ABSTRACT
The antitumor activity of Lycium barbarum polysaccharide (LBP) has been reported, but the structure–bioactivity relationship has still not been fully elucidated. In this study, four water-soluble LBP fractions with serial different molecular weights (MWs) were separated from LBP, designated LBP-2, LBP-3, LBP-4, and LBP-5. After a characteristic analysis, the relationship between MW and antitumor activity of LBP was investigated both in vitro using murine hepatoma H22 cells and in vivo using H22 tumor-bearing mice. In vitro, the results showed that all the LBP fractions had significant inhibition on H22 cells, in which LBP-3 had the best activity. LBP-3 could induce apoptosis, mitochondrial membrane potential destruction, and S phase arrest in H22 cells. In vivo, the results showed that LBP-2, LBP-3, LBP-4, and LBP-5 could inhibit the tumor growth in H22 tumor-bearing mice by 18.18%, 37.97%, 9.09%, and 14.44%, respectively. However, only LBP-3 was able to decrease the tumor weight significantly in H22 tumor-bearing mice. Meanwhile, all the LBP fractions did not show significant toxicity to murine splenocytes, thymus, and spleen. Taken together, these results demonstrated that the antitumor activity of LBP was closely related to its MW, and LBP-3 with medium MW (40–350 kDa) was the main active fraction.
KEYWORDS: Lycium barbarum polysaccharide, molecular weight, antitumor activity; H22 cells; flow cytometry; apoptosis
Introduction
Lycium barbarum polysaccharide (LBP), which is extracted from the fruit of a well-known Chinese herb Lycium barbarum, is found to have antitumor activity in recent years. In vitro study results showed that LBP treatment inhibited tumor cells growth. Zhang [1] found that LBP treatment inhibited the growth of QGY7703 cells via induction of S-phase cell-cycle arrest and calcium-related apoptosis. Similar studies showed that LBP treatment could inhibit the growth of mouse colon cancer cells, human gastric cancer MGC-803, and SGC-7901 cells, and arrest the cell cycles at the G0/G1 or S phases [2,3]. The anticancer effects of LBP were also demonstrated in tumor-bearing mice. Gan [4] revealed that a polysaccharide–protein complex from Lycium barbarum could significantly inhibit the growth of transplantable sarcoma S180 and improved the immune system in S180-bearing mice. Another study led by Zhang [5] found that LBP could obviously depress the death rate of H22 tumor-bearing mice and diminish gross tumor volume and weight.
Although the anticancer effect of LBP has been demonstrated both in vitro and in vivo, various LBP samples, obtained by different methods, have different structures and molecular weight (MW) [6]. Recent studies have indicated that biological activities of the polysaccharide partly depend on its MW [7]. LBP has an MW range of 10–2300 kDa, and its antitumor activity is probably related to MW [7]. Zhang [8] observed the effects of LBP fractions on human liver cancer cells (SMMC-7721) and found that LBP-a4 with an MW of 10.2 kDa could inhibit the proliferation of SMMC-7721 cells in a dose- and time-dependent manner, but LBP-p8 with an MW of 6.50 × 103 kDa could promote the growth of SMMC-7721 cells. However, the detailed relationship between the MWs and antitumor activities of LBP fractions is still not fully clear.
To further study the MW-antitumor activity relationship of LBP, LBP was separated into five fractions (LBP-1, LBP-2, LBP-3, LBP-4, and LBP-5) with serial different MWs by ultrafiltration membranes in this study. Then, the water-soluble LBP fractions (LBP-2, LBP-3, LBP-4, and LBP-5) were taken to investigate the antitumor activity both using murine hepatoma H22 cells in vitro and using H22 tumor-bearing mice in vivo.
Materials and methods
Materials
Penicillin–Streptomycin solution was purchased from Genom Biomedical Technology Co., Ltd (Hangzhou, China). Cisplatin Injection was purchased from NanJing Pharmaceutical Factory Co.,Ltd (Nanjing, China). Doxorubicin (Dox) for injection was purchased from Shenzhen Main Luck Pharmaceuticals Inc. (Shenzhen, China). Dimethyl sulfoxide (DMSO) was purchased from Tianjin Yongda Chemiacl Reagent Co., Ltd (Tianjin, China). Cyclophosphamide (Cy) for injection was purchased from Shanxi Powerdone Pharmaceutics Co., Ltd (Shanxi, China). RPMI-1640 was purchased from Corning; Rhodamine 123, propidium iodide (PI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), carbazole, galacturonic acid, and ribonuclease (RNase) were purchased from Sigma (St. Louis, USA). Annexin V-FITC/PI Apoptosis Detection Kit was purchased from eBioscience (Santa Clara, USA). Fetal bovine serum (FBS) was purchased from Biological Industries (Kibbutz Beit-Haemek, Israel).
Preparation and characterization analysis of LBP fractions
LBP was prepared with hot water as described previously [9]. The LBP fractions with different series MW were prepared according to the relevant reference [8]. Briefly, the LBP fractions were isolated by ultrafiltration membranes with an MW cutoff (MWCO) of 3, 8, 40, 350, and 400 kDa successively. Based on the MW, the fractions were named LBP-1, LBP-2, LBP-3, LBP-4, and LBP-5, respectively. The powder of the fractions was obtained by freeze-drying. The water-soluble LBP fractions (LBP-2, LBP-3, LBP-4, and LBP-5) were taken for investigation in the following experiments.
The characterization of LBP fractions was analyzed as described previously [10–13]. Briefly, the total sugar was determined by the phenol-sulfuric acid method [10]. Protein content was measured by the Bradford method [11]. Uronic acid was measured by the sulfuric acid carbazole colorimetry method, and galacturonic acid was used as the standard [12]. The vibrations of molecules and polar bonds between the different atoms in LBP fractions were analyzed using IR (Avatar 30FT-IR infrared spectrometer, Nicnet) [13].
Cell line and animals
Murine hepatoma H22 cells were cultured in RMPI-1640 with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37° with 5% CO2. The cells were subcultured until they reached the logarithmic growth phase.
Specific pathogen-free male BALB/c mice, weighing 20 ± 2 g, were purchased from the Guangdong Medical Laboratory Animal Center (Foshan, China). Animals were fed on a standard laboratory diet and water, and were kept in environmentally controlled quarters with temperature maintained at 25°C and a 12/12 h dark/light cycle. All experimental procedures were approved by the Animal Care and Use Committee of Guangzhou University of Chinese Medicine, PR China.
In vitro antitumor assay
Cell viability assay
The effect of LBP fractions on cell viability was measured using the MTT assay. H22 cells in logarithmic growth phase were plated in 96-well plates (1 × 104 cells/well) and treated with LBP-2, LBP-3, LBP-4, and LBP-5 (400, 800, 1200, and 1600 μg/mL) immediately for 24 h and 48 h. MTT (5 mg/mL) was added to each well at 24 h and 48 h respectively, and cells were incubated for another 4 h. After centrifugation at 300 × g for 10 min, the supernatant was removed, and 0.15 mL of DMSO was added to each well. The absorbance at 570 and 630 nm was measured on a microplate reader (Thermo Fisher Scientific, Waltham, USA). The percent viability of the treated cells was calculated using the following equation: relative viability (%) = (A 570 nm – A 630 nm) sample / (A 570 nm – A 630 nm) control × 100%.
In order to determine whether LBP fractions also have cytotoxicity to immune cells, the effects of LBP fractions on splenocytes viability were investigated in this study. Splenocytes were prepared as described previously with minor modifications [14]. Briefly, splenocytes were isolated from BALB/c mice, and the erythrocytes were lysed with red blood cell lysis buffer (0.829 mg/mL NH4Cl, 0.037 mg/mL Na2EDTA and 0.100 mg/mL KHCO3). After washing twice with precold phosphate-balanced solution (PBS), cells were resuspended in RMPI-1640 with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and seeded in 96-well plates (4 × 105 cells/well, sextuplicate wells in each group). After being treated with LBP fractions (400, 800, 1200, and 1600 μg/mL) for 72 h, the cell viability was measured by MTT assay as described above.
Cell-proliferation assay
The effect of LBP fractions on cell proliferation was detected by flow cytometry (FACSCanto II flow cytometer, BD Biosciences, Franklin Lakes, USA). H22 cells were treated with LBP-2, LBP-3, LBP-4, and LBP-5 (400, 800, 1200, and 1600 μg/mL) for 24, 48, and 72 h. Cells treated with RPMI-1640 medium were taken as negative controls, and those treated with cisplatin (10 μg/mL) were taken as positive controls. The cells were collected and washed twice with PBS before detection. Relative cell number was counted by flow cytometry for 1 min under a medium speed. The inhibition of LBP fractions on proliferation of H22 cells was calculated using the following equation: inhibition rate (%) = (cell number of negative control – cell number of sample) / cell number of negative control × 100%.
Apoptosis analysis
Cell apoptosis was analyzed using the Annexin V-FITC/PI apoptosis detection kit as described previously [15]. Briefly, after being treated with LBP-3 (400, 800, 1200 μg/mL) for 48 h, H22 cells were harvested and washed twice with PBS and stained with Annexin V-FITC/PI according to the manufacturer’s instructions. The samples were detected on an FACS Canto II flow cytometer (BD Biosciences) and analyzed using FACS Diva™ software.
MMP measurement
The MMP was detected by Rhodamine123 (Rho123), using flow cytometry and a fluorescence microscope (Nikon). After being treated with LBP-3 (400, 800, 1200 μg/mL) for 48 h, H22 cells were washed with PBS and dyed with Rho123 (10 μg/mL) at 37°C for 30 min. Morphological observations were carried out using a fluorescence microscope. The fluorescence intensity of cell was detected on an FACS Canto II flow cytometer (BD Biosciences) and analyzed with FACS Diva™ software.
Cell-cycle analysis
Cell cycles were analyzed by flow cytometry with PI staining as described previously [1]. Briefly, H22 cells were seeded in 24-well plates (5 × 104 cells/well) and treated with LBP-3 (400, 800, 1200 μg/mL) for 72 h. The cells were harvested, washed twice with PBS, and fixed with 70% ethanol for 24 h at −20°C. After two washes with PBS, cells were stained with PI (10 μg/mL) and RNase (10 μg/mL) at room temperature for 30 min in the dark. The samples were detected by flow cytometry and analyzed with ModFit LT cell-cycle-analysis software.
In vivo antitumor assay
Animal experiments and treatment protocol
To investigate the antitumor activity of LBP fractions in vivo, the murine H22 hepatocarcinoma model was used in this study. The animal experiments and treatment protocol were prepared as described previously with minor modifications [16]. Briefly, H22 cells (2 × 106 cells/mouse) were injected into the right armpit subcutaneously in BALB/c mice. Twenty-four hours after the tumor inoculation of H22 cells, the mice were divided into the model group, LBP-2 group, LBP-3 group, LBP-4 group, and LBP-5 group. Mice were treated with LBP fractions intragastrically at a dose of 250 mg/kg, except for the model group. The treatments were administered once daily for 10 days. To further investigate whether LBP-3 could inhibit tumor growth in a dose-dependent manner, mice were divided into the model group, cyclophosphamide (Cy, 20 mg/kg) treatment group, Doxorubicin (Dox, 10 mg/kg) treatment group, and three doses of LBP-3 (62.5, 125 and 250 mg/kg) treatment group. Cy was given intragastrically once daily for 10 days. Dox was injected intraperitoneally on day 1. LBP-3 was given intragastrically as described above.
Tumor inhibition rate and immune organ index
Twenty-four hours after the last administration, mice were killed immediately by cervical dislocation after anesthesia with chloral hydrate. The tumor, spleen, and thymus were immediately dissected and weighed. The tumor inhibition rate and immune organ indexes were calculated using the following equation respectively: tumor inhibition rate (%) = (the average tumor weight of model group – the average tumor weight of LBP fraction group) / the average tumor weight of model group × 100%, spleen or thymus index (mg/g) = the weight of spleen or thymus (mg) / body weight (g).
Statistical analysis
All values were expressed as mean ± SD. A one-way analysis of variance, followed by the Dunnett t test, was used to assess the statistical significance of differences between experimental groups. A P value of <0.05 was considered to be significant.
Results
Characterization of LBP fractions
The results showed that the total sugar contents of LBP-2, LBP-3, LBP-4, and LBP-5 were 66.88%, 54.47%, 56.53%, and 55.21%, respectively. The uronic acid, protein, and bound water were also found in the LBP fractions (Table 1).
Table 1.
Components of LBP fractions.
| Fractions | LBP-2 | LBP-3 | LBP-4 | LBP-5 |
|---|---|---|---|---|
| Molecular weights (kDa) | 350–400 | 40–350 | 8–40 | 3–8 |
| Total sugar (%) | 66.88 | 54.7 | 56.53 | 55.21 |
| Uronic acid (%) | 11.03 | 8.11 | 10.65 | 10.14 |
| Protein (%) | 0.27 | 1.14 | 0.11 | 0.34 |
| Bound water (%) | 12.80 | 14.59 | 14.88 | 15.85 |
| Fat (%) | 4.30 | 11.10 | 7.25 | 2.10 |
The carbohydrate-related molecules, such as C–H and C=C bonds, CH3 group, and glycosidic linkages, have characteristic absorptions in IR spectra [13]. Thus, IR spectra are usually used to identify the characteristic absorptions of polysaccharides. In the present study, the results showed that the IR spectra in all the LBP fractions appeared at about 3400, 2930, 1627, 1410, 1077, 918, 865, 818, and 778 cm−1 (Table 2). However, the absorption at 918 cm−1 was mainly found in LBP-4. The absorption peaks at about 3400 and 2930 cm−1 were due to hydroxyl stretching vibrations and C–H stretching vibrations, respectively [17,18]. The signal at about 1627 cm−1 was due to the bound water [17], consistent with the fact that the bound water was found in the LBP fractions. The absorption at 1410 and 1077 cm−1 was possibly due to nonsymmetric CH3 bending and C–O stretching vibration, respectively [13]. The small absorption band at 918 cm−1 was characteristic of β-glycosidic linkages between the sugars [18] and indicated that the β-glycosidic linkage mainly existed in the LBP-4. The characteristic absorption bands at 865 cm−1 and 818 cm−1 indicated that α-configuration and mannose existed in the LBP fractions [17,19]. The present results demonstrated that all the LBP fractions shared most of the characteristic absorptions.
Table 2.
Results of IR analysis of LBP fractions.
| Fractions | IR bands (cm−1) |
|---|---|
| LBP-2 | 3429, 2931, 1634, 1410, 1077, 865, 818, 778 |
| LBP-3 | 3405, 2932, 1627, 1410, 1077, 866, 818, 778 |
| LBP-4 | 3403, 2933, 1625, 1412, 1077, 918, 865, 818, 778 |
| LBP-5 | 3411, 2932, 1625, 1412, 1077, 865, 818, 778 |
LBP fractions were toxic to H22 cells, but not to murine splenocytes
To investigate the effect of the water-soluble LBP fractions (LBP-2, LBP-3, LBP-4, and LBP-5) on the viability of H22 cells, cells were measured by MTT assay after being treated with LBP fractions for 24 h and 48 h. The results showed that all the LBP fractions inhibited the viability of H22 cells in a dose-dependent manner (Figure 1(a,b)). Among these fractions, LBP-3 had the best inhibition with the lowest cell viability at the dose of 1600 μg/mL for 48 h.
To further confirm whether LBP fractions were also toxic to normal cells, the viability of murine splenocytes was measured by MTT assay after being treated with LBP fractions for 48 h. As shown in Figure 1(c), the results showed that LBP fractions were not toxic to murine splenocytes. On the contrary, LBP-3, LBP-4, and LBP-5 enhanced the cell viability. This was consistent with the previous studies that LBP could promote the proliferation of murine lymphocytes [20].
Figure 1.
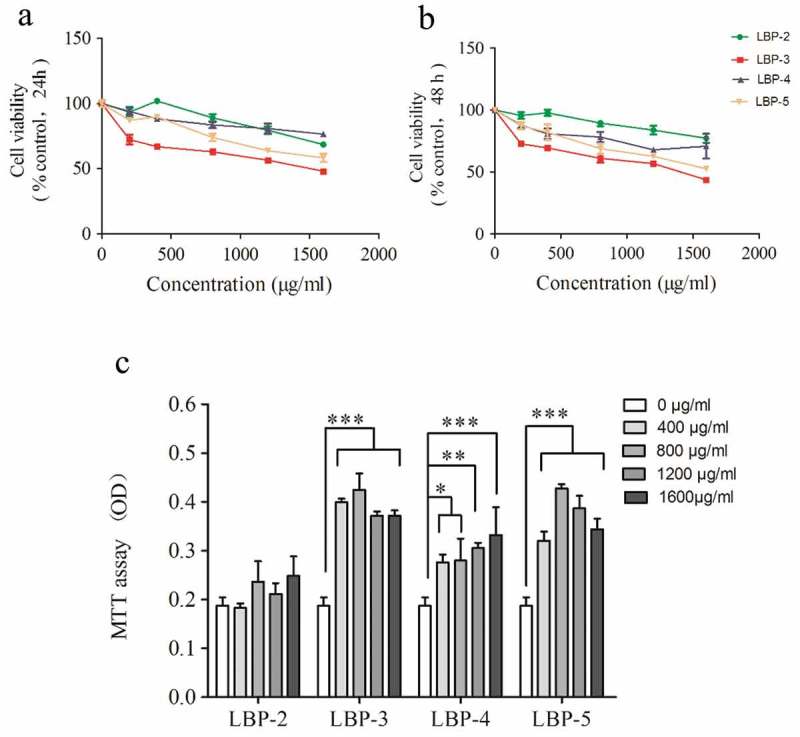
Effect of LBP fractions on the viability of H22 cells and splenocytes. H22 cells were treated with LBP fractions for 24 h (A) and 48 h (B). Splenocytes were treated with LBP fractions for 48 h (C). Cell viability was measured by MTT assay, and results are presented as percentage of control (untreated cells). Values are shown as the means ± SD of three replicates (n = 3) for H22 cells or six replicates (n = 6) for splenocytes. *P<0.05, **P<0.01 and ***P<0.001, compared with the control group (0 μg/mL).
LBP fractions inhibited the growth of H22 cells, and LBP-3 had the highest inhibition activity
The effect of LBP fractions on H22 cells proliferation were measured by FCM. The results (Figure 2(a)) showed that all the LBP fractions could inhibit the growth of H22 cells in a dose-dependent manner. Among the four fractions, LBP-3 had the highest inhibition activity, especially at the dose of 1600 μg/mL for 48 h where the percentage of inhibition was over 60%. Further study showed that LBP-3 inhibited growth of H22 cells in dose- and time-dependent manners (Figure 2(b)).
Figure 2.
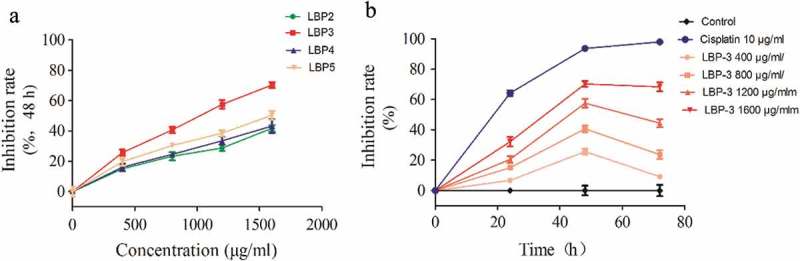
Effect of LBP fractions on H22 cells proliferation. (A) Cells were treated with LBP2-5 (400–1600 μg/mL) for 48 h. (B) Cells were treated with LBP-3 (400–1600 μg/mL) for 24–72 h. Cell numbers were counted by FCM, and results are presented as a percentage of control (untreated cells). Values are shown as the Means ± SD of three replicates (n = 3).
Since LBP-3 had the highest inhibition activity on H22 cells, the following in vitro experiments were focused on LBP-3.
LBP-3 induced apoptosis of H22 cells
Annexin V-FITC/PI apoptosis detection kit is a standard method that is used to assess cell apoptosis [21]. A previous study showed that LBP could induce apoptosis of human hepatoma SMMC-7721 cells [6]. To determine whether LBP-3 could induce apoptosis of H22 cells, the apoptosis of cells was measured using the Annexin V-FITC/PI apoptosis detection kit using FCM after the cells were treated with LBP-3 for 24 h and 48 h (Figure 3). The results showed that LBP-3 significantly induced apoptosis of H22 cells in a dose-dependent manner.
LBP-3 disrupted MMP on H22 cells
Since the mitochondrion is the powerhouse of the cell [22], disruption of the mitochondrial membrane potential (MMP) is one of the crucial factors in cell apoptosis [23] and has become a pharmacological target in cancer therapy for alterations in mitochondrial structure and functions that have long been observed in cancer cells [24]. Rhodamine-123 as a cationic fluorescent probe has been used for the measurement of MMP [25]. To investigate whether LBP-3-induced apoptosis of H22 cells was related to the MMP disruption, H22 cells were treated with different concentrations of LBP-3 for 48 h. Then, the MMP was measured using Rho123 as a fluorescent probe with fluorescence microscopy and FCM, respectively. The fluorescence microscopy results showed that Rho123 accumulation in H22 cells decreased significantly after treatment with LBP-3 (Figure 4(a)). The FCM analysis results showed that LBP-3 disrupted the MMP of H22 cells in a dose-dependent manner (Figure 4(b)).
Figure 3.
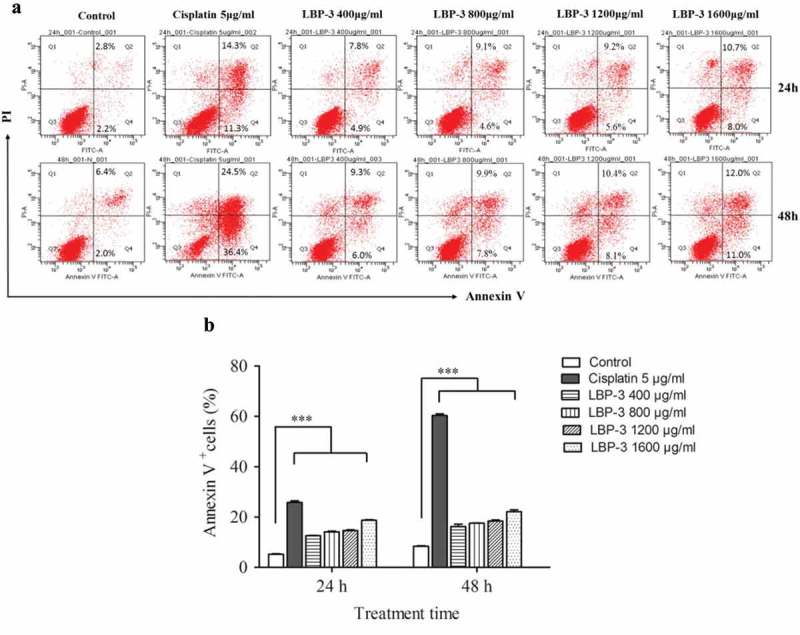
Effect of LBP-3 on H22 cells apoptosis. Cells were treated with LBP-3 (400–1600 μg/mL) for 24 and 48 h. Cells were stained by Annexin V/PI, and the apoptotic cell death (Annexin V+) was analyzed by FCM. Values are shown as the means ± SD of three replicates (n = 3). ***P<0.001, compared with the control group.
Figure 5.
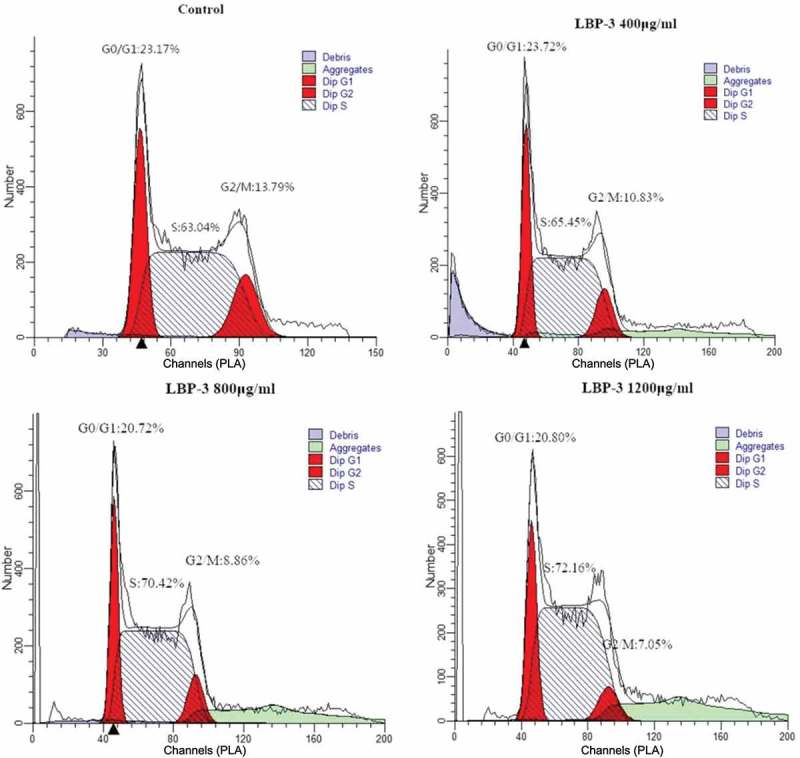
Inhibition of cell-cycle progress in H22 cells by treatment with LBP-3. H22 cells were treated with LBP-3 or medium for 72 h. Cells were fixed with 70% ethanol and stained with PI, and then the cell-cycle distribution was analyzed by flow cytometry.
Figure 4.
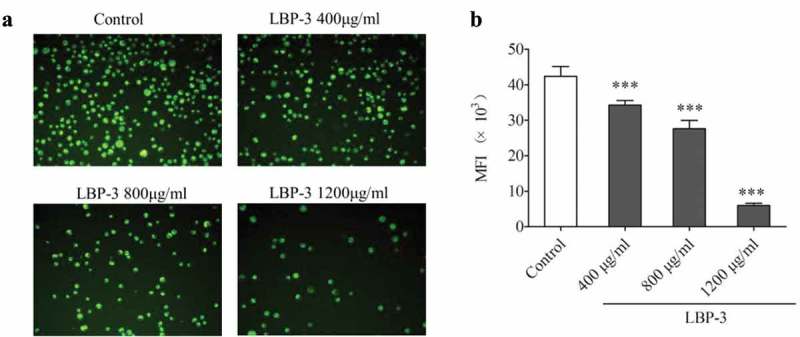
Effect of LBP-3 on H22 cells MMP. After being treated with LBP-3 (400–1200 μg/mL) for 48 h, cells were stained with Rho123 and observed in fluorescence microscopy with ×200 magnification (A). The Rho123 MFI of cells was measured by flow cytometry (B). Results of MMP are presented as mean of Rho123 MFI. Values were shown as the means ± SD of three replicates (n = 3). ***P<0.001, compared with the control group.
LBP-3 arrested H22 cells at the S phase
Previous studies had shown that LBP could arrest different human cancer cell lines at different phases [1–3]. To investigate whether LBP-3 inhibited H22 cell proliferation was related to arrest of cell-cycle progression, cell-cycle distribution of H22 cells was assayed by FCM after being treated with LBP-3 (400, 800, and 1200 μg/mL) for 72 h. The results showed that LBP-3 treatment caused a significant increase in cell number in the S phase (Table 3 and Figure 5), suggesting that LBP-3 could arrest H22 cells at the S phase directly.
Table 3.
Effect of LBP-3 on cell-cycle progression in H22 cells.
| Groups | Concentration (μg/mL) | G0/G1 phase (%) | S phase (%) | G2/M phase (%) |
|---|---|---|---|---|
| Control | 0 | 23.83 ± 0.58 | 63.7 ± 0.96 | 12.48 ± 1.32 |
| LBP-3 | 400 | 23.82 ± 0.09 | 65.86 ± 1.07* | 10.59 ± 0.67 |
| 800 | 21.49 ± 0.46*** | 70.41 ± 0.72*** | 8.03 ± 0.82*** | |
| 1200 | 21.17 ± 0.37*** | 72.25 ± 0.1*** | 6.58 ± 0.47*** |
Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group.
Effects of LBP fractions on tumor growth, thymus, and spleen index in H22 tumor-bearing mice
In order to confirm the results that LBP-3 had the highest antitumor activity as shown above among the LBP fractions, the effects of LBP fractions on tumor growth in H22 tumor-bearing mice were investigated. Since tumor-mass reduction often indicates a strong antitumor effect [16], tumor inhibition rate as an indicator was used to evaluate the antitumor activity of LBP fractions in the present study. The results showed that LBP-2, LBP-3, LBP-4, and LBP-5 could inhibit tumor growth in H22 tumor-bearing mice by 18.18%, 37.97%, 9.09%, and 14.44%, respectively. However, only LBP-3 significantly decreased the tumor weight in the mice (Table 4). In the further study, we found that LBP-3 inhibited the tumor growth in a dose-dependent manner (Table 5).
Table 4.
Effect of LBP fractions on tumor growth, thymus index, and spleen index in H22 tumor-bearing mice.
| Groups | Dose (mg/kg) | Tumor weight (g) | Inhibition rate (%) | Spleen index (mg/g) | Thymus index (mg/g) |
|---|---|---|---|---|---|
| Model | 0 | 1.87 ± 0.65 | – | 7.08 ± 1.05 | 1.32 ± 0.39 |
| LBP-2 | 250 | 1.53 ± 0.37 | 18.18 | 6.66 ± 1.59 | 1.35 ± 0.33 |
| LBP-3 | 250 | 1.16 ± 0.40* | 37.97 | 7.25 ± 1.81 | 1.44 ± 0.29 |
| LBP-4 | 250 | 1.70 ± 0.43 | 9.09 | 7.99 ± 0.43 | 1.52 ± 0.34 |
| LBP-5 | 250 | 1.60 ± 0.72 | 14.44 | 7.33 ± 1.17 | 1.41 ± 0.34 |
Data are shown as mean ± SD (n = 10). *P < 0.05 vs the model group.
Table 5.
Effect of LBP-3 on tumor growth and thymus index in H22 tumor-bearing mice.
| Groups | Dose (mg/kg) | Tumor weight (g) |
Inhibition rate (%) | Thymus index (mg/g) |
|---|---|---|---|---|
| Model | 1.63 ± 0.54 | – | 1.05 ± 0.27 | |
| Cy | 20 | 0.86 ± 0.25** | 47.24 | 0.38 ± 0.11*** |
| Dox | 10 | 0.24 ± 0.09*** | 85.28 | 0.15 ± 0.06*** |
| LBP-3 | 62.5 | 1.23 ± 0.35 | 24.54 | 1.04 ± 0.19 |
| 125 | 1.15 ± 0.51 | 29.45 | 1.16 ± 0.21 | |
| 250 | 0.90 ± 0.40** | 44.79 | 1.13 ± 0.30 |
Data are shown as mean ± SD (n = 8). **P < 0.01, ***P < 0.001 vs the control group.
Accumulating evidence had demonstrated that chemotherapy drugs often resulted in immunosuppressive side-effects [26–30]. To investigate whether LBP fractions could cause immunosuppressive side-effects in H22 tumor-bearing mice, the effects of LBP fractions on the spleen and thymus index were investigated in the present study. As expected, Dox and Cy significantly reduced the thymus index of the mice (Table 5). However, the LBP fractions had no significant effect on the spleen and thymus index of the mice (Tables 4 and 5). The results indicated that the LBP fraction did not cause immunosuppressive side-effects in the H22 tumor-bearing mice.
Discussion
In the present study, four water-soluble LBP fractions with serial different MW were isolated from LBP, in which we found that LBP-3 with medium MW had the highest antitumor activity in both in vitro and in vivo studies.
Biological activities of the polysaccharide partly depend on its MW [7]. Polysacchrides from Lycium barbarum have been demonstrated to have anticancer activity both in vitro and in vivo. However, LBP has an MW range of 10–2300 kDa [7], and the detailed relationship between the MW and antitumor activities of LBP fractions is still unclear. To clarify this, LBP were cut into five fractions with a series of different MWs, and the four water-soluble fractions were taken for investigation in the present study. Firstly, we investigated the effects of LBP fractions on viability and proliferation of H22 cells in vitro. Although all the LBP fractions could inhibit the viability and proliferation of H22 cells, LBP-3 had shown the best activity. Secondly, we investigated the effect of LBP fractions on tumor growth in H22 tumor-bearing mice to confirm the in vitro results. In accordance with the study in vitro, LBP3 had the best antitumor activity among the four LBP fractions in vivo. To our knowledge, this is the first investigation of antitumor activity in an LBP fraction with a series of different MWs both in vitro and in vivo at the same time. The results indicate that LBP-3 is the main active fraction of LBP in antitumor activity.
Apoptosis and cell-cycle arrest are both mechanisms for cytotoxic anticancer drugs work in cancer treatment [31,32]. Previous studies had shown that LBP could induce cancer cell apoptosis and cell-cycle arrest [1–5]. In our study, we found that LBP3 disrupted MMP and induced H22 cell apoptosis. The mitochondrion is the powerhouse of the cell [22], and we conclude that LBP-3 induced H22 cell apoptosis partly by disrupting MMP. Furthermore, LBP-3 could cause H22 cell arrest at the S phase. This agrees with several previous reports that LBP caused S phase arrest in human hepatoma QGY7703 cells [1] and gastric cancer SGC-7901 cells [3]. However, the other studies showed that LBP caused G0/G1 phase arrest in gastric cancer MGC-803 cells [3] and colon cancer cells [2]. These indicate that multiple mechanisms may be responsible for the anticancer effects of LBP in different types of cancer cells [2].
It is known that cytotoxic chemotherapy drugs not only kill cancer cells but also kill normal cells, which causes toxic side effects [33]. Myelosuppression and immunosuppression are common side effects for most chemotherapy drugs. Since the immune system plays an important role in preventing pathogen invasion, immunosuppression often leads to the development of opportunist infections, and even significant morbidity and mortality [30]. In our study, we found that none of the LBP fractions were cytotoxic to splenocytes, and furthermore LBP3, LBP4, and LBP5 could improve cell viability. In accordance with the in vitro study, none of the LBP fractions suppressed the thymus or spleen index in H22-tumor-bearing mice. In fact, LBP is extracted from the edible Chinese herb, Lycium barbarum. Previous studies have shown that LBP could enhance the proliferation of splenocytes in mice [34,35], activate macrophages [36], and promote both the phenotypic and functional maturation of DC [37]. Besides, LBP also has various other biological activities, such as neuroprotective effects [38,39], radioprotective activity [40], cardioprotective activity [41], and hepatoprotective effects [42,43]. Clinical trials have shown that LBP had remarkable protective effects in patients with type 2 diabetes [44]. These indicate that LBP-3 might be safe for cancer treatment. In order to support our conclusion, we further investigated the effect of LBP-3 on liver and kidney in H22-tumor-bearing mice. Results showed that LBP-3 did not cause damage in either liver or kidney tissue (Figure S1 in the supplemental file). Meanwhile, we found that LBP-3 did not cause the serum levels of AST, ALT or BUN increase (Table S1 in the supplemental file) which often indicates the dysfunction of liver and kidney, respectively. Taken together, we conclude that LBP-3 may be safe for antitumor therapy.
Conclusions
In the present study, the MW–antitumor activity relationship of LBP was investigated both in vitro and in vivo. In summary, four water-soluble LBP fractions (LBP-2, LBP-3, LBP-4, and LBP-5) with serial different MWs were separated from LBP by ultrafiltration membranes. In vitro, LBP-3 had the highest inhibition activity on H22 cells among the four fractions. LBP-3 induced apoptosis, MMP destruction, and S phase arrest in H22 cells, but it did not inhibit the cell viability of murine splenocytes. Consistent with the results in vitro, LBP-3 had the highest activity in inhibiting tumor growth and did not suppress the thymus and spleen index in H22-tumor-bearing mice. These results demonstrated that the antitumor activity of LBP was closely related to its MW. LBP-3 with medium MW was the main active fraction of LBP in antitumor therapy, and it seemed to be safe for use in antitumor therapy.
Supplementary Material
Acknowledgments
This study was supported by the Specialized Research Fund for the Doctoral Program of Higher Education [Grant No. 20124425110010]; The Special Funds from Central Finance of China in Support of the Development of Local Colleges and University [Educational finance Grant No. 276 (2014)]; and the project funded by the China Postdoctoral Science Foundation [Grant No. 2016M602548]. We also thank Prof. Xiao-Jun Zhang and Yang Chen for critical reading of the manuscript.
Funding Statement
This work was supported by the China Postdoctoral Science Foundation [2016M602548]; Specialized Research Fund for the Doctoral Program of Higher Education [20124425110010]; The Special Funds from Central Finance of China in Support of the Development of Local Colleges and University [276 (2014)].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed here.
References
- [1]. Zhang M, Chen H, Huang J, et al. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: inhibition of proliferation and induction of apoptosis. Life Sci. 2005;76:2115–10. [DOI] [PubMed] [Google Scholar]
- [2]. Mao F, Xiao B, Jiang Z, et al. Anticancer effect of lycium barbarum, polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol. 2011;28:121–126. [DOI] [PubMed] [Google Scholar]
- [3]. Miao Y, Xiao B, Jiang Z, et al. Growth inhibition and cell-cycle arrest of human gastric cancer cells by lycium barbarum polysaccharide. Med Oncol. 2010;27:785–790. [DOI] [PubMed] [Google Scholar]
- [4]. Gan L, Hua ZS, Liang YX, et al. Immunomodulation and antitumor activity by a polysaccharide-protein complex from lycium barbarum. Int Immunopharmacol. 2014;4:563–569. [DOI] [PubMed] [Google Scholar]
- [5]. Zhang MH, Wang XY, Wang XM, et al. Inhibition of Lycium barbarumpolysaccharide on transplanted liver cancer in mice. Chin Tradit Herbal Drugs. 2012;43:1142–1146. [Google Scholar]
- [6]. Qian Z, Lv X, Tao W, et al. Composition oflycium barbarumpolysaccharides and their apoptosis-inducing effect on human hepatoma SMMC-7721 cells. Food Nutr Res. 2015;59:28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Jin M, Huang Q, Zhao K, et al. Biological activities and potential health benefit effects of polysaccharides isolated from lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. [DOI] [PubMed] [Google Scholar]
- [8]. Zhang M, Tang X, Wang F, et al. Characterization of lycium barbarum polysaccharide and its effect on human hepatoma cells. Int J Biol Macromol. 2013;61:270–275. [DOI] [PubMed] [Google Scholar]
- [9]. Li W, Li Y, Wang Q, et al. Crude extracts from lycium barbarum suppress srebp-1c expression and prevent diet-induced fatty liver through ampk activation. Biomed Res Int. 2014. DOI: 10.1155/2014/196198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1955;28:350–356. [Google Scholar]
- [11]. Bradford MM. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- [12]. Bitter T, Muir H. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. [DOI] [PubMed] [Google Scholar]
- [13]. Li HS, Sun MN, Xu J, et al. Immunological response in h22 transplanted mice undergoing aconitum coreanum, polysaccharide treatment. Int J Biol Macromol. 2013;55:295–300. [DOI] [PubMed] [Google Scholar]
- [14]. Luo X, Zheng YY, Wen RY, et al. Effects of ceftriaxone induced intestinal dysbacteriosis on lymphocytes in different tissues in mice. Immunobiology. 2016;221:994–1000. [DOI] [PubMed] [Google Scholar]
- [15]. J Y S, Luo X, Zhang XJ, et al. Immunosuppressive activity of pogostone on T cells: blocking proliferation via S phase arrest. Int Immunopharmacol. 2015;26:328–337. [DOI] [PubMed] [Google Scholar]
- [16]. Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of astragalus membranaceus, polysaccharides in h22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287–290. [DOI] [PubMed] [Google Scholar]
- [17]. Sun Y, Liu J, Yue L, et al. One proteoglycan from the fruiting bodies of chroogomphis rutilus (schaeff.: fr.) o.k. miller: purification and structural features. Carbohydr Polym. 2011;86:1381–1384. [Google Scholar]
- [18]. Liang B, Jin M, Liu H. Water-soluble polysaccharide from dried Lycium barbarum fruits: isolation, structural features and antioxidant activity. Carbohydr Polym. 2011;83:1947–1951. [Google Scholar]
- [19]. Sheng X, Yan J, Meng Y, et al. Immunomodulatory effects of Hericium erinaceus derived polysaccharides are mediated by intestinal immunology. Food Funct. 2017;8:1020–1027. [DOI] [PubMed] [Google Scholar]
- [20]. Chen Z, Kwong HTB, Chan SH. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int Immunopharmacol. 2008;8:1663–1671. [DOI] [PubMed] [Google Scholar]
- [21]. Vermes I, Haanen C, Steffens-Nakken H, et al. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 1995;184:39–51. [DOI] [PubMed] [Google Scholar]
- [22]. Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Fang SQ, Wang YT, Wei JX, et al. Beneficial effects of chlorogenic acid on alcohol-induced damage in PC12 cells. Biomed Pharmacother. 2016;79:254–262. [DOI] [PubMed] [Google Scholar]
- [24]. Giannattasio S, Guaragnella N, Arbini AA, et al. Stress-related mitochondrial components and mitochondrial genome as targets of anticancer therapy. Chem Biol Drug Des. 2013;81:102–112. [DOI] [PubMed] [Google Scholar]
- [25]. Dai JQ, Huang YG, He AN. Dihydromethysticin kavalactone induces apoptosis in osteosarcoma cells through modulation of PI3K/Akt pathway, disruption of mitochondrial membrane potential and inducing cell cycle arrest. Int J Clin Exp Patho. 2015;8:4356–4366. [PMC free article] [PubMed] [Google Scholar]
- [26]. Berenbaum MC. Immunosuppression by platinum diamines. Brit J Cancer. 1971;25:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Sánchezsuárez P, Ostroskywegman P, Gallegoshernández F, et al. DNA damage in peripheral blood lymphocytes in patients during combined chemotherapy for breast cancer. Mutat Res-Fund Mol M. 2008;640(1–2):8–15. [DOI] [PubMed] [Google Scholar]
- [28]. Harris J, Sengar D, Stewart T, et al. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976;37:1058–1069. [DOI] [PubMed] [Google Scholar]
- [29]. Ozolins TR. Cyclophosphamide and the teratology society: an awkward marriage. Birth Defects Res B Dev Reprod Toxicol. 2010;89:289–299. [DOI] [PubMed] [Google Scholar]
- [30]. Becker MS, Schmezer P, Breuer R, et al. The traditional Chinese medical compound Rocaglamide protects nonmalignant primary cells from DNA damage-induced toxicity by inhibition of p53 expression. Cell Death Dis. 2014;5:e1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Hunter AM, Lacasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. [DOI] [PubMed] [Google Scholar]
- [32]. Visconti R, Monica RD, Grieco D. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. J Exp Clin Cancer Res. 2016;35:153–doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Levesque JP, Winkler IG. It takes nerves to recover from chemotherapy. Nat Med. 2013;19:669–671. [DOI] [PubMed] [Google Scholar]
- [34]. Bo R, Sun Y, Zhou S, et al. Simple nanoliposomes encapsulating Lycium barbarum polysaccharides as adjuvants improve humoral and cellular immunity in mice. Int J Nanomed. 2017;173:6289–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Peng XM, Huang LJ, Qi CH, et al. Studies on chemistry and immuno-modulating mechanism of a glycoconjugate from Lycium barbarum L. Chin J Chem. 2010;19:1190–1197. [Google Scholar]
- [36]. Zhang XR, Qi CH, Cheng JP, et al. Lycium barbarum polysaccharide LBPF4-OL may be a new Toll-like receptor 4/MD2-MAPK signaling pathway activator and inducer. Int Immunopharmacol. 2014;19:132–141. [DOI] [PubMed] [Google Scholar]
- [37]. Zhu J, Zhao LH, Zhao XP, et al. Lycium barbarum polysaccharides regulate phenotypic and functional maturation of murine dendritic cells. Cell Biol Int. 2007;31:615–619. [DOI] [PubMed] [Google Scholar]
- [38]. Ho YS, Yu MS, Yik SY, et al. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell Mol Neurobiol. 2009;29:1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Teng P, Li Y, Cheng W, et al. Neuroprotective effects of Lycium barbarum polysaccharides in lipopolysaccharide-induced BV2 microglial cells. Mol Med Rep. 2013;7:1977–1981. [DOI] [PubMed] [Google Scholar]
- [40]. Gong H, Shen P, Jin L, et al. Therapeutic effects of Lycium barbarum polysaccharide (LBP) on irradiation or chemotherapy-induced myelosuppressive mice. Chin J Inf Tradit Chin Med. 2005;20:155–162. [DOI] [PubMed] [Google Scholar]
- [41]. Xin YF, Wan LL, Peng JL, et al. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress [J]. Food Chem Toxicol. 2011;49:259–264. [DOI] [PubMed] [Google Scholar]
- [42]. Xiao J, Liong EC, Ching YP, et al. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr Diabetes. 2013;3:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Jia L, Li W, Li J, et al. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci Rep. 2016;6:36209-doi DOI: 10.1038/srep36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Cai H, Liu F, Zuo P, et al. Practical application of antidiabetic efficacy of lycium barbarum polysaccharide in patients with type 2 diabetes. Med Chem. 2015;11:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


