Abstract
Background
Galectin-3 (Gal-3) has been associated with heart failure (HF) and poor cardiovascular outcomes. However, the effect of longitudinal changes in Gal-3 on clinical outcomes remains unclear.
Objectives
We sought to study clinical determinants of change in Gal-3 among communitydwelling individuals. Further, we sought to examine the role of serial Gal-3 measurements in predicting risk of future HF, cardiovascular disease (CVD) and mortality.
Methods
A total of 2,477 participants of the Framingham Heart Study Offspring cohort underwent measurement of plasma Gal-3 levels at two exams (1995-1998 and 2005-2008). Linear regression was used to examine clinical correlates of change in Gal-3. Proportional hazards models were used to relate future clinical outcomes with change in Gal-3.
Results
The following clinical correlates were associated with greater longitudinal increases in Gal-3 levels: age, female gender, hypertension, diabetes, body-mass index, interim development of chronic kidney disease and HF (P<0.0001 for all in multivariable- model). Change in Gal-3 was associated with future HF (HR 1.39 per 1-SD increase, 95%CI 1.13-1.71), CVD (HR 1.29, 95%CI 1.11-1.51), and all-cause mortality (HR 1.30 95%CI 1.17-1.46). Change in Gal-3 was associated with both HF with preserved as well as reduced ejection fraction (P<0.05 for both).
Conclusions
Longitudinal changes in Gal-3 are associated with traditional cardiovascular risk factors and renal disease. In turn, change in Gal-3 predicts future HF, CVD, and mortality in the community. Future studies are needed to determine whether serial Gal-3 measures may be useful in disease prevention.
Keywords: Galectin-3, biomarker, change in Galectin-3, heart failure, cardiovascular disease, mortality
CONDENSED ABSTRACT:
Galectin-3 (Gal-3), a marker of cardiac fibrosis, is associated with incident heart failure (HF) and poor cardiovascular outcomes. We aimed to study clinical determinants of longitudinal change in Gal-3 and its effect on cardiovascular outcomes. We demonstrated that interim development of chronic kidney disease and heart failure as well as traditional cardiovascular risk factors predicted rise in Gal-3 over time. Further, longitudinal increase in Gal-3 levels was associated with significantly higher risk of developing HF, cardiovascular disease and mortality in the community. These findings highlight the potential role of serial Gal-3 measurements in predicting cardiovascular risk.
Introduction
Galectin-3 (Gal-3), a chimera-type β-galactoside-binding lectin, is an emerging biomarker of heart failure (HF) (1,2). Expressed by activated macrophages and other cells during myocardial stress, Gal-3 plays an important regulatory role in inflammation and fibrosis (2). Gal-3 binding sites are mainly located in myocardial extracellular matrix and cardiac fibroblast, where it induces fibroblast proliferation, collagen deposition and cardiac remodeling (3). In animal models, overexpression of myocardial Gal-3 during early pre-clinical stages of heart failure has been well documented (3,4). Further, intrapericardial infusion of recombinant Gal-3 in healthy animals promotes cardiac fibrosis and induces heart failure (3,4), while genetic disruption or pharmacological inhibition of Gal-3 prevents heart failure to develop in mice and rats (5). In human studies, higher levels of Gal-3 are associated with all-cause and cardiovascular mortality in patients with heart failure as well as in general population (6-9). In addition, circulating Gal-3 predicts incident HF after acute coronary syndrome in the community (10-13).
Despite relatively low biological variation (14), plasma Gal-3 levels do change over time (15) and Gal-3 trajectory might be independent of baseline levels (16). Systolic blood pressure and urinary excretion of albumin were shown to predict temporal changes in Gal-3 levels (17). Prior studies have shown that serial measurements of Gal-3 provides incremental prognostic information in patients with existing HF (8,18,19). Further, in a recent study, individuals with persistently elevated plasma Gal-3 concentrations had a significantly higher incidence of HF over an 8-year follow-up, with comparable signals for cardiovascular mortality, new onset atrial fibrillation (AF) and cardiovascular events, suggesting a role for repeated measurements of circulating Gal-3 (16).
The exact triggers that modulate upregulation of plasma Gal-3 levels remain unclear. In the present study, we aimed to investigate clinical predictors of longitudinal change in Gal-3 levels over the course of 10 years among participants of the Framingham Heart Study. We also sought to examine the effect of longitudinal changes in Gal-3 levels on clinical outcomes, including incident HF, cardiovascular disease (CVD), and all-cause mortality. We postulated that change in Gal-3 over time would be informative with respect to risk of of new-onset HF, CVD and all-cause mortality.
Methods
Study sample
The design and enrollment of the Framingham Heart Study (FHS) Offspring cohort has been described (20). In brief, in 1971, 5124 individuals consisting of children and spouses of the children of the FHS original cohort, were enrolled into a prospective, observational, community-based cohort, called FHS Offspring. Participants who attended two separate exams and had Gal-3 assayed at both timepoints were included in this analysis (earlier exam: 1995-1998, later exam: 2005-2008). Of 3532 attendees at the earlier exam, those with off-site exams (n = 120), missing Gal-3 (n = 55), outlier Gal-3 levels (n = 8), missing covariates (n = 198), and non-attendance at the later exam (n = 674: 380 deceased and 294 alive but did not attend later exam or did not have Gal-3 measured) were excluded, resulting in a final cohort of 2477 individuals. The Institutional Review Board at Boston University Medical Center approved the study protocols, and all participants provided written informed consent.
Gal-3 measurement
Plasma samples were collected after an overnight fast at each exam cycle, immediately centrifuged and stored at −80°C. Gal-3 concentrations were measured using an enzyme-linked immunosorbent assay (BG Medicine, Waltham, Massachusetts). Stability of Gal-3 measurement using enzyme-linked immunosorbent assay has been described previously (21-23). The assay shows consistent performance across lots. The respective lower and upper detection limits were 1.32 and 96.6 ng/ml for both exams. Respective within-run and total coefficients of variation were 2.1-5.7% and 4.2-12.0% for the earlier exam measurements, and 1.9-5.6% and 3.9-11.3% for the later exam measurements.
Clinical covariate
A thorough clinical assessment was performed at each exam cycle which included medical history and physical examination. Blood pressures were expressed as the average of two separate physician-obtained measurements. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medication(s). Body mass index (BMI) was calculated using weight and height at each exam cycle (kg/m2). Diabetes mellitus was defined as fasting glucose ≥126 mg/dl, random non-fasting blood glucose ≥200 mg/dl, use of oral hypoglycemic medication(s) or insulin, or prior history of diabetes. Participants smoking cigarettes regularly during the prior year for each exam were considered current smokers. Total and high-density lipoprotein (HLD) cholesterol levels were measured. Glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (24). Chronic kidney disease (CKD) was defined as eGFR ≤30 mL/min/1.73 m2. Left ventricular hypertrophy (LVH) was considered present if there was electrocardiographic evidence of LVH with strain. AF was diagnosed if there was evidence of atrial flutter or AF after reviewing all the available electrocardiograms or Holter monitor readings. B-type natriuretic peptide (BNP) levels were measured using high-sensitivity, noncompetitive immunoradiometric assays (Shionogi) (25).
Ascertainment of clinical endpoints
Participants of the FHS cohort undergo close surveillance for development of CVD and HF. Data obtained from medical records, physician visits and hospitalization are reviewed and new events are confirmed by a panel consisting of 3 FHS physicians, and are documented and updated in the surveillance datasets. FHS criteria were used to define heart failure (26). CVD was defined as myocardial infarction, coronary insufficiency (prolonged angina with documented ECG changes), coronary heart disease death, HF, or stroke. Interim events were considered new events that happened between the earlier and later exams (i.e., from 1995-1998 to 2005-2008). Incident events were defined as new events that happened after the later exam (i.e., from 2005-2008 onward).
Statistical analysis
Gal-3 concentrations were natural log-transformed due to skewed distribution. Change in Gal-3 between the earlier and later exams was calculated as log(Gal-3) at the later exam minus log(Gal-3) at the earlier exam. Baseline characteristics at each exam were summarized across quartiles of the change in Gal-3.
To identify clinical correlates of longitudinal change in Gal-3 (dependent variable), we employed linear regression models. We first assessed individual correlates in linear regression models adjusting for age, sex, and baseline levels of Gal-3 at the earlier exam. We then employed stepwise selection including all the correlates, forcing in age, sex and Gal-3 at the earlier exam. We included these clinical correlates ascertained at the earlier exam: systolic blood pressure, current use of antihypertensive medications, diabetes mellitus, BMI, high-density lipoproteins (HDL) to total cholesterol ratio, smoking, LVH, eGFR, prevalent CKD, CVD, and HF. We ran stepwise selection with and without interim development of CKD, CVD, and HF between the earlier and later exams.
To examine the effect of longitudinal change in Gal-3 levels on clinical outcomes, we used proportional hazard (Cox) regression models. In the primary analysis, we examined whether incident HF occurring after the later exam was associated with change in Gal-3 and. In secondary analyses, we examined associations with CVD and mortality. We also modeled change in Gal-3 by quartiles; furthermore, we dichotomized Gal-3 levels [at the clinical cut-off of 17.8 ng/ml (8)] to study whether outcomes were associated with persistently low (low-low), persistently high (high-high), progression from low to high levels (low-high), or progression from high to low levels (high-low). We used 2 models: a) adjusting for age, sex, and baseline levels of Gal-3; and b) adusting for age, sex, baseline levels of Gal-3, systolic blood pressure, current use of antihypertensive medications, diabetes mellitus, BMI, HDL to total cholesterol ratio, smoking, LVH, eGFR, prevalent CVD (except for the CVD models), and prevalent HF. Individuals with prevalent CVD or HF before the later exam were excluded from CVD and HF models respectively. In secondary analyses, we further adjusted for BNP in multivariable models.
Kaplan-Meier curves were used to depict the relation of change in Gal-3 by quartiles and by classes of change on clinical outcomes. In exploratory analyses, we examined change in Gal-3 levels vis a vis HF subtypes—including HF with preserved ejection fraction (HFpEF, defined as left ventricular ejection fraction > = 50%) and HF with reduced ejection fraction (HFrEF, left ventricular ejection fraction <50%). A 2-sided P-Value of <0.05 was deemed significant. All analyses were performed using SAS version 9.4 for Windows.
Results
Baseline characteristics
A total of 2,477 participants (mean age of 57 ± 9 years, 55% women) were included in our study. Gal-3 levels changed over time in the majority of participants, with >30% showing a >20% change from baseline. When examined using the dichotomous clinical cut-off of 17.8 ng/ml, we found that 6% of the participants changed from low to high and 6% from high to low Gal-3 levels. By contrast, 82% remained persistently low and 5% persistently high. Baseline characteristics of participants by quartiles of change in Gal-3 are summarized in Table 1. Participants with greater increase in Gal-3 were older at baseline, had higher systolic blood pressure, were more likely to be diabetic, and had greater BMI (p-value < 0.05 for all). In addition, participants with prevalent CVD or CKD had a higher increase in Gal-3 levels at the following exam (p-values 0.004 and 0.005, respectively).
Table 1.
Characteristics of participants at earlier exam by quartiles of longitudinal change in galectin-3
| Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
Total | P-value* | |
|---|---|---|---|---|---|---|
| n=619 | n=619 | n=620 | n=619 | n=2477 | ||
| Age, years | 55±8 | 56±8 | 57±9 | 59±9 | 57±9 | <0.0001 |
| Female | 330 (53) | 341 (55) | 351 (57) | 338 (55) | 1360 (55) | 0.70 |
| Systolic blood pressure, mmHg | 122±16 | 125±17 | 128±18 | 131±20 | 126±18 | <0.0001 |
| Antihypertensive treatment | 108 (18) | 117 (19) | 168 (27) | 197 (32) | 590 (24) | <0.0001 |
| Diabetes mellitus | 24 (4) | 27 (4) | 46 (7) | 81 (13) | 178 (7) | <0.0001 |
| Body mass index, kg/m2 | 27±5 | 27±5 | 28±5 | 29±5 | 28±5 | <0.0001 |
| Cholesterol, mg/dL | 208±38 | 206±37 | 205±38 | 206±3 8 | 206±105 | 0.12 |
| HDL, mg/dL | 52±17 | 52±16 | 53±16 | 51±16 | 52±16 | 0.50 |
| Smoking | 79 (13) | 100 (16) | 79 (13) | 95±15 | 353 (14) | 0.19 |
| Left ventricular hypertrophy | 1 (0.2) | 1 (0.2) | 2 (0.3) | 3 (0.5) | 7 (0.3) | 0.76 |
| eGFR, ml/min/1.73m2 | 98±146 | 92±49 | 91±39 | 89±38 | 92±82 | 0.31 |
| Prevalent CKD | 24 (4) | 29 (5) | 48 (8) | 46 (7) | 147 (6) | 0.005 |
| Prevalent CVD | 14 (2) | 19 (3) | 19 (3) | 36 (6) | 88 (4) | 0.004 |
| Prevalent HF | 2 (0.3) | 1 (0.2) | 0 (0) | 3 (0.5) | 6 (0.2) | 0.34 |
| Prevalent AF | 10 (2) | 7 (1) | 8 (1) | 15 (2) | 40 (2) | 0.27 |
Data shown are means±Standard Deviation for continuous variable and N (%) for binary variables Median (25th, 75th percentiles) for Gal-3 levels for each quartile were: Quartile 1: −3.4 (−4.4,−2.7) ng/ml, Quartile 2: −1.5 (−2.0,−1.1) ng/ml, Quartile 3: 0 (−0.4,0.4) ng/ml, Quartile 4: 2.4 (1.5,4) ng/ml, Total: −0.8 (−2.3,0.9) ng/ml.
HDL, high-density lipoprotein; eGFR, estimated glumerular filtration rate; CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure; AF, atrial fibrillation
P-values derive from statistical tests of equal means (or proportions) across quartiles using analysis of variance (ANOVA) for continuous variables and chi-squared test for categorical characteristics.
Clinical correlates of longitudinal change in Gal-3
In linear regression models adjusted for age, sex, and baseline Gal-3 levels, we found that older age, female sex, and lower baseline levels of Gal-3 were significantly associated with greater increases in Gal-3 over a 10-year period (Table 2). Traditional cardiovascular risk factors were similarly associated with greater increases in Gal-3 over time, including systolic blood pressure, anti-hypertensive use, diabetes, BMI, and smoking. Baseline CVD and CKD were significantly associated with greater increase in Gal-3, whereas prevalent HF was not. In a stepwise selection model, the following clinical covariates remained independent predictors of change in Gal-3: age, female sex, systolic blood pressure, antihypertensive medication use, diabetes, BMI, HDL to cholesterol ratio, eGFR, prevalent CKD and prevalent CVD (Table 2, model R-Square = 0.16).
Table 2.
Clinical correlates of longitudinal change in galectin-3 (n = 2477)
| Age, sex, baseline Gal-3-
adjusted model |
Multivariable- adjusted step-
wise model† |
|||||
|---|---|---|---|---|---|---|
| Trait | β* | SE | P-Value | β | SE | P-Value |
| Age | 0.0065 | 0.0005 | <.0001 | 0.0034 | 0.0005 | <0.0001 |
| Female | 0.0239 | 0.0084 | 0.0046 | 0.0423 | 0.0082 | <0.0001 |
| Systolic blood pressure | 0.0357 | 0.0045 | <.0001 | 0.0212 | 0.0045 | <0.0001 |
| Antihypertensive treatment | 0.0815 | 0.0100 | <.0001 | 0.0384 | 0.0103 | <0.0001 |
| Diabetes | 0.1226 | 0.0160 | <.0001 | 0.0679 | 0.0162 | <0.0001 |
| Body mass index | 0.0335 | 0.0042 | <.0001 | 0.0209 | 0.0042 | <0.0001 |
| HDL to cholesterol ratio | 0.0057 | 0.0029 | <0.0001 | |||
| Smoking | 0.0341 | 0.0119 | 0.0041 | |||
| Left ventricular hypertrophy | 0.0095 | 0.0777 | 0.90 | |||
| Glomerular filtration rate | −0.0039 | 0.0041 | 0.34 | |||
| Prevalent CKD | 0.0438 | 0.0179 | 0.01 | |||
| Prevalent CVD | 0.0767 | 0.0225 | 0.0007 | |||
| Prevalent HF | −0.0303 | 0.0838 | 0.71 | |||
| Interim development of CKD | 0.1945 | 0.0152 | <0.0001 | 0.1692 | 0.0149 | <0.0001 |
| Interim development of CVD | 0.1279 | 0.0201 | <0.0001 | |||
| Interim development of HF | 0.2457 | 0.0329 | <0.0001 | 0.1634 | 0.0338 | <0.0001 |
Estimated β coeficient represents the change in log-Gal3 between exam 1 and 2 in the presence of the trait at exam 1 (dichotomous variables) or per 1 standard deviation of the trait at exam 1 (continuous variables)
Step-wise selection forced age, sex, and baseline galectin-3 in the model and selected from all remaining variables listed in Table 2.
HDL, high-density lipoprotein; CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure
We examined interim events between the earlier and the later examinations, including 39 individuals who developed HF, 108 individuals who developed CVD and 198 who developed CKD. We found that the interim development of CHF and CKD was significantly associated with 17% and 19% increase in Gal-3 levels respectively (β±SE 0.16±0.01, p-value<0.0001 for CKD, and β±SE 0.16±0.03, p-values<0.0001 for HF). Interim development of CVD did not predict change in Gal-3 (p-value<0.05).
Change in Gal-3 over time is associated with incident cardiovascular disease
Heart failure
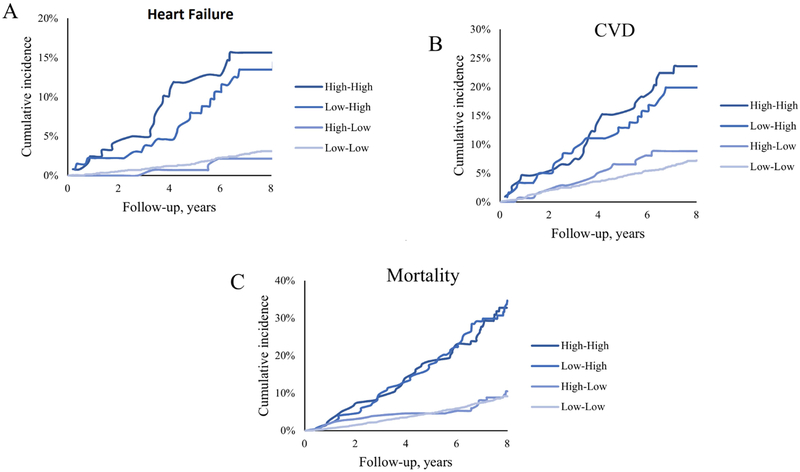
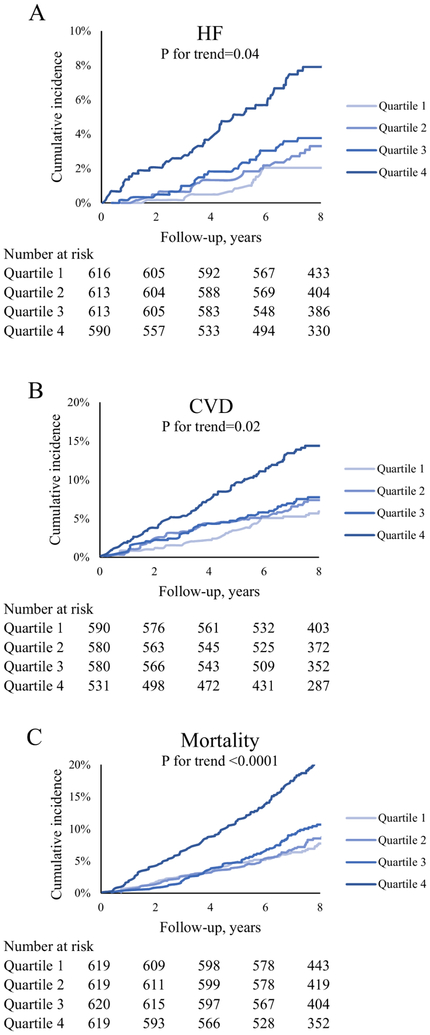
Of 2432 individuals free of HF at the later exam, 108 developed incident HF over a mean follow-up of 7.8 years. Whereas baseline Gal-3 was significantly associated with incident HF, longitudinal change in Gal-3 was significantly predictive of HF regardless of baseline Gal-3 levels (p-value <0.05) (Table 3; Online Table 1). In age and sex- adjusted models, a one standard devition (SD) longitudinal increase in log-Gal-3 was associated with 60% increase in risk of HF (HR (95% CI): 1.60 (1.34-1.93), p-value <0.0001) (Table 3). This association remained significant after adjusting for other risk factors of HF (p-value=0.0021). Further, longitudinal change in log-Gal-3 remained predictive of HF even after adjustment for baseline BNP levels (Online Table 2). Figure 1A depicts the cumulative incidence of HF across quartiles of change in Gal-3. A greater longitudinal increase in Gal-3 was associated with greater incidence of HF (p for trend 0.04 across quartiles) (Online Table 3). Individuals with persistently high levels of Gal-3 had 2.3 times higher risk of developing HF compared to those with persistently low levels (HR (95%CI): 2.28 (1.28-4.05), p-value = 0.005) (Online Table 4, Online Figure 1C; Figure 2A). Further, those with progression from low levels of Gal-3 at the earlier exam to high levels at the later exam (low-high) showed 82% increased risk of developing HF (HR (95%CI): 1.82 (1.03-3.22), p-value=0.02). By contrast, those who went from high to low Gal-3 levels on subsequent exam had a HR of 0.47, 95%CI: 0.15-1.51, p-value 0.21.
Table 3.
Association between incident outcomes, baseline galectin-3 and longitudinal change in galectin-3
| Baseline Gal-3 |
Change in Gal-3 |
|||
|---|---|---|---|---|
| Outcome/Model | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Incident heart failure | ||||
| Age, sex, baseline Gal-3- adjusted | 1.36 (1.10 - 1.68) | 0.0037 | 1.60 (1.34-1.93) | <0.0001 |
| Multivariable- adjusted* | 1.26 (1.00 - 1.59) | 0.048 | 1.39 (1.13-1.71) | 0.0021 |
| Incident cardiovascular disease | ||||
| Age, sex, baseline Gal-3- adjusted | 1.29 (1.12 - 1.50) | 0.0006 | 1.40 (1.29-1.61) | <0.0001 |
| Multivariable- adjusted* | 1.20 (1.02 - 1.41) | 0.0257 | 1.29 (1.11-1.51) | 0.001 |
| Mortality | ||||
| Age, sex, baseline Gal-3- adjusted | 1.38 (1.23 - 1.55) | <0.0001 | 1.38 (1.25-1.52) | <0.0001 |
| Multivariable- adjusted* | 1.42 (1.25 - 1.60) | <0.0001 | 1.30 (1.17 - 1.46) | <0.0001 |
Multivariable models adjusted for baseline galectin-3 levels, age, sex, systolic blood pressure, antihypertensive treatment, diabetes, body mass index, smoking, left ventricular hypertrophy, high-density lipoprotein (HDL) to cholesterol ratio, estimated glomerular filtration rate, prevalent cardiovascular disease (except for CVD model). Mortality analysis also adjusted for prevalent heart failure.
Hazard ratio and 95% confidence intervals for 1 standard deviation (SD) increase in log-Gal3 levels over a 10-year period. This corresponds to 3.3 ng/ml change in Gal-3.
Figure 1. Cumulative incidence of HF, CVD, and mortality by quartiles of longitudinal change in galectin-3.
Longitudinal rise in Gal-3 levels was associated with significantly higher risk of developing incident HF (A), incident CVD (B), and all-cause mortality (C). CVD = cardiovascular disease, HF = heart failure.
Figure 2. Cumulative incidence of HF, CVD, and mortality by classes of longitudinal change in galectin-3.
Persistently high levels of Gal-3 were associated with significantly higher risk of incident HF (A), incident CVD (B), and all-cause mortality (C). High-high, low-high, high-low, and low-low represent classes of change in Gal-3 between the earlier exam and the later exam using the clinical cut-off of 17.8 ng/ml. CVD = cardiovascular disease, HF = heart failure.
Heart failure subtypes
A total of 63 individuals developed HFpEF, and 38 developed HFrEF, with 7 unclassified HF cases. Longitudinal change in Gal-3 predicted both HFpEF and HFrEF (Table 4). Among patients with HFpEF, 6.3% had previous MI and 15.9% had major CVD prior to the incident HF event. By contrast, among patients with HFrEF, 10.5% had previous MI and 18.4% had antecedent major CVD. In age and sex- adjusted models, a 1-SD longitudinal increase in Gal-3 was associated with a 1.59-fold increased hazard of HFpEF, and a 1.73-fold increased risk of HFrEF (HR (95%CI): 1.59 (1.18-2.13), p-value 0.0022 and 1.73 (1.36-2.19), p-value<0.0001 for HFpEF and HFrEF, respectively). The results remained significant in multivariable adjusted models (p<0.05).
Table 4.
Association between incident HF subtype and longitudinal change in galectin-3
| HFpEF (63 events) |
HFrEF (38 events) |
|||
|---|---|---|---|---|
| Model | HR (95% CI) † | P-value | HR (95% CI) | P-value |
| Age, sex, baseline Gal-3- adjusted | 1.59 (1.18 - 2.13) | 0.0022 | 1.73 (1.36 - 2.19) | <0.0001 |
| Multivariable- adjusted* | 1.39 (1.05 - 1.84) | 0.0212 | 1.42 (1.02 - 1.96) | 0.03 |
Multivariable models adjusted for baseline galectin-3 levels, age, sex, systolic blood pressure, antihypertensive treatment, diabetes, body mass index, smoking, left ventricular hypertrophy, high-density lipoprotein (HDL) to cholesterol ratio, estimated glomerular filtration rate, prevalent cardiovascular disease (except for CVD model). Mortality analysis also adjusted for prevalent heart failure.
Hazard ratio and 95% confidence intervals for 1 standard devitaion (SD) increase in log-Gal3 levels over a 10-year period. This corresponds to 3.3 ng/ml change in Gal-3.
Cardiovascular disease
Of 2,281 individuals free of CVD at the later exam, 213 developed incident CVD over a mean follow-up of 7.7 years. One SD longitudinal increase in log-Gal3 was associated with 40% (p-value <0.0001) and 29% (p-value=0.001) increase in risk of CVD in age and sex- adjusted and multivariable- adjusted models respectively. Change in log-Gal3 predicted CVD even after further adjustment for baseline BNP levels (Online Table 2). Risk of incident CVD significantly increased across quartiles of change in Gal-3 (Figure 1B, p for trend = 0.02). Individuals in the fourth quartile (mean Gal-3 increase of 2.4 ng/ml) showed a 74% higher risk of developing CVD (HR (95%CI): 1.74 (1.12-2.71), p-value = 0.01). Participants with persistently high levels of Gal-3 (high-high) had an 80% higher risk of developing CVD compared with those with persistently low levels (HR (95% CI): 1.80 (1.13-2.86), p-value = 0.013) (Figure 2B). Though not significant, progression of Gal-3 levels from low to high levels was associated with 43% incease in CVD risk (HR (95%CI): 1.43 (0.89-2.28). The opposite was seen for individuals with high Gal-3 levels at the earlier exam and low Gal-3 levels at the later exam (HR (95%CI): 0.86 (0.47-1.57).
All-cause mortality
During a mean follow up of 7.9 years, 348 deaths were reported. Longitudinal change in Gal-3 was associated with higher mortality in both age/sex- and multivariable- adjusted models (HR (95%CI): 1.38 (1.25-1.52) and 1.30 (1.17-1.46) respectively, both p-values <0.0001). These results remained similar after further adjustment in baseline BNP levels (Online Table 2). The fourth quartile of the change in Gal-3 was associated with 94% increase in risk of death (p-value = 0.0002). Figure 1C demonstrates the Kaplan-Meier curves for mortality rate. Individuals with persistently high levels of Gal-3 (high-high) had over two-fold increased rate of mortality as compared to those with persistently low levels (HR 2.25, 95% CI (1.61 to 3.15, p-value <0.0001) (Figure 2C).
Discussion
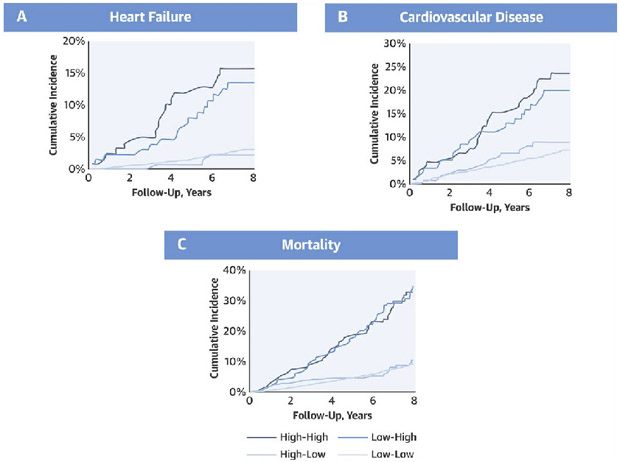
Our main study findings are 3-fold. First, while traditional cardiovascular risk factors including older age, hypertension, diabetes, and BMI are associated with rise in Gal-3 over time, the largest increases in Gal-3 occur with intervening events including the development of CKD and HF (Central Illustration). Second, we demonstrate that longitudinal change in Gal-3 above and beyond a single baseline measure is independently associated with the development of future HF, CVD, and all-cause mortality. Specifically, participants who had both persistently high Gal-3 levels, and those who had low baseline and high subsequent levels of Gal-3 had an over 2-fold increased hazard of future HF compared with individuals with persistently low Gal-3 levels. Third, we demonstrate that change in Gal-3 predicts both HFpEF and HFrEF. These data highlight the potential role of serial Gal-3 measurements in informing cardiovascular risk.
Central Illustration. Cumulative incidence of HF, CVD, and mortality by classes of longitudinal change in Galectin-3.
Persistently high levels of Gal-3 were associated with significantly higher risk of incident HF (A), incident CVD (B), and all-cause mortality (C). High-high, low-high, high-low, and low-low represent classes of change in Gal-3 between the earlier exam and the later exam using the clinical cut-off of 17.8 ng/ml. CVD = cardiovascular disease, HF = heart failure.
Clinical correlates of change in Gal-3 in our study are in keeping with two prior studies examining temporal changes in Gal-3 levels: Among patients with existing HF, Anand, et al found that lower baseline Gal-3 levels, lower eGFR, interim decrease in eGFR, female sex, and higher BMI were associated with greater increases in Gal-3 levels at 4 months (19). Similarly, in the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) study, investigators showed that in general population, systolic blood pressure >170 mmHg and urinary albumin excretion >30 mg/24 h were independent predictors of dynamic increase in Gal-3 levels across a time span of 9 years (17). We validate hypertension as a predictor, but extend these findings by showing that diabetes and obesity are also associated with a rise in Gal-3 over the span of about a decade. Interestingly, we found that women in the community have greater increases in Gal-3 over time compared with men. This is consistent with findings among women and men with HF (19). Whether Gal-3 potentially underlies some of the sex-differences observed in HF remains unclear.
Aside from traditional cardiovascular risk factors, we show that interim development of CKD and HF strongly influences change in Gal-3. Previous studies have demonstrated that baseline Gal-3 measures predict incident CKD in the general population independent of HF (27,28). Potential mechanisms could be aldosterone-induced vascular fibrosis mediated by Gal-3 as well as Gal-3 role in atherogenesis (29). In turn, we now show that the interim development of CKD further compounds future increases in Gal-3. Similarly, we found that interim development of HF was associated with subsequent increases in Gal-3. In our study, prevalent HF did not predict longitudinal change in Gal-3 given only 6 cases of prevalent HF. Further, prevalent (but not interim) CVD predicted change in Gal-3.
With respect to future outcomes, a single Gal-3 measurement has been shown to predict prognosis both among patients with existing HF and also development of future HF among those in the community (8,12). We now show that serial Gal-3 measures are informative above and beyond a single baseline measurement. Specifically, a steeper rise in Gal-3 over 10 years was independently associated with greater risk of future HF as well as CVD even after accounting for baseline Gal-3. Interestingly, when using the clinical cut-off of 17.8 ng/mL, we found that participants with persistently high levels of Gal-3 on serial exams (high-high) and those with progression of Gal-3 from low to high levels (low-high) showed a significantly greater risk of developing future heart failure. By contrast, participants with initial high levels and subsequent decline to low Gal-3 levels (high-low) showed no increased risk of future HF. Similar findings were previously reported in PREVEND, though associations were attenuated after adjusting for potential clinical confounders in the previous study (16). Finally, we demonstrate that the association of longitudinal change in Gal-3 and future HF extends to both HFpEF and HFrEF. Taken together, these findings highlight the added value of repeated measurement of Gal-3 in identification of individuals at risk for development of HF and CVD in the community.
The mechanism underlying the association of Gal-3 and HF may be multi-fold. First, traditional cardiovascular and metabolic risk factors may confound this association as they are both associated with Gal-3 trajectory and clinical outcomes (12,30). However, we show independent associations of change in Gal-3 and outcomes even after accounting for potential confounders. Increased Gal-3 expression has been shown to be an independent determinant of cardiac inflammation and fibrosis in experimental studies (2,31). Upregulation of Gal-3 in animal models is present prior to development of overt HF and leads to cardiac fibroblast activation and extracellular matrix deposition, and hence cardiac remodeling (2). Our study findings suggest that Gal-3 may contribute to the development of future HF in humans. Whether targeting the Gal-3 pathway may have clinical benefits with respect to disease prevention remains unknown (32).
Study limitations
Several limitations deserve mention. FHS participants are predominantly white, potentially limiting the generalizability of our findings to broader populations, particularly in light of known racial differences in the utility of Gal-3 and HF risk prediction (33). Gal-3 levels were ascertained at two timepoints nearly a decade apart, and participants with clinical events in the interim were excluded from survival analyses, potentially biasing our sample toward healthier participants.
Conclusions
In summary, traditional cardiovascular risk factors including older age, hypertension, diabetes, and BMI are associated with rise in Gal-3 levels over time, with the largest changes in Gal-3 in the context of interim development of CKD and HF. Further, change in Gal-3 over time is associated with the development of future HF, CVD, and all-cause mortality, and adds additional information beyond a single baseline measurement. These data highlight the potential role of serial Gal-3 measurements in informing cardiovascular risk. Future studies are needed to elucidate whether Gal-3 may represent a potential therapeutic target in both HF development and disease progression.
Supplementary Material
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Longitudinal rise in Gal- 3 predicts incident HF (all HF and both HFpEF and HFrEF subtypes), CVD, and all-cause mortality.
TRANSLATIONAL OUTLOOK: Future studies are required to examine the effect of Gal-3 inhibition on clinical outcomes.
Acknowledgments
Funding: This work was partially supported by the National Heart, Lung and Blood Institute (Framingham Heart Study, contract N01-HC25195 and HHSN268201500001I). Dr. Ho is supported by NIH grants K23-HL116780, R01-HL134893, R01-HL130224, and a Hassenfeld Research Scholar award from Massachusetts General Hospital, Boston, MA.
Abbreviaations and Acronyms
- BMI
body mass index
- BNP
B-type natriuretic peptide
- CKD
chronic kidney disease
- CVD
Cardiovascular disease
- eGFR
estimated glomerular filtration rate
- FHS
Framingham Heart Study
- Gal-3
Galectin-3
- HDL
high-density lipoproteins
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- LVH
left ventricular hypertrophy
Footnotes
Disclosures: Galectin-3 assays were provided by BG Medicine (Waltham, MA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 2009;11:811–7. [DOI] [PubMed] [Google Scholar]
- 2.de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 2010;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110:3121–8. [DOI] [PubMed] [Google Scholar]
- 4.Liu YH, D’Ambrosio M, Liao TD, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol 2009;296:H404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L, Ruifrok WP, Meissner M, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 2013;6:107–17. [DOI] [PubMed] [Google Scholar]
- 6.Chen A, Hou W, Zhang Y, Chen Y, He B. Prognostic value of serum galectin-3 in patients with heart failure: a meta-analysis. Int J Cardiol 2015;182:168–70. [DOI] [PubMed] [Google Scholar]
- 7.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. Am Heart J 2014;167:674–82 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Velde AR, Gullestad L, Ueland T, et al. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: data from CORONA and COACH. Circ Heart Fail 2013;6:219–26. [DOI] [PubMed] [Google Scholar]
- 9.de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 2012;272:55–64. [DOI] [PubMed] [Google Scholar]
- 10.Grandin EW, Jarolim P, Murphy SA, et al. Galectin-3 and the development of heart failure after acute coronary syndrome: pilot experience from PROVE IT-TIMI 22. Clin Chem 2012;58:267–73. [DOI] [PubMed] [Google Scholar]
- 11.Djousse L, Matsumoto C, Petrone A, Weir NL, Tsai MY, Gaziano JM. Plasma galectin 3 and heart failure risk in the Physicians’ Health Study. Eur J Heart Fail 2014;16:350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol 2012;60:1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagodzinski A, Havulinna AS, Appelbaum S, et al. Predictive value of galectin-3 for incident cardiovascular disease and heart failure in the population-based FINRISK 1997 cohort. Int J Cardiol 2015;192:33–9. [DOI] [PubMed] [Google Scholar]
- 14.Wu AH, Wians F, Jaffe A. Biological variation of galectin-3 and soluble ST2 for chronic heart failure: implication on interpretation of test results. Am Heart J 2013;165:995–9. [DOI] [PubMed] [Google Scholar]
- 15.Meijers WC, van der Velde AR, Muller Kobold AC, et al. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail 2017;19:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Velde AR, Meijers WC, Ho JE, et al. Serial galectin-3 and future cardiovascular disease in the general population. Heart 2016;102:1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Velde AR, Meijers WC, van den Heuvel ER, et al. Determinants of temporal changes in galectin-3 level in the general population: Data of PREVEND. Int J Cardiol 2016;222:385–90. [DOI] [PubMed] [Google Scholar]
- 18.Motiwala SR, Szymonifka J, Belcher A, et al. Serial measurement of galectin-3 in patients with chronic heart failure: results from the ProBNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) study. Eur J Heart Fail 2013; 15:1157–63. [DOI] [PubMed] [Google Scholar]
- 19.Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail 2013;15:511–8. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB FM, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979. 110:281–90. [DOI] [PubMed] [Google Scholar]
- 21.Christenson RH, Duh SH, Wu AH, et al. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem 2010;43:683–90. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Dieplinger B, Mueller T. One-year in vitro stability of cardiac troponins and galectin-3 in different sample types. Clin Chim Acta 2018;476:117–122. [DOI] [PubMed] [Google Scholar]
- 23.510(k) Substantial Equivalence Determination Decision Summary Assay Only Template. Available at: https://www.accessdata.fda.gov/cdrh_docs/reviews/K140436.pdf Accesed August 8, 2018.
- 24.Levey AS BJ, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 25.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–63. [DOI] [PubMed] [Google Scholar]
- 26.McKee PA CW, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 27.Rebholz CM, Selvin E, Liang M, et al. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol 2013;24:1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvier L, Martinez-Martinez E, Miana M, et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail 2015;3:59–67. [DOI] [PubMed] [Google Scholar]
- 30.Nayor M, Wang N, Larson MG, Vasan RS, Levy D, Ho JE. Circulating Galectin-3 Is Associated With Cardiometabolic Disease in the Community. J Am Heart Assoc 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Martinez E, Calvier L, Fernandez-Celis A, et al. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension 2015;66:767–75. [DOI] [PubMed] [Google Scholar]
- 32.Suthahar N, Meijers WC, Sillje HHW, Ho JE, Liu FT, de Boer RA. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018;8:593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEvoy JW, Chen Y, Halushka MK, et al. Galectin-3 and Risk of Heart Failure and Death in Blacks and Whites. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.