Abstract
Cardiovascular disease (CVD) including atherosclerosis is the leading cause of death worldwide. As CVDs and atherosclerosis develop, plaques begin to form in the blood vessels and become calcified. Calcification within the vasculature and atherosclerotic plaques have been correlated with rupture and consequently, acute myocardial infarction. However, current imaging methods to identify vascular calcification have limitations in determining plaque composition and structure. Nanoparticles can overcome these limitations due to their versatility and ability to incorporate a wide range of targeting and contrast agents. In this review, we summarize the current understanding of calcification in atherosclerosis, their role in instigating plaque instability, and clinical methodologies to detect and analyze vascular calcification. In addition, we highlight the potential of calcium-targeting ligands and nanoparticles to create novel calcium-detecting tools.
Keywords: Nanoparticle, Cardiovascular disease, Vascular calcification, Imaging, Drug delivery, Peptides
Lay Summary
Atherosclerosis is one of the major contributors of ischemic heart disease and stroke, which remain the world’s leading cause of death. Atherosclerosis is characterized by the chronic buildup of plaque and occlusion of the arteries. Over time, blood vessels become calcified, losing the elasticity and compliance that is critical to vascular health. Current diagnostic methods to detect calcification are limited to invasive imaging procedures with potentially fatal complications or methods with inadequate sensitivity in identifying plaque composition. Notably, recent work in calcium-detecting nanoparticles show promise as useful diagnostic tools for cardiovascular disease. In this review, we discuss the role of calcification in cardiovascular diseases, current imaging technologies for the detection of calcification, and the potential of nanoparticles as diagnostic and therapeutic agents.
1. Introduction
Cardiovascular disease (CVD) is a growing burden in the westernized world and is associated with events such as stroke and cardiac arrest [1]. In 2015, CVDs accounted for 31% of deaths globally and is estimated to claim 23.6 million lives in 2030 [2]. With CVDs affecting such a wide scope of the global population, the economic burden is tremendous. The total medical cost for CVD-related treatment is expected to be $1.1 trillion by 2035 [2]. Unfortunately, diagnostic techniques for CVDs are limited to blood tests and imaging procedures such as angiograms that often miss early stages of the disease. As such, it is crucial to invest in research focusing on the earlier diagnosis and reliable detection of CVDs in order to improve patient prognosis and quality of life.
A major CVD is atherosclerosis, a chronic, inflammatory disease of the arteries that leads to the buildup of plaques and occlusion of the vasculature. Atherosclerotic plaques initiate as low density lipoproteins, macrophages, and smooth muscle cells accumulate and migrate into the subendothelium. Eventually, the luminal surface of the blood vessel develops into fatty streaks that consist of lipid-loaded macrophages called foam cells. A layer of fibrous connective tissue, or fibrous cap, forms around the fatty streak and surrounds mature plaques that contain a necrotic core filled with apoptotic cells, lipids, cholesterol, and calcification. As atherosclerosis advances, plaques become susceptible to rupture and transition into “vulnerable plaques”. Vulnerable plaques are unstable plaques with a thin fibrous cap (<65 μm) that are prone to rupture (Fig. 1) [3]. When a fibrous cap ruptures, a combination of inflammatory reactions, macrophage accumulation, and thrombosis can result, which will signficantly exacerbate blood flow and can cause a myocardial infarction.
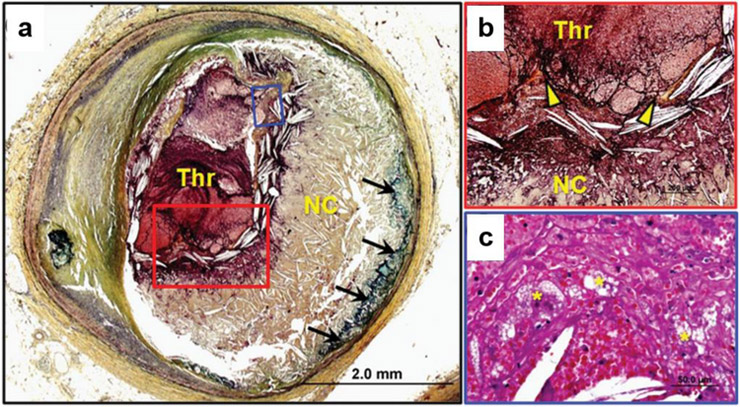
Fig. 1.
Plaque within the coronary artery has ruptured, causing an acute thrombus (Thr) that significantly occludes the vessel. The fibrous cap is almost nonexistent, with calcifications (black arrows) beneath the large necrotic core (NC), (a). Thrombus has formed where the thin fibrous cap (yellow arrow heads) is discontinued (b, red box). Foamy macrophages (yellow asterisks) migrate and accumulate in the thrombus, indicating inflammation (c, blue box). Reprinted with permission from Oxford University Press [4]
Although in the past few decades, studies have focused primarily on identifying fibrous cap thickness and the degree of luminal stenosis to predict the onset of fatal cardiac incidences, their correlation in inducing fatal cardiac incidences has become less clear in recent years [5]. In a study published by Stone et al., fibroatheromas with thin fibrous caps (<65 μm) were not at risk of rupture and were reported to account for only 4.9% of major adverse cardiac events within a 3.4 year follow-up period [6]. In addition, increasing evidence suggests that the degree of luminal stenosis alone is not a good indicator of rupture likelihood [7]. In many cases, adverse cardiac events occur in arteries with less than 50% stenosis, with the majority occurring in plaques with only 32% stenosis [3, 6]. As such, simple identification of a thin fibrous cap and the degree of luminal stenosis may not be sufficient to predict cardiac incidence or plaque rupture. Hence, investigations focusing on plaque composition and structure and its role in promoting rupture have been of recent interest.
In particular, over the past few decades, vascular calcification has been correlated with diminished cardiovascular health and considered a significant predictor of adverse cardiac events [8]. Calcium buildup within the vasculature can lead to serious diseases such as calcific aortic valve disease and atherosclerotic calcification [9]. Clinically, calcium is used as a gauge of atherosclerosis disease progression and recent assessment of atherosclerosis has correlated calcification with increased plaque instability and risk of myocardial infarctions [10-12].
Specifically, calcification within atherosclerotic plaques decreases mechanical stability [13]. In many cases, the orientation, size, and distribution of calcium nodules within the plaque can dictate the likelihood of rupture [12, 14, 15]. Consequently, the relationship between calcific plaque morphology and plaque rupture has gained much interest, but current clinical imaging methods have limitations in sensitivity and resolution for reliable calcium detection [16]. The problems associated with these methods include difficulty in distinguishing between noise and positive signals, as well as the inability to image calcium deposits that are deeply embedded within plaques.
Detection of cardiovascular calcification allows for clinicians to provide treatments and preventative therapies to mitigate further calcium formation [17-19]. Hormone therapy [18] and calcium channel blockers [17] have been shown to reduce coronary artery calcification in patients. Further, studies by Seo et al. and Khattab et al. utilizing drug-eluting stents reported decreases in incidences of myocardial infarction and death [20, 21]. Although treatment efficacy depends on the circumstances of each patient, diagnosis of arterial calcification early on could be used to prompt lifestyle modifications that improve patient outcomes [22]. As such, there is a need for accurate and reliable detection of atherosclerotic calcification in order to provide viable treatment options based on diagnoses.
Towards this goal, nanoparticles have emerged as promising tools for diagnostics and imaging. Nanoparticles are comprised of a variety of materials including lipids, metals, and polymers and can be engineered to have different size, shape, charge, and pharmacokinetic profiles [23-25]. Nanoparticles can have multimodal functionalities to simultaneously target and image a region of interest [26]. Increasingly, nanoparticles are being used as noninvasive drug delivery systems for targeted delivery of therapeutics [27]. Significant progress has been made in developing nanoparticles for targeting macrophages in atherosclerosis [28-31], for therapeutics and imaging of vascular disease [32-35], and treatment using endothelial cell targets [36, 37], which is further described elsewhere [38-42].
Calcium, or hydroxyapatite (HA), binding nanoparticles have been developed for a wide variety of purposes ranging from anticancer polymer drug carriers to bone-targeting gold nanoparticles [43, 44]. HA is a nonorganic calcium phosphate mineral that is prevalent in bone, teeth, and calcified vasculature. HA binding ligands including glutamic acid, phosphonic acid, and bisphosphonate, as well as peptides discovered through phage display or derived from bone-binding proteins have been used to functionalize nanoparticles to specifically target HA [43, 45]. Mineral-binding proteins commonly have polyacidic regions that facilitate electrostatic interactions with mineral surfaces [46]. Hence, several studies have included polyglutamic acid motifs in nanoparticles to facilitate HA binding [47-50]. Due to the parallels found in bone development and vascular calcification, bone-targeting agents can be adapted for use in vascular calcification disease for early detection and intervention.
In this review, we summarize the process of calcification in vascular disease and provide a brief overview of current clinical practice for imaging calcification. In addition, we highlight existing nanoparticles developed for vascular calcification detection, as well as discuss future approaches that can be adapted from bone applications.
2. Calcification in Vascular Disease
2.1. Vascular Smooth Muscle Cells in Calcification
Vascular calcification was previously believed to be an unregulated, passive process that occurred due to tissue degeneration [1]. However, recent evidence suggests that calcification is highly regulated through the activation of cellular signaling pathways such as Wnt signaling and the expression of Runt-related transcription factor 2 (Runx2) [1,9, 51-54]. Vascular calcification is marked by the growth of bone-like ossifications that propagate within the intima and medial layer of vessels [55]. The degree of vascular calcification increases with age, and ossification has been found in 15% of carotid atherosclerotic plaques [56]. However, vascular calcification is not limited to atherosclerosis and is also characteristic to chronic kidney disease and diabetes mellitus [57].
While not fully characterized, much of the pathogenesis of vascular calcification parallels signaling processes of skeletal bone formation [55]. During vascular calcification, vascular smooth muscle cells (VSMCs) differentiate into osteoblast-like phenotypes [9]. Inflammatory markers such as tumor necrosis factor-α (TNF-α) activate bone morphogenic proteins (BMP) which, subsequently, trigger the Msx2-Wnt signaling cascade [1, 58]. The Msx2-Wnt signaling cascade increases expression of Runx2, which promotes the production of bone formation regulators, osteocalcin, osterix, and osteopontin, as well as alkaline phosphatase activity [55, 59]. This leads to HA deposition by osteoblasts and VSMCs, creating calcified tissue [60].
In non-diseased states, the vasculature is regulated by mineralization inhibitors. One such inhibitor is matrix-Gla protein (MGP). Normally, healthy VSMCs express MGP which binds to intracellular calcium and inactivates BMP-2. However, MGP is downregulated during vascular calcification [61]. In MGP-knockout mice, severe vascular calcification and enhanced expression of Runx2 was found, indicating the critical role of MGP in inhibiting vascular calcification [62].
2.2. Extracellular Vesicles in Calcification
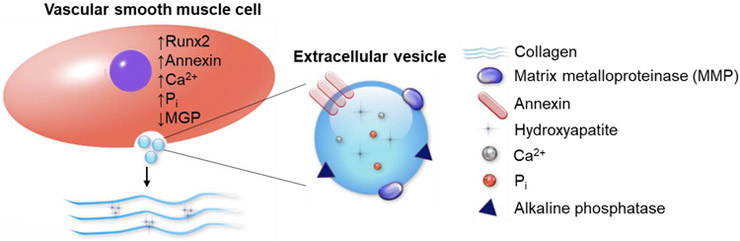
Throughout calcification, VSMCs release extracellular vesicles (EVs), typically 200 nm in diameter, that contain trace amounts of HA (Fig. 2) [63]. This mechanism of calcium release through EVs is believed to be a self-regulating process for cells to reduce high levels of intracellular calcium [64]. EVs contain approximately 79 different proteins including calcium-binding annexins. Annexins are triggered by high cytosolic calcium content and play a role in EV transportation and HA nucleation in the extracellular matrix (ECM) [54, 65-67]. These nucleation points start as spotty calcifications similar to those seen in Fig. 1a. Over time, EVs accumulate and aggregate into larger calcium nodules which result in hardened and calcified atherosclerotic plaques and vessels.
Fig. 2.
VSMCs release EVs that initiate and propagate vascular calcification in the ECM. Upregulated levels of Runx2, annexins, Ca2+, and inorganic phosphate (Pi) promote calcification. Low levels of calcification inhibitor MGP is found in calcifying VSMCs
Similarly, in skeletal bone formation, osteoclasts and osteoblasts remodel the bone through active deposition of unmineralized bone matrix and release of matrix vesicles [68]. Like osteoblasts, VSMCs release many proteins that are associated with bone formation such as BMPs, osteopontin, and osteocalcin [1, 69, 70]. Due to their similarities, understanding the mechanism of bone formation will also be beneficial in aiding the design and development of novel diagnostic and therapeutic technologies for vascular calcification.
2.3. Role of Calcification in Atherosclerotic Plaque Stability
Clinical evidence suggests a strong correlation between atherosclerotic plaque calcification and acute myocardial infarction. However, the morphology, shape, size, location and total mass of calcified nodules within an atherosclerotic plaque can individually affect plaque stability and determine the overall risk for acute myocardial infarction [71]. While any development of vascular calcification is detrimental to vascular health, some morphologies may be worse than others. Plaque instability from calcifications is hypothesized to occur due to the mismatch in tissue stresses between the soft noncalcified regions and hard calcified nodules within plaques [71]. These tissue interfaces have large circumferential stress, which upon exceeding the critical peak, can cause plaque rupture [72]. Total hard/soft tissue interface area between hard calcium nodules and soft tissue in an atherosclerotic plaque is affected by a combination of the size, morphology, structure, and location of calcified nodules.
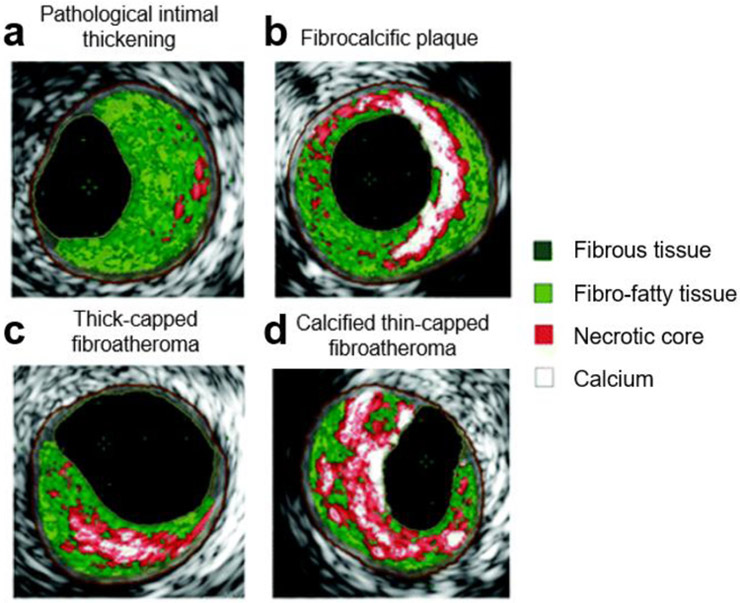
Spotty calcifications increase interfacial area and cause higher circumferential stress (Fig. 3c and d) [9]. When calcification nodules reach a size of 5-60 μm, local stresses can increase five-fold, destabilizing the plaques and predisposing them to rupture [74]. In a study by Maldonado et al., the fibrous cap of ruptured atheromas were embedded with microcalcifications smaller than 60 μm [75]. In contrast, larger, aggregated calcium nodules greater than 60 μm have less interfacial surface area as seen from the total perimeter surrounding the white regions in Fig. 3b compared to that of the spotty calcifications in Fig. 3d. Less interfacial area is hypothesized to cause lower levels of local stresses, which in turn stabilizes the plaque (Fig. 3b) [71]. In addition to size, the location of the calcification within the plaque can cause varying levels of stress. For instance, since pulsating arteries are subject to longitudinal stretching, smaller nodules less than 5 μm located at the longitudinal ends of the plaque have been reported to cause increased stress and rupture [63, 76]. However, determining the critical size and structure of calcifications that make plaques susceptible to rupture remains difficult clinically, due to the limitations in imaging technology and the lack of consensus in the field regarding the size and effects of calcifications [12, 63, 76-78]. As such, developing better tools to identify and visualize calcifications within the plaque can help bridge this gap.
Fig. 3.
Atherosclerotic plaque classifications based on virtual histology intravascular ultrasound (VH-IVUS). Plaques are categorized by intimal thickness and calcium growth (b, c, d). Calcium morphology and location can vary between types of plaques. Larger, bulk calcifications (b) have less interfacial area than spotty calcifications (c, d) causing lower circumferential stresses. The interfacial area can be assessed from the total perimeter between white and red regions in these cross-sectional images. Reprinted and adapted with permission from Wolters Kluwer Health [73, 3, 4]
3. Vascular Calcification Imaging and Diagnosis
In clinical practice, the presence of calcium, especially in the coronary arteries, is evaluated using varying methods that give equally varying results [76]. As such, diagnostic calcium scores can change depending on how the data is analyzed and interpreted [3]. Here, we discuss commonly used imaging modalities for vascular calcification detection such as computed tomography (CT), intravascular ultrasound (IVUS), magnetic resonance imaging (MRI), optical coherence tomography (OCT), and positron emission topography (PET).
3.1. Computed Tomography
Cardiac CT can be used to noninvasively image thin slices of the heart and coronary arteries through x-ray measurements. Cardiac CT can acquire 2.5- to 3-mm thick axial or horizontal cross-sectional images. Electron beam-CT, a form of CT designed specifically for imaging the heart, can measure aortic valve calcification [79]. However, EB-CT has been shown to have poor reproducibility between scans, with 14% to 51% variability, making accurate diagnoses difficult [80].
Currently, systematic assessment of calcium risk is determined by a coronary artery calcium score, or CAC, calculated from CT images [81]. Briefly, CAC is derived via the Agatston method which multiplies the affected area by a weighting factor based on the density of detected calcium deposits called the Hounsfield unit [80]. Because the Hounsfield unit density factor increases step-wise using values between 1 and 4, changes in the CAC may not accurately account for minor but destabilizing fluctuations in coronary calcium over time. Additionally, the CAC score does not take into account differences in thickness of the axial images that are taken [82]. In contrast, the calcium volume score (CVS), which represents the volume of calcium present, is considered to be a better representation of coronary calcium because it has better reproducibility and less variability than the Agatston score [83]. However, there are drawbacks to CVS as well, namely that it can be inaccurately inflated as the CT signal obtained is operator-dependent [82]. Hence, current scoring methods utilizing CT images may not accurately reflect the amount of existing vascular calcification.
3.2. Magnetic Resonance Imaging (MRI)
MRI is a noninvasive imaging method that does not expose the patient to ionizing radiation [84]. One study evaluating the ability of MRI to quantify carotid atherosclerotic plaque components in vivo confirmed histology-matching results showed 84% sensitivity and 91% specificity for calcification [85]. There was a strong correlation between MRI and histology area measurements for calcification (r = 0.74; P < 0.001). However, calcification measurements differed significantly from histology results when measured as a percentage of wall area (9.4 versus 5%, P < 0.001) [85]. Further, while some studies show MRI can differentiate the fibrous cap and lipid core of an atherosclerotic plaque, limited spatial resolution makes reliable identification of calcium structures difficult [86]. As such, MRI alone without calcium-targeting contrast agents may not be sufficient in accurately identifying vascular calcification.
3.3. Intravascular Ultrasound (IVUS)
Another medical imaging method for vascular disease is IVUS. IVUS is an invasive imaging method that allows for the visualization of calcium in plaques by using miniaturized probes attached to catheters. The ultrasound catheter is guided into the blood vessel and emits and receives reflected sound waves. IVUS has been used to visualize the amount of plaque in the arteries and the degree of luminal stenosis, and has been shown to have up to 90% sensitivity and 100% specificity for identifying coronary calcium [87]. IVUS has been reported to reliably and accurately identify atherosclerotic plaque components including spotty patterns of calcification (Fig. 3) [12]. However, the lengthy and costly procedure can trigger acute cardiac events due to the invasive approach [88].
3.4. Optical Coherence Tomography (OCT)
OCT is a catheter-based invasive imaging system that produces high resolution tomographic images of internal vessel microstructure based on the echo time delay and magnitude of backscattered light. Because the wavelength of the imaging light is much shorter than ultrasound, OCT offers significantly improved imaging resolution over IVUS (10 – 40 μm axial resolution vs. 100-300 μm, respectively; Table 1) [89]. In one study assessing the accuracy of OCT imaging for characterizing atherosclerotic plaque, 357 OCT images obtained at autopsy demonstrated a sensitivity of 96% and a specificity of 97% for the detection of fibrocalcific plaques [90]. In addition, OCT images showed more accurate plaque microstructure and degree of calcification when compared to IVUS imaging [91]. While IVUS cannot penetrate calcified deposits, OCT has the ability to do so; hence, OCT is able to assess calcium thickness, area, and volume. OCT provides a higher resolution of imaging compared to other imaging modalities, but the invasive nature of the imaging procedure makes it less than ideal for use in high risk patients with vulnerable plaques.
Table 1.
Clinical imaging modalities for atherosclerotic plaque and vascular calcification.
| EB-CT | MRI | OCT | IVUS | PET | |
|---|---|---|---|---|---|
| Spatial resolution |
0.3 - 1.5 mm | 0.5 - 2 mm | 10 - 40 μm | 100 - 300 μm |
0.8 - 2.5 mm |
| Calcium detection |
+ | − | + | + | − |
| Luminal stenosis |
+ | + | + | + | − |
| Plaque composition |
− | + | + | + | + |
| Cost | Low | High | Low | High | High |
| Required time |
Low | High | Low | Low | High |
| Advantage | Functional and anatomical imaging | Functional and anatomical imaging, high resolution | High resolution | Good imaging of calcium and differences between hard and soft plaques | High sensitivity, high availability of molecular probes, quantitative |
| Disadvantage | Significant restrictions for continuous volume scan | Low sensitivity, cannot use metal, semi-quantitative | Invasive, cannot provide optimal arterial wall images in large vessels, poor tissue penetration | Invasive and requires use of catheters, difficult to distinguish structures | Moderate resolution, short-lived isotopes |
3.5. Positron Emission Topography (PET)
PET measures gamma ray emissions from a positron-emitting tracer molecule that is injected into subjects. For imaging atherosclerosis, the most common radioactive tracers used are 2-deoxy-2-18F-fluoro-D-glucose (18F-FDG) and fluorine 18-sodium fluoride (18F-NaF). These tracers are able to accumulate in atherosclerotic plaques because it is taken up but not metabolized by metabolically active cells [92, 93]. In a study by Derlin et al., 18F-NaF uptake correlated with calcification in 88% of lesions [93]. In another study, PET was used to assess calcification in the aortic valves of 120 subjects using both 18F-NaF and 18F-FDG as tracers. The study found that activity of both tracers was higher in patients with aortic stenosis compared to the controls [94]. Specifically, 18F-NaF uptake demonstrated a strong correlation with increases in the severity of aortic stenosis. Tracer activity steadily increased with higher levels of calcification and inflammation related to disease severity [94]. Unfortunately, PET images are subject to partial-volume effects due to low resolution, which may lead to underestimation of tracer accumulation [93]. Using PET scanning concurrently with another imaging method such as EB-CT could potentially remedy the effects of low resolution [95]. Nanoparticles can also be developed to meet clinical needs and overcome the limitations associated with these imaging technologies.
4. Detecting Calcium using Nanoparticles
4.1. Calcium Detection with Nanoparticles in Atherosclerosis
Nanoparticles functionalized with targeting and imaging modalities have been proposed to detect vascular calcification. To date, nanoparticles designed for atherosclerosis have been successful in identifying different stages of inflammation by targeting macrophages as well as in delivering treatments such as microRNA therapies to prevent atheroma formation [96-98]. Nanoparticles that have been designed to specifically detect vascular calcification have been developed by applying MRI, near infrared fluorescence (NIRF), and PET approaches. They can be advantageous by specifically targeting HA, reducing the margin of error in defining calcium nodules in the vasculature that is common in clinical calcium detection methods such as ultrasound. Further, nanoparticles can be administered intravenously, eliminating the potential risks of atherosclerotic plaque rupture and myocardial infarction presented by the invasive imaging methods including IVUS and OCT.
To that end, a noninvasive imaging approach using fluorescence imaging was developed by Lee et al [45]. In this study, a molecular optical imaging probe that binds to HA was designed in order to selectively detect calcification [45, 99]. A 19-mer peptide, CγEPRRγEVAγELγEPRRγEVAγEL, derived from osteocalcin, was labeled with a Cy5.5 fluorescent dye to create a fluorescent label for HA detection [45]. The study confirmed successful binding to HA via fluorescence microscopy. Further, comparisons of fluorescence images and μCT images demonstrated HA targeting and binding in excised, calcified human carotid arteries and aortic valves. While promising, the studies did not progress into in vivo imaging due to the limitations of optical imaging such as low penetration depths and spatial resolution [99, 100]. Optical fluorescence imaging has very high resolutions (~10 μm), but poor tissue penetration (<1 cm) makes it difficult to use for deep tissue imaging.
In contrast, others have characterized nanoparticle systems and their efficacy in calcification detection using in vivo models. For example, Aikawa et al. tested a multimodal molecular imaging nanoparticle that can detect early inflammation and osteogenesis in aortic valve disease within murine models [101]. The authors created a magnetofluorescent nanoparticle (MNFP) with an outer layer of dextran and a superparamagnetic iron oxide core for MRI. MNFPs were functionalized with the far red fluorochrome, VT680, and peptides targeting vascular cell adhesion molecule-1 (VCAM-1) through very late antigen-4 (VLA-4). Upon systemic injection in apolipoprotein E-deficient (ApoE−/−) mice, the main murine model of atherosclerosis, MFNPs were trapped by inflamed, VCAM-1-expressing endothelial cells and were readily internalized by macrophages. To further characterize the mechanisms involved in calcification, the authors injected commercially available, protease-activatable, NIRF agents MMPSense680, and calcium-binding bisphosphonate-conjugated NIRF, OsteoSense780. Dual injection of protease- activatable, NIRF agents and MNFPs identified high levels of proteolytic activity localized near macrophages. Furthermore, results confirmed the involvement of proteases in matrix degradation and structural changes that became nucleation sites for HA formation. Imaging and tracking of early proteolytic activity involved in HA formation was performed using fluorescence microscopy and MRI via both ex vivo and in vivo analysis. In this study, while high resolution MRI was used for ex vivo analysis with spatial resolutions in the sub-millimeter range, the best imaging resolution that could be achieved to visualize cellular processes in vivo was 1 mm. Nonetheless, Aikawa demonstrated MFNPs have potential in detecting cellular activity responsible for early calcification.
A more recent study highlighted the development of iron oxide nanoparticles functionalized with neridronate, a bisphosphonate drug used for bone disease, for HA targeting in plaques [102]. Bisphosphonates have high affinity to HA because of the structural similarities to pyrophosphates, compounds found in blood plasma and urine that are known to inhibit HA formation [103]. These magnetic iron oxide nanoparticles were 7.5 nm in diameter, and upon intravenous injection in ApoE−/− mice, the nanoparticles accumulated significantly in atherosclerotic plaques as determined by MRI and histology. In ex vivo analysis, calcium deposits were stained with Von Kossa, iron with Prussian blue, and macrophages with antibodies against F4/80. Colocalization was found among macrophages, calcium, and the neridronate- functionalized iron oxide nanoparticle in the aorta. Thus, this study demonstrated the promise of combining MRI and bisphosphonate on nanoparticles for targeting atherosclerotic calcium.
In addition to detection, nanoparticles have been reported to deliver therapeutics against calcification. One therapeutic molecule gaining popularity is the calcium-chelating agent disodium ethylenediaminetetraacetic acid (EDTA) which binds to calcium and can prevent its precipitation. Lei et al. loaded EDTA into 150-200 nm sized bovine serum albumin (BSA) nanoparticles coated with elastin antibodies to target medial arterial calcification [104]. The study showed the elastin nanoparticles successfully bound to damaged aorta in a CaCl2-induced vascular calcification injury rat model with a three-fold increase in accumulation when compared to a non-targeting IgG antibody. EDTA was released slowly over the course of five days at the target site and ex vivo analysis showed a reversal in calcification. Atomic absorption spectrometry analysis confirmed mice treated with elastin antibody nanoparticles loaded with EDTA had reduced levels of calcium (5.25 μg of calcium per 10 mg aortic tissue), while non-targeting IgG nanoparticles loaded with EDTA had more calcium (28.18 μg of calcium per 10 mg aortic tissue). These initial results show promising efficacy of the EDTA-BSA nanoparticles, but a long term efficacy study is needed to assess the therapeutic potential against calcification reformation.
4.2. Calcium-Targeting in Bones for Potential Use in Vascular Applications
Given the similarities, calcium-targeting moieties proposed for bone and teeth applications also have potential to be adapted for applications in vascular calcification [105-108]. For instance, Hengst et al. developed bone-targeting liposomes functionalized with a bisphosphonate-derivative, cholesteryl-trisoxyehtylene-bisphosphonic acid (CHOL-TOE-BP), as the targeting moiety [109]. When DiD fluorescently-labelled liposomes were incubated with synthetic HA overnight, the CHOL-TOE-BP liposomes bound to HA with close to 100% efficiency compared to almost no binding using a non-targeting control. This shows the potential for use of CHOL-TOE-BP to detect vascular calcification.
In addition, gold nanoparticles functionalized with glutamic acid, phosphonic acid and alendronate, have been developed to target mineral crystals for microdamage assessment in bone tissue [43]. Polyglutamic acid sequences, alendronate, and phosphonic acids have all been previously demonstrated to have specific binding to HA [48, 110, 111]. Gold is a contrast agent that demonstrates high X-ray attenuation and biocompatibility which is optimal for imaging. Ross et al. reported that gold nanoparticles functionalized with alendronate had the best binding affinity with an equilibrium binding constant, K (mg/L), of 3.40 and six-fold greater binding affinity when compared to glutamic acid only. As HA is the main mineralizing calcium in vascular calcification, the bone-targeting nanoparticles have the potential to be altered and repurposed for vascular diseases.
4.3. Calcium-Binding Peptides
As described, nanoparticles have the capability to actively target substrates using surface ligands, antibodies, or peptides [112]. Peptides have proven to be favorable ligands for the active targeting of nanoparticles owing to their biocompatibility, biodegradability, and tunable characteristics such as size and secondary structure. To date, several peptides have been identified to selectively bind to HA [113, 114]. In particular, Roy et al. identified that the sequence SVSVGMKPSPRP binds to HA with a relatively small KD of 14.1 μM [114, 115]. The authors report the amino acid motif, SVSV, to be the significant contributor to the binding interaction. It is hypothesized that the α-helical secondary structure of the peptide may serve as a scaffolding mechanism to promote stability upon binding HA due to the alignment of acidic side-chains of the peptide with the basic HA [114, 116]. Others have hypothesized that the charge-based interactions of this peptide and HA reduce the reliance of specificity that is common with protein-protein interactions [117]. However, molecular dynamic studies regarding the precise intermolecular interactions between amino acid residues and calcium mineral is needed to elucidate their binding mechanism. Moreover, the binding affinity of this peptide for calcium in the context of vascular calcification should be evaluated to harness its full potential for CVDs.
In addition to SVSV, a polyglutamate peptide sequence has been reported to be successful in binding bone grafts [118-120]. Varying numbers of glutamate were added to the osteoinductive peptide derived from collagen, Asp-Gly-Glu-Ala (DGEA), to create diglutamate (E2DGEA), tetraglutamate (E4DGEA) and heptaglutamate (E7DGEA) conjugates. Interestingly, all conjugated peptides showed a higher degree of binding to the bone grafts when compared to DGEA alone. In particular, E7DGEA demonstrated the best binding to bone grafts in vitro and in vivo. E7DGEA-coated bone grafts were subcutaneously implanted into mice and even after 2 months post-implantation, E7DGEA remained adhered to the bone.
Murphy et al. conjugated negatively-charged amino acids that showed calcium-targeting abilities to semi-random peptides, GNAE or GNAEGNAR. The peptides were modified with eight glutamic acid residues (Glu8) or eight aspartic acids (Asp8), or pamidronate, a nitrogen- containing bisphosphonate, to test their ability to enhance binding to HA [111]. In vitro binding studies showed approximately 60% binding to HA for peptides conjugated with Asp8 or Glu8, and 50% binding for pamidronate-conjugated peptides after 30 minutes of incubation. Hence, modifications with polyglutamic acid, polyaspartic acid, or pamidronate can be used as a strategy to enhance HA targeting abilities, and can be incorporated into future nanoparticle systems with the goal of targeting vascular calcification.
Notably, using a customized surface plasmon resonance (SPR) imaging system, the affinity of HA binding moieties to the HA mineral surface can be determined [115]. However, for those without such a customized SPR system, assessing binding affinity remains difficult. To assess specificity to HA, some have compared binding to HA with other calcium phosphate derivatives [45, 99, 114]. These studies can provide a general idea regarding the binding affinity and specificity of targeting systems to HA in vitro, but additional work needs to be done in vivo as HA minerals are not limited to regions of vasculature and nanoparticles can be subject to off-target binding. In addition, there has been limited work studying the molecular dynamics, interactions, and forces that dictate HA binding and reversibility, prompting the need for additional investigations. Developing nanoparticle systems that can successfully identify vascular calcification will facilitate accurate treatment for patients. Future studies should focus on incorporating theranostic agents that can simultaneously provide treatment at the diseased sites.
5. Conclusion
Vascular calcification and plaque rupture are predicted to cause acute myocardial infarction and adverse cardiac events. As such, nanoparticles that detect vascular calcification to directly assess the risk of plaque rupture and acute cardiac events can be clinically beneficial. As calcification is often associated with bones, many nanoparticles for calcium detection or binding for orthopedic applications may be repurposed for applications in vascular calcification and overcome the limitations of current clinical imaging modalities. In addition, calcium-targeting nanoparticle imaging systems can incorporate therapeutic agents that can immediately unload treatment at the site of disease. As such, nanoparticles fabricated for calcium diagnostics have great future potential. While we highlighted atherosclerotic calcification in this review, calcification is not limited to atherosclerosis and arteries, but are also present in other diseases such as chronic kidney disease. As such, developing novel diagnostic nanoparticle tools for calcium detection may have a broad and significant impact on other pathologies.
Acknowledgements
The authors would like to acknowledge the financial support from the Women in Science and Engineering program at University of Southern California (USC) for undergraduate research awarded to SC and Gabilan Assistant Professorship awarded to EJC. In addition, we acknowledge the financial support from the L. K. Whittier Foundation and the National Heart, Lung, and Blood Institute (NHLBI), R00HL124279, awarded to EJC.
References
- 1.Leopold JA. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med. 2015;25(4):267–74. doi: 10.1016/j.tcm.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1772–8. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 4.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2013;34(10):719–28. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Pasterkamp G. Requiem for the 'vulnerable plaque'. Eur Heart J. 2015;36(43):2984–7. doi: 10.1093/eurheartj/ehv349. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 7.Pasterkamp G, Schoneveld AH, van der Wal AC, Haudenschild CC, Clarijs RJ, Becker AE et al. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol. 1998;32(3):655–62. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D, Mackenzie NC, Farquharson C, Macrae VE. Mechanisms and clinical consequences of vascular calcification. Front Endocrinol (Lausanne). 2012;3:95. doi: 10.3389/fendo.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24(7):1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 10.Yu PJ, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G et al. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009;138(1):196–9. doi: 10.1016/j.jtcvs.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–33. [DOI] [PubMed] [Google Scholar]
- 12.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110(22):3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J et al. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297(2):H802–10. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutgens E, van Suylen RJ, Faber BC, Gijbels MJ, Eurlings PM, Bijnens AP et al. Atherosclerotic plaque rupture: local or systemic process? Arterioscler Thromb Vasc Biol. 2003;23(12):2123–30. doi: 10.1161/01.ATV.0000097783.01596.E2. [DOI] [PubMed] [Google Scholar]
- 15.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7(9):528–36. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8(4):461–71. doi: 10.1016/j.jcmg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension. 2001;37(6):1410–3. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 19.de Ribamar Costa J, Mintz GS Jr., Carlier SG, Mehran R, Teirstein P, Sano K et al. Nonrandomized comparison of coronary stenting under intravascular ultrasound guidance of direct stenting without predilation versus conventional predilation with a semi-compliant balloon versus predilation with a new scoring balloon. Am J Cardiol. 2007;100(5):812–7. doi: 10.1016/j.amjcard.2007.03.100. [DOI] [PubMed] [Google Scholar]
- 20.Seo A, Fujii T, Inoue T, Onoda S, Koga A, Tanaka Y et al. Initial and long-term outcomes of sirolimus-eluting stents for calcified lesions compared with bare-metal stents. Int Heart J. 2007;48(2):137–47. [DOI] [PubMed] [Google Scholar]
- 21.Khattab AA, Otto A, Hochadel M, Toelg R, Geist V, Richardt G. Drug-eluting stents versus bare metal stents following rotational atherectomy for heavily calcified coronary lesions: late angiographic and clinical follow-up results. J Interv Cardiol. 2007;20(2):100–6. doi: 10.1111/j.1540-8183.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 22.Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63(17):1703–14. doi: 10.1016/j.jacc.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Biju V Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43(3):744–64. doi: 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- 24.Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans A Math Phys Eng Sci. 2010;368(1915):1333–83. doi: 10.1098/rsta.2009.0273. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY et al. Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005;5(3):473–7. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 26.Bogart LK, Pourroy G, Murphy CJ, Puntes V, Pellegrino T, Rosenblum D et al. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano. 2014;8(4):3107–22. doi: 10.1021/nn500962q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma G, She ZG, Valenta DT, Stallcup WB, Smith JW. Targeting of Macrophage Foam Cells in Atherosclerotic Plaque Using Oligonucleotide-Functionalized Nanoparticles. Nano Life. 2010;1(3-4):207–14. doi: 10.1142/S1793984410000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz A, Dengler MA, van der Kuip H, Yildiz H, Rosch S, Klumpp S et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2013;34(6):462–75. doi: 10.1093/eurheartj/ehs366. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Gaytan BL, Fay F, Lobatto ME, Tang J, Ouimet M, Kim Y et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26(3):443–51. doi: 10.1021/bc500517k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu M, Naguib YW, Aldayel AM, Shi YC, Hursting SD, Hersh MA et al. Biodistribution and in vivo activities of tumor-associated macrophage-targeting nanoparticles incorporated with doxorubicin. Mol Pharm. 2014;11(12):4425–36. doi: 10.1021/mp500565q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo SP, Pineda F, Barrett JC, Poon C, Tirrell M, Chung EJ. Gadolinium-Functionalized Peptide Amphiphile Micelles for Multimodal Imaging of Atherosclerotic Lesions. ACS Omega. 2016;1(5):996–1003. doi: 10.1021/acsomega.6b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anselmo AC, Modery-Pawlowski CL, Menegatti S, Kumar S, Vogus DR, Tian LL et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–53. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Dahlman JE, Barnes C, Khan O, Thiriot A, Jhunjunwala S, Shaw TE et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–55. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsourkas A, Shinde-Patil VR, Kelly KA, Patel P, Wolley A, Allport JR et al. In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug Chem. 2005;16(3):576–81. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 37.Bruckman MA, Jiang K, Simpson EJ, Randolph LN, Luyt LG, Yu X et al. Dual-modal magnetic resonance and fluorescence imaging of atherosclerotic plaques in vivo using VCAM-1 targeted tobacco mosaic virus. Nano Lett. 2014;14(3):1551–8. doi: 10.1021/nl404816m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117(3):379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung EJ, Tirrell M. Recent Advances in Targeted, Self-Assembling Nanoparticles to Address Vascular Damage Due to Atherosclerosis. Adv Healthc Mater. 2015;4(16):2408–22. doi: 10.1002/adhm.201500126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khodabandehlou K, Masehi-Lano JJ, Poon C, Wang J, Chung EJ. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp Biol Med (Maywood). 2017;242(8):799–812. doi: 10.1177/1535370217693116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S, Kim CW, Simmons RD, Jo H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol. 2014;34(10):2206–16. doi: 10.1161/ATVBAHA.114.303425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsigkas N, Kidambi S, Tawakol A, Chatzizisis YS. Drug-loaded particles: "Trojan horses" in the therapy of atherosclerosis. Atherosclerosis. 2016;251:528–30. doi: 10.1016/j.atherosclerosis.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 43.Ross RD, Roeder RK. Binding affinity of surface functionalized gold nanoparticles to hydroxyapatite. J Biomed Mater Res A. 2011;99(1):58–66. doi: 10.1002/jbm.a.33165. [DOI] [PubMed] [Google Scholar]
- 44.de Miguel L, Popa I, Noiray M, Caudron E, Arpinati L, Desmaele D et al. Osteotropic polypeptide nanoparticles with dual hydroxyapatite binding properties and controlled cisplatin delivery. Pharm Res. 2015;32(5):1794–803. doi: 10.1007/s11095-014-1576-z. [DOI] [PubMed] [Google Scholar]
- 45.Lee JS, Tung CH. Osteocalcin biomimic recognizes bone hydroxyapatite. Chembiochem. 2011;12(11):1669–73. doi: 10.1002/cbic.201100162. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM et al. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J Biol Chem. 2000;275(21):16213–8. doi: 10.1074/jbc.M001773200. [DOI] [PubMed] [Google Scholar]
- 47.Fujisawa R, Kuboki Y. Preferential adsorption of dentin and bone acidic proteins on the (100) face of hydroxyapatite crystals. Biochim Biophys Acta. 1991;1075(1):56–60. [DOI] [PubMed] [Google Scholar]
- 48.Fujisawa R, Wada Y, Nodasaka Y, Kuboki Y. Acidic amino acid-rich sequences as binding sites of osteonectin to hydroxyapatite crystals. Biochim Biophys Acta. 1996;1292(1):53–60. [DOI] [PubMed] [Google Scholar]
- 49.Johnsson M, Levine MJ, Nancollas GH. Hydroxyapatite binding domains in salivary proteins. Crit Rev Oral Biol Med. 1993;4(3-4):371–8. [DOI] [PubMed] [Google Scholar]
- 50.Boskey AL. Osteopontin and related phosphorylated sialoproteins: effects on mineralization. Ann N Y Acad Sci. 1995;760:249–56. [DOI] [PubMed] [Google Scholar]
- 51.Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23(11):1798–805. doi: 10.1359/jbmr.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demer LL, Watson KE, Bostrom K. Mechanism of calcification in atherosclerosis. Trends Cardiovasc Med. 1994;4(1):45–9. doi: 10.1016/1050-1738(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 53.Hsu JJ, Lim J, Tintut Y, Demer LL. Cell-matrix mechanics and pattern formation in inflammatory cardiovascular calcification. Heart. 2016;102(21):1710–5. doi: 10.1136/heartjnl-2016-309667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109(1):e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 55.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109(5):564–77. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunt JL, Fairman R, Mitchell ME, Carpenter JP, Golden M, Khalapyan T et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33(5):1214–9. [DOI] [PubMed] [Google Scholar]
- 57.Schlieper G, Schurgers L, Brandenburg V, Reutelingsperger C, Floege J. Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant. 2016;31(1):31–9. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 58.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 59.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC Jr., et al. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-regulated kinase 1/2. J Thorac Cardiovasc Surg. 2009;138(4):1008–15. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 60.Choi ST, Kim JH, Kang EJ, Lee SW, Park MC, Park YB et al. Osteopontin might be involved in bone remodelling rather than in inflammation in ankylosing spondylitis. Rheumatology (Oxford). 2008;47(12):1775–9. doi: 10.1093/rheumatology/ken385. [DOI] [PubMed] [Google Scholar]
- 61.Lomashvili KA, Wang X, Wallin R, O'Neill WC. Matrix Gla protein metabolism in vascular smooth muscle and role in uremic vascular calcification. J Biol Chem. 2011;286(33):28715–22. doi: 10.1074/jbc.M111.251462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104(6):733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15(3):335–43. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34(4):715–23. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Genge BR, Wu LNY, Wuthier RE. Differential Fractionation of Matrix Vesicle Proteins - Further Characterization of the Acidic Phospholipid-Dependent Ca-2+-Binding Proteins. J Biol Chem. 1990;265(8):4703–10. [PubMed] [Google Scholar]
- 66.Kapustin AN, Shanahan CM. Calcium Regulation of Vascular Smooth Muscle Cell-Derived Matrix Vesicles. Trends Cardiovas Med. 2012;22(5):133–7. doi:PII S1050-1738(12)00222-8 DOI 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Krohn JB, Hutcheson JD, Martinez-Martinez E, Aikawa E. Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J Physiol. 2016;594(11):2895–903. doi: 10.1113/JP271338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Persy V, D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15(9):405–16. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 69.F Patrick Ross JC, Alvarez Jose I, Sander Diane, Butler WIlliam T, Farach-Carson Mary C, Mintz Keith A., Robey Pamela Gehron, Teitelbaum Steven L., Cheresh David A. Interactions between the Bone Matrix Proteins Osteopontin and Bone Sialoprotein and the Osteoclast Integration Potentiate Bone Resorption. The Journal of Biological Chemistry. 1993;268, 13(May 5):9901–7. [PubMed] [Google Scholar]
- 70.Guiqian Chen CD, Yi-Ping Li. TGF-Beta and BMP Signaling in Osteoblastt Differentiation and Bone Formation. Int J Biol Sci. 2012;8(2):272–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hutcheson JD, Maldonado N, Aikawa E. Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability. Curr Opin Lipidol. 2014;25(5):327–32. doi: 10.1097/MOL.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richardson PD, Davies MJ, Born GV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989;2(8669):941–4. [DOI] [PubMed] [Google Scholar]
- 73.Calvert PA, Liew TV, Gorenne I, Clarke M, Costopoulos C, Obaid DR et al. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol. 2011;31(9):2157–64. doi: 10.1161/ATVBAHA.111.229237. [DOI] [PubMed] [Google Scholar]
- 74.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A. 2013;110(26):10741–6. doi: 10.1073/pnas.1308814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maldonado N, Kelly-Arnold A, Vengrenyuk Y, Laudier D, Fallon JT, Virmani R et al. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: potential implications for plaque rupture. Am J Physiol Heart Circ Physiol. 2012;303(5):H619–28. doi: 10.1152/ajpheart.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demer LL, Tintut Y, Nguyen KL, Hsiai T, Lee JT. Rigor and Reproducibility in Analysis of Vascular Calcification. Circ Res. 2017;120(8):1240–2. doi: 10.1161/CIRCRESAHA.116.310326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bittencourt MS, Cerci RJ. Statin effects on atherosclerotic plaques: regression or healing? BMC Med. 2015;13:260. doi: 10.1186/s12916-015-0499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imoto K, Hiro T, Fujii T, Murashige A, Fukumoto Y, Hashimoto G et al. Longitudinal structural determinants of atherosclerotic plaque vulnerability: a computational analysis of stress distribution using vessel models and three-dimensional intravascular ultrasound imaging. J Am Coll Cardiol. 2005;46(8):1507–15. doi: 10.1016/j.jacc.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 79.Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF et al. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004;110(3):356–62. doi: 10.1161/01.CIR.0000135469.82545.D0. [DOI] [PubMed] [Google Scholar]
- 80.Hecht HS, Budoff MJ, Berman DS, Ehrlich J, Rumberger JA. Coronary artery calcium scanning: Clinical paradigms for cardiac risk assessment and treatment. Am Heart J. 2006;151(6):1139–46. doi: 10.1016/j.ahj.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 81.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Rumberger JA, Kaufman L. A rosetta stone for coronary calcium risk stratification: agatston, volume, and mass scores in 11,490 individuals. AJR Am J Roentgenol. 2003;181(3):743–8. doi: 10.2214/ajr.181.3.1810743. [DOI] [PubMed] [Google Scholar]
- 83.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208(3):807–14. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 84.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–7. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 85.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25(1):234–9. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 86.Kramer CM, Anderson JD. MRI of atherosclerosis: diagnosis and monitoring therapy. Expert Rev Cardiovasc Ther. 2007;5(1):69–80. doi: 10.1586/14779072.5.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedrich GJ, Moes NY, Muhlberger VA, Gabl C, Mikuz G, Hausmann D et al. Detection of intralesional calcium by intracoronary ultrasound depends on the histologic pattern. Am Heart J. 1994;128(3):435–41. [DOI] [PubMed] [Google Scholar]
- 88.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99(10):1044–59. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 89.Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401–15. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 90.Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106(13):1640–5. [DOI] [PubMed] [Google Scholar]
- 91.Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39(4):604–9. [DOI] [PubMed] [Google Scholar]
- 92.Orbay H, Hong H, Zhang Y, Cai W. Positron emission tomography imaging of atherosclerosis. Theranostics. 2013;3(11):894–902. doi: 10.7150/thno.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med. 2010;51(6):862–5. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 94.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125(1):76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 95.Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E et al. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography--a novel concept. Hell J Nucl Med. 2011;14(2):114–20. [PubMed] [Google Scholar]
- 96.Allen S, Liu YG, Scott E. Engineering nanomaterials to address cell-mediated inflammation in atherosclerosis. Regen Eng Transl Med. 2016;2(1):37–50. doi: 10.1007/s40883-016-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7(2):68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kheirolomoom A, Kim CW, Seo JW, Kumar S, Son DJ, Gagnon MK et al. Multifunctional Nanoparticles Facilitate Molecular Targeting and miRNA Delivery to Inhibit Atherosclerosis in ApoE(−/−) Mice. ACS Nano. 2015;9(9):8885–97. doi: 10.1021/acsnano.5b02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JS, Morrisett JD, Tung CH. Detection of hydroxyapatite in calcified cardiovascular tissues. Atherosclerosis. 2012;224(2):340–7. doi: 10.1016/j.atherosclerosis.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulz RB, Semmler W. Fundamentals of optical imaging. Handb Exp Pharmacol. 2008(185 Pt 1):3–22. doi: 10.1007/978-3-540-72718-7_1. [DOI] [PubMed] [Google Scholar]
- 101.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–86. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 102.Pellicoab AVL-V J, Benitoa M, García-Segurab JM, Fusterc V, Ruiz-Cabelloab J and Herranz F. Microwave-driven synthesis of bisphosphonate nanoparticles allows in vivo visualisation of atherosclerotic plaque. Royal Society of Chemistry Advances. 2014;5:1661–5. doi: 10.1039/C4RA13824D. [DOI] [Google Scholar]
- 103.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265–70. [DOI] [PubMed] [Google Scholar]
- 104.Lei Y, Nosoudi N, Vyavahare N. Targeted chelation therapy with EDTA-loaded albumin nanoparticles regresses arterial calcification without causing systemic side effects. J Control Release. 2014;196:79–86. doi: 10.1016/j.jconrel.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li N, Song J, Zhu G, Shi X, Wang Y. Alendronate conjugated nanoparticles for calcification targeting. Colloids Surf B Biointerfaces. 2016;142:344–50. doi: 10.1016/j.colsurfb.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 106.Choi SW, Kim JH. Design of surface-modified poly(D,L-lactide-co-glycolide) nanoparticles for targeted drug delivery to bone. J Control Release. 2007;122(1):24–30. doi: 10.1016/j.jconrel.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 107.Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials. 2012;33(29):7164–73. doi: 10.1016/j.biomaterials.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 108.Swami A, Reagan MR, Basto P, Mishima Y, Kamaly N, Glavey S et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci U S A. 2014;111(28):10287–92. doi: 10.1073/pnas.1401337111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hengst V, Oussoren C, Kissel T, Storm G. Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int J Pharm. 2007;331(2):224–7. doi: 10.1016/j.ijpharm.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 110.Sanchez F, Zhang L. Molecular dynamics modeling of the interface between surface functionalized graphitic structures and calcium-silicate-hydrate: interaction energies, structure, and dynamics. J Colloid Interface Sci. 2008;323(2):349–58. doi: 10.1016/j.jcis.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 111.Murphy MB, Hartgerink JD, Goepferich A, Mikos AG. Synthesis and in vitro hydroxyapatite binding of peptides conjugated to calcium-binding moieties. Biomacromolecules. 2007;8(7):2237–43. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- 112.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–55. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Mao J, Shi X, Wu YB, Gong SQ. Identification of Specific Hydroxyapatite {001} Binding Heptapeptide by Phage Display and Its Nucleation Effect. Materials (Basel). 2016;9(8). doi: 10.3390/ma9080700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roy SKS Marc D., Amis Eric J., Becker Matthew L. Identification of a Highly Specific Hydroxyapatite-binding Peptide using Phage Display. Advanced Materials. 2008;20(10):1830–6. [Google Scholar]
- 115.Weiger MC, Park JJ, Roy MD, Stafford CM, Karim A, Becker ML. Quantification of the binding affinity of a specific hydroxyapatite binding peptide. Biomaterials. 2010;31(11):2955–63. doi: 10.1016/j.biomaterials.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 116.Hauschka PV, Carr SA. Calcium-dependent alpha-helical structure in osteocalcin. Biochemistry. 1982;21(10):2538–47. [DOI] [PubMed] [Google Scholar]
- 117.Meisel CL, Bainbridge P, Mitsouras D, Wong JY. Targeted Nanoparticle Binding to Hydroxyapatite in a High Serum Environment for Early Detection of Heart Disease. ACS Applied Nano Materials. 2018. doi: 10.1021/acsanm.8b01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bain JL, Culpepper BK, Reddy MS, Bellis SL. Comparing variable-length polyglutamate domains to anchor an osteoinductive collagen-mimetic peptide to diverse bone grafting materials. Int J Oral Maxillofac Implants. 2014;29(6):1437–45. doi: 10.11607/jomi.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bain JL, Bonvallet PP, Abou-Arraj RV, Schupbach P, Reddy MS, Bellis SL. Enhancement of the Regenerative Potential of Anorganic Bovine Bone Graft Utilizing a Polyglutamate-Modified BMP2 Peptide with Improved Binding to Calcium-Containing Materials. Tissue Eng Part A. 2015;21(17–18):2426–36. doi: 10.1089/ten.TEA.2015.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Culpepper BK, Bonvallet PP, Reddy MS, Ponnazhagan S, Bellis SL. Polyglutamate directed coupling of bioactive peptides for the delivery of osteoinductive signals on allograft bone. Biomaterials. 2013;34(5):1506–13. doi: 10.1016/j.biomaterials.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]