Abstract
Objective
To summarize the best available evidence regarding various topics related to primary care management of opioid use disorder (OUD).
Data sources
MEDLINE, Cochrane Library, Google, and the references of included studies and relevant guidelines.
Study selection
Published systematic reviews and newer randomized controlled trials from the past 5 to 10 years that investigated patient-oriented outcomes related to managing OUD in primary care, diagnosis, pharmacotherapies (including buprenorphine, methadone, and naltrexone), tapering strategies, psychosocial interventions, prescribing practices, and management of comorbidities.
Synthesis
From 8626 articles, 39 systematic reviews and an additional 26 randomized controlled trials were included. New meta-analyses were performed where possible. One cohort study suggests 1 case-finding tool might be reasonable to assist with diagnosis (positive likelihood ratio of 10.3). Meta-analysis demonstrated that retention in treatment improves when buprenorphine or methadone are used (64% to 73% vs 22% to 39% for control), when OUD is treated in primary care (86% vs 67% in specialty care, risk ratio [RR] of 1.25, 95% CI 1.07 to 1.47), and when counseling is added to pharmacotherapy (74% vs 62% for controls, RR = 1.20, 95% CI 1.06 to 1.36). Retention was also improved with naltrexone (33% vs 25% for controls, RR = 1.35, 95% CI 1.11 to 1.64) and reduced with medication-related contingency management (eg, loss of take-home doses as a punitive measure; 68% vs 77% for no contingency, RR = 0.86, 95% CI 0.76 to 0.99).
Conclusion
There is reasonable evidence that patients with OUD should be managed in the primary care setting. Diagnostic criteria for OUD remain elusive, with 1 reasonable case-finding tool. Methadone and buprenorphine improve treatment retention, while medication-related contingency methods could worsen retention. Counseling is beneficial when added to pharmacotherapy.
Résumé
Objectif
Résumer les meilleures données probantes disponibles concernant divers sujets liés à la prise en charge du trouble de consommation d’opioïdes (TCO) dans les soins primaires.
Sources de l’information
MEDLINE, Bibliothèque Cochrane, Google, de même que les références des études incluses et les lignes directrices pertinentes.
Sélection des études
Les revues systématiques et les plus récentes études randomisées contrôlées, publiées au cours des 5 à 10 dernières années, qui portaient sur des paramètres axés sur le patient en lien avec la prise en charge du TCO dans les soins primaires, le diagnostic, la pharmacothérapie (y compris la buprénorphine, la méthadone et la naltrexone), les stratégies de traitement dégressif, les interventions psychosociales, les pratiques relatives aux prescriptions et la prise en charge des comorbidités.
Synthèse
Au nombre des 8626 articles, on a retenu 39 revues systématiques et 26 études randomisées contrôlées supplémentaires. Si possible, de nouvelles méta-analyses étaient effectuées. Une étude de cohortes fait valoir qu’un outil de dépistage serait raisonnablement utile pour aider au diagnostic (rapport de vraisemblance de 10,3). Des méta-analyses ont démontré que le maintien du traitement s’améliore lorsque la buprénorphine ou la méthadone sont utilisées (64 à 73 % c. 22 à 39 % dans le groupe témoin), lorsque le TCO est traité dans les soins primaires (86 c. 67 % en soins spécialisés, rapport de risque [RR] de 1,25, IC à 95 % de 1,07 à 1,47), et si le counseling accompagne la pharmacothérapie (74 c. 62 % dans le groupe témoin, RR = 1,20, IC à 95 % de 1,06 à 1,36). Le maintien était aussi amélioré avec la naltrexone (33 c. 25 % dans le groupe témoin, RR = 1,35, IC à 95 % de 1,11 à 1,64), mais réduit selon les mesures de contingence liées aux médicaments (p. ex. refus des doses à emporter par mesure punitive; 68 c. 77 % dans le groupe sans mesure punitive, RR = 0,86, IC à 95 % de 0,76 à 0,99).
Conclusion
Des données probantes étayent bien la pertinence du traitement des patients ayant un TOC dans le contexte des soins primaires. Les critères diagnostiques du TCO demeurent vagues, sauf pour 1 outil de dépistage raisonnablement utile. La méthadone et la buprénorphine améliorent le maintien du traitement, tandis que les mesures de contingence liées aux médicaments pourraient le réduire. Le counseling est bénéfique en accompagnement de la pharmacothérapie.
Opioid use disorder (OUD) is an important public health concern.1 While various organizations have responded to this crisis with a variety of guidelines and educational resources, none has done so with an exclusive primary care audience in mind or with the information necessary to allow for shared, informed decision making.2,3 In order to provide comprehensive care, primary care clinicians require information on all aspects of OUD management (such as treatment agreements and urine drug testing) and management of comorbidities (such as anxiety and pain). In some cases, access to more comprehensive supports might be limited owing to physical or financial barriers, furthering the need to provide clinicians with accessible evidence-based information.
We completed 17 systematic reviews to answer key clinical questions originating from a committee tasked with writing an OUD guideline for primary care (page 321).4 The systematic reviews were related to the following:
management of OUD in primary care;
diagnosis of OUD;
- treatment, including
- -pharmacotherapeutic management of OUD (buprenorphine, methadone, naltrexone, and cannabinoids),
- -prescribing practices (use of daily witnessed ingestion, urine drug testing, and treatment agreements),
- -tapering off drug therapy in OUD (tapering off opioids, tapering off opioid agonist therapy [OAT] compared with long-term maintenance, and fast vs slow tapering regimens in patients discontinuing OAT),
- -psychosocial interventions for OUD (counseling, motivational interviewing, cognitive-behavioural therapy, contingency management, and technology-based psychosocial interventions),
- -residential treatment programs; and
management of comorbidities in patients with OUD (acute pain, chronic pain, insomnia, anxiety, and attention deficit hyperactivity disorder).
The full list of questions appears in an appendix, available from CFPlus.*
Two additional topics (the role of OAT without any additional supports and the use of sustained-release oral morphine) were also investigated with abbreviated systematic searches.
METHODS
To complete this review, we followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and the protocol for systematic review of systematic reviews. 5,6
Data sources
The evidence team created a search strategy with guidance from an experienced librarian for each of the clinical questions created. Two authors (D.P., J.T.) performed the search for systematic reviews and randomized controlled trials (RCTs) for each clinical question with no language restrictions. The search was restricted to nonanimal studies. The databases and resources used to search for relevant systematic reviews included MEDLINE, Cochrane Library, Google, published guidelines on OUD, and reference lists of the included systematic reviews. The search included any articles up to June 2018, but was generally limited to the past 5 to 10 years. Key words opioid or opiate were used for all searches. Specifics for each question and the corresponding key words, timelines, and search strategies used can be found in the appendix (CFPlus).* After the search for systematic reviews was complete, an additional search of MEDLINE was undertaken to find RCTs published since the most recent systematic review for each clinical question. Reference lists of included articles were hand searched to identify potentially missed articles.
Study selection
Beyond systematic reviews and newer RCTs, inclusion criteria were studies of adult patients with OUD reporting on at least 1 of the following outcomes: morbidity and mortality, social outcomes, quality of life and symptoms, or opioid use outcomes (these are defined in Table 1). Systematic reviews of observational studies were included; however, observational data were only considered when RCTs did not exist. Individual observational studies were not used to inform recommendations. Exclusion criteria were studies on detoxification from opioids; studies in pediatric, pregnant, or cancer patients; and studies completed within a prison setting. Any exceptions made were recorded in the appendix (CFPlus).*
Table 1.
Outcomes considered relevant for study inclusion
| OUTCOME | WHAT THE OUTCOME INCLUDES |
|---|---|
| Morbidity and mortality | Mortality, fatal and nonfatal overdose, suicide, hospitalization or emergency department visits, and acquiring infections such as hepatitis B and C |
| Societal outcomes | Crime, incarceration, employment, housing, and transmission of infections such as hepatitis B and C |
| Quality of life and symptoms | Incidence of adverse events, withdrawal symptoms, patient satisfaction, quality-of-life scales, and scales related to guideline questions (eg, pain, anxiety) |
| Opioid use and treatment retention | Ongoing opioid use (from urine toxicology preferentially) and remaining in treatment |
Dual title, abstract, and full-text review were completed for all systematic review and RCT searches to determine study eligibility. Single review was completed for guidelines and their references, with dual assessment if full-text review was required. Disagreements over inclusion were resolved by consensus.
Data extraction
Dual data extraction was completed using templates created by 2 authors (C.R.F., J.T.), one specifically for systematic reviews and one for RCTs. For systematic reviews, data extracted included author, year, title, study design, general characteristics, setting, sex, mean age, mean duration, duration range, outcomes reported (along with number of studies, RCTs, and patients for each outcome), values associated with the outcomes, the intervention, and the control. If no usable data were found in a given systematic review, authors attempted to obtain that data from the included trials.
Following extraction, data tables of systematic reviews and RCTs were created with headings for total studies, age, population, relevant studies, duration of studies, intervention, outcomes, and risk-of-bias quality assessment. The data tables created can be found in the appendix (CFPlus).*
Risk-of-bias assessment
Risk of bias was assessed using a modified AMSTAR (A Measurement Tool to Assess Systematic Reviews) rubric for systematic reviews, focusing on the 6 most relevant questions7,8:
Was study selection and data extraction performed by dual reviewers?
Was the literature search comprehensive?
Were the included study characteristics described?
Was the quality of the included studies assessed and reported?
Were the methods used to combine results appropriate?
Were conflicts of interest reported?
For systematic reviews, each question was scored as 1 (completed) or 0 (not completed). These individual scores were then summated, with a higher total score suggesting a lower risk of bias. For RCTs, the Jadad 5-point scoring rubric was used.9 The risk-of-bias assessment for each article was completed by at least 2 independent authors, and disagreement was resolved by consensus or a third author.
Analysis
Following data extraction, we used study outcomes and meta-analyses to answer each clinical question. We reported study characteristics and outcomes descriptively using means and other statistical results as per original papers. We prioritized systematic reviews of RCTs and individual RCT results over systematic reviews of observational data. Where outcomes were measured in various ways, we preferentially reported on the more objective outcomes. For example, for the outcome of continued opioid use in studies of pharmacotherapy, we report on the results of urine drug tests over self-report outcomes.
Performing new meta-analyses
If no relevant meta-analyses existed or if relevant RCTs had been published since the most recent systematic review, a new meta-analysis was completed using the RevMan 5 software. We used a Mantel-Haenszel statistical method and focused on reporting risk ratios (RRs) when appropriate. Not wanting to overweigh smaller studies, we chose a fixed-effects analysis if there was no reason to speculate that the effect of the intervention would deviate meaningfully between studies. We assessed heterogeneity using the I2 statistic. Values greater than 50% were indicative of “high heterogeneity” and suggested a sensitivity analysis be completed to determine the cause of the heterogeneity. Additionally, we performed an exploratory meta-analysis of the effects of buprenorphine, methadone, and naltrexone on mortality. Owing to the low event rate, mortality events from the 3 treatments were combined and meta-analysis was completed using the exact method with odds ratios. 10
SYNTHESIS
Details of the study flow (PRISMA) are provided in the appendix (CFPlus).* All searches combined identified a total of 8626 articles, with 39 systematic reviews and an additional 26 RCTs (29 publications) being included. The characteristics of the included systematic reviews and RCTs, reasons for exclusion of systematic reviews after full-text review, and modified AMSTAR scores and Jadad scores are provided in the appendix (CFPlus).*
We preferentially report meta-analysis for treatment retention, ongoing drug use, and select key outcomes. All other outcomes, as well as details of individual RCTs that contributed to each meta-analysis, are available in the appendix (CFPlus).*
No RCT data available
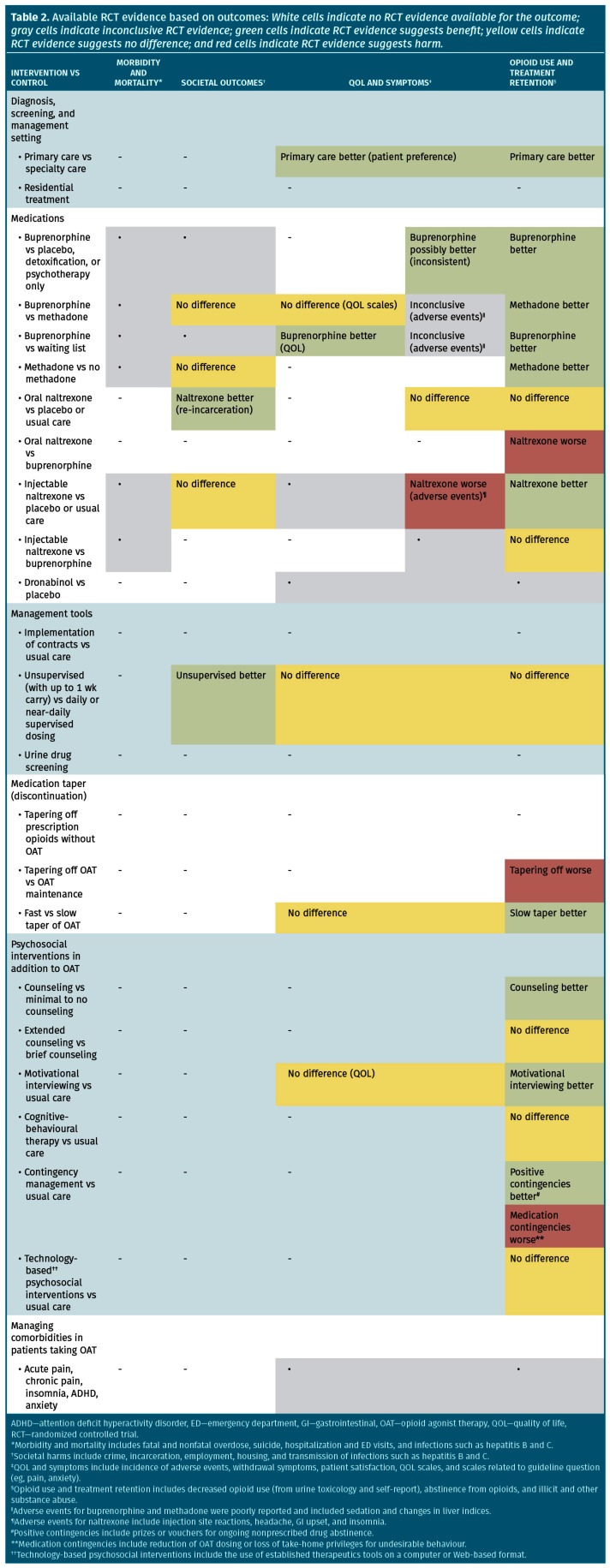
Overall, 9 of the 17 systematic reviews we completed had either no RCT data available for the specified outcomes or the data were considered inconclusive (Box 1). No systematic review or RCT had data to support all outcomes, and no individual systematic review or RCT provided adequate data on morbidity and mortality (Table 2).
Box 1. Systematic reviews with no or inconclusive RCT evidence for any outcome.
The following topics had no or inconclusive RCT evidence:
Residential treatment
Cannabinoids for OUD
Implementation of contracts vs usual care
Urine drug screening
Management of acute pain in patients with OUD
Management of chronic pain in patients with OUD
Management of insomnia in patients with OUD
Management of ADHD in patients with OUD
Management of anxiety in patients with OUD
ADHD—attention deficit hyperactivity disorder, OUD—opioid use disorder, RCT—randomized controlled trial.
Table 2.
Available RCT evidence based on outcomes: White cells indicate no RCT evidence available for the outcome; gray cells indicate inconclusive RCT evidence; green cells indicate RCT evidence suggests benefit; yellow cells indicate RCT evidence suggests no difference; and red cells indicate RCT evidence suggests harm.
Management of OUD in primary care
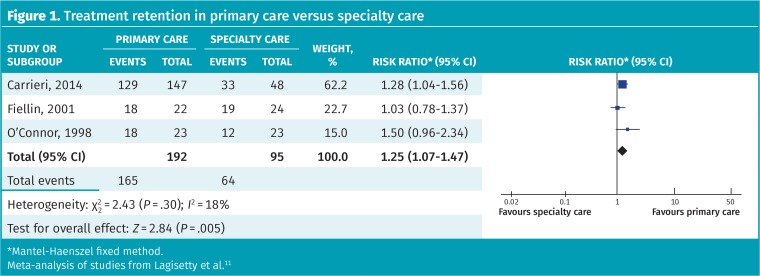
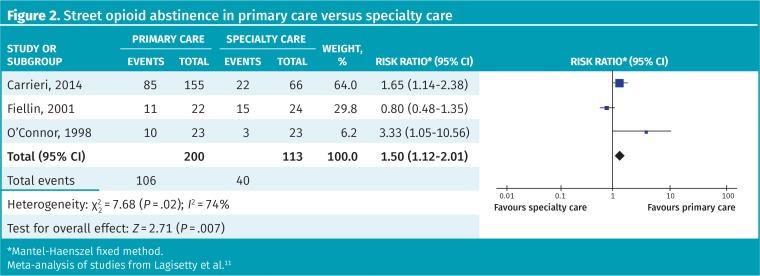
No previous meta-analysis was available; however, 4 RCTs compared the management of OUD in primary care with that in specialty care (numbers of participants ranged from 46 to 221). Three of these looked at patient satisfaction rates and found statistically significantly higher rates (ie, more satisfaction) with primary care (eg, 77% vs 38%). We performed a meta-analysis of the effect of treatment setting on retention and found program retention was 86% in primary care versus 67% in a specialty clinic (RR = 1.25, 95% CI 1.07 to 1.47, I2 = 18%) (Figure 1).11 Street opioid abstinence was also higher in primary care settings (53% vs 35%, RR = 1.50, 95% CI 1.12 to 2.01, I2 = 74%); however, heterogeneity was high and this included both self-reported and urine-confirmed data (Figure 2).11
Figure 1.
Treatment retention in primary care versus specialty care
*Mantel-Haenszel fixed method.
Meta-analysis of studies from Lagisetty et al.11
Figure 2.
Street opioid abstinence in primary care versus specialty care
*Mantel-Haenszel fixed method.
Meta-analysis of studies from Lagisetty et al.11
Diagnosis
Fourteen systematic reviews on identifying patients with OUD were found, but none assessed diagnostic criteria. Two case-finding tools were compared with the Diagnostic and Statistical Manual of Mental Disorders (4th or 5th edition) criteria: the COMM (Current Opioid Misuse Measure), a 17-question scale, and the POMI (Prescription Opioid Misuse Index), a 6-question checklist. Both have been assessed in only 1 cohort study each, reporting positive likelihood ratios of 3.35 and 10.3, respectively (CFPlus).*
Treatment
Pharmacotherapy
Buprenorphine: We found 2 systematic reviews and an additional 6 RCTs (as 9 publications) of buprenorphine alone or combined with naloxone. Compared with placebo, buprenorphine significantly retained more patients in treatment (64% vs 39% for placebo, number needed to treat [NNT] of 4 at 30 days to 52 weeks; RR = 1.66, 95% CI 1.52 to 1.82, I2 = 86%) (CFPlus).*
Methadone: One systematic review and 1 RCT of methadone were found. Retention in treatment was higher with methadone compared with no methadone (73% vs 22% for controls, NNT = 2 at 45 days to 2 years; RR = 3.37, 95% CI 2.83 to 4.02, I2 = 73%) (CFPlus).*
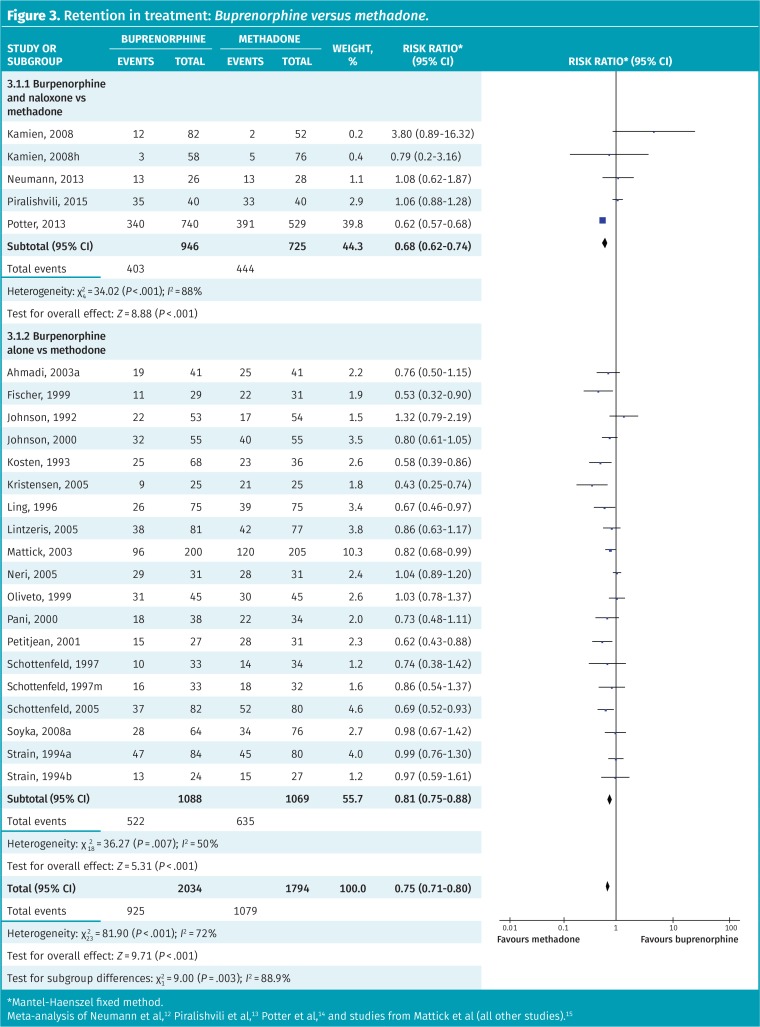
Our meta-analysis of 24 RCTs directly comparing buprenorphine with methadone revealed higher retention rates with methadone (45% vs 60% with methadone, NNT=7; RR = 0.75, 95% CI 0.71 to 0.80) (Figure 3).12–15 However, substantial heterogeneity was present (I2 = 72%). This also differed from a published systematic review that found no difference in retention rates between buprenorphine and methadone.16 Neilsen and colleagues’ systematic review meta-analyzed subgroups of patients from 3 of the above studies who used prescription opioids rather than heroin.16
Figure 3.
Retention in treatment: Buprenorphine versus methadone.
*Mantel-Haenszel fixed method.
Meta-analysis of Neumann et al,12 Piralishvili et al,13 Potter et al,14 and studies from Mattick et al (all other studies).15
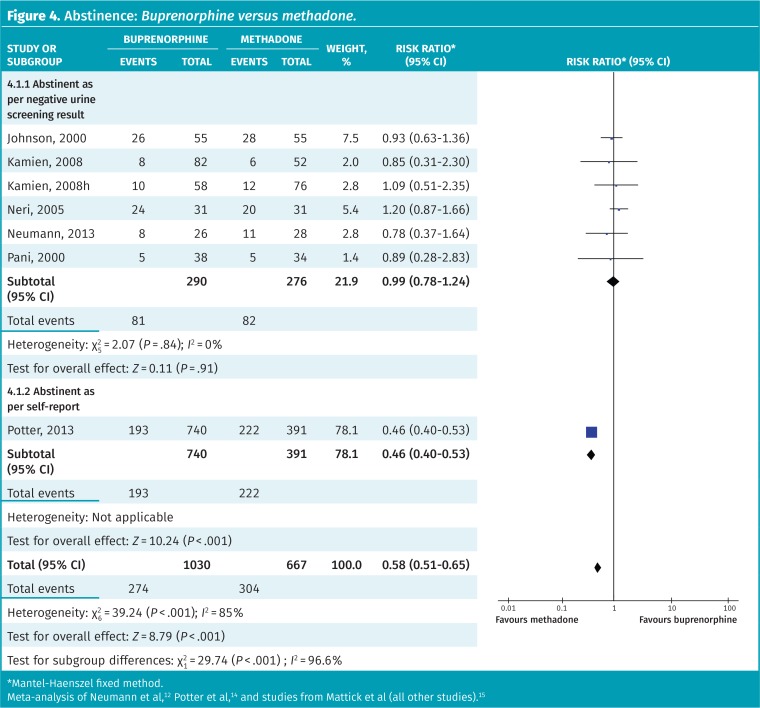
Overall, opioid abstinence appears higher with methadone than with buprenorphine (Figure 4).12,14,15 However, there was a statistically significant difference between subgroups of studies that measured abstinence objectively and those that relied on self-report (P < .001). If only studies that used objective measures are included, there is no difference in abstinence between buprenorphine and methadone (RR = 0.99, 95% CI 0.78 to 1.24, I2 = 0%).
Figure 4.
Abstinence: Buprenorphine versus methadone.
*Mantel-Haenszel fixed method.
Meta-analysis of Neumann et al,12 Potter et al,14 and studies from Mattick et al (all other studies).15
Adverse effects were poorly reported in both the buprenorphine and the methadone literature. Two RCTs found no difference between the drugs, except for more sedation with methadone (58% vs 26% with buprenorphine) in 1 RCT. Two RCTs found fewer adverse effects with buprenorphine than in controls (CFPlus).*
Naltrexone: Two systematic reviews and 5 RCTs were found on the opioid antagonist naltrexone. Indirect comparison reveals lower rates of retention than with OAT, but naltrexone is still better than placebo or usual care (33% vs 25% for controls, RR = 1.35, 95% CI 1.11 to 1.64, I2 = 0%) (CFPlus).* Although results of subgroup analysis of oral naltrexone were not statistically significant (RR = 1.32, 95% CI 0.97 to 1.79), they were numerically similar to the results for injectable naltrexone, and results of the test for subgroup differences between oral and injectable forms were not significant (P = .86). Naltrexone also increased abstinence from opioids (39% vs 27% for controls, RR = 1.48, 95% CI 1.11 to 1.98, I2 = 63%) (CFPlus).* Based on 4 small RCTs, naltrexone decreases re-incarceration (24% vs 33% for controls, RR = 0.69, 95% CI 0.51 to 0.94, I2 = 0%) (CFPlus).*
Buprenorphine, methadone, and naltrexone combined event rates: As mortality rates were very low across buprenorphine, methadone, and naltrexone studies, we performed an exploratory meta-analysis combining event rates for all 3 drugs and found a statistically significant reduction in overall mortality with the use of pharmacotherapy in patients with OUD (odds ratio of 0.34, 95% CI 0.10 to 0.95, 7 RCTs).
Prescribing practices
Daily witnessed ingestion (vs take-home doses or “carries”): Both treatment retention and continued drug use are no different between daily witnessed and unsupervised ingestion (CFPlus).* However, none of the included RCTs had a completely unsupervised arm; rather, they compared various levels of supervision (eg, 2 vs 5 times per week) (CFPlus).*
Urine drug testing: No RCTs were found (CFPlus).*
Treatment agreements: All RCTs of treatment agreements in patients with OUD incorporated contingency management. Therefore, it is not possible to differentiate the effects of contracts from those of the contingencies on patient outcomes.
Tapering.
There were no systematic reviews or RCTs of tapering off opioids versus the use of OAT for treating OUD. Three RCTs compared tapering off OAT compared with long-term maintenance. Abstinence was not reported; however, the group that was maintained on treatment had a greater number of opioid-negative urine test results in 1 RCT (53% vs 35% for those tapered; significance was not reported). Opioid use was also higher in the tapering arm of a different RCT (numbers not reported, P < .05) (CFPlus).*
Psychosocial supports.
Eight systematic reviews were identified on psychosocial supports. There was substantial variation with regard to inclusion criteria and analysis; thus, we prioritized 5 key interventions and assessed individual RCTs identified from the systematic reviews.
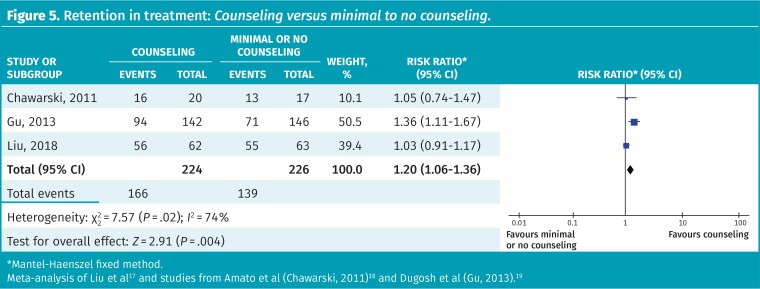
The addition of standard counseling to OAT is more effective in retaining people in treatment than no or minimal counseling (74% vs 62% for controls, NNT = 8; RR = 1.20, 95% CI 1.06 to 1.36, 3 RCTs); however, the heterogeneity was high (I2 = 74%) (Figure 5).17–19 No difference was noted between extended counseling sessions (45 to 60 minutes) compared with “standard” sessions of 15 to 20 minutes (RR = 1.19, 95% CI 0.88 to 1.62) (CFPlus).*
Figure 5.
Retention in treatment: Counseling versus minimal to no counseling.
*Mantel-Haenszel fixed method.
Meta-analysis of Liu et al17 and studies from Amato et al (Chawarski, 2011)18 and Dugosh et al (Gu, 2013).19
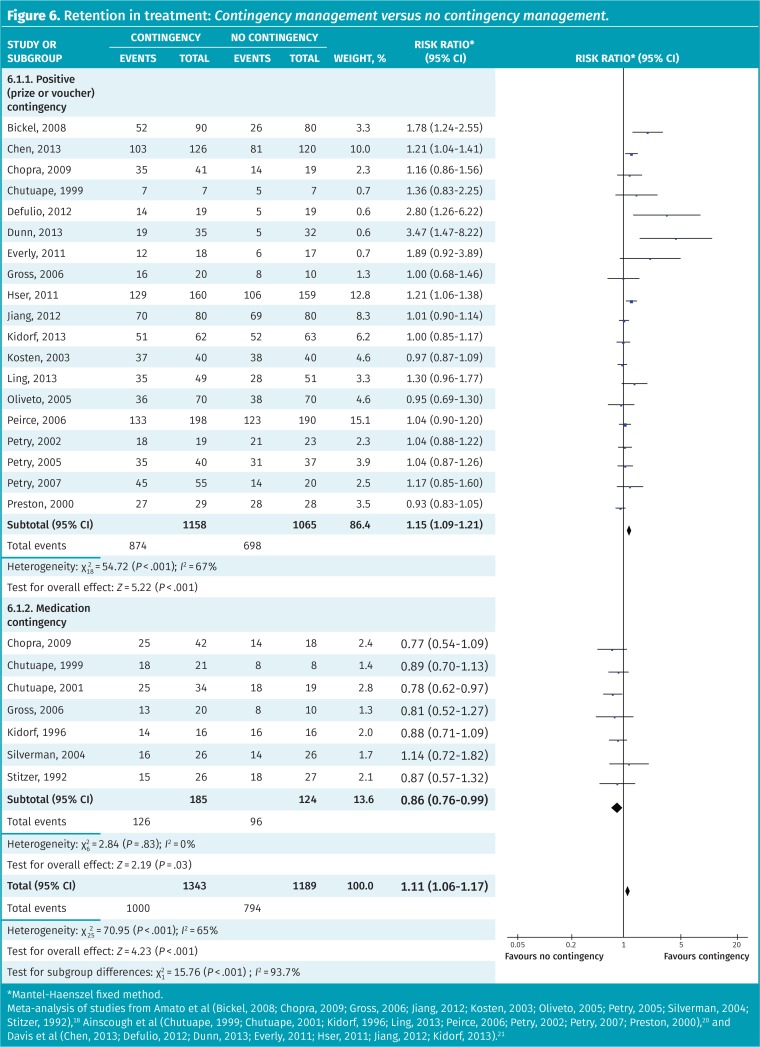
The use of contingency management, defined as either “rewards” for desired behaviour (eg, vouchers or prizes) or loss of privileges for undesired behaviour (eg, loss of medication carries for positive urine drug screening results), increases retention in treatment (RR = 1.11, 95% CI 1.06 to 1.17) (Figure 6).18,20,21 Subgroup analysis suggests the benefits are primarily from positive contingencies (RR = 1.15, 95% CI 1.09 to 1.21), with negative or medication-related contingencies worsening retention (68% vs 77% for no contingency, RR = 0.86, 95% CI 0.76 to 0.99) (test for subgroup difference P < .001). Methods of reporting opioid use were too heterogeneous to be meta-analyzed.
Figure 6.
Retention in treatment: Contingency management versus no contingency management.
*Mantel-Haenszel fixed method.
Meta-analysis of studies from Amato et al (Bickel, 2008; Chopra, 2009; Gross, 2006; Jiang, 2012; Kosten, 2003; Oliveto, 2005; Petry, 2005; Silverman, 2004; Stitzer, 1992),18 Ainscough et al (Chutuape, 1999; Chutuape, 2001; Kidorf, 1996; Ling, 2013; Peirce, 2006; Petry, 2002; Petry, 2007; Preston, 2000),20 and Davis et al (Chen, 2013; Defulio, 2012; Dunn, 2013; Everly, 2011; Hser, 2011; Jiang, 2012; Kidorf, 2013).21
Management of comorbidities in patients with OUD.
There was inadequate RCT evidence in all searched areas (CFPlus).*
Other topics.
Results of other systematic reviews on other topics, such as residential treatment, cannabinoids, fast versus slow tapering, motivational interviewing, cognitive-behavioural therapy, and technology-based psychosocial interventions are available in the appendix (CFPlus).*
DISCUSSION
There is a surprising lack of RCT data for a variety of topics important to the management of OUD in primary care. Nine of the 17 systematic reviews we completed had either no RCT evidence or RCT evidence that was impossible to make conclusive statements on.
While systematic reviews of observational data suggest that ongoing use of OAT results in a reduction in mortality,22,23 we found no RCT powered to investigate this outcome. Our exploratory meta-analysis of the combined effects of buprenorphine, methadone, and naltrexone suggests that medication-assisted treatment might reduce mortality. However, adequately powered RCTs are needed for confirmation. Methadone might be superior to buprenorphine for treatment retention, but opioid abstinence rates do not differ between methadone and buprenorphine when objective reporting measures are used. Most patients in pharmacotherapy studies were using heroin, not prescription opioids. Thus, outcomes in patients using prescription opioids might vary from what we have reported. One meta-analysis using subgroups of patients taking prescription opioids found no difference in retention rates between methadone and buprenorphine.16 Some provinces maintain prescribing restrictions on methadone, and methadone typically requires more supervision to achieve therapeutic doses. Randomized controlled trials of naltrexone typically only included patients who had undergone complete detoxification from opioids before enrolment. This limits its use as a first-line agent in primary care.
Despite finding numerous systematic reviews on the diagnosis of OUD, only one questionnaire with strong predictive ability for OUD that might be useful in primary care settings (POMI) was identified. The currently used Diagnostic and Statistical Manual of Mental Disorders, 5th edition, criteria for OUD are difficult to apply to patients taking prescription opioids for the management of chronic pain.24 Diagnosis of OUD in these patients remains challenging.
Primary care is an appropriate setting for the management of OUD, with improved patient outcomes compared with specialty care. While most of the included RCTs provided some type of supportive team or training, other RCTs have shown that OAT alone, without additional supports, also improves outcomes, particularly retention in treatment (CFPlus).*
Our results for counseling and contingency management differ considerably from other systematic reviews. The most frequently cited systematic review of contingency management combined RCTs of both positive and negative contingencies, reporting no benefit on retention in treatment.18 As negative or medication-related contingencies might be viewed as a disciplinary measure, it might be more appropriate to meta-analyze positive and negative contingencies separately. When analyzed separately, positive contingencies (eg, being given the opportunity to work on days where urine drug screening results are negative) are noted to improve treatment retention, whereas negative or medication-related contingencies (eg, loss of medication carries or lowering OAT doses) negatively affect retention in treatment. This is relevant for optimal OUD management, as negative contingencies are often used when patients are “caught” using opioids. It is notable that complete abstinence was rarely achieved even in carefully monitored trials, and positive urine samples might be a sign of suboptimal treatment. Best practices need to be carefully balanced with the safety of the patient and public in a nonpunitive manner.
Limitations
Limitations of this review included a lack of consistent terminology regarding OUD (eg, heroin abuse, opioid use, addiction, opioid dependency), which might have affected our ability to identify all relevant studies. Treatment studies were generally open label, suffered from high dropout rates, and included primarily patients using heroin as opposed to prescription opioids. Most studies were not designed to determine effects on morbidity and mortality, but instead focused on drug use outcomes and retention, which were inconsistently assessed and reported across trials.
Conclusion
Evidence supports primary care as a treatment setting for OUD. While diagnosing OUD remains a challenge for patients taking chronic prescription opioids for pain, the POMI might be a useful case-finding tool to identify patients with OUD. Buprenorphine and methadone might help patients stay in treatment, particularly if used long term; however, the optimal length of treatment is unknown. The addition of counseling, even brief sessions, to OAT helps patients stay in treatment even longer. Punitive measures should be avoided for ongoing drug use. Rather, changes to treatment might be required to help the patient reach his or her treatment goals, or to ensure the safety of the patient and the public.
Acknowledgments
We thank Janice Kung, MLIS, for her assistance with creating the search strategy, our peer reviewers for their valuable feedback, and the Alberta College of Family Physicians for its continued support. This project was funded by Alberta Health through the Primary Health Care Opioid Response Initiative.
Editor’s key points
▸ This systematic review of systematic reviews and randomized controlled trials was developed with a primary care focus to inform simplified guidelines for managing opioid use disorder (OUD) in the primary care setting.
▸ Evidence supports primary care as a treatment setting for OUD. While diagnosing OUD remains a challenge for patients taking chronic prescription opioids for pain, the POMI (Prescription Opioid Misuse Index) might be a useful case-finding tool to identify patients with OUD. Buprenorphine and methadone might help patients stay in treatment, particularly if used long term; however, the optimal length of treatment is unknown.
▸ The addition of counseling, even brief sessions, to opioid agonist therapy helps patients stay in treatment longer. Punitive measures should be avoided for ongoing drug use. Rather, changes to treatment might be required to help the patient reach his or her treatment goals, or to ensure the safety of the patient and the public.
Points de repère du rédacteur
▸ Cette revue systématique des revues systématiques et des études randomisées contrôlées a été conçue dans l’optique des soins primaires, dans le but d’éclairer la simplification des lignes directrices relatives à la prise en charge du trouble de consommation d’opioïdes (TCO) dans le contexte des soins primaires.
▸ Des données probantes étayent la pertinence des soins primaires comme milieu de traitement du TCO. Il demeure difficile de diagnostiquer un TCO chez des patients qui prennent des opioïdes sur une base chronique contre la douleur, mais l’indice POMI (Prescription Opioid Misuse Index) peut se révéler un outil utile de dépistage des patients souffrant d’un TCO. La buprénorphine et la méthadone peuvent aider les patients à poursuivre leur traitement, en particulier s’ils sont utilisés à long terme; par ailleurs, la durée optimale de la thérapie reste à déterminer.
▸ L’ajout de counseling à la thérapie aux agonistes opioïdes, même sous forme de séances brèves, aide les patients à suivre leur traitement plus longtemps. Il faut éviter les mesures punitives à l’égard de l’usage continu de drogues. Il pourrait plutôt être nécessaire d’apporter des changements au traitement afin d’aider les patients à atteindre leurs objectifs thérapeutiques, ou pour assurer la sécurité du patient et du public.
Footnotes
Results of the systematic reviews and abbreviated systematic reviews, the full list of questions, search details, exceptions to the exclusion criteria, the data tables, study flow details, modified AMSTAR and Jadad scores, and details about individual randomized controlled trials, as well as authors’ full disclosure of competing interests, are available at www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors were part of the Evidence Review Team and contributed to preparing the manuscript for submission.
Competing interests
None of the authors has a financial conflict of interest to declare. The full disclosure is available from CFPlus.*
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Special Advisory Committee on the Epidemic of Opioid Overdoses. National report: apparent opioid-related deaths in Canada (January 2016 to March 2018). Ottawa, ON: Public Health Agency of Canada; 2018. [Google Scholar]
- 2.Government of Canada. Strengthening Canada’s approach to substance use issues. Ottawa, ON: Government of Canada; 2018. Available from: www.canada.ca/en/health-canada/services/substance-use/canadian-drugs-substances-strategy/strengthening-canada-approach-substance-use-issue.html. Accessed 2019 Mar 20. [Google Scholar]
- 3.Bruneau J, Ahamad K, Goyer MÈ, Poulin G, Selby P, Fischer B, et al. Management of opioid use disorders: a national clinical practice guideline. CMAJ. 2018;190(9):E247–57. doi: 10.1503/cmaj.170958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korownyk C, Perry D, Ton J, Kolber MR, Garrison S, Thomas B, et al. Managing opioid use disorder in primary care. PEER simplified guideline. Can Fam Physician. 2019;65:321–30. (Eng), e173–84 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339 b2535. [PMC free article] [PubMed] [Google Scholar]
- 6.Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan GM, Finley CR, Ton J, Perry D, Ramji J, Crawford K, et al. Systematic review of systematic reviews for medical cannabinoids. Pain, nausea and vomiting, spasticity, and harms. Can Fam Physician. 2018;64:e78–94. Available from: www.cfp.ca/content/cfp/64/2/e78.full.pdf. Accessed 2019 Apr 8. [PMC free article] [PubMed] [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Mehta CR, Patel NR, Gray R. Computing and exact confidence interval for the common odds ratio in several 2 × 2 contingency tables. J Am Stat Assoc. 1985;80:969–73. [Google Scholar]
- 11.Lagisetty P, Klasa K, Bush C, Heisler M, Chopra V, Bohnert A. Primary care models for treating opioid use disorders: what actually works? A systematic review. PLoS One. 2017;12(10):e0186315. doi: 10.1371/journal.pone.0186315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann AM, Blondell RD, Jaanimägi U, Giambrone AK, Homish GG, Lozano JR, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and co-existent opioid addiction. J Addict Dis. 2013;32(1):68–78. doi: 10.1080/10550887.2012.759872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piralishvili G, Otiashvili D, Sikharulidze Z, Kamkamidze G, Poole S, Woody GW. Opioid addicted buprenorphine injectors: drug use during and after 12-weeks of buprenorphine-naloxone or methadone in the Republic of Georgia. J Subst Abuse Treat. 2015;50:32–7. doi: 10.1016/j.jsat.2014.10.003. Epub 2014 Oct 22. [DOI] [PubMed] [Google Scholar]
- 14.Potter JS, Marino EN, Hillhouse M, Nielsen S, Wiest K, Canamar CP, et al. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from Starting Treatment with Agonist Replacement Therapies (START). J Stud Alcohol Drugs. 2013;74(4):605–13. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database System Rev. 2014;(3):CD002209. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Neilsen S, Larance B, Degenhardt L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database System Rev. 2016;(5):CD011117. doi: 10.1002/14651858.CD011117.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Liu P, Song R, Zhang Y, Liu C, Cai B, Liu X, et al. Educational and behavioural counselling in a methadone maintenance treatment program in China: a randomized controlled trial. Front Psychiatry. 2018;4(9):113. doi: 10.3389/fpsyt.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2011;(10):CD004147. doi: 10.1002/14651858.CD004147.pub4. [DOI] [PubMed] [Google Scholar]
- 19.Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict Med. 2016;10(2):93–103. doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainscough TS, McNeill A, Strang J, Calder R, Brose LS. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318–39. doi: 10.1016/j.drugalcdep.2017.05.028. Epub 2017 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev Med. 2016;92:36–46. doi: 10.1016/j.ypmed.2016.08.008. Epub 2016 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma J, Bao YP, Wang RJ, Su MK, Liu MX, Li JQ, et al. Effects of medication-assisted treatment on mortality among opioid users: a systematic review and meta-analysis. Mol Psychiatry. 2018 Jun 22; doi: 10.1038/s41380-018-0094-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:i1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ton J, Korownyk C, Allan GM. Does this patient taking prescription opioids have opioid use disorder? Edmonton, AB: Tools for Practice; 2018. Available from: gomainpro.ca/wp-content/uploads/tools-for-practice/1539789463_tfp222opioidscreeningfv.pdf. Accessed 2019 Jan 31. [Google Scholar]