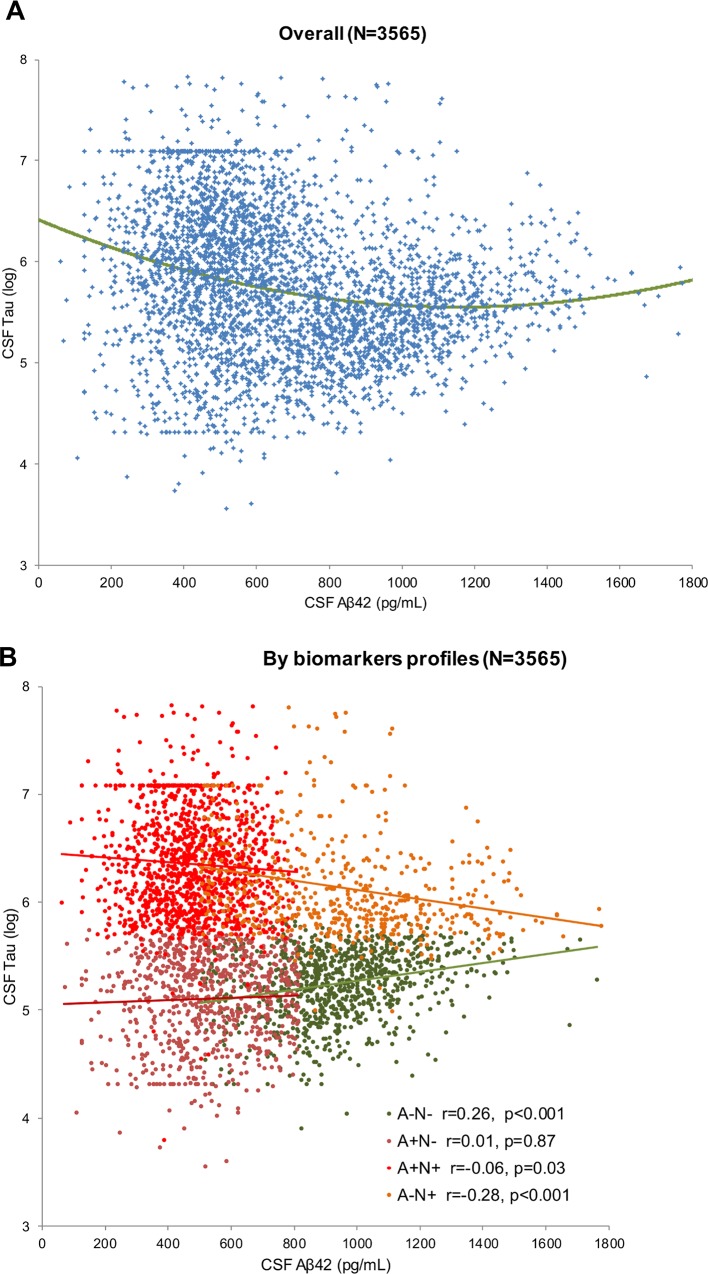
Comparisons of linear and quadratic models for the association between CSF Tau and CSF Aβ42 are presented in S1 Table. The quadratic model fitted the data better in the total study population (delta AIC = 43). However, this was not the case in all profile sub-groups as the quadratic model was inferior to the linear model for A-N-, A+N- and A+N+ profiles (delta AIC = -2), and was only better than the linear model in A-N+ patients (delta AIC = 8). The scatter plot of CSF Aβ42 and CSF Tau is presented in Fig 1, in the total study population and in the profiles defined by biomarkers.
Fig 1. Scatter plot of CSF Aβ42 and log transformed CSF Tau.
A. In the total study population. The quadratic model is presented in green. B. In analysis separately in each profile. Green: A-N-, brown: A+N-, orange: A-N+, red: A+N+. Linear regression models are presented and Spearman correlation coefficients.
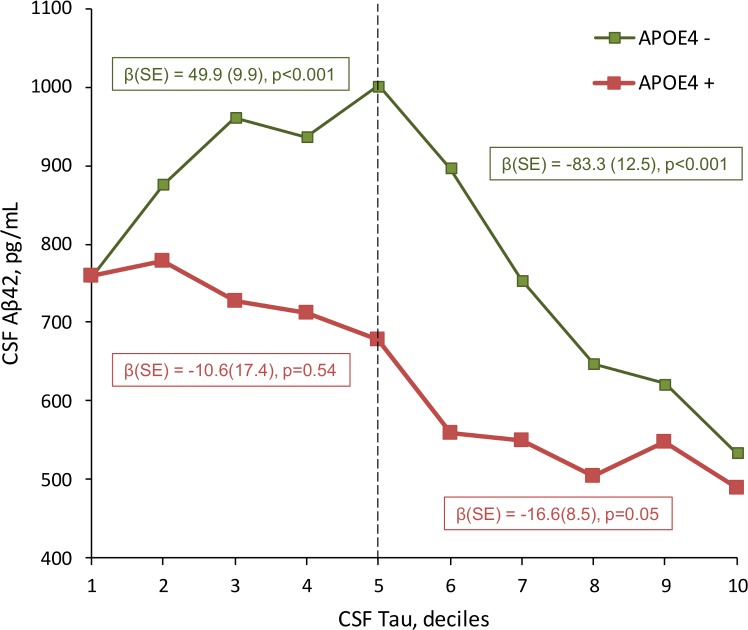
Fig 2 shows the mean CSF Aβ42 value as a function of deciles of CSF Tau in APOE4 positive and negative participants. In APOE4 negative subjects, the mean value of CSF Aβ42 increased from the first to the 5th decile of CSF Tau (β[SE] = 49.9[9.9], p<0.001), and then decreased for subjects with higher CSF Tau levels (β[SE] = -83.3[12.5], p<0.001). For APOE4 positive subjects, there was no association between CSF Aβ42 and CSF Tau stable until the 5th decile (β[SE] = -10.6[17.4], p = 0.54), and then decreased for those with higher CSF Tau values (β[SE] = -16.6[8.5], p = 0.05).
Fig 2. Mean CSF Aβ42 by deciles of CSF Tau, as a function of APOE4 status.
Green line: No APOE ε4 allele. Red line: at least one APOE ε4 allele. The linear regression coefficients show analysis between CSF Aβ42 and deciles of CSF Tau are presented by APOE4 status, stratified by the median of CSF Tau.
Finally, Table 3 presents the evolution of profiles among the 70 patients who had 2 lumbar punctures, over a median follow-up of 2.1 years. Of the 23 patients initially classified as A+N- profile, 61% of them remained A+N- at the 2d assessment, and 13% of them evolved to A+N+ profile. For the 14 patients initially classified as A-N+ profile, 50% had the same profile and 36% evolved to an A+N+ profile.
Table 3. Change over time in profiles defined by CSF biomarkers profiles in 70 patients with 2 assessments of biomarkers.
Profiles are based on CSF Aβ42, CSF Tau, and CSF p-Tau (NIA-AA classification).
| Profile at time 2 | |||||
|---|---|---|---|---|---|
| Profile at time 1 | A-N- | A+N- | A+N+ | A-N+ | Total |
| A-N- | 4 (30) | 7 (54) | 1 (8) | 1 (8) | 13 |
| A+N- | 5 (22) | 14 (61) | 3 (13) | 1 (4) | 23 |
| A+N+ | 0 (0) | 2 (10) | 16 (80) | 2 (10) | 20 |
| A-N+ | 1 (7) | 1 (7) | 5 (36) | 7 (50) | 14 |
Discussion
We report the following findings on the relationship between CSF Tau and CSF Aβ42 in a large, multicentric cohort of 3565 patients assessed for cognitive disorders:
We confirmed the previously reported quadratic relationship [22] between these 2 biomarkers in the total population not stratified by biomarker profiles.
Further analysis revealed that the association between CSF Tau and CSF Aβ42 is strongly dependant on biomarkers profiles. This association was positive in those with a normal profile, no association was found in amyloidosis only subjects, and an inverse association in those with SNAP and AD profiles. The association between CSF Tau and CSF Aβ42 was linear and not quadratic when examined separately in each profile group.
Analysis of change in biomarkers showed that 36% of those who were initially classified as A-N+ evolved to an A+N+ profile, compared to 13% for those who were initially classified as A+N-.
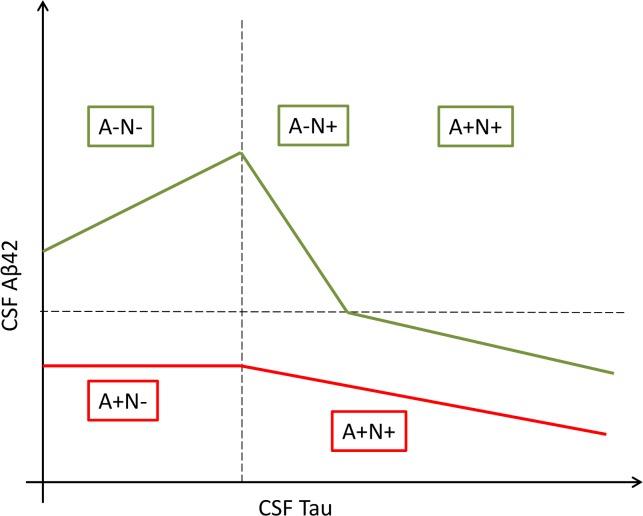
We propose a theoretical framework to explain these results which suggest two distinct patterns, illustrated by Fig 3. The first pattern is characterized by biomarkers in normal range where level of Aβ42 in the CSF increases with level of CSF Tau reflecting early stage of AD. This stage corresponds to an increase in production and/or decrease in degradation of Aβ42 in the brain which is still being compensated by an increase in its clearance into the CSF. At some point in disease progression systems responsible for clearing Aβ42 from the brain into the CSF become overwhelmed [31] and Aβ42 accumulates in plaques in the brain and CSF Aβ42 levels decrease while CSF Tau continues to increase to abnormal levels, reflecting the progression of tau pathology. Within this pattern, some A-N+ patients evolve to future AD profile as they are simply in the phase that follows the initial increase of CSF Aβ42. This would explain why one third of our patients initially classified as A-N+ finally evolve to an A+N+ profile with biomarkers assessed at the second lumbar puncture. In the second pattern shown in Fig 3, level of CSF Aβ42 remains low and stable during the initial progression of Tau pathology (A+N- patients) to the point where CSF Aβ42 begins to decrease inversely with CSF Tau (A+N+). Interestingly, these two patterns of change in biomarkers reflect that we found in analyses stratified by APOEe4 status; patients without APOE4 allele correspond to the first pattern: initial increase and then decrease of CSF Aβ42 with progression of CSF tau, while patients with at least one APOE4 allele correspond to the second pattern: initial stability (A+N-) and then decrease of CSF Aβ42 with progression of tau pathology.
Fig 3. Two distinct patterns: Theoretical framework of the association between CSF Aβ42 and CSF Tau.
In green, patients with normal profiles have an increase of CSF Aβ42 followed by a decrease after the progression of tau pathology. In red, patients with isolated low CSF Aβ42 had a stable level before declining.
The kinetics of CSF Aβ42 in very early stages of sporadic AD remains largely unknown. Data from studies on carriers of autosomal-dominant AD mutations,[32, 33] as well as from experimental studies [34], suggest that CSF Aβ42 levels increase before and during the early phase of brain amyloid deposition. In their study on biomarker changes in dominantly inherited Alzheimer’s disease, Bateman et al. reported that CSF Aβ42 levels were higher in persons with autosomal dominant AD 20 to 30 years before the clinical onset of the disease [32], the earliest observed phenomenon associated with AD.
In the recently proposed biological diagnosis of AD based on the A/T/N classification, A-T+ profiles are considered to be non-AD pathologic change [28]. Our findings suggest that this conclusion may need to be tempered in light of our findings that some of these subjects also evolve towards an AD profile. The neuropathological concept of “primary age-related tauopathy” (PART) has recently emerged to characterize the many patients with mainly neurofibrillary pathology and only few or none Aβ deposits [35, 36]. Whether this condition represents an independent disease remains the subject of debate; several authors have suggested that PART belongs to the AD spectrum, with some patients beginning their disease by an initial spreading of tau lesions that precedes Aβ accumulation [4, 37]. CSF biomarkers represents an opportunity to test in-vivo these hypothesis and the fact that one third of our patients initially classified as A-N+ evolved to an A+N+ profile supports this hypothesis. Furthermore, many neuropathological studies have reported that neurofibrillary tangles accumulation is a common feature of ageing [38–40]. Current methods lack sensitivity to detect minimal tangle presence in the entorhinal area and hippocampus, more sensitive methods for tau detection may in the future increase the proportion of A-N+ patients.
This study has several strengths, including its large size which allowed us to cover a wide spectrum of AD pathology, the inclusion of data from 18 memory clinics, and the fact that the analyses of CSF were performed in a single biochemistry department which prevents intersite variability [29]. One of the main limitations is that most of the patients had only one CSF biomarkers assessment, which allows only cross-sectional analysis. We however checked the evolution of biomarkers profiles in 70 patients who had 2 lumbar punctures. Future large scale studies with repeated CSF biomarkers assessments and large duration of follow-up will be helpful to better understand the kinetics of these biomarkers, especially at the early stage of the disease. Another limitation is that the four profiles were defined using CSF biomarkers rather than independent criteria (PET-imaging, neuropathology).
In conclusion, this report describes the relationship between CSF Aβ42 and CSF Tau in a large and multicentric population of patients who were referred to memory clinics for further assessment of cognitive disorders. We found that the nature of the association between these biomarkers was dependent of the biomarker based profile of the patients: positive association in normal profile, negative association in SNAP and AD profiles, lack of association in amyloidosis only subjects. These results plead for an increase of CSF Aβ42 early in the disease, before its decline, especially among non-APOE4 subjects. This pattern may explain why some patients with neurodegeneration only markers convert to AD profiles only over time.
Supporting information
Profiles are based on CSF Aβ42, CSF Tau, and CSF p-Tau (NIA-AA classification).
(DOCX)
Acknowledgments
We gratefully acknowledge and thank all the patients and their relatives for their time and contributions.
Data Availability
Data are available at: https://figshare.com/articles/CSF_biomarkers_profiles_in_AD_pdf/7868594.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2012;8(1):1–13. Epub 2012/01/24. 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science (New York, NY). 1992;256(5054):184–5. Epub 1992/04/10. . [DOI] [PubMed] [Google Scholar]
- 3.Reitz C. Alzheimer's disease and the amyloid cascade hypothesis: a critical review. International journal of Alzheimer's disease. 2012;2012:369808 Epub 2012/04/17. 10.1155/2012/369808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta neuropathologica. 2014;128(6):767–72. Epub 2014/11/02. 10.1007/s00401-014-1356-1 . [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain: a journal of neurology. 2015;138(Pt 10):2814–33. Epub 2015/08/19. 10.1093/brain/awv236 . [DOI] [PubMed] [Google Scholar]
- 6.Zetterberg H, Lautner R, Skillback T, Rosen C, Shahim P, Mattsson N, et al. CSF in Alzheimer's disease. Advances in clinical chemistry. 2014;65:143–72. Epub 2014/09/23. . [DOI] [PubMed] [Google Scholar]
- 7.Blennow K. A Review of Fluid Biomarkers for Alzheimer's Disease: Moving from CSF to Blood. Neurology and therapy. 2017;6(Suppl 1):15–24. Epub 2017/07/25. 10.1007/s40120-017-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimmer T, Riemenschneider M, Forstl H, Henriksen G, Klunk WE, Mathis CA, et al. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biological psychiatry. 2009;65(11):927–34. Epub 2009/03/10. 10.1016/j.biopsych.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of neurology. 2009;66(3):382–9. Epub 2009/03/11. 10.1001/archneurol.2008.596 . [DOI] [PubMed] [Google Scholar]
- 10.Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. The Lancet Neurology. 2010;9(1):119–28. Epub 2010/01/20. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2011;7(3):280–92. Epub 2011/04/26. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR Jr., Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Annals of neurology. 2012;71(6):765–75. Epub 2012/04/11. 10.1002/ana.22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR Jr., Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, et al. Suspected non-Alzheimer disease pathophysiology—concept and controversy. Nature reviews Neurology. 2016;12(2):117–24. Epub 2016/01/20. 10.1038/nrneurol.2015.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caroli A, Prestia A, Galluzzi S, Ferrari C, van der Flier WM, Ossenkoppele R, et al. Mild cognitive impairment with suspected nonamyloid pathology (SNAP): Prediction of progression. Neurology. 2015;84(5):508–15. Epub 2015/01/09. 10.1212/WNL.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prestia A, Caroli A, van der Flier WM, Ossenkoppele R, Van Berckel B, Barkhof F, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80(11):1048–56. Epub 2013/02/08. 10.1212/WNL.0b013e3182872830 . [DOI] [PubMed] [Google Scholar]
- 16.Vos SJ, Verhey F, Frolich L, Kornhuber J, Wiltfang J, Maier W, et al. Prevalence and prognosis of Alzheimer's disease at the mild cognitive impairment stage. Brain: a journal of neurology. 2015;138(Pt 5):1327–38. Epub 2015/02/20. 10.1093/brain/awv029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung JK, Plitman E, Nakajima S, Caravaggio F, Shinagawa S, Iwata Y, et al. The Effects of Cortical Hypometabolism and Hippocampal Atrophy on Clinical Trajectories in Mild Cognitive Impairment with Suspected Non-Alzheimer's Pathology: A Brief Report. Journal of Alzheimer's disease: JAD. 2017;60(2):341–7. Epub 2017/08/23. 10.3233/JAD-170098 . [DOI] [PubMed] [Google Scholar]
- 18.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–47. Epub 2016/07/03. 10.1212/WNL.0000000000002923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer's disease pathophysiology (SNAP) or Alzheimer's disease pathology: a longitudinal study. Lancet Neurol. 2016;15(10):1044–53. Epub 2016/07/28. 10.1016/S1474-4422(16)30125-9 . [DOI] [PubMed] [Google Scholar]
- 20.Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, et al. Longitudinal beta-Amyloid Deposition and Hippocampal Volume in Preclinical Alzheimer Disease and Suspected Non-Alzheimer Disease Pathophysiology. JAMA neurology. 2016;73(10):1192–200. Epub 2016/08/23. 10.1001/jamaneurol.2016.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormino EC, Papp KV, Rentz DM, Schultz AP, LaPoint M, Amariglio R, et al. Heterogeneity in Suspected Non-Alzheimer Disease Pathophysiology Among Clinically Normal Older Individuals. JAMA neurology. 2016;73(10):1185–91. Epub 2016/08/23. 10.1001/jamaneurol.2016.2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Leon MJ, Pirraglia E, Osorio RS, Glodzik L, Saint-Louis L, Kim HJ, et al. The nonlinear relationship between cerebrospinal fluid Abeta42 and tau in preclinical Alzheimer's disease. PloS one. 2018;13(2):e0191240 Epub 2018/02/08. 10.1371/journal.pone.0191240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouton-Liger F, Wallon D, Troussiere AC, Yatimi R, Dumurgier J, Magnin E, et al. Impact of cerebro-spinal fluid biomarkers of Alzheimer's disease in clinical practice: a multicentric study. Journal of neurology. 2014;261(1):144–51. Epub 2013/10/29. 10.1007/s00415-013-7160-3 . [DOI] [PubMed] [Google Scholar]
- 24.Troussiere AC, Wallon D, Mouton-Liger F, Yatimi R, Robert P, Hugon J, et al. Who needs cerebrospinal biomarkers? A national survey in clinical practice. Journal of Alzheimer's disease: JAD. 2014;40(4):857–61. Epub 2014/03/01. 10.3233/JAD-132672 . [DOI] [PubMed] [Google Scholar]
- 25.Paquet C, Bouaziz-Amar E, Cognat E, Volpe-Gillot L, Haddad V, Mahieux F, et al. Distribution of Cerebrospinal Fluid Biomarker Profiles in Patients Explored for Cognitive Disorders. Journal of Alzheimer's disease: JAD. 2018. Epub 2018/07/04. 10.3233/jad-180240 . [DOI] [PubMed] [Google Scholar]
- 26.Boumenir A, Cognat E, Sabia S, Hourregue C, Lilamand M, Dugravot A, et al. CSF level of β-amyloid peptide predicts mortality in Alzheimer’s disease. Alzheimer's Research & Therapy. 2019;11(1):29 10.1186/s13195-019-0481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF biomarker variability in the Alzheimer's Association quality control program. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9(3):251–61. Epub 2013/04/30. 10.1016/j.jalz.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2018;14(4):535–62. Epub 2018/04/15. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumurgier J, Vercruysse O, Paquet C, Bombois S, Chaulet C, Laplanche JL, et al. Intersite variability of CSF Alzheimer's disease biomarkers in clinical setting. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9(4):406–13. Epub 2012/11/13. 10.1016/j.jalz.2012.06.006 . [DOI] [PubMed] [Google Scholar]
- 30.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. Journal of pharmacokinetics and biopharmaceutics. 1978;6(2):165–75. Epub 1978/04/01. . [DOI] [PubMed] [Google Scholar]
- 31.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nature reviews Neurology. 2015;11(8):457–70. Epub 2015/07/22. 10.1038/nrneurol.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. The New England journal of medicine. 2012;367(9):795–804. Epub 2012/07/13. 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease. Science translational medicine. 2014;6(226):226ra30 Epub 2014/03/07. 10.1126/scitranslmed.3007901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maia LF, Kaeser SA, Reichwald J, Lambert M, Obermuller U, Schelle J, et al. Increased CSF Abeta during the very early phase of cerebral Abeta deposition in mouse models. EMBO molecular medicine. 2015;7(7):895–903. Epub 2015/05/17. 10.15252/emmm.201505026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta neuropathologica. 2014;128(6):755–66. Epub 2014/10/29. 10.1007/s00401-014-1349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta neuropathologica. 2015;129(5):757–62. Epub 2015/03/18. 10.1007/s00401-015-1407-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M, et al. PART is part of Alzheimer disease. Acta neuropathologica. 2015;129(5):749–56. Epub 2015/01/30. 10.1007/s00401-015-1390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cerebral cortex (New York, NY: 1991). 1994;4(2):138–50. Epub 1994/03/01. . [DOI] [PubMed] [Google Scholar]
- 39.Hyman BT, Gomez-Isla T. The natural history of Alzheimer neurofibrillary tangles and amyloid deposits. Neurobiology of aging. 1997;18(4):386–7; discussion 9–92. Epub 1997/07/01. . [DOI] [PubMed] [Google Scholar]
- 40.Tsartsalis S, Xekardaki A, Hof PR, Kovari E, Bouras C. Early Alzheimer-type lesions in cognitively normal subjects. Neurobiology of aging. 2018;62:34–44. Epub 2017/11/07. 10.1016/j.neurobiolaging.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Profiles are based on CSF Aβ42, CSF Tau, and CSF p-Tau (NIA-AA classification).
(DOCX)
Data Availability Statement
Data are available at: https://figshare.com/articles/CSF_biomarkers_profiles_in_AD_pdf/7868594.