Abstract
Striatal dopamine strongly regulates how individuals use time to guide behavior. Dopamine acts on D1-and D2-dopamine receptors in the striatum. However, the relative role of these receptors in the temporal control of behavior is unclear. To assess this, we trained rats on a task in which they decided to start and stop a series of responses based on the passage of time and evaluated how blocking D1 or D2-dopamine receptors in the dorsomedial or dorsolateral striatum impacted performance. D2 blockade delayed the decision to start and stop responding in both regions, and this effect was larger in the dorsomedial striatum. By contrast, dorsomedial D1 blockade delayed stop times, without significantly delaying start times, whereas dorsolateral D1 blockade produced no detectable effects. These findings suggest that striatal dopamine may tune decision thresholds during timing tasks. Furthermore, our data indicate that the dorsomedial striatum plays a key role in temporal control, which may be useful for localizing neural circuits that mediate the temporal control of action.
Keywords: Striatum, dopamine, interval timing, time perception, decision making, decision threshold
1. Introduction
Controlling behavior based on the passage of time is critical for engaging in optimal decision-making strategies [1]. Despite its importance, how the brain represents time is largely unknown. Several lines of evidence suggest that dopamine in the striatum is crucial for timing [2]. For example, Parkinson’s disease, which depletes striatal dopamine, frequently causes timing impairments [3]. Consistent with this, lesioning or optogenetically manipulating the substantia nigra pars compacta — the primary source of striatal dopamine —profoundly impacts performance during timing tasks [4,5]. Furthermore, transient over-expression of striatal D2 receptors disrupts timing behavior [6].
These findings provide a foundation for our understanding of striatal dopamine’s role in timing. However, two questions remain open. First, dopamine tunes striatal output by modulating distinct pools of neurons that express either D1-or D2-type dopamine receptors. These subpopulations have distinct downstream targets and are thought to play differential roles in behavior, yet their relative roles in timing have not been assessed. Second, whether the dorsomedial and dorsolateral striatum play different roles in timing, as they do in action-selection or goal-directed vs. habit-based behavior remains unclear [7,8].
We addressed these questions by training rats on a timing task referred to as the ‘peak-interval procedure’. During this task, subjects are presented with a cue that indicates reward can be earned for responding after a specified duration elapses (e.g., pressing a lever after 6 seconds have passed). Probe trials are also included in which the cue remains on much longer than normal and no reward is given. When averaged across trials, probe trial responding resembles a Gaussian distribution. The peak of the response distribution (i.e., peak-time) provides a general measure of overall timing accuracy, and the spread gives an estimate of timing precision [9]. However, during individual probe trials, rats emit an abrupt burst of responses that clusters around the time of reward. The times at which rats decide to start and stop emitting responses during single trials can be measured, giving a more detailed depiction of timing processes [10].
Once rats were trained, we infused either a D1 or D2 antagonist into the dorsomedial or dorsolateral striatum. We tested two predictions. First, systemic pharmacological data suggest that D2 receptors play a more prominent role in time-keeping than D1 receptors [11]. Therefore, we expected a larger impact of striatal D2 blockade on performance. Second, association cortices regulate timing (e.g., parietal [12], frontal [13]) and project to the dorsomedial striatum in rodents. Therefore, we predicted that D1 and D2 blockade would have larger effects in the dorsomedial striatum, relative to the dorsolateral striatum.
Experimental procedures were carried out in accordance with the University of Iowa’s Institutional Animal Care and Use Committee guidelines and the National Institutes of Health guide for the care and use of laboratory animals. Twenty-one Male Long-Evans rats— approximately 3 months of age at the start of the experiment—served as subjects. Rats were placed on modest water restriction to increase motivation, receiving approximately 10ml of water per day. Training and testing took place in standard operant conditioning chambers (Med Associates), described in detail previously [13].
Rats were initially trained on the peak interval procedure, which consisted of three phases. First, rats were trained to insert their snout into a nosepoke in order to receive water reward (4 sessions), delivered through a dispenser located on the opposite wall of the chamber. Any snout-insertion resulted in water delivery (0.17 ml). Sessions lasted 1.5 hours or until rats received 60 rewards. During the second ‘fixed-interval (FI) training’ phase (8 sessions), trials began with the illumination of a houselight located above the nosepoke. Reward delivery occurred following the first response after the cue had been present for 6s, whereas responses prior to this time had no consequence. When a rewarded response occurred, the cue terminated. Trials were followed by a dark, 30–45 second inter-trial interval (uniformly distributed), after which the next trial began. ‘Peak-interval training’ (14 sessions) was identical to FI training, except that non-reinforced probe trials were interspersed with FI trials. Similar to FI trials, probe trials began with the illumination of the houselight. However, reward was not delivered and the cue terminated after 18–24 seconds passed (uniformly distributed), independent of responding. Probe trials initially comprised 25% of trials within a session (8 sessions). Then, we increased this proportion to 50% (6 sessions).
After training, we implanted cannulae bilaterally into the dorsomedial (n = 10) or dorsolateral striatum (n = 11). Anesthesia was initiated with isofluorane and maintained with ketamine (100mg/kg) and xylazine (10mg/kg). Surgeries began by retracting the scalp and leveling the skull between bregma and lambda. Craniotomies were placed over the surgical coordinates, and four screws were inserted along the edges of the skull. Cannulae were then lowered into either the dorsomedial (from bregma: AP +0.0, ML +/−2.4, DV −4.2, relative to skull surface) or dorsolateral (AP +0.7, ML +/−3.6, DV −4.4) striatum. Our coordinates for the dorsolateral striatum matched previous work [14,15]. The dorsomedial coordinate was slightly more posterior in order to target the central portion of the dorsomedial striatum [8,13,15]. Craniotomies were sealed with cyanoacrylate (SloZap, Pacer Technologies), accelerated by ZipKicker (Pacer Technologies), and then further secured with methyl methacrylate (AM Systems).
Following 1 week of post-surgical recovery, rats were retrained for 7 sessions before receiving drug infusions. Rats were extensively acclimated to infusions under hand restraint, which took place in a 76cm X 51cm X 36cm plexiglass container. Following the first retraining session, rats were placed in the container for one minute, without restraint. After the second and third sessions, rats were restrained by hand for 3 and 5 minutes, respectively. For the fourth and fifth sessions, rats were restrained for 5 minutes prior to beginning each session. Before the sixth session, rats underwent a mock infusion. No restraint was given during the seventh session, as all critical infusions sessions were always preceded by normal behavioral recovery days. During infusion sessions, rats were infused bilaterally with 0.5 µl of either saline, a D1-antagonist (SCH-23390; 1 µg / µl), or a D2-antagonist (Sulpiride; 1 µg / µl) via an infusion cannula (randomized order). Infusions were conducted at a rate of 0.5 µl / min. Once complete, the infusion cannula was left in place for 1 minute to allow for diffusion. Sessions began 30 minutes following all infusions. We conducted one session for each drug type, and infusion sessions contained both rewarded and probe trials, to make them indistinguishable from normal training sessions.
Data analysis was conducted by grouping responses during probe trials into 1s bins. Peak times were identified by fitting a Gaussian + Kurtosis parameter function to average probe-trial response rates [16]:
Y0 is the baseline; S is a scaling factor; PT is the mean (i.e., peak-time); SD is the standard deviation; and K is the kurtosis parameter.
Peak-time estimates are usually emphasized when using this task. Traditionally, they are assumed to approximate the temporal expectation associated with a cue, providing an estimate of timing accuracy. However, shifts in peak-times can be driven by selective effects on start or stop times on a single-trial basis, making them a coarse measure of timing function [17]. Therefore, to fully evaluate the nature of any peak time shifts we observed, we also identified start and stop times during individual probe trials using ‘single-trials analysis’, described in detail previously [10]. This method identifies start and stop times by fitting three flat lines (first low, second high, and third low) to individual probe trial response rates, minimizing absolute residuals. The left and right edges of the higher line are taken as the start and stop time, respectively. Peak, start, and stop times for each drug-type and area were compared to saline sessions using mixed-model ANOVAs. Area (dorsomedial vs. dorsolateral striatum) served as a between subject variable, and Drug (saline vs. D1/D2 antagonist) served as a within subject variable. Planned-comparisons were conducted using paired-or independent-samples t-tests.
Following experiments, rats were sacrificed with pentobarbital (100mg/kg), and perfused transcardially with 4% paraformaldehyde. Brains were post-fixed with 4% paraformaldehyde for 24 hours, and then, transferred to a 20% sucrose solution for a minimum of 24 hours. Brains were then sliced, mounted on gelatin-subbed slides, and stained using Cresyl violet. Cannula placement was confirmed via stereological microscopy. Cannula placements for all rats are depicted in Figure 1.
Figure 1:
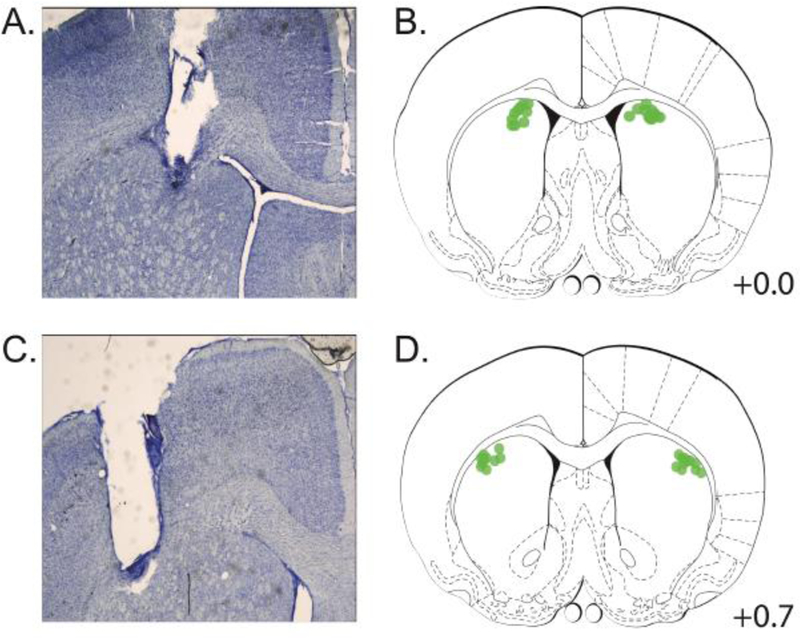
Cannula location. A) Nissl-stained coronal section showing cannula placement in the dorsomedial striatum (right hemisphere). B) Cannula placements (in green) from all rats in the dorsomedial striatum group (n = 10). C) Nissl-stained coronal section showing cannula placement in dorsolateral striatum (right hemisphere). D) Cannula placements for all rats in the dorsolateral striatum group (n = 11).
Average probe trial response rates during infusion sessions in the dorsomedial and dorsolateral striatum are plotted in Figure 2. In the dorsomedial striatum, D1 blockade appeared to selectively delay when rats stopped responding during probe trials. In contrast, this effect appears to be absent in the dorsolateral striatum. Consistent with this, an ANOVA revealed no main effects or interactions for either area on either peak [Saline: M = 7.8 +/−0.3s SEM, D1 blockade: M = 8.9 +/−0.6s SEM; Fs(1,19) < 3.6] or start times [Fs(1,19) < 3.4]. Importantly, when stop times were evaluated, we found a significant Drug X Area interaction [F(1,19) = 5.67 p < 0.05], as D1 blockade significantly delayed stop times in the dorsomedial [t(9) = 2.51, p < 0.05], but not dorsolateral [t(9) = 0.64], striatum.
Figure 2: D2 blockade affects start and stop times, and D1 blockade affects stop times in the dorsomedial striatum.
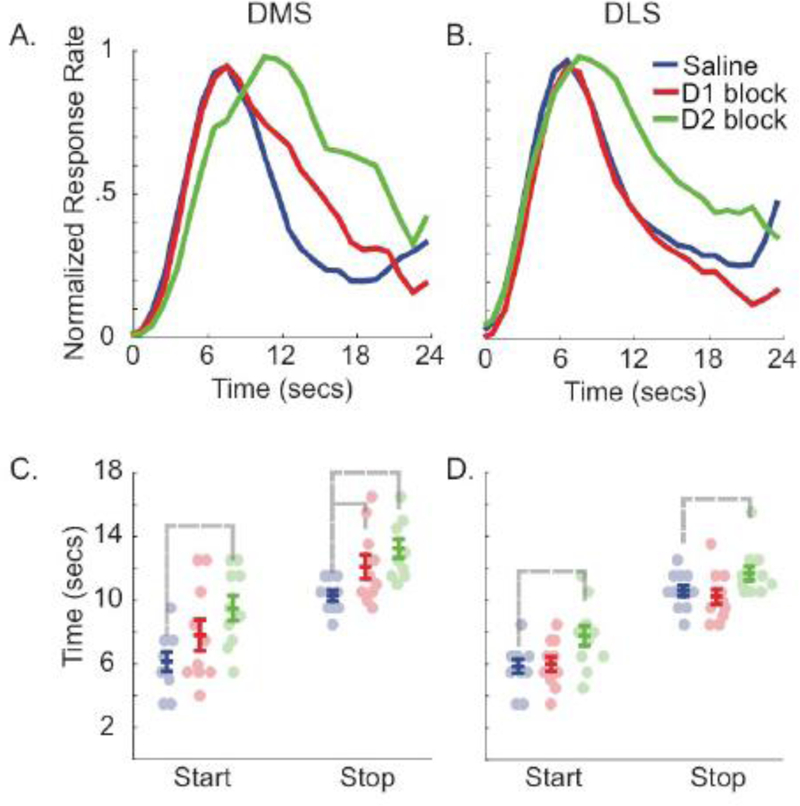
A and B show normalized probe trial response rate during infusion sessions in the dorsomedial and dorsolateral striatum, respectively. C and D show start and stop times obtained via single-trial analysis in the dorsomedial striatum (n = 10) and dorsolateral striatum (n = 11), respectively. Circles indicate individual subject medians; colored lines show mean ± standard error for each infusion type; and gray lines indicate significant differences (p < 0.05).
Unlike D1 blockade, infusing a D2 antagonist in both the dorsomedial and dorsolateral striatum appeared to delay when rats started and stopped responding during probe trials. However, these effects appear to be larger in the dorsomedial striatum. This was confirmed by analyzing start and stop times. Analysis of start times revealed a significant main effect of Drug [F(1,19) = 77.68, p < 0.001]. Importantly, while start times were rightward shifted in both areas [dorsomedial striatum: t(9) = 6.77, p < 0.001, dorsolateral: t(10) = 5.47, p < 0.001], the effect size was larger in the dorsomedial striatum [Drug X Area, F(1,19) = 5.83, p < 0.03]. Stop times showed a similar pattern, with a main effect of Drug [F(1,19) = 36.41, p < 0.001], due to a rightward shift in both areas [dorsomedial striatum: t(9) = 5.57, p < .001, dorsolateral: t(10) = 2.60, p < 0.03], with this effect being larger in the dorsomedial striatum [Drug X Area, F(1,19) = 7.70, p < 0.02]. However, analysis of peak times only revealed a main effect of Drug [Saline: M = 7.8 +/−0.3s SEM, D2 blockade: M = 11.45 +/−0.68s SEM; F(1,19) = 30.74, p < 0.001], driven by an overall rightward shift across both areas [Drug X Area interaction, F(1,19) < 1.20].
To summarize, blocking D1 receptors in the dorsomedial striatum significantly delayed the decision to stop responding, yet produced no apparent effects in the dorsolateral striatum. By contrast, blocking D2 receptors delayed start and stop times in both regions, yet to a larger extent in the dorsomedial striatum. Neither drug flattened the response distribution during probe trials, suggesting temporal discrimination, per se, was not disrupted as seen following striatal lesions [4]. Rather, D1 and D2 blockade appeared to modulate components of the timing system. These data help elucidate the role of striatal dopamine role in timing.
First, similar to our results, systemic injections of D2 antagonists often delay start and stop times during the peak-interval procedure [18]. Traditionally, this is interpreted as D2 blockade slowing internal ‘clock-speed’, causing subjects to respond later than normal as a result [19]. This explanation may account for our D2 blockade results. However, it is more difficult to apply this interpretation to the apparent selective effect of D1 blockade on stop times, as clock-speed effects should delay when subjects both start and stop responding [17]. While it is possible that D1 blockade produced in non-linear clock-speed effect that increasingly slowed the clock as time progressed, such an effect has not been documented to our knowledge.
The null effect of D1 blockade on start times may not necessarily imply a lack of an effect, as there may be sub-threshold shifts that we were unable to detect. However, if the effect is indeed selective for stop times alone, it would open the alternative explanation that striatal dopamine tunes decision thresholds that control when to start and stop responding during probe trials. Under this account, D1 blockade would selectively increase the stop threshold, thereby delaying when rats stop responding, yet leaving start times unaffected. In contrast, D2 blockade could have increased start and stop thresholds, rather than clock speed. Alternatively, D2 blockade could have increased the start threshold alone, which may have—by default—delayed stop times.
This explanation would be consistent with broader models of decision-making that postulate the striatum sets decision thresholds in a variety of contexts [20]. For example, using a non-timing task, Jin and Costa [21] recently found a subpopulation of striatal neurons that selectively fired around the decision to initiate and terminate a series of responses.
A decision-threshold account also aligns with more recent work on the relationship between dopamine and timing. Specifically, increasing dopamine levels via systemic agonist injections [22] or genetic manipulations [23] often causes animals to start responding earlier without impacting stop times. Conversely, decreasing dopamine levels delays start times, yet still leaves stop times unaffected [17]. These asymmetric effects on start and stop times are more consistent with a decision-threshold interpretation than one based on clock-speed, and our results suggest they may be due to dorsostriatal dopamine levels specifically.
Some attribute these effects to dopamine’s impact on motivation. Specifically, higher and lower dopamine levels tend to increase and decrease motivation, respectively. Correspondingly, manipulations that increase motivation (e.g., food deprivation) cause subjects to start responding earlier in anticipation of reward, whereas manipulations that decrease motivation (e.g., pre-feeding, reward devaluation, etc.) produce the opposite effect [for review see 17]. Typically, neither of these manipulations affect stop times. These results suggest that dopamine impacts timing performance via interactions between motivational and timing processes [17].
This motivational account is consistent with some aspects of our data (see supplemental information). For example, dopamine blockade decreased response rates in many cases, indicating a decrease in motivation. Furthermore, we observed improved temporal precision in start and stop times following both D1 and D2 blockade, even in cases where reliable shifts in timing measures were not present (e.g., start times under D1 blockade). This is potentially consistent with a decrease in noisy or impulsive responses that are known to contaminate single-trial data [24]. The main problem with this interpretation is that, as noted above, motivational manipulations typically shift start times alone, which is at odds with D1 blockade’s potentially selective effect on stop times and D2 blockade’s effect on both start and stop times. Nonetheless, further evaluating whether striatal dopamine impacts performance by producing interactions between motivation and timing is a critical direction for future work.
Our results extend findings indicating that the dorsomedial and dorsolateral striatum play distinct roles in behavior. Neuroanatomically, these areas primarily differ with respect to the cortical input they receive, with association cortices projecting to the dorsomedial striatum and sensorimotor cortices project to the dorsolateral striatum. Most findings suggest that rodent association cortices, such as the medial frontal cortex, play a prominent role in timing [13]. These results extend this work by showing that D2 blockade produced stronger effects in the dorsomedial striatum, and D1 blockade only showed significant effects in this area.
However, prior work has shown that the dorsolateral striatum mediates well-rehearsed, ‘habit-based’ behaviors [14]. Some research has shown that performance during the peak-interval procedure can become habit-based, although this appears to take much longer than normal tasks (e.g., > 120 sessions [25]). Nonetheless, future work could evaluate whether the effects of dorsolateral dopamine blockade grow as a function of training.
These findings open several other avenues for future work on the role of striatal dopamine in timing. For example, we only evaluated a single dose of each drug in the present study. Obtaining a full dose-response curve might reveal further differences or similarities between D1-and D2-blockade. Second, pharmacological agents can be non-specific and work on the timescale of hours. More selective and temporally specific methods could be used, such as optogenetic targeting of D1 vs. D2 striatal neurons. Finally, we only used a single interval in the present work. This helped maximize the amount of data we were able to collect during a single session, but makes it difficult to fully dissociate timing from non-timing related effects. For instance, D2-blockade may have produced a motor delay, which would account for the rightward shift in start/stop times we observed in both areas of the striatum. D1-blockade’s potentially selective effect on stop times would be more difficult to explain as a purely motoric effect. Nonetheless, this effect could have been due to perseveration.
We have two recommendations to help further rule out these accounts. The first is to run the same task, yet using two cues that are associated with distinct intervals (e.g., cue 1 −6s / cue 2–18s), using either a within-or between-subjects design. Timing effects typically produce proportional shifts in behavioral measures (e.g., 20% shifts in start times, regardless of a cue’s duration [23]). Therefore, if dopamine-blockade impacts timing, one would expect that start and/or stop times would shift in proportion to the interval being timed. In contrast, motor-delays or perseveration would be more likely to produce absolute shifts in timing-measures (e.g., a 1s delay in start times, regardless of the cue’s interval).
Second, a temporal bisection task might address motor confounds. The bisection task requires subjects to categorize the length of a cue as short or long. As subjects make a single ‘choice’ in this task, it would be more difficult to explain performance effects using motor or perseverative explanations. However, it would be more difficult to assess decision-threshold vs. clock-speed effects with this procedure alone [5].
To summarize, we found that D2 blockade in both striatal regions increased start and stop times, particularly in the dorsomedial striatum, whereas D1 blockade delayed stop times only in the dorsomedial striatum. These effects are most consistent with striatal dopamine modulating decision thresholds. This parallels recent work using systemic dopaminergic manipulations. Our findings indicate that the locus of these effects may lie in the dorsal striatum, with dopamine in the dorsomedial region being particularly critical for the temporal control of action.
Supplementary Material
Highlights.
Striatal dopamine modulates how behavior is guided by the passage of time
Dopamine tunes striatal output via D1 and D2 type dopamine receptors
We show that striatal D1 vs. D2 receptor blockade differentially impacts timing behavior
Effects were larger in the dorsomedial striatum, relative to the dorsolateral striatum
Acknowledgments
This work was supported by the National Institutes of Health [R01MH116043 awarded to NSN, F31 NS106737 to BJD, and T32-NS007421 awarded to BJD], the Alfred P. Sloan foundation (scholarship awarded to BJD), and a Kwak-Ferguson fellowship awarded to BJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors have no competing interests to declare
References
- [1].Gür E, Balcı F, Mice optimize timed decisions about probabilistic outcomes under deadlines, Anim. Cogn 20 (2017) 473–484. [DOI] [PubMed] [Google Scholar]
- [2].Howard CD, Li H, Geddes CE, Jin X, Dynamic Nigrostriatal Dopamine Biases Action Selection, Neuron 93 (2017) 1436–1450.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gu BM, Jurkowski A, Malapani C, Lake J, Meck W, in: Vatakis A, Allman M (Eds.), Time distortions in mind: Temporal processing in clinical populations, Bayesian Models of Interval Timing and Distortions in Temporal Memory as a Function of Parkinson’s Disease and Dopamine-Related Error Processing, Brill, 2015. [Google Scholar]
- [4].Meck WH, Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems, Brain Res 1109 (2006) 93–107. [DOI] [PubMed] [Google Scholar]
- [5].Soares S, Atallah BV, Paton JJ, Midbrain dopamine neurons control judgment of time, Science 354 (2016) 1273–1277. [DOI] [PubMed] [Google Scholar]
- [6].Drew M, Simpson E, Kellendonk C, Herzberg W, Lipatova O, Fairhurst S, Kandel E, C. Operant Motivation and Interval Timing, J. Neurosci 27 (2007) 7731–7739. Operant Motivation and Interval Timing, J. Neurosci. 27 (2007) 7731–7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ito M, Doya K, Distinct neural representation in the dorsolateral, dorsomedial, and ventral parts of the striatum during fixed-and free-choice tasks, J. Neurosci. Off. J. Soc. Neurosci 35 (2015) 3499–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin HH, Ostlund SB, Knowlton BJ, Balleine BW, The role of the dorsomedial striatum in instrumental conditioning, Eur. J. Neurosci 22 (2005) 513–523. [DOI] [PubMed] [Google Scholar]
- [9].Matthews AR, He OH, Buhusi M, Buhusi CV, Dissociation of the role of the prelimbic cortex in interval timing and resource allocation: beneficial effect of norepinephrine and dopamine reuptake inhibitor nomifensine on anxiety-inducing distraction, Front. Integr. Neurosci 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Church RM, Meck WH, Gibbon J, Application of scalar timing theory to individual trials, J. Exp. Psychol. Anim. Behav. Process 20 (1994) 135–155. [DOI] [PubMed] [Google Scholar]
- [11].Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD, Effects of dopamine antagonists on the timing of two intervals, Pharmacol. Biochem. Behav 75 (2003) 9–15. [DOI] [PubMed] [Google Scholar]
- [12].Jazayeri M, Shadlen M, A Neural Mechanism for Sensing and Reproducing a Time Interval, Curr. Biol 25 (2015) 2599–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Emmons EB, Corte BJD, Kim Y, Parker KL, Matell MS, Narayanan NS, Rodent medial frontal control of temporal processing in the dorsomedial striatum, J. Neurosci (2017) 1376–17. [DOI] [PMC free article] [PubMed]
- [14].Yin HH, Knowlton BJ, Balleine BW, Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning, Eur. J. Neurosci 19 (2004) 181–189. [DOI] [PubMed] [Google Scholar]
- [15].Stalnaker TA, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G, Neural Correlates of Stimulus–Response and Response–Outcome Associations in Dorsolateral Versus Dorsomedial Striatum, Front. Integr. Neurosci 4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matell MS, De Corte B, Kerrigan T, DeLussey CM, Temporal Averaging in Response to Change, Timing Amp Time Percept 4 (2016) 223–247. [Google Scholar]
- [17].Balcı F, Interval Timing, Dopamine, and Motivation, Timing Amp Time Percept 2 (2014) 379–410. [Google Scholar]
- [18].MacDonald CJ, Meck WH, Differential effects of clozapine and haloperidol on interval timing in the supraseconds range, Psychopharmacology (Berl.) 182 (2005) 232–244. [DOI] [PubMed] [Google Scholar]
- [19].Meck WH, Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock, Pharmacol. Biochem. Behav 25 (1986) 1185–1189. [DOI] [PubMed] [Google Scholar]
- [20].Lo CC, Wang XJ, Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks, Nat. Neurosci 9 (2006) 956–63. [DOI] [PubMed] [Google Scholar]
- [21].Jin X, Costa RM, Start/Stop Signals Emerge in Nigrostriatal Circuits during Sequence Learning, Nature 466 (2010) 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Taylor KM, Horvitz JC, Balsam PD, Amphetamine affects the start of responding in the peak interval timing task, Behav. Processes 74 (2007) 168–175. [DOI] [PubMed] [Google Scholar]
- [23].Balci F, Ludvig EA, Abner R, Zhuang X, Poon P, Brunner D, Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice, Brain Res 1325 (2010) 89–99. [DOI] [PubMed] [Google Scholar]
- [24].Matell MS, Portugal GS, Impulsive responding on the peak-interval procedure, Behav. Processes 74 (2007) 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cheng R-K, Hakak OL, Meck WH, Habit formation and the loss of control of an internal clock: inverse relationship between the level of baseline training and the clock-speed enhancing effects of methamphetamine, Psychopharmacology (Berl.) 193 (2007) 351–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


