Abstract
Systemic lupus erythematosus (SLE) severity correlates with elevated serum levels of type I interferons (IFN), cytokines produced in large quantities by pDC in response to engagement of TLR7 and TLR9 with endocytosed nucleic acids. B cell adaptor for PI3K (BCAP) promoted many aspects of TLR7-driven lupus-like disease including Isg15 and Ifit1 expression in blood and an immature pDC phenotype associated with higher IFN production. BCAP−/− mice produced significantly less serum IFNα than WT mice after injection of TLR9 agonist, and BCAP promoted TLR7 and TLR9-induced IFNα production specifically in pDC. TLR-induced IFNα production in pDC requires DOCK2-mediated activation of Rac1 leading to activation of IKKα, a mechanism we show was dependent on BCAP. BCAP−/− pDC had decreased actin polymerization, Rac1 activation, and reduced IKKα phosphorylation upon TLR9 stimulation. We show a novel role for BCAP in promoting TLR-induced IFNα production in pDC and in SLE pathogenesis.
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by the loss of tolerance to self-antigens and dysregulated cytokine activities. Disease activity and severity is correlated with elevated serum levels of type I interferons (IFN), specifically IFNα (1). Type I IFN are a cytokine family that induce an anti-viral state and enhance innate and adaptive immune responses. Type I IFN levels are tightly regulated because the pleiotropic effects of their immune activating properties can have deleterious consequences (1). Though all cells can produce small amounts of type I IFN, plasmacytoid dendritic cells (pDC) constitutively express endosomal TLR7 and TLR9 to fulfill their prime function of swift and robust secretion of IFNα in response to nucleic acid sensing (2). There is increasing evidence that pDC are directly implicated in the pathogenesis of SLE and are an important source of IFNα in this disease (3–5), though the precise mechanisms by which TLR7/9-induced IFNα production is controlled remains undefined.
pDC activation occurs upon RNA or DNA recognition by TLR7 or TLR9, respectively, leading to stimulation of two divergent signaling pathways to induce production of either IFNα or inflammatory cytokines (2). The proinflammatory cytokine pathway requires MyD88 to activate NF-κB and elicit transcription of inflammatory cytokines. The Type I IFN response requires activation of MyD88, phosphorylation of inhibitor of NF-κB kinase-α (IKKα), and subsequent activation and translocation of IRF7 into the nucleus (6). IRAK1, TRAF3, DOCK2, Rac1, AP3, and numerous other signaling molecules are specifically required for IFNα induction (2, 6, 7). Interestingly, phosphatidylinositol-3 kinase (PI3K) is essential for IRF7 activation and IFNα production but dispensable for the inflammatory cytokine response in pDC (8). TLR-induced PI3K activation in macrophages requires B cell adaptor for PI3K (BCAP), an adaptor protein broadly expressed in leukocytes that is capable of recruiting PI3K through phosphorylation of its four YxxM tyrosines (9, 10). Because of the link between BCAP and TLR signaling in macrophages and the importance of PI3K in pDC IFNα production, we investigated the role of BCAP in regulating the TLR-induced IFNα production in pDC and in TLR7-driven lupus-like disease.
Materials and Methods
Mice
BCAP−/− mice (Pik3ap1−/−) were backcrossed >9 generations to C57BL/6 mice (9, 11, 12), TLR7.1 mice were provided by S. Bolland (NIH) (13), BDCA2-DTR mice were obtained from JAX (14). Mice were bred and housed at Benaroya Research Institute. All experiments were performed under approved IACUC protocols.
Phenotyping lupus-like disease in TLR7.1 mice
Male and female mice were used at 10–13 wk of age. Leukocyte expansion and activation was measured by flow cytometry. Serum anti-RNA Abs were quantified by anti-RNA ELISA (15). Frozen kidney cryosections (5 μm) were stained with anti-IgG-FITC. Raw integrated density/area was calculated via ImageJ. Paraffin sections were stained with H&E and PAS and were analyzed for kidney pathology in a blinded manner.
pDC depletion in TLR7.1/BDCA2-DTR mice
5 wk old TLR7.1 or TLR7.1/BDCA2-DTR mice were injected with 10 ng/g body weight DT every 48 h for 3 weeks and euthanized 24 h after the last DT injection.
pDC
Bone marrow (BM) cells were cultured in pDC media (RPMI,10%FCS, P/S, L-glutamate, NEAA, Hepes, Sodium Pyruvate, β-mercaptoethanol) containing 200 ng/ml Flt3L for 7 d and purified by sorting as CD11b-MHCII-CD11c+PDCA1+. pDC harvested directly ex vivo from BM were enriched by MACS depletion followed by sorting.
pDC TLR-ligand stimulation
30,000–50,000 purified pDC per well were plated in 96-well plates in pDC media with 200 ng/ml Flt3L. Cells were rested for 2 h and then stimulated for 12–15 h: R848 (1 μg/ml) (Invivogen), CpG-A ODN1585 (2 μM) (Invivogen), CpG-C ODN2395 (2–5 μM) (IDT). Immune complexes were formed by combining CG50 DNA (30 ng/ml) with mAb PA4 (10 μg/ml) for 1 h at 37°C (16), then added to pDC for 12–15 h. pDC were treated with VSV (MOI 3) for 12–15 h. Alexa647-labled CpG-C (IDT) was incubated with Flt3L-cultured pDC for indicated times and internalization measured by flow cytometry.
In vivo CpG-ODN
10 μg CpG-A or CpG-C in 100 μl of PBS was mixed with 20 μg DOTAP (Avanti) in 100 μl PBS in a polystyrene tube at RT for 10 min, then 200 μl DOTAP-CpG mixture/mouse was injected i.v. Serum was collected at 3 h post injection.
Cytokine quantification
Type I IFN was quantified by incubating supernatant or serum with L929-ISRE reporter cells for 6 h with rmIFNα standard (PBL), followed by luminescence measurement. IL-6 was quantified by ELISA (eBioscience).
Quantitative RT-PCR
Blood RNA was generated using RNeasy protect animal tubes and blood kit (Qiagen). cDNA was synthesized using Qiagen reagents with random hexamers and oligo(dT) primers. Quantitative PCR was performed using SYBR Green reagents (TaKaRa Bio) on a 7500 fast real-time PCR system (Applied Biosystems). Primers were as in (17).
Immunoblotting and co-Immunoprecipitation
Sorted pDC from Flt3L culture were rested overnight and stimulated with CpG-C (1–5 μM). Cytoplasmic extracts were run on SDS-Page, and phosphorylation of IKKα (Ser176/180; 2697; Cell Signaling Technology) was analyzed by immunoblot. For co-immunoprecipitation, HEK-293 T cells were transfected with DOCK2 (22) and BCAP-FLAG vectors. Cells were lysed and immunoprecipitated with anti-FLAG (M2; Sigma-Aldrich). Immunoprecipitates and lysates were blotted for DOCK2 (09-454; EMD Millipore) and FLAG.
Rac1 activation and actin remodeling assay
Sorted pDC were rested for 3 h in low serum medium containing 200 ng/ml Flt3L, then stimulated with 1 μM CpG-C. In some cases, pDC were pretreated for 30 min with 1 μM ZSTK474 (Tocris). Rac1 activation in cell lysates was measured by Rac-1 GLISA (Cytoskeleton, Inc.). For actin polymerization, cells were fixed with 4% PFA, permeabilized with 0.1% Triton-X100, 3% BSA in PBS, and stained with A488-Phalloidin (Cytoskeleton, Inc.) for 30 min, and analyzed by fluorescence microscopy. MFI for each cell was analyzed using Image J.
Statistics
Statistical analysis was performed with Prism 8 (Graphpad) as specified in figure legends.
Results
BCAP promotes TLR7-driven lupus-like disease
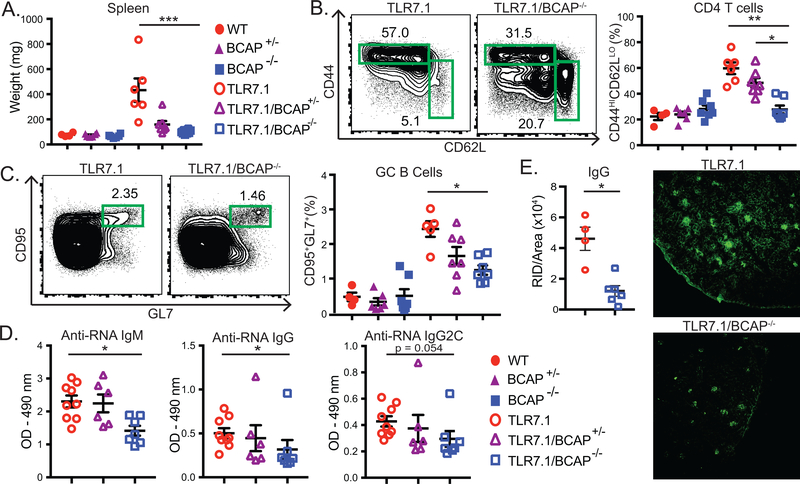
Increased TLR7 dosage induces systemic autoimmunity and immune activation in TLR7.1 transgenic mice, which express 8–16 fold higher TLR7 mRNA than wildtype mice (13). To evaluate the contribution of BCAP to TLR7-driven lupus-like disease, we crossed the TLR7.1 transgene into the BCAP−/− background. TLR7.1/BCAP−/− mice developed significantly less severe lupus like-disease compared to TLR7.1 mice, with a significant reduction of splenomegaly and of CD11b+CD11c+ myeloid expansion in the blood of TLR7.1/BCAP−/− mice compared to TLR7.1 mice (Fig. 1A, Supplemental Fig. 1A-B)(13). We also found a reduced frequency of activated/effector CD44+CD62L- and recently activated CD69+ populations in both CD4+ (Fig. 1B, Supplemental Fig. 1C-D) and CD8+ T cell (Supplemental Fig. 1E) subsets in TLR7.1/BCAP−/− mice. We also observed a significant reduction in CD95+GL7+ germinal center B cells and CD23+CD21low follicular B cells in TLR7.1/BCAP−/− mice with an increase of CD23-CD21High marginal zone B cells (Fig. 1C, Supplemental Fig. 1F-G). TLR7.1/BCAP−/− mice produced significantly less IgM, IgG and IgG2c anti-RNA autoantibodies (Fig. 1D). As disease progresses, TLR7.1 mice develop immune complex deposition in the kidney (Fig. 1E) and mild kidney pathology (Supplemental Fig. 1H), which was reduced in TLR7.1/BCAP−/− mice. Overall, our data show that BCAP is a critical regulator of lupus-like disease progression in TLR7.1 mice.
Figure 1. BCAP promotes lupus-like disease in TLR7.1 mice.
Spleens from male and female 10–13 wk old mice were analyzed. (A) Spleen weight. (B) Percent of activated/effector CD4+TCRβ+ T cells (gated as CD44+CD62L-). (C) Percent of TCRβ-B220+ cells with a germinal center phenotype (gated as CD95+GL7+). (D) Serum anti-RNA antibodies of indicated isotypes. (E) Representative IgG staining of kidney sections with quantitation. (A-E) Each symbol represents an individual mouse (n=4–9 mice per group) from 3 independent experiments, mean +/− SEM are shown. * p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Kruskal Wallis with Dunn’s multiple comparison post test (A-D) or Mann-Whitney test (E).
BCAP regulates TLR-induced IFNα responses in vivo
Dysregulation of IFNα secretion in response to innate recognition of nuclear antigens contributes to SLE pathogenesis (1). The amount of type I IFN or IFN signature in the blood of SLE patients correlates with disease severity, pathogenic autoantibodies and clinical manifestations of disease. TLR7.1 mice have a type I IFN signature (13, 17), and TLR7.1 pDC constitutively express type I IFN mRNA (17). To determine a role for pDC in TLR7.1 mice, we used the BDCA2-DTR transgene (14) to deplete pDC from 5 week old TLR7.1/BDCA2-DTR+ mice using diphtheria toxin injection for 3 weeks. Transient pDC depletion reduced aspects of early lupus-like disease in TLR7.1 mice, including splenomegaly, early activated CD69+ CD4 and CD8 T cells, and effector/memory CD8 T cells (Supplemental Fig. 2), showing an important role for pDC early in TLR7.1 disease.
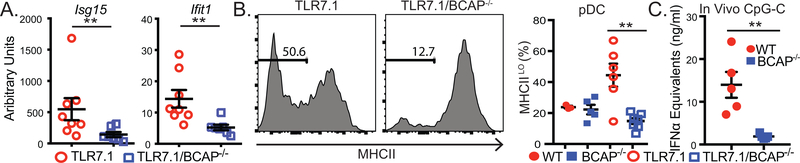
TLR7.1/BCAP−/− mice had reduced mRNA for the ISGs Ifit1 and Isg15 in the blood (Fig. 2A), suggesting BCAP may be important in pDC activation. We found that TLR7.1/BCAP−/− mice had a reduced population of MHCIILO pDC compared to TLR7.1 mice (Fig. 2B), a population that has been associated with IFNα production (18, 19), consistent with a role for BCAP in pDC function during chronic TLR7 signaling. To examine the role of BCAP in regulating TLR-driven IFNα production in vivo in a non-transgenic model, we injected WT and BCAP−/− mice with TLR9 agonists complexed with DOTAP, which induce IFNα in vivo in a pDC-dependent manner (20). BCAP−/− mice produced significantly less serum IFNα, but comparable quantities of IL-6, after injection of CpG-A and CpG-C (Fig. 2C, Supplemental Fig. 3A-B), consistent with a pDC IFNα defect. The decreased Ifit1 and Isg15 expression and decreased percentage of immature MHCIILO pDC in TLR7.1/BCAP−/− mice, and the reduced IFNα in serum after TLR9 agonist injection in BCAP−/− mice led us to hypothesize BCAP promotes TLR-induced IFNα in pDC
Figure 2. BCAP regulates IFNα responses in vivo.
(A) Isg15 and Ifit1 levels in whole blood measured by qPCR. (B) pDC were gated as CD11b-PDCA-1+CD11c+ cells. (C) Mice were injected with DOTAP-CpG-C mixture and serum was collected 3 hours post injection. Each symbol represents an individual mouse (n=3–8 per group) from 3 independent experiments, mean +/− SEM are shown. **p < 0.01, ***p < 0.001, (A) Mann-Whitney test, (B) Kruskal Wallis with Dunn’s multiple comparison post test, (C) Mann-Whitney test.
BCAP-deficient pDC produce less IFNα when stimulated via TLR7 and TLR9
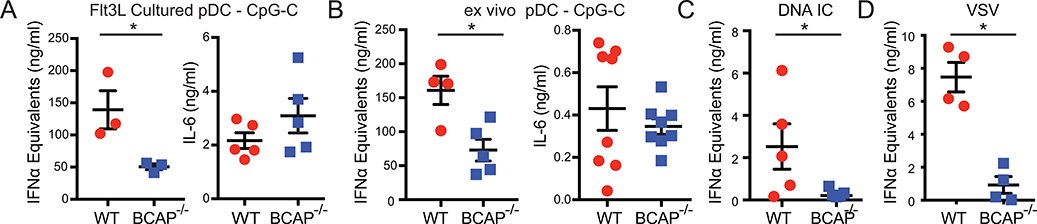
We confirmed that BCAP does not affect pDC development or homeostasis, because BCAP−/− mice had similar numbers and phenotype of pDC in the BM and spleen (Supplemental Fig. 3C). pDC generated from Flt3L-cultured BCAP−/− BM had similar frequency, but reduced total number compared to WT BM. To assess whether BCAP promotes TLR-induced IFNα production specifically in pDC, we first used pDC grown from BM with Flt3L. BCAP−/− pDC secreted significantly less IFNα in response to CpG-C and R848, but similar amounts of IL-6 (Fig. 3A, Supplemental Fig. 3D). Similar results were seen with pDC harvested directly ex vivo with CpG-C or CpG-A (Fig. 3B, Supplemental Fig. 3E). Thus, BCAP−/− pDC had an impaired ability to induce TLR9 and TLR7-driven IFNα, but not IL-6.
Figure 3. BCAP-deficient pDC produce less IFNα.
(A, C, D) pDC grown in Flt3L-containing media from whole BM and (B) mature pDC harvested directly ex vivo from BM were stimulated with CpG-C (A, B), DNA-containing IC (C), or VSV (D). IFNα was quantified by ISRE reporter assay and IL-6 was quantified by ELISA. Each symbol represents cells from an individual mouse (n=3–5 per group), mean +/− SEM are shown. Representative of 3 independent experiments. * p < 0.05, **p < 0.01, unpaired Student’s t-test (A, B), Mann-Whitney (C, D). In all cases IFNα and IL-6 secretion were not detectable from unstimulated pDC.
In SLE, immune complexes (IC) of autoantibodies and self-nucleic acids are internalized by Fc receptors to activate endosomal TLR signaling and IFNα secretion. Therefore, we stimulated purified pDC with DNA-containing IC. BCAP−/− pDC produced significantly less IFNα when stimulated with ICs (Fig. 3C). Similar results were obtained when pDC were stimulated with the RNA virus Vesicular stomatitis virus (VSV), an activator of TLR7 signaling (Fig. 3D). Thus, BCAP selectively promoted TLR-induced IFNα activation in pDC.
BCAP associates with DOCK2 and promotes Rac1 and IKKα activation
Nucleic acid-induced IFNα production in pDC requires two signaling pathways that converge upon IKKα activation. At the cell surface, nucleic acid recognition initiates a cascade leading to DOCK2-mediated activation of Rac1 that occurs at the plasma membrane and is independent of TLR nucleic acid binding (7, 21). In the endosome, internalized RNA or DNA is recognized by TLR7 or TLR9, respectively, leading to MyD88 activation. Both pathways are required for IKKα phosphorylation and subsequent IRF7 activation and IFNα production. DOCK2 activation of Rac1 and phosphorylation of IKKα are required for IFNα, but not inflammatory cytokine, production in pDC (7). Because BCAP−/− pDC had a similar phenotype to Dock2−/− pDC, we hypothesized BCAP regulates TLR-induced IFNα production through DOCK2 activation of Rac1. Importantly, BCAP−/− mice showed no evidence of described Dock2 genomic mutations (22) (not shown) and BCAP−/− pDC have normal DOCK2 expression (Supplemental Fig. 3F). Furthermore, our proteomics data in macrophages suggested a potential interaction between BCAP and DOCK2 (Ni and Hamerman, unpublished observations).
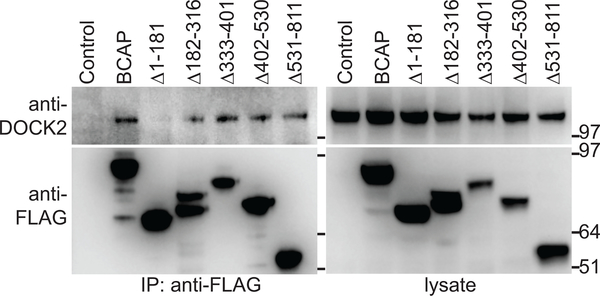
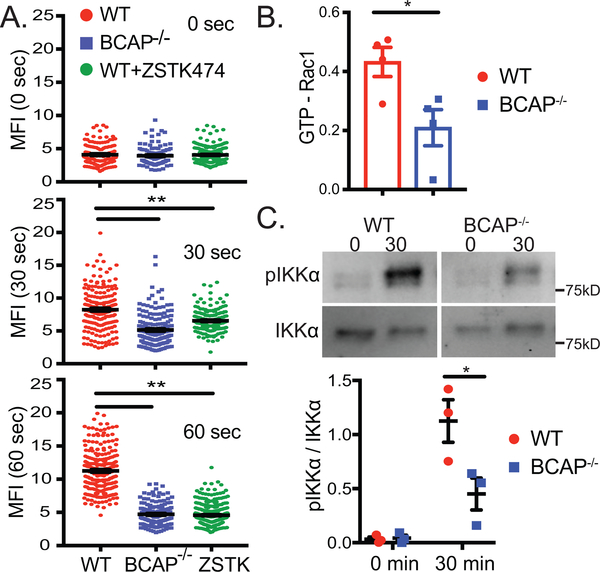
We confirmed that BCAP and DOCK2 interact using co-transfection studies and found that BCAP binds to DOCK2 through its N-terminal domain (Fig. 4, Supplemental Fig. 3G). In pDC, DOCK2 activates Rac1, a member of the Rho family GTPases that regulates cytoskeleton organization including actin polymerization (7). BCAP−/− pDC had reduced CpG-induced actin polymerization (Fig. 5A, Supplemental Fig. 3H), an indirect readout for Rac1 activation. Inhibiting class I PI3K in WT pDC phenocopied BCAP-deficiency. This result was not due to decreased DNA internalization as there was no difference in CpG uptake in WT vs BCAP−/− pDC (Supplemental Fig. 3I). We directly tested whether BCAP promotes Rac1 activation by quantifying the active GTP-bound form of Rac after CpG-C stimulation. BCAP−/− pDC had reduced CpG-induced Rac1 activation (Fig. 5B), showing that BCAP is upstream of CpG-induced Rac1 activation. Nucleic acid-induced IFNα production in pDC requires both DOCK2-mediated Rac1 activation and MyD88 activation for the phosphorylation and activation of IKKα. Consistent with reduced Rac1 activation, BCAP−/− pDC had reduced IKKα phosphorylation in response to CpG-C stimulation (Fig. 5C).
Figure 4. BCAP associates with DOCK2 using the N-terminal domain.
(A) 293T cells were transfected with DOCK2 and BCAP-FLAG or BCAP-FLAG mutants as indicated. Lysates were immunoprecipitated with anti-FLAG Ab and blotted for DOCK2 or FLAG. Schematic of mutants is shown in Supplemental Fig. 3G.
Figure 5. BCAP promotes actin polymerization, Rac1 activation and IKKα phosphorylation.
pDC grown from BM in Flt3L were sorted and stimulated with CpG-C. (A) Actin polymerization was visualized by phalloidin staining followed by confocal microscopy and quantification of MFI per cell. Each symbol represents an individual cell analyzed in one experiment (n=100–200 per group) and is representative of 3 experiments. Where indicated, pDC were pre-treated with ZSTK474 for 30 min before CpG-C. (B) Quantification of Rac1 activation measured by GTP-bound Rac1. Each symbol represents an independent experiment (n=4), and mean +/− SEM is shown. (C) Phosphorylation of IKKα was quantified by western blot, panels shown are from the same exposure cropped to exclude other treatment groups. Representative blot and quantification of 3 independent experiments is shown as mean +/− SEM. *p < 0.05,**p < 0.01, one-way ANOVA with Tukey's posttest (A), unpaired Student’s t-test (B and C).
Discussion
Here, we show BCAP promotes TLR7-driven lupus-like disease in vivo and TLR-induced IFNα production in pDC. We found that TLR7.1/BCAP−/− mice have reduced disease, but whether this alleviation of disease is dependent specifically on expression of BCAP in pDC, or in other cells such as B cells, activated T cells, or other myeloid cells requires further investigation (11, 23, 24). There is reason to believe the role of BCAP in promoting pDC IFNα expression could be significant for disease reduction. First, we show here that transient pDC depletion ameliorated TLR7.1 disease. Second, others have shown protection from or reduced severity of lupus-like disease in various mouse models either by acutely or constitutively reducing pDC number or diminishing the capacity of pDC to induce TLR7/9 induced IFNα (3–5). Third, all B cell responses are not lacking in TLR7.1/BCAP−/− mice consistent with prior reports (25, 26). TLR7.1/BCAP−/− mice have more germinal center B cells than WT mice without the TLR7.1 transgene, and TLR7.1/BCAP−/− mice produce anti-RNA IgM and IgG, suggesting their B cell-T cell interactions are at least partially intact. Together, this suggests that disease reduction in TLR7.1/BCAP−/− mice is attributed to something more than just a B cell defect. Our results point to a reduction of pDC IFNα production as one part of the protection of TLR7.1/BCAP−/− mice from lupus-like disease. However, we do not rule out a contribution of other pDC functions as well as defects in other innate or adaptive immune cells that express BCAP (9, 10, 12, 23–27).
We found that BCAP promotes TLR-induced IFNα production in pDC and that BCAP−/− pDC have reduced F-actin polymerization, Rac1 activation, and phosphorylation of IKKα, and IFNα secretion upon stimulation with nucleic acids. We propose that BCAP regulates IFNα production upstream of DOCK2-Rac1 signaling, which are independent of nucleic acid engagement of TLR7 and TLR9 (7) (Supplemental Fig. 4). The mechanism by which nucleic acid recognition activates DOCK2 and Rac1 remains unknown. Receptor for advanced glycation end-products (RAGE) can bind and internalize nucleic acids for delivery to TLR7/9 containing endosomes, and can activate Rac1, thus RAGE may be one receptor activating this pathway (28–30). The mechanism by which Rac1 leads to IKKα activation also remains undefined, however there is evidence that Rac-mediated production of ROS may control IKKα phosphorylation (31). Additionally, ROS and the PI3K product PIP3 are required for conjugation of LC3 to LC3-associated phagosomes (LAPosomes). In macrophages after nucleic acid stimulation, LC3 is recruited to LAPosomes to anchor IKKα and bring the kinase to IRF7, where it is phosphorylated and translocated into the nucleus to transcribe type I IFN genes (32). We propose that BCAP, both through direct binding to DOCK2 and through induction of class I PI3K activity, participates in activation of DOCK2 and Rac1, and subsequent downstream signals leading to IKKα phosphorylation. This is supported by studies showing CpG DNA-induced PI3K activation occurs independently of nucleic acid engagement of TLR (33).Further studies are required to determine if BCAP mediates ROS production via Rac1 upon nucleic acid recognition in pDC, and any potential role for BCAP in parts of this signaling pathway downstream of MyD88, which were not investigated in this study.
There is increasing evidence that pDC secretion of IFNα contributes to pathogenesis of systemic autoimmune diseases such as SLE and Sjogren’s disease (1). Understanding the molecular mechanisms that regulate IFNα production while leaving the NF-kB dependent inflammatory cytokine response intact could reveal novel ways to selectively target the IFNα pathway, this could prove to be significant for maintaining antigen presentation and regulatory aspects of pDC.
Supplementary Material
Key Points.
TLR7-driven lupus-like disease is ameliorated in the absence of BCAP.
BCAP promotes TLR7/9-induced IFNα, but not IL-6, in pDC.
BCAP associates with DOCK2 and promotes CpG DNA-induced Rac1 and IKKα activation.
Acknowledgements
We thank A. Marshak-Rothstein for IC reagents, D. Campbell for VSV, D. Stetson for L929-ISRE cells, K. Campbell and T. Kurosaki for BCAP−/− mice, vivarium staff, flow cytometry and histology core at Benaroya Research Institute for support, and J.M Duggan and M. Acharya for knowledge and insights.
This work was supported by NIH grants F31AI120505 (T.C.), T32 AI10667 (T.C.), GM095421 (T.C.), R21AR068679 (J.A.H), R01AI113325 (J.A.H), R56AI132808 (J.A.H) and R01AI124693 (to J.A.H).
References
- 1.Elkon KB, and Wiedeman A. 2012. Type I IFN system in the development and manifestations of SLE: Current Opinion in Rheumatology 24: 499–505. [DOI] [PubMed] [Google Scholar]
- 2.Swiecki M, and Colonna M. 2015. The multifaceted biology of plasmacytoid dendritic cells. Nature Reviews Immunology 15: 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, Beutler B, and Theofilopoulos AN. 2013. Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus. Proceedings of the National Academy of Sciences 110: 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D’Agati V, Elkon KB, and Reizis B. 2014. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med 211: 1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, and Colonna M. 2014. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. The Journal of Experimental Medicine 211: 1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino K, Sugiyama T, Matsumoto M, Tanaka T, Saito M, Hemmi H, Ohara O, Akira S, and Kaisho T. 2006. IκB kinase-α is critical for interferon-α production induced by Toll-like receptors 7 and 9. Nature 440: 949–953. [DOI] [PubMed] [Google Scholar]
- 7.Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, Ishikawa T, Hoshino K, Uruno T, Cao Q, Higashi S, Kawaguchi Y, Enjoji M, Takayanagi R, Kaisho T, Yoshikai Y, and Fukui Y. 2010. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. The Journal of Experimental Medicine 207: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guiducci C, Ghirelli C, Marloie-Provost M-A, Matray T, Coffman RL, Liu Y-J, Barrat FJ, and Soumelis V. 2008. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. The Journal of Experimental Medicine 205: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni M, MacFarlane AW, Toft M, Lowell CA, Campbell KS, and Hamerman JA. 2012. B-cell adaptor for PI3K (BCAP) negatively regulates Toll-like receptor signaling through activation of PI3K. Proceedings of the National Academy of Sciences 109: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troutman TD, Hu W, Fulenchek S, Yamazaki T, Kurosaki T, Bazan JF, and Pasare C. 2012. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc Natl Acad Sci U S A 109: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki T, Takeda K, Gotoh K, Takeshima H, Akira S, and Kurosaki T. 2002. Essential Immunoregulatory Role for BCAP in B Cell Development and Function. Journal of Experimental Medicine 195: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacFarlane AW, Yamazaki T, Fang M, Sigal LJ, Kurosaki T, and Campbell KS. 2008. Enhanced NK-cell development and function in BCAP-deficient mice. Blood 112: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, and Bolland S. 2007. Control of Toll-like Receptor 7 Expression Is Essential to Restrict Autoimmunity and Dendritic Cell Proliferation. Immunity 27: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swiecki M, Gilfillan S, Vermi W, Wang Y, and Colonna M. 2010. Plasmacytoid Dendritic Cell Ablation Impacts Early Interferon Responses and Antiviral NK and CD8+ T Cell Accrual. Immunity 33: 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giltiay NV, Chappell CP, Sun X, Kolhatkar N, Teal TH, Wiedeman AE, Kim J, Tanaka L, Buechler MB, Hamerman JA, Imanishi-Kari T, Clark EA, and Elkon KB. 2013. Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. Journal of Experimental Medicine 210: 2773–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda K, Richez C, Uccellini MB, Richards RJ, Bonegio RG, Akira S, Monestier M, Corley RB, Viglianti GA, Marshak-Rothstein A, and Rifkin IR. 2009. Requirement for DNA CpG Content in TLR9-Dependent Dendritic Cell Activation Induced by DNA-Containing Immune Complexes. The Journal of Immunology 183: 3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buechler MB, Teal TH, Elkon KB, and Hamerman JA. 2013. Type I IFN drives emergency myelopoiesis and peripheral myeloid expansion during chronic Toll-like receptor 7 signaling. J Immunol 190: 886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanning SL, George TC, Feng D, Feldman SB, Megjugorac NJ, Izaguirre AG, and Fitzgerald-Bocarsly P. 2006. Receptor Cross-Linking on Human Plasmacytoid Dendritic Cells Leads to the Regulation of IFN-α Production. The Journal of Immunology 177: 5829–5839. [DOI] [PubMed] [Google Scholar]
- 19.Grouard G, Rissoan M-C, Filgueira L, Durand I, Banchereau J, and Liu Y-J. 1997. The Enigmatic Plasmacytoid T Cells Develop into Dendritic Cells with Interleukin (IL)-3 and CD40-Ligand. The Journal of Experimental Medicine 185: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, and Colonna M. 2011. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. Journal of Experimental Medicine 208: 2367–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanjuan MA, Rao N, Lai K-TA, Gu Y, Sun S, Fuchs A, Fung-Leung W-P, Colonna M, and Karlsson L. 2006. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol 172: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purtha WE, Swiecki M, Colonna M, Diamond MS, and Bhattacharya D. 2012. Spontaneous mutation of the Dock2 gene in Irf5−/− mice complicates interpretation of type I interferon production and antibody responses. Proc Natl Acad Sci U S A 109: E898–E904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deason K, Troutman TD, Jain A, Challa DK, Mandraju R, Brewer T, Ward ES, and Pasare C. 2018. BCAP links IL-1R to the PI3K–mTOR pathway and regulates pathogenic Th17 cell differentiation. Journal of Experimental Medicine 215: 2413–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh MD, Ni M, Sullivan JM, Hamerman JA, and Campbell DJ. 2018. B cell adaptor for PI3-kinase (BCAP) modulates CD8+ effector and memory T cell differentiation. Journal of Experimental Medicine 215: 2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamazaki T, and Kurosaki T. 2003. Contribution of BCAP to maintenance of mature B cells through c-Rel. Nature Immunology 4: 780–786. [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Maeda A, Iwamatsu A, Gotoh K, and Kurosaki T. 2000. BCAP: The Tyrosine Kinase Substrate that Connects B Cell Receptor to Phosphoinositide 3-Kinase Activation. Immunity 13: 817–827. [DOI] [PubMed] [Google Scholar]
- 27.Duggan JM, Buechler MB, Olson RM, Hohl TM, and Hamerman JA. 2017. BCAP inhibits proliferation and differentiation of myeloid progenitors in the steady state and during demand situations. Blood 129: 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson BI, Kalea AZ, del M. Arriero M, Harja E, Boulanger E, D’Agati V, and Schmidt AM. 2008. Interaction of the RAGE Cytoplasmic Domain with Diaphanous-1 Is Required for Ligand-stimulated Cellular Migration through Activation of Rac1 and Cdc42. J. Biol. Chem 283: 34457–34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertheloot D, Naumovski AL, Langhoff P, Horvath GL, Jin T, Xiao TS, Garbi N, Agrawal S, Kolbeck R, and Latz E. 2016. RAGE Enhances TLR Responses through Binding and Internalization of RNA. The Journal of Immunology 197: 4118–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sirois CM, Jin T, Miller AL, Bertheloot D, Nakamura H, Horvath GL, Mian A, Jiang J, Schrum J, Bossaller L, Pelka K, Garbi N, Brewah Y, Tian J, Chang C, Chowdhury PS, Sims GP, Kolbeck R, Coyle AJ, Humbles AA, Xiao TS, and Latz E. 2013. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. The Journal of Experimental Medicine 210: 2447–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, and Engelhardt JF. 2006. Interleukin-1β Induction of NFκB Is Partially Regulated by H2O2-mediated Activation of NFκB-inducing Kinase. J. Biol. Chem 281: 1495–1505. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi K, Taura M, and Iwasaki A. 2018. The interaction between IKKα and LC3 promotes type I interferon production through the TLR9-containing LAPosome. Sci. Signal 11: eaan4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma C, Muranyi M, Chu CH, Zhang J, and Chu W-M. 2013. Involvement of DNA-PKcs in the IL-6 and IL-12 Response to CpG-ODN Is Mediated by Its Interaction with TRAF6 in Dendritic Cells. PLOS ONE 8: e58072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floc’h AL, Tanaka Y, Bantilan NS, Voisinne G, Altan-Bonnet G, Fukui Y, and Huse M. 2013. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. Journal of Experimental Medicine 210: 2721–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe M, Terasawa M, Miyano K, Yanagihara T, Uruno T, Sanematsu F, Nishikimi A, Côté J-F, Sumimoto H, and Fukui Y. 2014. DOCK2 and DOCK5 Act Additively in Neutrophils To Regulate Chemotaxis, Superoxide Production, and Extracellular Trap Formation. The Journal of Immunology 193: 5660–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.